Abstract
Hydrogels, due to their unique biocompatibility, flexible methods of synthesis, range of constituents, and desirable physical characteristics, have been the material of choice for many applications in regenerative medicine. They can serve as scaffolds that provide structural integrity to tissue constructs, control drug and protein delivery to tissues and cultures, and serve as adhesives or barriers between tissue and material surfaces. In this work, the properties of hydrogels that are important for tissue engineering applications and the inherent material design constraints and challenges are discussed. Recent research involving several different hydrogels polymerized from a variety of synthetic and natural monomers using typical and novel synthetic methods are highlighted. Finally, special attention is given to the microfabrication techniques that are currently resulting in important advances in the field.
1. Introduction
Since the seminal tissue engineering work by Vacanti et al.[1] in the late 1980s, many important advancements have brought this field ever closer to achieving its potential as a life-saving and life-improving option for countless patients, where truly suitable medical treatments do not yet exist. The means by which tissue engineering or regenerative medicine is beginning to reach its potential are through the delivery of cell and tissue constructs to the body and the direction and therapeutic assistance of innate healing responses. Ultimately, replacement or repaired tissues should be indistinguishable from normal, healthy tissues in structure and function.
The need for success in this field is tragically abundant. It is reported that while about 77 people receive transplants in the US each day, nearly 20 die because of shortages.[2] Currently, there are over 98 000 patients waiting on lists, with an average time to transplant of more than three years.[2] The situation is understandably worse in parts of the undeveloped world where the availability of surgical services is often lacking, the infrastructure for rapidly linking patients to compatible donors may be nonexistent, and the necessary follow-up care and access to required lifelong therapeutics are wholly unrealistic.
Regenerative medicine holds promise not only as a means to compensate for donor shortages, but also as a means to improve the standard of care. In many cases, transplants or medical prosthetics are currently available, but they only offer a partial solution in comparison to the healthy, undamaged physiological state. Therefore it is important for researchers in regenerative medicine to stay mindful of current medical options and continually attempt to improve upon them. In this regard, the physical building blocks utilized in tissue engineering must be as inherently safe and as similar to nature as is achievable.
In terms of material requirements in regenerative medicine, such as those needed for tissue scaffolds or as therapeutic delivery systems, hydrogels have long received attention because of their innate structural and compositional similarities to the extracellular matrix and their extensive framework for cellular proliferation and survival. Many hydrogel types with vastly different chemical and physical properties have been developed over the last several decades from a wide variety of chemical building blocks and using an array of synthetic techniques. This expanse of hydrogel knowledge allows for scaffold properties, such as cellular attachment, molecular response, structural integrity, biodegradability, biocompatibility, and solute transport to be carefully engineered to meet the proliferative demands of the construct.
2. Network Structure and Properties
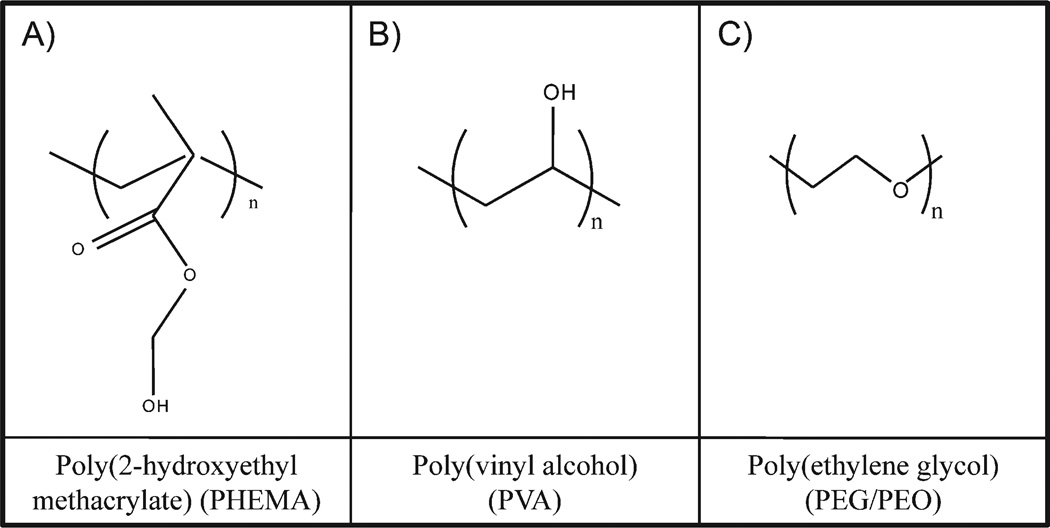
Hydrogels are three-dimensional networks formed from hydrophilic homopolymers, copolymers, or macromers (preformed macromolecular chains) crosslinked to form insoluble polymer matrices. These polymers, generally used above their glass transition temperature (Tg), are typically soft and elastic due to their thermodynamic compatibility with water and have found use in many biomedical applications.[3] Synthetic monomers used in tissue engineering include, among others, poly(ethylene glycol) (PEG), poly(vinyl alcohol) (PVA), and polyacrylates such as poly(2-hydroxyethyl methacrylate) (PHEMA). Biological hydrogels have been formed from agarose, alginate, chitosan, hyaluronan, fibrin, and collagen, as well as many others.[4,5]
2.1. Physical Structure
In general, the crosslinked structure of hydrogels is characterized by junctions or tie points, which may be formed from strong chemical linkages (such as covalent and ionic bonds), permanent or temporary physical entanglements, microcrystallite formation, and weak interactions (such as hydrogen bonds).[6] For crosslinking and network formation, several options for preparation have been developed. For example, homopolymers and their combinations may be chemically crosslinked with glutaraldehyde to form PVA networks or ethylene glycol dimethacrylate (EGDMA) to form poly(acrylic acid) (PAA) hydrogels. Polymers can be prepared and combined in the form of blends, copolymers, and interpenetrating networks (IPNs). Hydrogels based on blends, for example, have been prepared via a freeze–thaw process where the uncrosslinked polymer is repeatedly frozen and thawed in cycles to form a crosslinked network.[7,8] IPNs may be synthesized by sequentially polymerizing and crosslinking a monomer in the presence of an already crosslinked polymer network or, simultaneously, if two polymer chains are polymerized by significantly different processes. Ultimate network morphology of a hydrogel can be amorphous, semicrystalline, H-bonded, supramolecular, or consisting of hydrocolloidal aggregates.[6] The chains comprising the network may be based on natural, synthetic, or hybrid combinations of these materials. The physical structure and characteristics of hydrogels depend upon starting monomers and macromers, synthesis and fabrication methods, solvent conditions, degradation, and mechanical loading history.
In terms of ionic charge, hydrogels can be neutral, cationic, anionic, or ampholytic as determined by pendant groups incorporated into the gel backbone. Much of the success with synthetic hydrogels in tissue engineering is due to work with PHEMA, a neutrally charged gel. Molecular structures of some neutrally charged synthetic repeat units that are typically used in tissue engineering are shown in Figure 1.
Figure 1.
Neutrally charged synthetic monomers typically used in tissue engineering.
When providing a substrate for cellular proliferation, synthetic hydrophilic scaffolds using charged gels would tend to facilitate better cellular attachment compared to uncharged gels. Furthermore, more hydrogels used in regenerative medicine are being synthesized from natural macromers, which are typically ionic or ionizable. As this trend continues, consideration must be given to the inherent differences in solute transport and cell and protein adherence compared to neutral gels.[9]
Several molecular parameters can be used to quantitatively describe the network structure of hydrogels. These include υ2,s, the polymer volume fraction in the swollen state (the amount of polymer within the gel); M̅C, the average molecular weight between crosslinks; and ξ, the related measure of distance between crosslinks (i.e., mesh size). The two prominent theoretical treatments used to describe the network structure of hydrogels and to determine these parameters are derived from equilibrium swelling theory and rubber elasticity theory.[10]
2.2. Equilibrium Swelling Theory and Network Characteristics
With neutral gels, Flory–Rehner theory[11] is useful for analysis. This theory describes swelling by stating that crosslinked polymers will reach equilibrium in a fluidic environment by the thermodynamic force to reduce entropy via mixing as opposed to by the elastic or retractile force of the polymer chains themselves to contract. Analytically, this is shown with Gibbs free energy as indicated in Equation 1 below.
| (1) |
With ionic gels, the situation is further complicated by the addition of a term to account for the total free energy contribution due to the ionic properties of the network. This modification is shown in Equation 2.
| (2) |
In these equations, the mixing term, ΔGmixing, is a quantitative description of the compatibility between the polymer and solvent, water in the case of hydrogels, and is often expressed as the polymer–solvent interaction parameter, χ1. Differentiation with respect to the moles of solvent molecules at constant temperature and pressure results in expressions of Equation 1 and 2 in terms of chemical potentials (not shown). At equilibrium conditions, the net chemical potential between the solvent within the gel and the surrounding solution is zero. This zero net chemical potential equates the elastic and mixing potentials. Flory–Rehner theory leads to an expression for molecular weight between crosslinks, M̅C, if the hydrogel is prepared in the absence of a solvent. This expression is shown in Equation 3.
Peppas and Merrill[12] modified this theory, as shown in Equation 4, for hydrogels prepared in the presence of a solvent by considering changes in the elastic potential due to the solvent.
| (3) |
| (4) |
Here, M̅N is the average molecular weight of the polymer chains prepared in the absence of a crosslinker. This implies that M̅N should be the value of the original homo- or copolymer average molecular weight when that polymer is crosslinked with an added crosslinking agent. Also, V1 is the molar volume of the water and υ is the specific volume of the water. The terms υ2,r and υ2,s denote the polymer volume fraction in the “relaxed” and fully swollen states, respectively. These are denoted by the subscripts r and s shown in these terms. The relaxed state refers to state of the polymer immediately after crosslinking, but before any additional swelling occurs. It should be noted that if the gel is prepared in the absence of a solvent, the polymer volume fraction in the relaxed state becomes one (υ2,r=1), which causes Equation 4 to simplify to Equation 3. The swollen state fraction, υ2,s, in both equations refers to the polymer volume fraction when the hydrogel is fully swollen in the presence of pure water.
For ionic gels, Brannon-Peppas and Peppas[9] derived a more complex equation that results in two separate, but equivalent expressions for anionic and cationic gels, as shown in Equations 5 and 6, respectively.[9] Utilization of these equations for average molecular weight between crosslinks additionally requires ionic strength, I, and dissociation constants, Ka and Kb.
| (5) |
| (6) |
For solute transport, an important measure for determining the maximum size of solutes that can diffuse in a gel is porosity, which can be described by the mesh size, ξ (i.e., correlation length), and quantifies the average linear distance between crosslinks. If the root-mean-square end-to-end distance of the polymer chains between crosslinks (free of long-range interactions), which is called the unperturbed distance , is known, then Equation 7 can be utilized to determine mesh size.[13] From Equation 7, the unperturbed distance is the ratio of mesh size to the extension ratio. The extension ratio, α, can be determined from the swollen polymer volume fraction, υ2,s, as shown in Equation 8.
| (7) |
| (8) |
To predict mesh size without the unperturbed distance, Equation 9 may be used if the following parameters can be determined: bond length of the polymer backbone, l, which is often 1.54 Å; the characteristic ratio, CN, (ratio of the square of the unperturbed distance to the square of the random flight end-to-end distance), which is available for many polymers; the average molecular weight between crosslinks, M̅C; the swollen polymer volume fraction, υ2,s; and Mr, or the molecular weight of the repeat units.[14,15]
| (9) |
In hydrogel applications in regenerative medicine, the mesh size may require determination under realistic conditions, such as in solution containing salts, ions, nutrients, etc. Therefore, it must be noted here that when we determine the mesh size in real systems, the swollen polymer volume fraction, υ2,s, as used in Equation 8 must be determined in the physiological solution in which the gel is swollen, not pure water.
