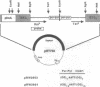
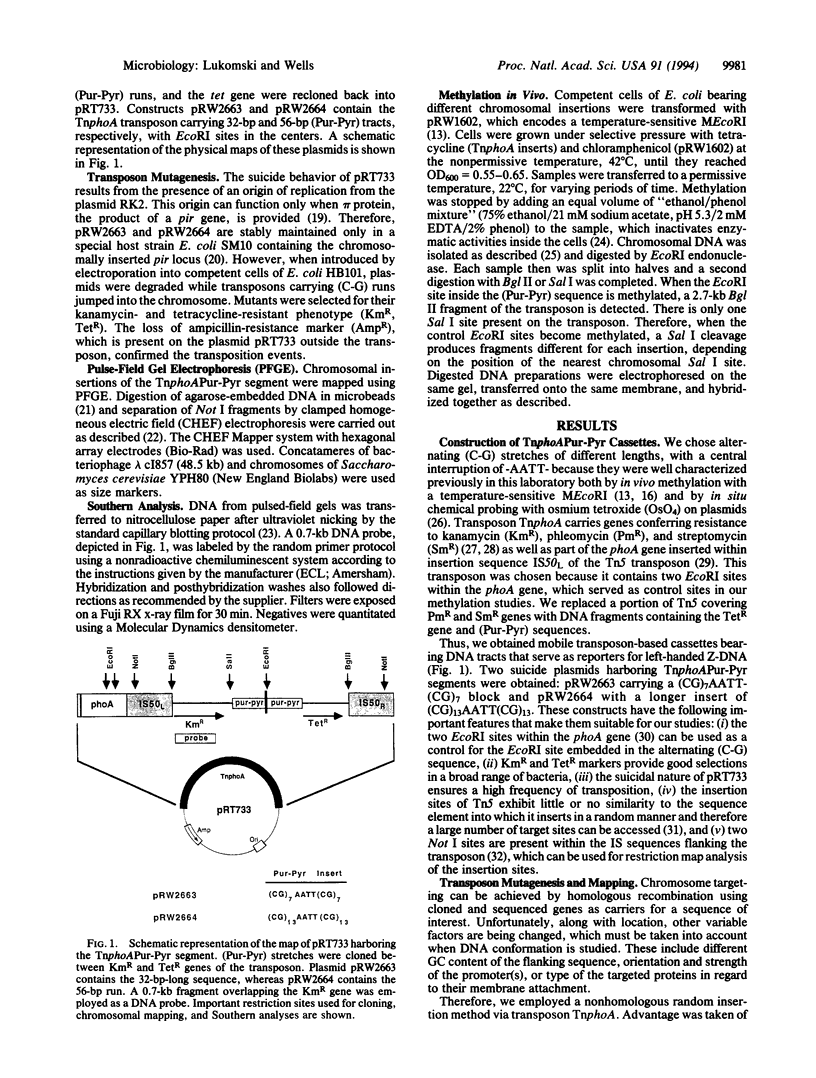
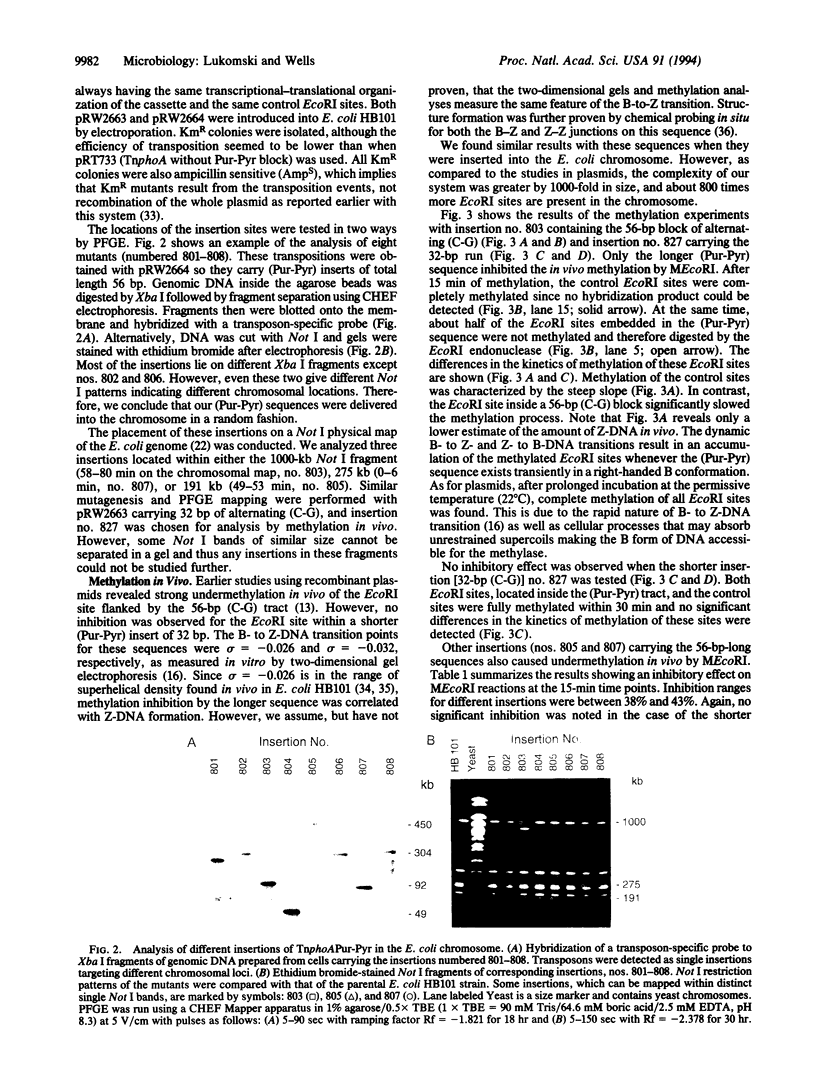
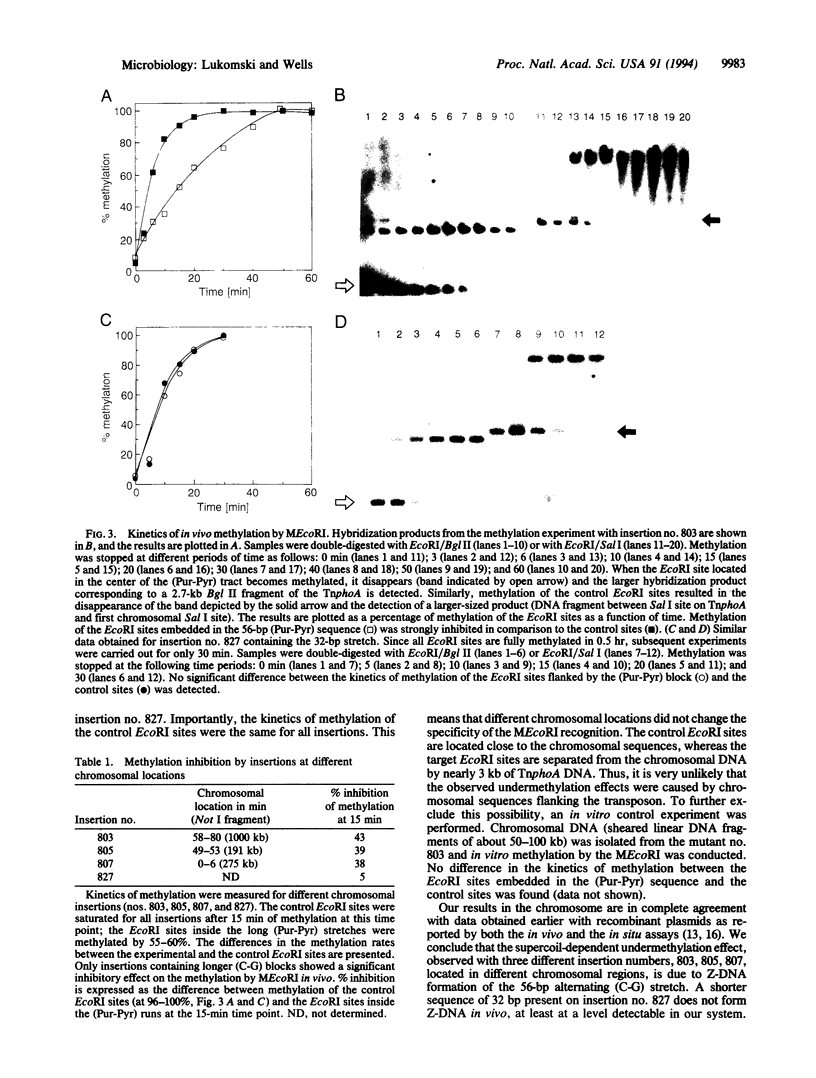
Abstract
A system for studying Z-DNA formation in the Escherichia coli chromosome was developed. Prior investigations in recombinant plasmids showed that alternating (Pur-Pyr) sequences can adopt a left-handed Z-DNA conformation both in vitro and in vivo. We constructed mobile, transposon-based cassettes carrying cloned (Pur-Pyr) sequences containing an EcoRI site in the center. These cassettes were subsequently inserted into different locations in the E. coli chromosome in a random fashion. A number of stable insertions were characterized by Southern analysis and pulsed-field gel electrophoresis mapping. A cloned temperature-sensitive MEcoRI methylase was expressed in trans as the probe to study Z-DNA formation in vivo. In this system, the control EcoRI sites were quickly methylated when cells were placed at the permissive temperature. Strong inhibition of the methylation was observed, however, only for the EcoRI sites embedded in a 56-bp run of (C-G). In contrast, the shorter sequence of 32 bp did not show this behavior. Prior in vitro determinations revealed that the longer tract required less energy to stabilize the Z-helix than the shorter block. We conclude that the observed inhibition of methylation is due to Z-DNA formation in the E. coli chromosome. In vitro, these sequences undergo the B- to Z-DNA transition at a supercoil density of -0.026 for the 56-bp insert and -0.032 for the 32-bp block. Since only the longer (C-G) tract but not the shorter run adopted the left-handed conformation in the chromosome, we propose that these densities establish the boundaries in the different chromosomal loci investigated; these boundaries are in good agreement with the extremes found in plasmids.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arndt-Jovin D. J., Udvardy A., Garner M. M., Ritter S., Jovin T. M. Z-DNA binding and inhibition by GTP of Drosophila topoisomerase II. Biochemistry. 1993 May 11;32(18):4862–4872. doi: 10.1021/bi00069a023. [DOI] [PubMed] [Google Scholar]
- Auerswald E. A., Ludwig G., Schaller H. Structural analysis of Tn5. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):107–113. doi: 10.1101/sqb.1981.045.01.019. [DOI] [PubMed] [Google Scholar]