2.3. Rubber Elasticity
Hydrogels under mechanical stress can exhibit a range of responses from rapid, elastic recovery following an applied stress or strain to a time-dependent recovery approaching viscous behavior. At temperatures below the Tg, a transition away from the rubbery state would tend to drive the behavior of the gel toward viscoelasticity due to a slow rearrangement of polymer segments under deformation. In this regime, mechanical considerations such as creep, stress relaxation, and dynamic loading may become important.[16] However since hydrogel scaffolds for tissue engineering are typically water-swollen to maintain proliferating cells, the aqueous penetrant tends to plasticize the gel and induce a reduction in the polymer’s glass transition temperature. This would typically decrease Tg to well below the environmental temperature of 37 °C as required for human cell cultures and thus subsequently establishes a transition from the glassy state to the rubbery, elastic regime.
Peppas and Merrill[12] modified the original theories for polymer elasticity developed by Flory,[17] again to account for hydrogels prepared in the presence of a solvent. Shown in Equation 10, τ is the applied stress as a function of elongation, where ρ is the polymer density, R is the universal gas constant, and T is absolute temperature. This offers an alternative means to determine the average molecular weight between crosslinks, M̅C, by measurement of the applied stress, τ, using a test specimen. As with mesh size calculations, the swollen polymer volume fraction refers to the solution in which the gel is swollen.
| (10) |
Since both equilibrium swelling theory and rubber elasticity theory rely on experimentally determined gel properties to determine average molecular weight between crosslinks, these models are useful for noncovalently crosslinked hydrogels as well. Hydrogels used in regenerative medicine may be formed by physical entanglements, microcrystallites, or hydrogen-bonded structures. In a general way, these theories may be extended to systems of this type by treating the junctions or tie points that cause gel formation as physical crosslinks with equivalent behavior as the covalent crosslinks intended with the above theories. Typically, the same equations are used, but the M̅C term is replaced by the terms M̅J or M̅e to indicate the gel is not covalently crosslinked. Here, M̅J is the number average molecular weight between junctions, and M̅e is the number average molecular weight between entanglements. Figure 2 illustrates the structural similarities between physical and chemical crosslinks in gel networks. It must be noted however that gels containing extensive crystallites represent a significant deviation from the rubber elasticity assumptions used to derive the theories.
Figure 2.
Network structure of a hydrogel showing junctions, entanglements, and covalent linkages. Not all tie points (crosslink) types shown here are necessarily present in a given hydrogel.
2.4. Solute Transport
Effective solute transport is one of the most critical design parameters for hydrogels in regenerative medicine. Mass transport parameters determine how nutrients, gasses, waste products, and bioactive agents, such as growth factors to stimulate natural tissue growth,[18] are exchanged within scaffolds or are delivered by the gel. Except in hydrogels with very large micropores or forced flow conditions, convection usually does not play a significant role in the movement of solutes through hydrogel matrices. Diffusion alone is regarded as the driving transport phenomenon. Analysis of drug and protein diffusion by Ende et al.[19] in ionic gels revealed that mesh size and environmental conditions, including pH and temperature, are all critically important in solute diffusion. They further concluded that hydrogels might be tailor-made for the release of a specific drug, protein, or peptide. Other studies showing the effect of pH on drug transport from ionized hydrogels done by Brannon-Peppas and Peppas[20] showed that pH-dependent hydrogels could be prepared to exhibit zero-order or near zero-order release, an important benchmark for many drug delivery systems.
With biological systems, where both the polymer and the solute are frequently ionized, the interactions between the polymer and solute themselves become an important factor in determining transport behavior. Collins and Ramirez[21] studied this relationship directly and showed that polymer–solute interactions tend to decrease transport of solute molecules. Further work by Gudeman and Peppas[22] examined these interactions in well-characterized IPNs of PVA and PAA by varying the ionic content in the membrane (by varying the amount of PAA) and then testing transport at pH values above and below the pKa of the ionic component. This work further confirmed the effect of polymer–solute interactions and demonstrated that permeation is controlled by size exclusion.
Because virtually all solute transport models involving hydrogels are based primarily on diffusion, computational analysis utilizes Fick’s law as shown in the general vector form below.[23,24] In Equation 11, ci is the concentration of the species i and Dig is the concentration-dependant diffusion coefficient of species i in the gel.
| (11) |
Since water-swollen hydrogels would typically be in the rubbery regime due to a reduction in glass transition temperature, Fickian diffusion is applicable for most applications in tissue engineering provided that gel is principally amorphous. This analysis is insufficient if the gel is significantly heterogeneous with regard to structural discontinuities (such as localized crystallization or phase separations), nonswollen glassy regions, interpenetrating structures, or composite formations (such as fibrous inclusions).
Some of the earliest work in solute transport through hydrogels that compared experimental observation to theoretical prediction was done by Renkin,[25] who studied solute diffusion through porous cellulose membranes based on Fick’s law for a diffusion rate in one dimension, as shown in Equation 12.
| (12) |
Here is the solute diffusion rate for a given species i, D is the diffusion coefficient, A is the apparent diffusion area, and is the concentration gradient across the membrane. In Renkin’s diffusion experiments, the rate of diffusion for a variety of solutes was measured through inert porous membranes. His experimental results were found to be in close agreement with the theory proposed earlier by Pappenheimer.[26] This was one the first demonstrations of solute diffusion as a function of pore and solute size.
Early theoretical models that have been applied in the design of modern tissue engineering scaffolds were based on transport in microporous systems (with pore radii of r≤1 µm). Anderson and Quinn[27] studied the hydrodynamic equations governing transport to account for Brownian motion and steric restrictions. They showed that a one-dimensional diffusion–convection analysis could be used for such systems, and they developed a series of equations to account for the effect of the pore wall itself on the solute–solvent drag.
Peppas and Reinhart[28] developed a model based on free volume theory for a three-component system of water, solute, and polymer. This model predicted the dependence of the solute diffusion coefficient on solute size, mesh size, degree of swelling, and other structural characteristics of the hydrogels.
| (13) |
Here, DSM and DSW are the diffusion coefficients of the solutes in the membrane and water respectively, and this ratio is referred to as the normalized diffusion coefficient. The other terms in this equation not previously defined are k1 and k2, which are structural parameters of the polymer–water complex; , which is the average critical molecular weight between crosslinks at which diffusion is precluded; rs, which is the Stokes hydrodynamic radius of the solute; and Qm, which is the degree of swelling of the membrane. This theory was developed for diffusion in highly swollen membranes. Well-characterized, amorphous PVA membranes[29] later validated this theory with experimental data.
Prausnitz and collaborators[30] used Monte Carlo simulations to develop a modified size exclusion theory based on the statistical distribution of chains in the network. This theory, however, does not consider ionic interactions or the effects of side groups on the structure, but instead focuses on chains in a large region of free space. The intention of their theory was to provide a general understanding of partially ionized polyelectrolytes so that other theories could be built upon it.
While it is impossible to span the full array of transport research involving hydrogels with the potential for scaffold design applicability, the reader is referred to the sources already mentioned and to the following works: Reinhart and Peppas[29] for studies on the structure, characteristics, and solute diffusion behavior with PVA; Ende et al.[31] for work on the characteristics of PAA showing pH-dependent solute diffusion; and Gudeman[32] who showed solute diffusion through PVA/PAA membranes as a function of ionic strength and pH of the swelling agent. Reviews by Amsden,[33] Muhr and Blanshard,[34] and Meadows and Peppas[35] have compared and characterized many of the models developed for diffusion coefficients by gel type and the underlying modeling techniques utilized.
3. Applications of Hydrogels in Tissue Engineering
There are several applications in regenerative medicine where hydrogels have found utility. Langer and Vacanti[36] were among the first to elucidate the basic techniques used in tissue engineering to repair damaged tissues, as well as the ways polymer gels are utilized in these techniques. To date, hydrogels in regenerative medicine have been used as scaffolds to provide structural integrity and bulk for cellular organization and morphogenic guidance, to serve as tissue barriers and bioadhesives, to act as drug depots, to deliver bioactive agents that encourage the natural reparative process, and to encapsulate and deliver cells.
3.1. Hydrogels as Scaffold Materials
As previously mentioned, hydrogels are an attractive scaffolding material because their mechanical properties can be tailored to mimic those of natural tissues. As scaffolds, hydrogels are used to provide bulk and mechanical constitution to a tissue construct, whether cells are adhered to or suspended within the 3D gel framework. When cellular adhesion directly to the gel is favored over suspension within the scaffold, incorporation of various peptide domains into the hydrogel structure can dramatically increase the tendency for cellular attachment. A particularly successful strategy to mediate cellular attachment is the inclusion of the RGD adhesion peptide sequence (arginine–glycine–aspartic acid). Cells that have been shown to favorably bind to RGD include fibroblasts, endothelial cells (ECs), smooth muscle cells (SMCs), osteoblasts, and chondrocytes. RGD in hydrogels, which can be incorporated on the surface or throughout the bulk of the gel, has shown enhanced cellular migration, proliferation, growth, and organization in tissue regeneration applications.[37,38]
The fundamental obligation of a tissue scaffold is to maintain cellular proliferation and desired cellular distribution throughout the expected service life of the construct. In many cases, the life of the scaffold would be until degradation is complete. Therefore a critical design consideration for hydrogels in regenerative medicine is the transition in functional dependence between the scaffold and the emergent tissue during scaffold biodegradation and the healing process. Further, the importance of scaffold degradation in tissue cultures has been demonstrated by examining cellular viability in nondegradable scaffolds. For example, poly(ethylene glycol)-dimethacrylate (PEGDMA) and PEG have been photopolymerized to form hydrogel networks with encapsulated bovine and ovine chondrocytes for cartilage regeneration.[39,40] After photopolymerization, cells within the scaffold remained viable and evenly dispersed, but due to the nondegradable nature of these scaffolds, cell counts tended to decrease significantly over time.
Biodegradable poly(propylene fumarate-co-ethylene glycol) (P(PF-co-EG)) has been photopolymerized with implanted endothelial cells to form hydrogel scaffolds for vascular cell growth.[41] In these studies, it was shown that the cells were distributed throughout the hydrogel and were actively proliferating. Mann et al.[42] utilized PEG-diacrylate derivatives with grafted RGD-peptides to fabricate photopolymerized hydrogels as scaffolds for vascular smooth muscle cells. These cells remained viable in scaffolds, proliferated, and produced matrix proteins. Cells were shown to spread and migrate in proteolytically degradable scaffolds, but they were spherical and grouped in clusters in nondegradable hydrogels. It was shown that in proteolytically degradable hydrogels, cells had an increased rate of proliferation and extracellular matrix production over cells in nondegradable PEG-diacrylate scaffolds. Although much success has been achieved with the use of hydrogel scaffolds for tissue regeneration and replacement, these gels should generally be biodegradable to maximize the ability of scaffolds to foster proliferating replacement tissues.[43]
3.2. Hydrogels as Barriers
To improve the healing response following tissue injury, hydrogels have been used as barriers in order to prevent restenosis or thrombosis due to post-operative adhesion formation.[44–47] It has been shown that forming a thin hydrogel layer intravascularly via interfacial photopolymerization will prevent restenosis by reducing intimal thickening and thrombosis.[44,45]
The thin hydrogel layer is able to reduce intimal thickening because it provides a barrier to prevent platelets, coagulation factors, and plasma proteins from contacting the vascular wall. The contact of these factors to vessel walls stimulates smooth muscle cell proliferation, migration, and matrix synthesis events that lead to restenosis. Hydrogel barriers have additionally been used to prevent post-operative adhesion formation. In one example, poly(ethylene glycol-co-lactic acid) diacrylate hydrogels were formed by bulk photopolymerization on intraperitoneal surfaces. These hydrogel barriers functioned to prevent fibrin deposition and fibroblast attachment at the tissue surface.[46,47] In addition, biodegradable poly(ethylene glycol-co-lactic acid) diacrylate macromers were coated onto tissue surfaces to form barriers, which functioned to resist protein adsorption and diffusion as well as minimize cell adhesion.
3.3. Hydrogels with Drug Delivery Capabilities
Hydrogels are often used as localized drug depots because they are hydrophilic, biocompatible, and their drug release rates can be controlled[48–50] and triggered intelligently by interactions with biomolecular stimuli.[51–53] Macromolecular drugs, such as proteins or oligonucleotides that are hydrophilic, are inherently compatible with hydrogels. By controlling the degree of swelling, crosslinking density, and degradation rate, delivery kinetics can be engineered according to the desired drug release schedule. Furthermore, photopolymerized hydrogels are especially attractive for localized drug delivery because they can adhere and conform to targeted tissue when formed in situ. Drug delivery aspects in hydrogels may be used to function simultaneously with the barrier role of hydrogels to deliver therapeutic agents locally while preventing post-operative adhesion formation.
Biodegradable photopolymerized hydrogels have been formed on intraperitoneal tissues to locally release tissue plasminogen activator, urokinase plasminogen activator, and ancrod.[43,46] These systems show significant reductions in adhesion formation compared to using intraperitoneal injections or hydrogel barriers alone.
Additionally, hydrogels formed on the inner surface of blood vessels via interfacial photopolymerization have been utilized for intravascular drug delivery.[43] These gels can be formed in bilayers, where the innermost (luminal) layer is less permeable than the outer (intimal) layer near the vessel wall. A lower molecular weight polymer precursor is used to form the luminal layer, making it less permeable. The function of this bilayer hydrogel structure is to enhance the delivery of released proteins into the arterial media. Layered matrix devices are also useful in releasing drugs with non-uniform concentration profiles, where varying the thickness and solute diffusion coefficient of each layer allows for non-uniformity of therapeutic release.[50] In addition, different drug concentrations can be entrapped into each layer during synthesis of a multilaminated matrix device to achieve optimal release behavior. Recent work by Ladet et al.[54] showed that layered, multimembrane hydrogels could be produced from alginate and chitosan using start–stop, interrupted gelation techniques. These so-called ‘onion’ structures (Fig. 3) that were formed may hold promise in tissue engineering because various layers for different drug concentrations, cellular encapsulation, bioadhesives, and barriers could be incorporated sequentially.
Figure 3.
Multimembrane gels: a) Macroscopic vessel. b) Microscopic capsule. c) Macroscopic onionlike structure section. Reproduced with permission from [54]. Copyright 2008 Nature Publishing Group.
3.4. Hydrogels for Cell Encapsulation
Cell transplantation can be achieved with hydrogels because they can provide immunoisolation while still allowing oxygen, nutrients, and metabolic products to diffuse easily into the hydrogel. For the development of a bio-artificial endocrine pancreas, photopolymerized PEG diacrylate (PEGDA) hydrogels have been fabricated to transplant islets of Langerhans.[55] In these studies, islet cells were suspended in a photopolymerizable PEGDA prepolymer solution, and the solution was used to formulate PEG-based microspheres that entrapped the islets. The first formulation of these microspheres provided sufficient immunoisolation, however the diffusion of nutrients to the entrapped cells was limited. The next formulation included a reduction in thickness of the interfacially photopolymerized hydrogels in order to increase the diffusion of nutrients to the encapsulated islets. By reducing thickness, encapsulated islets remained viable for prolonged periods and the hydrogel retained its immunoisolation function.[55]
4. Design Considerations
The extracellular matrix (ECM), the collective interstitial space and basement membrane, provides the mechanical framework for natural tissues. This is one of the most important guides for scaffold designs and accordingly has been the ideal model for many material pursuits in tissue engineering. The ECM itself is a hydrophilic microscale 3D matrix with two main solid structures: collagen fibers and proteoglycan filaments. The collagen fibers are formed as bundles and extend through interstitium, providing durability and tensile strength for the surrounding tissue. Proteoglycan filaments are coiled structures made from protein and hyaluronic acid (HA). Together with the entrapped interstitial fluid, which resembles plasma, but at a lower protein concentration, ECM exhibits a gel-like consistency.[56]
While mimicking the natural cellular environment is an essential advantage in many regards, it is also important to consider the inherent differences between normal tissue growth and replacement tissues resulting from medical intervention. These may include the absence of many co-proliferating neighboring cellular structures during construct cultivation, the implantation process, and the compensatory physical demands of the scaffold once in place. Further considerations must be made if the scaffold is designed for intentional biodegradation so that cellular ingrowth gradually assumes a complete functional role.
As a minimum with regard to ECM similarity, tissue engineering scaffolds should provide a 3D environment for cell growth. This architecture better mimics natural tissue and allows for gene expression and morphology that cannot be achieved in 2D. It must also be kept in mind that the primary purpose of a tissue scaffold is to promote tissue regeneration, with or without the presence of cells adhered in the scaffold. While the hydrogel must possess unique physical properties suited to the type of tissue it is applied in, all hydrogels must first satisfy the basic requirement of biocompatibility for any function to be realized in clinical use. Beyond this, they must also provide the appropriate macroenvironment and microenvironment for tissue ingrowth and cellular proliferation. Meeting these goals requires both physicochemical and biological cues applied with spatiotemporal control.
4.1. Biocompatibility
There are many definitions for biocompatibility that attempt to illustrate the notion of a harmonious existence between self and nonself. An important design consideration for engineered tissue constructs is that no or limited deleterious immunological, toxic, or foreign body responses should occur as a result of regenerative medical intervention. Williams[57] defines biocompatibility as “the ability of a material to perform with an appropriate host response in a specific application.” This definition is particularly relevant in tissue engineering since the nature of tissue constructs is to continuously interact with the body through the healing and cellular regeneration process as well as during scaffold degradation. If this requirement is not met, the hydrogel can be fouled or there may be damage and scarring to connected tissues, whether those tissues are immediately adjacent or linked by vasculature.
Toxic chemicals that may be used in the polymerization of synthetic hydrogels present a challenge for in vivo biocompatibility if conversion is not 100%. Furthermore, initiators, organic solvents, stabilizers, emulsifiers, unreacted monomers, crosslinkers, and the like used in hydrogel polymerizations or during processing may be toxic to host cells if they seep out to tissues or encapsulated cells. For example, Irgacure 2959, a typical photoinitiator used in many free radical photopolymerizations, has been shown to decrease cell viability when used in concentrations upwards of 0.1%.[58] To remove hazardous chemicals from preformed gels, synthesis should be typically followed by various purification processes, such as solvent washing or dialysis. In situ gelation of scaffolds, usually with oligomers and prepolymers, presents a special challenge since reactants used to synthesize the gel are injected into the body while still in a prepolymer solution. Utilizing this technique, ideal for its minimal invasiveness, requires special caution to ensure all components are safe and reasonably nontoxic.
While naturally derived polymers are frequently regarded as having superior biocompatibility over synthetic polymers, it must be noted that the presence of synthetic crosslinkers and initiators used in the polymerizations of naturally derived monomers and prepolymers are subject to the same toxicity concerns as purely synthetic gels.
4.2. Vascularization
With the exception of a small minority of tissue types (e.g., cartilage), most tissue is vascularized. This provides a conduit for nutrient exchange and the elimination of waste products by perfusion. Neovascularization, the formation of new blood vessels in adult tissue, is therefore an important consideration for most tissue engineering initiatives. Providing the right scaffold for new blood vessels to grow is a significant challenge. The scaffold must provide appropriate porosity, pore size(s), and allowances for vascular remodeling to occur as tissues mature. As scaffold designs further evolve and tissue constructs increase in complexity, scaffolds may similarly need to accommodate for lymphangiogenesis and neurogenesis as well.
In certain tissue engineering applications, hydrogels have been very successful as vascularizable scaffolds. For example, Stevens et al.[59] showed that alginate-based hydrogel scaffolds could be used in vivo to recreate vascularized bone. This scaffold required no additional growth factors or ECM molecules. Thus far, this approach has been limited to bone tissue regeneration.
There are three general strategies to enhance the vascularization of tissue engineering scaffolds.[60] The first is to incorporate regulatory factors that motivate the growth of vasculature from surrounding tissues or recruit endothelial progenitor cells (EPCs). The second is seeding the scaffold with ECs or EPCs. The third strategy is prevascularization in vivo. Alginate-,[61,62] gelatin-,[63] HA-,[64,65] PHEMA-,[66] and PEG-based[67] hydrogels loaded with vasculogenic growth factors have been shown to successfully induce microvessel growth following implantation. In order to spatially control the growth of new vasculature, Golden and Tien[68] designed EC-seeded microfluidic channels in collagen and fibrin hydrogel scaffolds. To date, most of the progress in EC-seeding has been limited to in vitro studies. Relying on EPCs and the native vasculature to invade implanted scaffolds is a process dependent upon the presence of significant quantities of circulating progenitor cells and can take days to occur. In general, cells cannot survive more than a few hundred micrometers from a blood vessel. If other cell types are encapsulated within the scaffold, they may become necrotic while waiting for vascular ingrowth.
Embryonic stem cells (ESCs) are a potentially limitless source of cells for in vitro prevascularization. But this requires that their differentiation (and lack thereof) be controlled. Langer and collaborators designed dextran-based hydrogels incorporated with growth factors that could enhance, but not control the vascular differentiation of human ESCs (hESCs).[69] Later they discovered that hyaluronic acid hydrogels could maintain hESCs in their undifferentiated state until vascular differentiation was desired.[70] This may be related to HA’s prominence in early fetal development[71] and its ability to suppress vascularization.[72]
4.3. Degradation
The human body is in a constant state of turnover. The homeostasis of bone tissue, for example, is maintained by the destructive and regenerative actions of both osteoclasts and osteoblasts, respectively. Wound healing is known to involve the controlled breakdown and synthesis of ECM. A loss of either of these processes is associated with pathology.[73] A more biomimetic approach to hydrogel scaffolds may be one that can undergo the same kind of controlled breakdown as most living tissue. An entirely degradable scaffold can be progressively relieved of function and replaced by new tissue as degradation progresses. Complete degradation would alleviate many concerns about long-term implant stability and integrity. When designing biodegradable hydrogels, the rate of degradation and breakdown of products must be considered. Certain tissue engineering applications may not require complete scaffold degradation, such as with articular cartilage or corneal replacement. For these applications, a well-integrated, but permanent or semipermanent scaffold may be the best choice to replace the basic function of lost or damaged tissue.
Degradable hydrogels can be made by incorporating cleavable groups into the polymer backbone or crosslinks. These groups can be cleaved nonselectively via processes such as hydrolysis. Biodegradation is achieved when the network structure can be broken down through biological processes, such as enzymatic digestion.[74] Bryant and Anseth[75] incorporated hydrolytically cleavable groups into PEG networks and found a correlation between the degradation profile and the production and distribution of collagen from encapsulated chondrocytes. Additionally, hydrogels can be made with incorporated ECM components, such as collagen and HA, which are naturally biodegradable and lead to mimicking of the natural tissue growth environment during cellular proliferation.
When these materials come from natural sources, using them may carry the risk of batch-to-batch variation, and control over their physicochemical properties is limited. Hubbell and collaborators[76–79] developed an innovative approach to making biodegradable hydrogels using ECM fragments. They synthesized PEG hydrogels with ligands for cell attachment and peptide fragments that function as ECM metalloproteinase (MMP) substrates. The native ECM acts not only as a substrate for cell growth and attachment, but also as reservoir for regulatory factors. Mimicking this function, it was possible[67,80] to incorporate growth factors into the networks that could be released in a ‘cell-demanded’ fashion. The allure of this technique is that it allowed for proliferating cells to remodel the scaffold as needed both spatially and temporally. In this way, the macroscopic properties of the hydrogel were tuned to provide control over the microenvironment of the actual cells.