- Belas R., Erskine D., Flaherty D. Transposon mutagenesis in Proteus mirabilis. J Bacteriol. 1991 Oct;173(19):6289–6293. doi: 10.1128/jb.173.19.6289-6293.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg P. E. Cloning and characterization of the Escherichia coli gene coding for alkaline phosphatase. J Bacteriol. 1981 May;146(2):660–667. doi: 10.1128/jb.146.2.660-667.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi A., Wells R. D., Heintz N. H., Caddle M. S. Sequences near the origin of replication of the DHFR locus of Chinese hamster ovary cells adopt left-handed Z-DNA and triplex structures. J Biol Chem. 1990 Dec 15;265(35):21789–21796. [PubMed] [Google Scholar]
- Blaho J. A., Wells R. D. Left-handed Z-DNA binding by the recA protein of Escherichia coli. J Biol Chem. 1987 May 5;262(13):6082–6088. [PubMed] [Google Scholar]
- Bliska J. B., Cozzarelli N. R. Use of site-specific recombination as a probe of DNA structure and metabolism in vivo. J Mol Biol. 1987 Mar 20;194(2):205–218. doi: 10.1016/0022-2836(87)90369-x. [DOI] [PubMed] [Google Scholar]
- Borowiec J. A., Gralla J. D. All three elements of the lac ps promoter mediate its transcriptional response to DNA supercoiling. J Mol Biol. 1987 May 5;195(1):89–97. doi: 10.1016/0022-2836(87)90329-9. [DOI] [PubMed] [Google Scholar]
- Collis C. M., Hall R. M. Identification of a Tn5 determinant conferring resistance to phleomycins, bleomycins, and tallysomycins. Plasmid. 1985 Sep;14(2):143–151. doi: 10.1016/0147-619x(85)90074-5. [DOI] [PubMed] [Google Scholar]
- Drlica K., Franco R. J., Steck T. R. Rifampin and rpoB mutations can alter DNA supercoiling in Escherichia coli. J Bacteriol. 1988 Oct;170(10):4983–4985. doi: 10.1128/jb.170.10.4983-4985.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund A. M., Bichara M., Fuchs R. P. Z-DNA-forming sequences are spontaneous deletion hot spots. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7465–7469. doi: 10.1073/pnas.86.19.7465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glikin G. C., Jovin T. M., Arndt-Jovin D. J. Interactions of Drosophila DNA topoisomerase II with left-handed Z-DNA in supercoiled minicircles. Nucleic Acids Res. 1991 Dec;19(25):7139–7144. doi: 10.1093/nar/19.25.7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski A., Blaho J. A., Larson J. E., Shimizu M., Wells R. D. Tetracycline promoter mutations decrease non-B DNA structural transitions, negative linking differences and deletions in recombinant plasmids in Escherichia coli. J Mol Biol. 1989 Jun 5;207(3):513–526. doi: 10.1016/0022-2836(89)90461-0. [DOI] [PubMed] [Google Scholar]