4.4. Macroenvironment
Many synthetic hydrogels provide a blank slate for the design of tissue engineering scaffolds and thus can be used as a platform for biological cues. Hydrogels possess mechanical and physicochemical properties that can be tuned to control cell growth and proliferation in the same way that biochemical and physical cues are commonly used. Understanding how these properties are controlled and how they change in various tissue engineering applications is crucial for success.
The mechanical properties of hydrogels as tissue engineering scaffolds can have a profound effect on attached or encapsulated cells. It is well known the ECM maintains a level of isometric tension between cells in a given tissue. This level of stabilizing pre-stress differs by tissue type and can be altered in disease processes. The response of individual cells to changes in these stresses can vary from morphological changes to changes in gene expression.[81] Because of this, hydrogel scaffolds may need to be designed with tissue specific mechanical properties. Engler and co-workers[82] showed that the stiffness of polyacrylamide gels was more important for smooth muscle cell spreading than the concentration of cell adhesion ligands. They also showed that gel stiffness can be used to control the differentiation of mesenchymal stem cells.[83]
A principle of polymer mechanics is that crosslinking density can be used to control the properties of polymer networks, such as mechanical compliance, swelling, and mesh size. Crosslinking density can also be used to affect cells encapsulated within hydrogels. For example, Bryant et al.[84,85] showed that changes in PEG hydrogel crosslinking density caused changes in cell growth and morphology. They also found that the amount and composition of ECM secreted by encapsulated cells depended on other gel properties such as mesh size and hydrophilicity.[86,87]
Tuning porosity has shown to be significantly successful in the assimilation of scaffolds with host tissues. Early work on porous scaffolds showed that implants with interconnected pores between 0.8–8 µm lack a fibrous capsule and had dramatically more neovascularization than implants with smaller or larger holes.[88,89] This pore size allowed for the infiltration of host cells and has been linked to the long-term success of synthetic hydrogels for cornea replacement.[90]
4.5. Microenvironment
Cellular growth and proliferation is driven by both intrinsic and extrinsic cues. Extrinsic cues can be provided by the ECM, cell–cell adhesion, and soluble factors. Many of these signals function due to their spatiotemporal distribution. This is known as the cellular microenvironment. For example, the differentiation of hematopoietic stem cells (HSCs) is determined by their spatial location within bone marrow. A gradient pattern of differentiation is established by the proximity of individual HSCs to the bone surface, where they receive regulatory factors from bone cells that inhibit their differentiation.[91] Damage to tissue can cause a change in the microenvironment that may be exploited. For example, the injured central nervous system forms a local wound response known as a glial scar that inhibits axonal regeneration.[92] Blocking the inhibitory cues in this scar has led to greater recovery of function after spinal injury.[93]
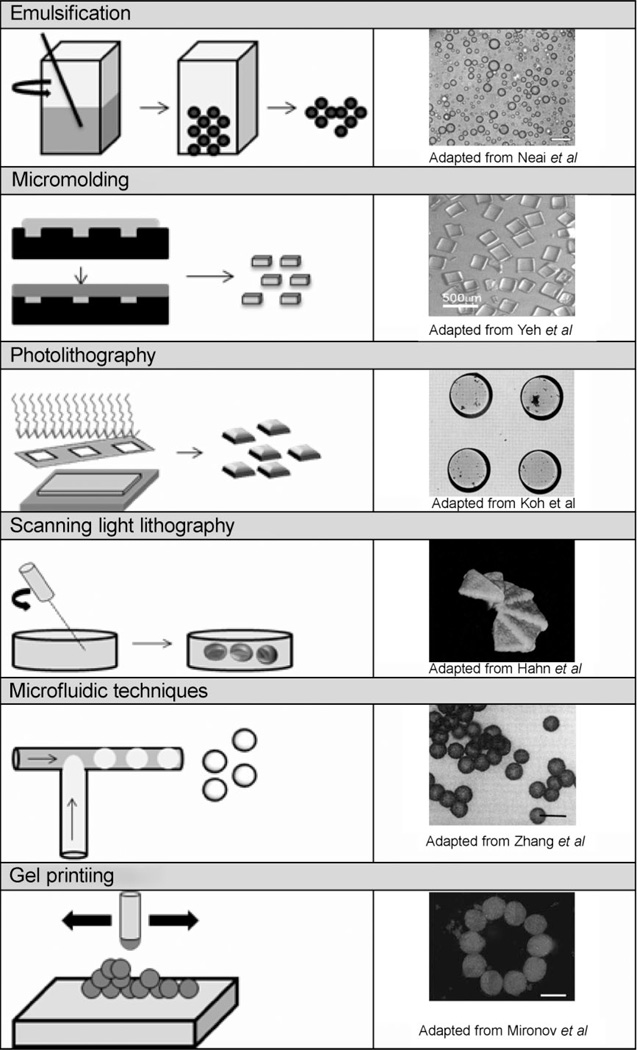
In earlier designs of tissue engineering constructs, there was a disregard for the individual cell environment in favor of bulk properties. This is changing as it becomes more apparent that both are important. A number of recent publications have addressed the importance of controlling the cellular microenvironment.[94–97] There has also been a tendency to assume that the success or failure of a scaffold hinges on the fate of a single cell type, but most natural tissue is composed of multiple types. With the advent of novel micro- and nanoscale fabrication, it is now possible to create hydrogel scaffolds with a directed spatial distribution of cells[98,99] and regulatory factors,[100] to exert more control over microenvironments.
5. Types of Hydrogels in Tissue Engineering
Synthetic hydrogels, such as those based on PHEMA, were some of the earliest biomaterials used as tissue engineering scaffolds and helped lay the foundation for current work. Understanding both past failures and current successes of these materials may help novice researchers avoid pitfalls in the design of newer, better hydrogels. While many gels based on natural macromers are increasing in popularity due to their inherent biocompatibility, synthetic gels have advantages that are important in regenerative medicine. These advantages include easier large- scale production and highly tunable and consistent properties. Control of these material properties helped to advance the understanding of cellular interactions with synthetic substrates and the body’s response to foreign materials. While being mostly biocompatible, many synthetic gels are made using harsh synthetic chemistry. This requires care to ensure that contaminants and unreacted reagents present during synthesis are subsequently removed. Here, we overview prevalent synthesis methods and some examples of synthetic hydrogels that have been successfully used for tissue engineering to date. Table 1 summarizes many hydrogel applications in tissue engineering covered in this review. There are also several excellent reviews in the literature that summarize the many uses of synthetic hydrogels in broader biomedical applications.[15,143–145]
Table 1.
Summary of selected hydrogel applications in tissue engineering.
| Intended tissue | Cell type(s) studied | Hydrogel type(s) | Hydrogel function(s) | Section | Ref. |
|---|---|---|---|---|---|
| Bone | Osteoblasts | PEG–PLA [a] | Drug delivery, Encapsulation | 5.4 | [101,102] |
| Bone | Osteoblasts | Peptide amphiphile–Ti composite | Encapsulation, Implant | 5.9 | [103] |
| Bone | Fibroblasts | PEG | Scaffold | 4.3 | [76] |
| Cardiovascular | Bone marrow cells | Fibrin | Cell delivery, Scaffold | 5.6 | [104] |
| Cardiovascular | Embryonic carcinoma | PEG | Encapsulation | 4.3 | [77] |
| Cardiovascular | Cardiomyocyte, Endothelial, ESCs | SAP | Encapsulation, Scaffold | 5.9 | [105] |
| Cardiovascular | Hepatocytes | HA, Alginate, Carboxymethylcellulose | Scaffold | 5.5 | [106] |
| Cartilage | Chondrocytes | Fibrin | Cell delivery, Scaffold | 5.6 | [107] |
| Cartilage | Chondrocytes | PEO Semi-IPN | Drug delivery, Encapsulation | 3.1 | [39,40] |
| Cartilage | Chondrocytes | PEG | Drug delivery, Encapsulation | 5.4 | [108] |
| Cartilage | ESCs | PEG | Drug delivery, Encapsulation | 5.4 | [109–112] |
| Cartilage | Chondrocytes | PVA | Encapsulation | 4.1 | [58] |
| Cartilage | Chondrocytes | PEG | Encapsulation | 4.3 | [79] |
| Cartilage | Chondrocytes | PEG | Encapsulation | 4.4 | [84] |
| Cartilage | Chondrocytes | PEG | Encapsulation | 4.4 | [85] |
| Cartilage | Chondrocytes | PEG–PLA–PVA [a] | Encapsulation | 5.3 | [113] |
| Cartilage | Chondrocytes | Alginate | Encapsulation | 5.7 | [114] |
| Cartilage | Chondrocytes | Collagen | Encapsulation | 5.8 | [115] |
| Cartilage | Chondrocytes | Collagen, HA | Encapsulation | 5.8 | [116] |
| Cartilage | Chondrocytes | PEG–PLA [a] | Encapsulation, Scaffold | 4.3–4.4 | [75,86,87] |
| Cartilage | MSCs | PEG | Encapsulation, Scaffold | 5.4 | [117] |
| Cartilage | Chondrocytes, MSCs | PEG | Encapsulation, Scaffold | 5.4 | [118,119] |
| Cartilage | Chondrocytes | PLLA [b], Agar, Gelatin | Encapsulation, Scaffold | 5.5 | [120] |
| Cartilage | Chondrocytes | HA, Collagen | Encapsulation, Scaffold | 5.5 | [121] |
| Cartilage | Chondrocytes | Fibrin | Encapsulation, Scaffold | 5.6 | [122] |
| Cartilage | Chondrocytes | SAP | Encapsulation, Scaffold | 5.9 | [123] |
| Cartilage/Bone | – | Alginate, HA | Bioreactor, Scaffold | 4.2 | [59] |
| Connective Tissue | Fibroblasts | HA | Encapsulation, Scaffold | 5.1 | [124] |
| ECM | Fibroblasts | HA, Chondroitin Sulfate, Gelatin | Encapsulation, Scaffold | 5.5 | [125] |
| Eye | – | HA | Barrier, Scaffold | 5.5 | [126] |
| Eye | – | PHEMA | Scaffold | 5.2 | [127] |
| Facial | Chondrocytes | Alginate | Encapsulation, Implant | 5.7 | [128] |
| Facial | – | HA | Space-Filler | 5.5 | [129] |
| Intraperitoneal | – | HA | Barrier | 5.5 | [130] |
| Intraperitoneal | – | PEG, PEG/PLA [a] | Barrier, Drug delivery | 3.2–3.3 | [46,47] |
| Intraperitoneal | – | PEG | Drug delivery | 3.3 | [49] |
| Neural | – | Collagen | Drug delivery | 5.8 | [131] |
| Neural | Neuroprogenitors | SAP | Entrapment, Scaffold | 5.9 | [132] |
| Neural | – | PHEMA–MMA | Scaffold | 5.2 | [133] |
| Pancreatic | Islet of Langerhans | PEG | Encapsulation | 3.4 | [55] |
| Pancreatic | Islet of Langerhans | PEG–PLA [a] | Encapsulation | 5.4 | [134] |
| Skeletal Muscle | Myoblasts | PHEMA | Scaffold | 5.2 | [135] |
| Skin | – | Chondroitin sulfate, HA | Barrier | 5.5 | [136] |
| Skin | – | Collagen | Drug delivery | 5.8 | [137] |
| Skin | – | Fibrin | Glue | 5.6 | [138] |
| Skin | Fibroblasts | HA | Scaffold | 5.1 | [139] |
| Spinal cord | – | PHEMA | Drug delivery, Scaffold | 4.2 | [66] |
| Spinal cord | Astroglial cells | Collagen | Encapsulation | 5.8 | [140] |
| Vascular | – | PEG | Barrier | 3.2 | [44,45] |
| Vascular | – | PEGDA | Drug delivery | 3.3 | [48] |
| Vascular | – | Alginate | Drug delivery | 4.2 | [61,62] |
| Vascular | – | Gelatin | Drug delivery | 4.2 | [63] |
| Vascular | – | HA | Drug delivery | 4.2 | [63,64] |
| Vascular | – | PEG | Drug delivery, Scaffold | 4.2 | [67] |
| Vascular | hESCs | HA | Encapsulation | 4.2 | [70] |
| Vascular | MSCs, Primary smooth muscle | PEG | Encapsulation | 4.3 | [78] |
| Vascular | Endothelial cells | P(PF-co-EG) | Encapsulation | 3.1 | [41] |
| Vascular | hESCs | Dextran | Encapsulation, Drug delivery | 4.2 | [69] |
| Vascular | Smooth muscle cells | PEG | Scaffold | 3.1 | [42] |
| Vascular | Endothelial cells | PEG | Scaffold | 4.3 | [80] |
| Vocal Cord | – | HA–Gelatin | Scaffold | 5.1 | [141] |
| Vocal Cord | – | Collagen, Alginate | Scaffold | 5.8 | [142] |
PLA = poly(lactic acid).