- Jaworski A., Higgins N. P., Wells R. D., Zacharias W. Topoisomerase mutants and physiological conditions control supercoiling and Z-DNA formation in vivo. J Biol Chem. 1991 Feb 5;266(4):2576–2581. [PubMed] [Google Scholar]
- Jaworski A., Hsieh W. T., Blaho J. A., Larson J. E., Wells R. D. Left-handed DNA in vivo. Science. 1987 Nov 6;238(4828):773–777. doi: 10.1126/science.3313728. [DOI] [PubMed] [Google Scholar]
- Jorgensen R. A., Rothstein S. J., Reznikoff W. S. A restriction enzyme cleavage map of Tn5 and location of a region encoding neomycin resistance. Mol Gen Genet. 1979;177(1):65–72. doi: 10.1007/BF00267254. [DOI] [PubMed] [Google Scholar]
- Kolter R., Inuzuka M., Helinski D. R. Trans-complementation-dependent replication of a low molecular weight origin fragment from plasmid R6K. Cell. 1978 Dec;15(4):1199–1208. doi: 10.1016/0092-8674(78)90046-6. [DOI] [PubMed] [Google Scholar]
- Koob M., Szybalski W. Cleaving yeast and Escherichia coli genomes at a single site. Science. 1990 Oct 12;250(4978):271–273. doi: 10.1126/science.2218529. [DOI] [PubMed] [Google Scholar]
- Lafer E. M., Sousa R. J., Rich A. Z-DNA-binding proteins in Escherichia coli purification, generation of monoclonal antibodies and gene isolation. J Mol Biol. 1988 Sep 20;203(2):511–516. doi: 10.1016/0022-2836(88)90017-4. [DOI] [PubMed] [Google Scholar]
- Lodge J. K., Berg D. E. Mutations that affect Tn5 insertion into pBR322: importance of local DNA supercoiling. J Bacteriol. 1990 Oct;172(10):5956–5960. doi: 10.1128/jb.172.10.5956-5960.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoil C., Beckwith J. TnphoA: a transposon probe for protein export signals. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8129–8133. doi: 10.1073/pnas.82.23.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazodier P., Cossart P., Giraud E., Gasser F. Completion of the nucleotide sequence of the central region of Tn5 confirms the presence of three resistance genes. Nucleic Acids Res. 1985 Jan 11;13(1):195–205. doi: 10.1093/nar/13.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W. G., Simons R. W. Chromosomal supercoiling in Escherichia coli. Mol Microbiol. 1993 Nov;10(3):675–684. doi: 10.1111/j.1365-2958.1993.tb00939.x. [DOI] [PubMed] [Google Scholar]