PLLA = poly(L-lactic acid).
5.1. Synthesis
Conventional reaction schemes for synthetic hydrogels rely on the presence of a multifunctional crosslinking agent during polymer synthesis. Free radical polymerization is a widely used method where a polymer chain propagates through the consumption of vinyl monomers. Free radical polymerization is advantageous in many tissue engineering applications because of its convenience for in situ polymerization and its well-characterized gelation kinetics.[146] Preformed chains, or macromers, can be used to create hydrogels by introducing vinyl or other functional groups onto them. In this way synthetic chemistry can be used to create hydrogels from naturally derived macromers, such as HA and chitosan. Acrylate-based derivatives are common functional groups that can be polymerized with the help of thermal or photoinitiated free radical initiators (Fig. 4A). The reaction occurs very quickly and uncontrollably. This can result in a wide distribution of molecular weights between crosslinks and other inhomogeneous properties throughout the hydrogel. Controlled radical polymerizations, such as atom transfer radical polymerization (ATRP), are an attractive way to gain more controlled properties, but as of yet have not shown much success in tissue engineering applications. The added control in these reactions typically comes at the cost of time efficiency and requires the use of toxic transition metals which must be removed.
Figure 4.
Three reaction schemes that can be used to make synthetic hydrogels from hydrophilic macromers. A) A diacrylate macromer undergoes free radical polymerization. B) A thiol and acrylate group undergo conjugate addition to crosslink two macromers. C) A pendant alkyne and azide ‘click’ together to crosslink two macromers via a 1,2,3-triazole group.
An alternative synthesis method is Michael (conjugate) addition.[67,139,141,147–150] A typical conjugate addition reaction scheme involves mixing an acrylated macromer with a thiolated macromer (Fig. 4B). The reaction is rapid and highly specific, and it does not require initiation. Conjugate addition reactions based on other β-unsaturated esters or amides combined with thiols can be used, but with much slower reaction kinetics.[124,150] The conjugate addition polymerization of hydrogels in the presence of biological compounds and live cells does carry the risk of side reactions due to the presence of competing nucleophiles. Both radical polymerization and conjugate addition are useful in high throughput screening due to their fast kinetics. For example, Langer and co-workers used microarrays to simultaneously screen hundreds of photopolymerized diacrylate networks and conjugate adducts for tissue engineering and gene delivery applications.[151–154]
Click chemistry is another attractive method to crosslink macromers. Click chemistry provides mild (e.g., physiological) reaction conditions with high chemical selectivity, similar to many naturally occurring chemical reactions. The click reaction between terminal azides and acetylenes is highly specific, results in high yields, and occurs dependably in the presence of competing functional groups (Fig. 4C).[155] Macromers that are combined using this chemistry, or ‘clicked’ together, can result in hydrogels with properties not possible using conventional methods. Malkoch and co-workers[156] compared PEGgels prepared by click chemistry with those prepared using radical polymerization and found striking differences in mechanical properties. The clicked gels could sustain up to ≈30× the amount of tensile stress as compared to conventional PEG gels. Crescenzi and co-workers[157] used the click reaction to prepare HA hydrogels that were biocompatible and served as drug delivery reservoirs. These results suggest that the range of properties for synthetic hydrogels is much wider than previously thought.
5.2. Poly(2-hydroxethyl methacrylate)
Poly(2-hydroxyethyl methacrylate) (Fig. 1A) hydrogels have been used as implant materials for almost half a century.[158] PHEMA networks can be polymerized from 2-hydroxyethyl methacrylate using free radical precipitation polymerization. The resultant hydrogel is a relatively weak material that is biologically inert. It is also highly resistant to protein adsorption and, consequently, cell adhesion. Its commercially available monomer often comes contaminated with a small fraction of ethylene glycol dimethacrylate. This makes an uncrosslinked macromer nearly impossible to obtain without further purification.
One of the earliest uses of PHEMA was as an artificial cornea, or keratoprosthesis. Chirila and co-workers evaluated biphasic PHEMA keratophostheses with a homogeneous core and porous skirts for long-term cornea replacement.[90] The success of the prostheses depended on the porosity of the skirt region, with micrometer-size pores causing a unique host response devoid of fibrous encapsulation. In a Phase I clinical trial, the implants were retained for up to 2.5 years.[159] PHEMA implants have been shown to undergo delayed, episodic calcification in vivo.[127,133] However, to what degree contaminants, such as methacrylic acid (MMA), enhance calcification has not been established. Methacrylic-acid- and acrylic-acid-based hydrogels have a high affinity for calcium and other alkaline earth metals, making them more prone to calcification.[160–162] Recently, Bryant et al.[135] developed a biodegradable PHEMA scaffold with controlled porosity. Collagen was covalently bound to the pendant hydroxyl groups to enhance myoblast growth and proliferation. Biodegradable PHEMA scaffolds are an important development given that the primary obstacle to the success of PHEMA gels is the lack of a means for elimination.
5.3. Poly(vinyl alcohol)
Poly(vinyl alcohol) (Fig. 1B) is prepared from the partial hydrolysis of poly(vinyl acetate). It can be crosslinked into a gel either physically or chemically (e.g., via treatment with monoaldehydes). In recent studies, PVA was photocured to produce hydrogels as an alternative to chemical crosslinking. The potential toxic environments, which are created from chemical crosslinking, are harmful to cells; thus researchers are attempting to stay away from this method.[163] PVA is similar to PHEMA in having available pendant alcohol groups that act as attachment sites for biological molecules. In addition to having multiple attachment sites, PVA is also elastic and thus can induce cell orientation or matrix synthesis by enhancing the transmission of mechanical stimuli to seeded cells.[163] PVA, like other neutral hydrogels, is nonadhesive to cells and proteins, but can be made so by conjugation with biological factors. Martens et al.[113] have successfully copolymerized PEG and PVA to produce a biodegradable hydrogel. In this study it was found that the degradation rate of PEG/PVA copolymer hydrogels was faster than the degradation rate of PEG hydrogels, but was much slower than the degradation rate of PVA homopolymer hydrogels.
One of the most successful tissue engineering applications for PVA has been for avascular tissue. PVA hydrogels are stronger than most other synthetic gels, have a low coefficient of friction, and have structural properties similar to natural cartilage.[164] Oka and collaborators[165,166] investigated PVA hydrogels for articular cartilage replacements by examining physical aspects including lubrication, load bearing, biocompatibility, and attachment of the material to bone. They used high molecular weight PVA along with a novel annealing process to enhance the tensile strength of PVA hydrogels up to 17 MPa, mimicking that of normal human articular cartilage. This new material was found to have excellent biocompatibility and physical properties. They showed that PVA artificial knee menisci in rabbits lasted beyond two years with no loss in integrity or mechanical properties.[167] Currently a PVA hydrogel known as Salubria™ (Salumedic, Atlanta, GA) is marketed in Europe and Canada for articular cartilage replacement.
5.4. Poly(ethylene glycol)
Poly(ethylene glycol) (Fig. 1C) is hydrophilic and biocompatible, with properties that limit immunogenicity, antigenicity, protein binding, and cell adhesion.[40,168] Chains above ≈10 kDa are known as poly(ethylene oxide) due to the negligible concentration of end groups. PEG homopolymer is a polyether than can be polymerized from ethylene oxide by condensation. These chains possess terminal hydroxyl groups which are frequently derivatized to make PEG macromers for use in a wide variety of reaction schemes. In the 1970s PEG first became popular as a surface coating for biomaterials due to its intriguing ability to block serum protein adsorption.[169] This phenomenon is still not completely understood but some proposed explanations suggest the ‘exclusion volume’ of hydrated, highly mobile PEG chains as an important contributing factor.[170] The nonadsorptive properties of PEG may also be explained by the lack of protein binding sites on the polymer chain. One thing that sets PEG apart from PHEMA and PVA is the lack of hydrogen bond donating groups, a feature that has been shown to be critical in reducing protein binding.[171] For a comprehensive review of the physical properties of PEO that contribute to its biocompatibility, the reader is referred to a review by Lee et al.[172]
PEG hydrogels have been the most successful synthetic gels for tissue engineering applications to date. For example, photocurable hydrogels that are PEG-based are widely used to encapsulate cells into scaffolds because of their inert nature. PEG’s stealth characteristics are widely known, and thus, these gels may be used in scaffolds with encapsulated cells to prevent undesired interactions between the polymer and the encapsulated cells.[75,101,102,108,117,134] However, scaffolds with incorporated PEG chains can be modified with bioactive peptides to do the reverse, i.e., induce cellular behavior, such as adhesion to proteins.[39,40,118] Further, PEG can be used as a mediator in the immobilization of the RGD sequence. PEG with a molecular weight (MW) of 3500 Da has a special characteristic length of 35 Å, which is the effective distance found between RGD and a substrate and thus makes the sequence available to cells.[173]
Terminal hydroxyl groups can be derivatized to create PEG macromers that can participate in chain or step polymerizations. PEG macromers have low toxicity, and therefore these hydrogels can be formed in situ to fill irregular defect sites. The use of highly reactive terminal acrylate groups allows gels to be reacted quickly with free thiol groups or photopolymerized with ultraviolet or visible light. Langer and co-workers showed that photopolymerization of PEG gels can even be done transdermally.[118] PEG macromers can also be coupled with peptides and growth factors and incorporated with biodegradable units. For example, Elisseeff and co-workers[109–112,119] designed growth-factor-loaded PEG diacrylate-based hydrogels with biodegradable crosslinks and covalently attached cell adhesive molecules. These gels were successful as stem cell delivery vehicles for cartilage growth in vivo. As with many other synthetic hydrogels, PEG scaffolds have been most successful in tissue engineering applications that do not require the scaffold to be vascularized, such as skin and cartilage.
5.5. Hyaluronic Acid and Natural Materials
Naturally derived hydrogels are widely thought to have an edge over synthetic biomaterials where biocompatibility is concerned since natural gels may offer better chemical and morphological cues to cells. Many of the components used in their synthesis comprise much of the in vivo structure and, hence, can also offer environmental advantages as ECM-mimics for cell-based devices. Langer and collaborators have used a number of naturally derived materials, including HA, alginate, collagen, chitosan, and others, thus spurring the growth of materials for tissue engineering over the last decade. The macromer repeat units utilized in these gels are shown in Figure 5. Issues that should be addressed in materials research with natural gels include the predictability of degradation behavior, batch-to-batch variability, and recent concerns regarding possible denaturation during fabrication and processing, such as electrospinning for nanofibers.[174]
Figure 5.