- Pavitt G. D., Higgins C. F. Chromosomal domains of supercoiling in Salmonella typhimurium. Mol Microbiol. 1993 Nov;10(3):685–696. doi: 10.1111/j.1365-2958.1993.tb00940.x. [DOI] [PubMed] [Google Scholar]
- Peck L. J., Wang J. C. Transcriptional block caused by a negative supercoiling induced structural change in an alternating CG sequence. Cell. 1985 Jan;40(1):129–137. doi: 10.1016/0092-8674(85)90316-2. [DOI] [PubMed] [Google Scholar]
- Pestov D. G., Dayn A., Siyanova EYu, George D. L., Mirkin S. M. H-DNA and Z-DNA in the mouse c-Ki-ras promoter. Nucleic Acids Res. 1991 Dec 11;19(23):6527–6532. doi: 10.1093/nar/19.23.6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmouni A. R., Wells R. D. Stabilization of Z DNA in vivo by localized supercoiling. Science. 1989 Oct 20;246(4928):358–363. doi: 10.1126/science.2678475. [DOI] [PubMed] [Google Scholar]
- Rahmouni A. R., Wells R. D. Stabilization of Z DNA in vivo by localized supercoiling. Science. 1989 Oct 20;246(4928):358–363. doi: 10.1126/science.2678475. [DOI] [PubMed] [Google Scholar]
- Schroth G. P., Chou P. J., Ho P. S. Mapping Z-DNA in the human genome. Computer-aided mapping reveals a nonrandom distribution of potential Z-DNA-forming sequences in human genes. J Biol Chem. 1992 Jun 15;267(17):11846–11855. [PubMed] [Google Scholar]
- Sinden R. R., Pettijohn D. E. Chromosomes in living Escherichia coli cells are segregated into domains of supercoiling. Proc Natl Acad Sci U S A. 1981 Jan;78(1):224–228. doi: 10.1073/pnas.78.1.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. L., Econome J. G., Schutt A., Klco S., Cantor C. R. A physical map of the Escherichia coli K12 genome. Science. 1987 Jun 12;236(4807):1448–1453. doi: 10.1126/science.3296194. [DOI] [PubMed] [Google Scholar]
- Taylor R. K., Manoil C., Mekalanos J. J. Broad-host-range vectors for delivery of TnphoA: use in genetic analysis of secreted virulence determinants of Vibrio cholerae. J Bacteriol. 1989 Apr;171(4):1870–1878. doi: 10.1128/jb.171.4.1870-1878.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifonov E. N., Konopka A. K., Jovin T. M. Unusual frequencies of certain alternating purine-pyrimidine runs in natural DNA sequences: relation to Z-DNA. FEBS Lett. 1985 Jun 3;185(1):197–202. doi: 10.1016/0014-5793(85)80769-9. [DOI] [PubMed] [Google Scholar]