Molecular structure of typical natural macromer repeat units used in hydrogels for tissue engineering: A) Hyaluronic acid is composed of disaccharide repeat units of d-glucuronic acid and d-N-acetylglucosamine, linked together via alternating β-1,4 and β-1,3 glycosidic bonds. B) Chitosan is composed of randomly distributed N-acetyl-d-glucosamine (acetylated unit) and β-1,4-linked d-glucosamine (deacetylated unit), where x is usually much smaller than y. Chitosan is a partial deacetylation product of chitin which is composed entirely of N-acetyl-d-glucosamine (acetylated units). C) Agarose is an alternating 1,4-linked 3,6-anhydro-α-l-galactopyranose and 1,3-linked β-d-galactopyranose. Some residues are replaced by methylated, sulphated, or other sugar units, which affect gel formation. D) Alginate consists of varying number of α-l-guluronic acid (x) and β-d-mannuronic acid (y) residues connected via 1,4-linkages.
Hyaluronic acid (Fig. 5A), is a high molecular weight glycosaminoglycan present in all mammals with repeating disaccharide units composed of (β-1,4)-linked d-glucuronic acid and (β-1,3)-linked N-acetyl-d-glucosamine. HA in the body occurs in salt form as hyaluronate and is found in high concentrations in several soft connective tissues, including skin, umbilical cord, synovial fluid, and vitreous humor. In commercial production, HA is commonly extracted from rooster comb and human umbilical cord or is manufactured in large quantities by bacterial fermentation.[175] HA is degraded in the body by hyaluronidase (hyase) into smaller oligosaccharides, while d-glucuronidase and N-acetyl-hexosaminidase further degrade the oligosaccharide fragments by removing nonreducing terminal sugars. In addition to enzymatic degradation, HA can also be degraded by reactive oxygen intermediates, a mechanism that has been implicated as a source of HA fragments at sites of inflammation.[176] Unmodified HA is subject to rapid degradation which leads to it getting cleared from the site of administration. HA, therefore, offers innate degradation as an important advantage.
HA is particularly good for tissue engineering applications because of its high viscoelasticity and space filling properties. This rheological feature is directly exploited in the application of hyaluronan for ophthalmic surgery[126] and in the treatment of osteoarthritis.[177] Moreover, as a result of its ability to form hydrated, expanded matrices, HA has also been successfully used in cosmetic applications such as soft tissue augmentation.[129] Recent work suggests that even more opportunities lie in the exploitation of its biological characteristics, such as in wound healing applications.[130,136] Recently, HA has been used by Langer and colleagues to make micromolds for cell encapsulation.[178,179]Here, cells encapsulated by the micromold could later be recovered by enzymatic degradation.
To reduce the rapid in vivo enzymatic digestion of HA by hyase, it is necessary to introduce synthetic crosslinks. Crosslinking slows the release of drugs from the gel in therapeutic delivery applications due to changes in solute transport characteristics as previously described. This simple polysaccharide offers multiple sites for modification via its carboxyl and multiple hydroxyl groups. Different crosslinking strategies can be used to tailor it for a desired application. HA can be functionalized with a methacrylate group,[180] heterobifunctional crosslinkers such as 1-ethyl-3- (3-dimethylaminopropyl) carbodiimide hydrochloride (EDC),[181] or homobifunctional crosslinkers such as PEGDA. Dithiobis (propanoic dihydrazide) (DTPH), divinyl sulfone, glutaraldehyde α-β-poly-(N-2-hydroxyethyl) (2-aminoethylcarbamate)-d, and l-aspartamide (PHEA–EDA, which is synthetic, biocompatible, water-soluble and has a proteinlike structure) have also been used.[182] HA gets damaged easily at temperatures exceeding 90 °C, and it cannot be effectively dehydrated, therefore preventing the use of dehydrothermal treatments.[121]
Each glucuronic acid unit on HA contains a carboxyl group, giving rise to HA’s polyanionic character at physiological pH. Its hydrophilic nature makes it well-suited for applications requiring minimal cellular adhesion, such as post-surgical adhesion barriers. It can, however, be modified with peptides to create a biomaterial that supports cell attachment, spreading and proliferation. Thiol-modified HA can be modified with the RGD sequence (Arg-Gly-Asp), which is known to be a cell recognition site for numerous adhesive proteins present in the ECM and in blood.[124] For example, Shu et al.[125] have incorporated RGD into hyaluronan hydrogels. They showed that while RGD promoted better attachment and proliferation at the surface, it was inefficient at recruiting cells for adhesion within the hydrogel. Additionally, Park and Hubbell[183] have incorporated RGD into a HA-based hydrogel through interpenetrating networks and found that the RGD did have significant influence on the recruitment and proliferation of fibroblasts. The extent of cell attachment can be influenced by varying the concentration and structure of the peptides and the length of the PEG spacer used.
HA composites, such as HA–DTPH, PEGDA, and peptide hydrogels have been used as injectable constructs for in vivo tissue formation. The gels crosslink in situ and may be seeded with cells prior to injection. Cell-loaded hydrogels have shown by immunohistochemistry that the encapsulated cells retain their phenotype and secrete ECM in vivo.[150] Other combinations of HA with natural gel-forming materials (e.g., chondroitin sulfate, gelatin,[125] alginate, carboxymethylcellulose,[106] and collagen) have also found utility in tissue engineering applications.[121,184]
5.6. Fibrin Hydrogels for Tissue Engineering
Fibrin is another naturally occurring material that has shown promise in recent years as a cell delivery vehicle[122,185] and injectable scaffold.[104] The main advantage of fibrin gels is that fibrinogen can be obtained autologously from the plasma, thereby reducing the risks of a foreign body reaction. Furthermore, fibrin has been used in conjunction with other gels, such as HA-based gels, to deliver chondrocytes in a knee injury model.[107]
Traditionally, fibrin has found utility in the medical field as glue composed of fibrinogen and thrombin solutions that form a clot when mixed together. The primary usage of fibrin glue is to control bleeding and adhere tissues during surgery.[186] It has also demonstrated improved results with skin grafts, particularly difficult skin grafts, and the delivery of exogenous growth factors to speed wound healing time.[138]
5.7. Alginate in Hydrogels for Tissue Engineering
Alginate is a linear block copolymer of d-mannuronic acid (M) and l-guluronic acid (G) residues (Fig. 5D) that has been widely used for cell encapsulation.[187] The relative amount of each depends on the source of alginate. The sequence in the blocks can be either similar or alternating (MMMM, GGGG, or GMGM).[187] Commercially available alginate is extracted from brown seaweed algae. Blocks of l-guluronic acid are stiffer than d-mannuronic or alternating blocks due to the diaxial linking between residues. The viscosity of an alginate solution and its overall stiffness once gelled depend on the concentration of the polymer and its molecular weight distribution.[188] The higher the M/G ratio, the smaller the average pore size of alginate gels is.[189,190] Crosslinking between polymer chains depends on the amount of l-guluronic and multivalent cations (e.g., Ca2+, Ba2+) which can interact with carboxylic acid groups in the sugars.[191] Gelling conditions, such as temperature, also affect the network structure. For example, Ca2+ diffuses slowly leading to a more ordered crosslinked polymer at lower temperatures.[192,193] As a less popular alternative to ionic crosslinking, many diamines and dihydrazides have been used to covalently crosslink alginate.
As a biomaterial, alginate is used because of its biocompatibility, non-immunogenicity, and hydrophilic nature. It is also convenient since it can be injected with an ionic solution to produce a hydrogel around a defect.[128] However, alginate cannot be enzymatically broken down and has poorly regulated degradation. Some concern has also been expressed over the immunogenicity of some forms of alginate, which are high in d-mannuronic acid content.[194] Furthermore, cells cannot adhere to alginate unless it is modified with cellular adhesion molecules (e.g., laminin, fibronectin, collagen, and RGD sequences, which allow more specific interactions). It has been shown that the phenotype of myoblasts encapsulated in alginate gels can be controlled by varying RGD concentration and M/G ratio.[195,196] Adhesive interactions between cells and peptides coupled to alginate polymers have also shown to enhance the strength of the gels.[197]
In order to promote the degradation of alginate gels, a variety of approaches can be used. Gamma irradiation breaks high molecular weight chains into shorter chains, thus allowing these polymers to be cleared faster in vivo.[198] Partial oxidation of alginate with sodium periodate makes the chains more susceptible to degradation through hydrolysis.[199] Gels can also be formed by crosslinking either with poly(guluronate) or partially oxidized alginate (poly(aldehyde guluronate)).[200]
Alginate gels have also been used in drug delivery applications. To reduce the diffusion of hydrophilic drugs through alginate gels, drugs can be trapped in the polymer through ionic complexation. Alginate gels have also been used to encapsulate cells (e.g., osteoblasts and chondrocytes) for cartilage repair and solutions of chondrocytes and alginate have been injection-molded into anatomically shaped implants.[128] Stevens et al. have used alginate gels to culture explants of periosteum for cartilage tissue engineering.[114] Alginate has further been utilized in surgical dressings,[201] and even for suppressing the absorption of radioactive strontium in the body.[202]
5.8. Collagen
Collagen fibers are strong and form through self-aggregation and crosslinking, making them popular in biomedical applications. A schematic showing the composition of collagen fibrils, starting from its amino acids sequences is shown in Figure 6.
Figure 6.
Schematic diagram showing the hierarchical structure of collagen fibrils. Three polypeptide strands (A) form a right-handed triple helix of collagen type II (B), and these helical molecules are interconnected with pyridinium crosslinks (C). The collagen fibrils (D) are mainly composed of staggered collagen type II molecules which are connected with other fibrils via collagen type IX molecules. A–C adapted with permission from [203]. Copyright 2007 IOP Publishing. Figure 6D reproduced with permission from [204]. Copyright 1998 Wiley-Liss.
Collagen gels can be formed in situ and can be easily manipulated as a natural delivery device for cells and growth factors. While many applications use unmodified collagen, chemical crosslinkers can be used to inhibit in vivo absorption of collagen in applications which require slow degrading constructs, such as drug delivery. Collagen type I is found in fibrocartilage. Collagen type II constitutes the bulk of the collagen found in articular cartilage (along with small amounts of collagen type XI), where 50–80% of the dry mass is contributed by collagen fibrils. In the native environment, negatively charged proteoglycans are physically immobilized in this fibrillar network to aid in load-bearing. Interfibrillar connections help maintain the network structure against the swelling pressure of water and are likely due collagen type IX.[205] The tensile strength of individual collagen fibrils depends on the diameter of the fibril as well as the extent of crosslinking between collagen strands in the collagen triple helix.[206]
Collagen has been used to make hydrogels for vocal cord regeneration,[142] spinal cord conduit repair,[140] and cartilage defects.[115,116,204] Cartilage has very little capacity for spontaneous healing because it is avascular. However, the transplantation of primary cells (e.g., chondrocytes extracted with collagenase II from joint cartilage) into scaffolds is an approach that has demonstrated clinical potential. Cells are suspended in collagen solutions, fibrillogenesis occurs at 37 °C forming gels, and then the cells are cultured in the hydrogels for 1–6 weeks. The efficacy of the resultant scaffold is gauged by the glycosaminglycan (GAG) content and number of cells.