- Vardimon L., Rich A. In Z-DNA the sequence G-C-G-C is neither methylated by Hha I methyltransferase nor cleaved by Hha I restriction endonuclease. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3268–3272. doi: 10.1073/pnas.81.11.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittig B., Dorbic T., Rich A. The level of Z-DNA in metabolically active, permeabilized mammalian cell nuclei is regulated by torsional strain. J Cell Biol. 1989 Mar;108(3):755–764. doi: 10.1083/jcb.108.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittig B., Wölfl S., Dorbic T., Vahrson W., Rich A. Transcription of human c-myc in permeabilized nuclei is associated with formation of Z-DNA in three discrete regions of the gene. EMBO J. 1992 Dec;11(12):4653–4663. doi: 10.1002/j.1460-2075.1992.tb05567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worcel A., Burgi E. On the structure of the folded chromosome of Escherichia coli. J Mol Biol. 1972 Nov 14;71(2):127–147. doi: 10.1016/0022-2836(72)90342-7. [DOI] [PubMed] [Google Scholar]
- Zacharias W., Jaworski A., Larson J. E., Wells R. D. The B- to Z-DNA equilibrium in vivo is perturbed by biological processes. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7069–7073. doi: 10.1073/pnas.85.19.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharias W., Larson J. E., Kilpatrick M. W., Wells R. D. HhaI methylase and restriction endonuclease as probes for B to Z DNA conformational changes in d(GCGC) sequences. Nucleic Acids Res. 1984 Oct 25;12(20):7677–7692. doi: 10.1093/nar/12.20.7677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Lockshin C., Herbert A., Winter E., Rich A. Zuotin, a putative Z-DNA binding protein in Saccharomyces cerevisiae. EMBO J. 1992 Oct;11(10):3787–3796. doi: 10.1002/j.1460-2075.1992.tb05464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng G. X., Kochel T., Hoepfner R. W., Timmons S. E., Sinden R. R. Torsionally tuned cruciform and Z-DNA probes for measuring unrestrained supercoiling at specific sites in DNA of living cells. J Mol Biol. 1991 Sep 5;221(1):107–122. doi: 10.1016/0022-2836(91)80208-c. [DOI] [PubMed] [Google Scholar]