In order to help stabilize chondrocyte phenotype and increase proteoglycan synthesis, researchers have tried composites of collagen with hyaluronic acid, and found that HA increases the amount of ECM deposited by chondrocytes.[116] However, in a study on collagen–alginate and collagen–hyaluronan composites for restoring appropriate shape and pliability to scarred vocal folds, collagen–alginate hydrogels not only supported more ECM synthesis, but also showed less mass loss, or prolonged augmentation, which is a desirable feature for vocal fold regeneration.[142]
It is important for scaffolds to have open-pore geometry to encourage cell-ingrowth. In addition, different cell types show selectivity for different pore sizes.[207] For bone tissue engineering, pore sizes greater than 150µm promote new bone formation.[208] In this effort, collagen type I has been cast into sacrificial molds of wax (indirect solid free-form fabrication with polypropylene fumarate)[116] as well as molds fabricated with 3D phase change inkjet printers (indirect rapid prototyping), which produce a series of connected channels of a known diameter for mass and fluidic transport.[209]
Collagen has also been used in drug delivery systems in ophthalmology,[210] as a controlling material for transdermal delivery,[137] microparticles for drug delivery,[211] tablets for protein delivery,[212] and in sponges for wounds in skin replacement.[213] In addition, collagen has been used as artificial blood vessels. Sefton and co-workers[214–218] have developed sub-micrometer diameter collagen gels coated with endothelial cells and have found that the constructs are nonthrombogenic in in vitro studies.
Collagen can serve as a good model system for transport in the brain. Saltzman and co-workers has used this feature to study the rate of transport of nerve growth factor (NGF) in collagen gels.[219]Mahoney and Anseth[220] have used encapsulated collagen and exogenously added a growth factor in PEG hydrogels seeded with fetal forebrain cells for improving neural cell survival and metabolic activity. Collagen gels are also being used as nerve guidance materials in the spinal cord and peripheral nervous system.[131,140]
5.9. Self-Assembled Peptides
Another important class of hydrogels are those made from self-assembled peptides (SAPs).[103,221–223] Self-assembled peptides are polypeptides that assemble under specific conditions to form fibers or other kinds of nanoscale structures.[223,224] Typically, these fibers assemble in hydrophilic environments such that they form specific structures from a variety of different assembly methods.
For example, Stupp and collaborators[224–228] have developed a class of self-assembled peptides made from amphiphilic molecules, which are made from a class of polypeptides linked to a polycarbon chain (Fig. 7). The polypeptide region is typically hydrophilic while the hydrocarbon chain is hydrophobic. These polypeptides can self-assemble into rodlike shapes. This occurs partially due to the assembly of the hydrophobic regions as well as the charge shielding of the hydrophilic end groups by salts and ionic molecules in the solution.[228] A number of studies have demonstrated that amphiphilic self-assembled peptides can be used for a variety of tissue engineering applications.[103,229,230] These molecules can be decorated with a variety of functional units to increase cellular adhesion and allow signaling to cell surface receptors. For example, laminin and fibronectin peptide domains can be attached onto the self-assembled peptides that are made using these amphiphilic molecules.[230] Furthermore, it is possible to encapsulate molecules and allow their sustained release from hydrogels made with self-assembled peptides.
Figure 7.
Structure of peptide amphiphile nanofiber for self-assembly into a fibrous crosslinked scaffold for bone tissue engineering applications. Reproduced with permission from [229]. Copyright 2001 American Association for the Advancement of Science.
Zhang and collaborators[105,123,231,232] fabricated another class of SAPs where peptides self-assembled into beta sheets that can subsequently form hydrogels. It has been demonstrated that a variety of cell types can be encapsulated within these hydrogels and that they can be used for generating three-dimensional environments for cell culture and tissue engineering applications.[105,123] For example, it has been found that adult stem cells can be cultured within these self-assembling peptides.[132] More recently, a number of other self-assembling peptides have been generated which assemble based on similar approaches.[123] These self-assembled peptides provide a number of unique advantages, such as the ability to form gels and relatively easy gel functionalization.[232] However, these gels are typically mechanically weak, and cannot be used for tissue engineering applications that require high mechanical integrity of the resulting gel structures. The data in literature suggests that self-assembled peptides are potentially a powerful approach to generate tissuelike, engineered structures. Recently, it has been demonstrated that the controlled assembly of self-assembled peptides, along with molecules such as HA domains, can be used to form strong membranes that can potentially be useful for generating tissue engineered structures, such as blood vessels and membranes. It is envisioned that future developments in this area could be beneficial for generating artificial tissues.
6. Advanced Fabrication Methods
As stated before, one of the main challenges for using hydrogels for tissue engineering is to recreate the complex microarchitecture and vascularization of native tissues.[4,94,233–235] Recently, the use of microscale technologies has been proposed as a method of addressing a number of challenges associated with difficulties in making tissue constructs that mimic the function and appearance of tissues in the body.[15] To engineer hydrogels with microscale resolution, a number of techniques have been developed.[234] Current approaches that are used for making hydrogels with controlled features can be categorized based on the technologies used to crosslink the gel during fabrication, including approaches such as emulsification, micromolding, photolithography, and microfluidic techniques. These techniques are shown schematically in Figure 8.
Figure 8.
Various microfabrication techniques for hydrogels. Images adapted with permission from [179,236–240]. From top to bottom respectively, copyright 2005 Royal Society of Chemistry, 2006 Elsevier, 2001 American Chemical Society (ACS), 2006 Wiley-VCH, 2006 ACS, and 2003 Elsevier.
6.1. Bottom-Up Approach
To use microfabricated gels for tissue engineering, it is important to create tissue complexity within engineered tissues.[234] Hydrogel microfabrication approaches for developing tissue engineering materials can be categorized into two types, namely “bottom-up” and “top-down.” Each of these approaches utilizes a unique way of engineering tissue complexity within hydrogel-based tissue scaffolds.
The bottom-up approach to using hydrogels for tissue engineering utilizes individual hydrogel building blocks. These hydrogel blocks can range from tens to hundreds of micrometers and typically comprise cells encapsulated within each block. This approach aims to use the concept of repeating functional units that are present in native tissues.
For example, in hepatic tissue, a repeat unit that performs much of the function of the liver is the lobule. Thus, if one can microengineer a lobule, which is a hexagonal-shaped structure, then it may be possible to duplicate a major portion of liver function. These structures can be assembled in packed beds and thus have natural tissuelike complexity and function.[234]
Early work in this area was performed by Sefton and colleagues[215] in which a packed bed of collagen rods was coated with endothelial cells and encapsulated hepatocytes. In this approach, the collagen rods were packed inside a larger tube to generate a miniature liverlike structure. It was demonstrated that the presence of endothelial cells on the packed bed of collagen rods delayed blood coagulation. However, one challenge with these devices was the difficulty in generating more complex structures comprising different cell types. To address this challenge, a number of approaches have been developed. For example, it has been proposed that by using cell printers, individual building blocks made from cells and hydrogels can be deposited on top of each other in a controllable manner.[238,241] More recently, approaches based on self-assembly or directed assembly of hydrogels have been developed. In one example, two phase reactors consisting of hydrophobic and hydrophilic components were used to assemble hydrogels of controlled shapes in a predetermined manner.[242] In this approach, it was demonstrated that lock- and- key-shaped hydrogels can be induced to assemble into defined structures by using the surface tension between the oil and the hydrogel phase as the driving force. As the hydrogel phase minimizes its interactions with the oil phase, it will self-assemble into structures that minimize overall energy. It was demonstrated that the cells within these types of structures can maintain their viability for tissue engineering applications.
Although bottom-up tissue engineering shows significant potential in addressing some major challenges in regenerative medicine, a significant number remain. One of the main challenges is the lack of appropriate ways to generate large tissues in a reproducible manner. Currently, cell and tissue printing techniques still suffer from a number of technical issues such as cell blockages within the structures.[243] Furthermore, other techniques such as the random packing or the directed assembly of microgels are still under active investigation and further work is required to determine whether they will be feasible in generating 3D tissues.
6.2. Top-Down Approach
The top-down approach is derived from attempts to create microvasculature within polymeric scaffolds. This approach makes possible the synthesis of gels for generating tissue constructs with defined structures. This can be done using a number of different systems. One variation of this approach uses laser fabrication or photolithography to generate scaffolds with controlled porosity. The scaffold porosity can be used to generate interconnected pores as in standard tissue scaffolds. Alternatively, this approach can be used to engineer capillary structures that can be used to mimic tissue vascular beds and have comparable transport characteristics.[244–246] Additionally, it has been observed that radial diffusion from a microfluidic channel in a hydrogel results in predictable cell viability as a function of distance.[244] Collagen channels have been lined with endothelial cells to mimic native vessel structures.[68] This is accomplished either by stacking layers or by rolling individual sheets to generate 3D tissue constructs with embedded vasculature. Alternative top-down approaches, such as leaching of polymeric beads or other types of porogens, can also be used to generate controlled hydrogel structures.[247]
Future work in this area appears extremely promising, especially if scale-up can be improved and other microfabricated bioengineering devices (e.g., biosensors[248] and therapeutic delivery systems) can be incorporated to provide integrated solutions in regenerative medicine.[249]
6.3. Emulsification
Emulsification has historically been used to fabricate hydrogel microspheres by putting hydrogel precursors in a hydrophobic medium (such as oil) and breaking up the hydrogel phase into small droplets by agitation. Based on the agitation conditions, the size of the microgels can be controlled. Spherical microgels made by emulsification have been used for variety of microencapsulation techniques including immunoisolation.[250,251] In these approaches, the microgel is used to isolate transplanted cells from the host’s immune system, while enabling the exchange of oxygen and nutrients as well as cell-secreted metabolic products between the hydrogel and the surrounding environment. Emulsification can be also used to encapsulate ESCs within microgels as an in vitro culture to generate more controllable environments for differentiation.[252,253] Although emulsification is a relatively simple process, it does contain a number of potential limitations. For example, the shape of the resulting gels is usually limited to spheres, and despite the ability to control the resulting sizes, there will always be some degree of heterogeneity in the resulting spherical gels.
6.4. Molding
To enable additional control of the size and heterogeneity of gels, micromolding can be used. Micromolding is an approach through which a prefabricated mold is used to shape and then crosslink hydrogel precursors into desired forms.[178,179,254] The emergence of biological microelectromechanical system (Bio-MEMS) technology have led to the increased adaptation of micromolding. In these approaches, microprocessor fabrication technologies widely used in the microelectronics industry, such as lithography, etching, and deposition, have been used to fabricate micromolded hydrogels with desired features.[15,234,255] Micromolding has been mostly used to fabricate structures, such as heat-crosslinkable polymers including collagen,[256] agarose,[244] and gelatin[68,257] as well as photocrosslinkable polymers (e.g., methacrylated PEG and HA[179]). Cells have been able to remain viable inside both of these types of structures.[237,258]
One challenge with micromolding has been to fabricate harvestable microstructures from chemically crosslinkable hydrogels, such as alginate and chitosan. Although researchers have demonstrated that alginate can be micromolded,[246] the generation of small free floating alginate structures has been difficult to attain using molds from poly(dimethylsiloxane) (PDMS). To address this challenge, a technique has been developed based on micromolding of hydrogels using other gels as templates.[259] In this approach, the gel precursor is initially formed using the hydrogel mold and a crosslinking agent is then added across the mold to crosslink the resulting structures into a new gel. Using this approach, alginate and chitosan microstructures were fabricated and have shown to encapsulate cells within controlled structures.
Molding has also been used to fabricate nanoscale hydrogel structures. To do this, it is important to use molds that can dehydrate the hydrogels in regions that make contact between the mold and the substrate. This has been achieved by using fluorinated polymers that can be subsequently crosslinked to generate nanoscale molds.[260] These molds can be used to fabricate nanoscale materials with controlled shapes and sizes in a reproducible manner.[261] Interestingly, it has been demonstrated that the shape of nanoparticles can be used to regulate the targeting of drug carriers,[262] and thus the ability to control the shape of nanoscale microgels may be of clinical benefit for drug delivery.
6.5. Photolithography
Another approach to fabricate hydrogels is by photolithography.[237,263,264] Photolithography is a technique in which photocrosslinkable hydrogels are placed underneath a mask that controls the exposure of light to particular regions of a film of hydrogel precursors. Where the light is exposed, the photocrosslinkable hydrogel will crosslink to generate structures that are in the shape of the mask. With photolithography, structures spanning sub-micrometer- to millimeter-scale can be generated. Photolithography has been shown to be compatible with variety of polymers.[237,263,265,266]
As previously discussed, an area of caution with photocrosslinkable gels to create cell-loaded structures is the cytotoxicity of the photoinitator and UV light exposure. A number of studies have systematically examined the effects of UV dosage, as well as photoinitiator and hydrogel precursor concentration on cell behavior.[179] More recently, the development of photocrosslinkable systems that utilize blue light for crosslinking has further advanced and improved the safety of this technology for tissue engineering applications.[58]
In addition to photolithography, other methods have also been developed to generate controllable 3D shapes by focusing and scanning light. In one approach known as laser scanning lithography, focused laser light has been used to crosslink photoactive hydrophilic polymers in specific regions.[236] By using similar approaches, it is possible to build complex tissue scaffolds one layer at a time.[267] Furthermore, it is possible to use focused light to conjugate bioactive molecules within prefabricated gels.[268] Such an approach has been used to pattern photoactive RGD peptides within agarose gels to generate adhesive pathways that enabled directed cell migration into gels.
6.6. Microfluidics
A final method to create microscale structures from hydrogels is by using microfluidics. A variety of approaches have been recently used to create microscale hydrogels by creating single or multi-phase flows within microfluidic channels. Often, hydrogel precursors and cells flow through a microchannel that controls the resulting shape of the generated hydrogel.[269–271] By layering these cell-laden microgels on each other, intricate 3D structures have been generated, in which multiple cell types can be patterned relative to each other to recreate tissuelike complexity. Also, the ability to generate distinct fluidic features, such as concentration gradients, has been used to generate materials with spatially distinct features.[272] For example, microfluidic generators have been used to create hydrogels with concentration gradients of bioactive molecules[100] or substrate elasticities.[273] Additionally, two-phase systems composed of hydrophilic droplets in a hydrophobic medium are used to generate hydrogel droplets with controllable physical properties. As the hydrogel precursor suspension flows through the channel, exposure to a crosslinking agent forms a gel.
Finally, the ability to integrate approaches such as microfluidics and photolithography has been used to engineer unique hydrogels. In one example, microfluidic channels were used to generate microengineered hydrogels using processes in which a stream of gel precursors was exposed to light that passed through a mask and was focused with a microscope.[274] As the fluid is exposed to the light, the hydrogel will crosslink to form microgels that will be subsequently collected at the microchannel outlet. By using this process, it is possible to encapsulate cells in hydrogels of carefully controlled shapes.[275]
7. Concluding Remarks
Hydrogels are fundamentally biocompatible due to their hydrophilicity and intrinsic similarity to our own anatomical framework or ECM. They are highly customizable as 3D networks, with a very large selection of available constituents, synthesis techniques, and fabrication methodologies. For these reasons, hydrogels have been used extensively in many biological and clinical applications, including drug delivery and tissue engineering.
In this review, we have attempted to give the necessary fundamentals of hydrogel properties, synthesis, and fabrication options to illustrate what is really achievable from a materials standpoint. We have also attempted to define the corresponding uses of hydrogels in tissue engineering, or regenerative medicine in the larger sense, by covering the most essential concepts and recent discoveries currently driving this highly multidisciplinary field.
Future advances in tissue engineering, as well as in related fields, will require thoughtful integration to ensure regenerative medicine lives up to its true clinical potential. Johnson et al.[276] systematically identified the strategic directions for tissue engineering in 2007. According to their findings, particular focus in stem cell science, angiogenesis, and molecular biology are among the most important areas where increased attention may help guide the most significant leaps to come. We further suggest that the inclusion of ‘intelligence’ or self-guided features should emerge in materials for regenerative medicine. Eventually, functional flexibility for dynamic biological demands can be incorporated into tissue constructs and thus enhance the integration of the surrounding tissues and the tissue cultures. Additionally, greater attention to microfabrication techniques should continue so that organlike complexity in engineered tissues becomes a reality.
Acknowledgements
This work is dedicated to Professor Robert Langer of MIT on the occasion of his 60th birthday. Dr. Langer is a long-time collaborator and personal friend of the senior author, Nicholas A. Peppas. He is also a continuing source of inspiration for two other authors, Omar Z. Fisher and Ali Khademhosseini, who have worked in Dr. Langer’s laboratories. This work is supported in part by grants DGE-0333080 from the National Science Foundation and EB-000246 from the National Institute of Health. This article is part of a special issue on Regenerative Medicine that is published in honor of Prof. Robert Langer on the occasion of his 60th birthday.
Biography

Nicholas A. Peppas is the Fletcher S. Pratt Chair in Chemical Engineering, Biomedical Engineering, and Pharmacy, and is the director of the Center for Biomaterials, Drug Delivery and Bionanotechnology at the University of Texas at Austin. He is a member of the National Academy of Engineering, the Institute of Medicine of the National Academies, and the National Academy of Pharmacy of France. He received his Diploma in Engineering (D. Eng.) from the National Technical University of Athens, Greece in 1971 and his Sc.D. from MIT in 1973, both in chemical engineering.
Contributor Information
Brandon V. Slaughter, Department of Biomedical Engineering, C0800, The University of Texas at Austin, Austin, TX 78712 (USA)
Shahana S. Khurshid, Department of Biomedical Engineering, C0800, The University of Texas at Austin, Austin, TX 78712 (USA)
Omar Z. Fisher, Department of Biomedical Engineering, C0800, The University of Texas at Austin, Austin, TX 78712 (USA)
Ali Khademhosseini, Center for Biomedical Engineering, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Harvard-MIT Division of Health Sciences and Technology, Massachusetts, Institute of Technology, Cambridge, MA 02139 (USA).
Nicholas A. Peppas, Email: peppas@che.utexas.edu, Biomaterials, Drug Delivery, Bionanotechnology, and Molecular, Recognition Laboratories, Department of Chemical Engineering, C0400, The University of Texas at Austin, Austin, TX 78712 (USA); Department of Pharmaceutics, C0400, The University of Texas at Austin, Austin, TX 78712 (USA); Department of Biomedical Engineering, C0800, The University of Texas at Austin, Austin, TX 78712 (USA).
References
- 1.Vacanti JP, Morse MA, Saltzman WM, Domb AJ, Perezatayde A, Langer R. J Pediatr. Surg. 1988;23:3. doi: 10.1016/s0022-3468(88)80529-3. [DOI] [PubMed] [Google Scholar]
- 2.Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 1997–2006, Department of Health and Human Services (HHS), Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation, Rockville, MD 2007. The data and analyses reported in this report have been supplied by the United Network for Organ Sharing, VA and Arbor Research Collaborative for Health, MI under contract with HHS. The authors alone are responsible for reporting and interpreting these data.
- 3.Peppas NA, Huang Y, Torres-Lugo M, Ward JH, Zhang J. Annu. Rev. Biomed. Eng. 2000;2:9. doi: 10.1146/annurev.bioeng.2.1.9. [DOI] [PubMed] [Google Scholar]
- 4.Lee KY, Mooney DJ. Chem. Rev. 2001;101:1869. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 5.Malafaya PB, Silva GA, Reis RL. Adv. Drug Delivery Rev. 2007;59:207. doi: 10.1016/j.addr.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Peppas NA. Hydrogels in Medicine and Pharmacy. I. Boca Raton, FL: CRC Press; 1987. p. 180. [Google Scholar]
- 7.Hassan CM, Peppas NA. Biopolymers/PVA Hydrogels/Anionic Polymerisation Nanocomposites. Berlin: Springer; 2004. pp. 37–65. [Google Scholar]
- 8.Hassan CM, Stewart JE, Peppas NA. Eur. J. Pharm. Biopharm. 2000;49:161. doi: 10.1016/s0939-6411(99)00056-9. [DOI] [PubMed] [Google Scholar]
- 9.Brannon-Peppas L, Peppas NA. Chem. Eng. Sci. 1991;46:715. [Google Scholar]
- 10.Lowman AM, Dziubla TD, Bures P, Peppas NA. Molecular and Cellular Foundations of Biomaterials. In: Sefton M, Peppas NA, editors. Advances in Chemical Engineering. Vol. 29. New York: Academic Press; 2004. p. 248. [Google Scholar]
- 11.Flory PJ, Rehner J. J Chem. Phys. 1943;11:521. [Google Scholar]
- 12.Peppas NA, Merrill EW. J Appl. Polym. Sci. 1977;21:1763. [Google Scholar]
- 13.Canal T, Peppas NA. J Biomed. Mater. Res. 1989;23:1183. doi: 10.1002/jbm.820231007. [DOI] [PubMed] [Google Scholar]
- 14.Billmeyer FW. Textbook of Polymer Science. New York: Wiley; 1984. [Google Scholar]
- 15.Peppas NA, Hilt JZ, Khademhosseini A, Langer R. Adv. Mater. 2006;18:1345. [Google Scholar]
- 16.Anseth KS, Bowman CN, Brannon-Peppas L. Biomaterials. 1996;17:1647. doi: 10.1016/0142-9612(96)87644-7. [DOI] [PubMed] [Google Scholar]
- 17.Flory PJ. Principles of Polymer Chemistry. Ithaca, NY: Cornell University Press; 1963. [Google Scholar]
- 18.Chen RR, Mooney DJ. Pharm. Res. 2003;20:1103. doi: 10.1023/a:1025034925152. [DOI] [PubMed] [Google Scholar]
- 19.Ende MTA, Hariharan D, Peppas NA. React. Polym. 1995;25:127. [Google Scholar]
- 20.Brannon-Peppas L, Peppas NA. J Controlled Release. 1989;8:267. [Google Scholar]
- 21.Collins MC, Ramirez WF. J Phys. Chem. 1979;83:2294. [Google Scholar]
- 22.Gudeman LF, Peppas NA. J Membr. Sci. 1995;107:239. [Google Scholar]
- 23.Crank J. The Mathematics of Diffusion. 2nd ed. Oxford: Oxford Science Publications; 1975. [Google Scholar]
- 24.Crank GS, Park J. Diffusion in Polymers. New York: Academic; 1968. p. 452. [Google Scholar]
- 25.Renkin LF. J Gen. Physiol. 1954;38:225. [PMC free article] [PubMed] [Google Scholar]
- 26.Pappenheimer JR. J Physiol. Rev. 1953;33:387. doi: 10.1152/physrev.1953.33.3.387. [DOI] [PubMed] [Google Scholar]
- 27.Anderson JL, Quinn JA. Biophys. J. 1974;14:130. doi: 10.1016/S0006-3495(74)70005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peppas NA, Reinhart CT. J Membr. Sci. 1983;15:275. [Google Scholar]
- 29.Reinhart CT, Peppas NA. J Membr. Sci. 1984;18:227. [Google Scholar]
- 30.Sassi AP, Blanch HW, Prausnitz JM. J Appl. Polym. Sci. 1996;59:1337. [Google Scholar]
- 31.Ende MTA, Peppas NA. J Appl. Polym. Sci. 1996;59:673. [Google Scholar]
- 32.Gudeman LF, Peppas NA. J Membr. Sci. 1995;107:239. [Google Scholar]
- 33.Amsden B. Macromolecules. 1998;31:8382. [Google Scholar]
- 34.Muhr AH, Blanshard VJM. Polymer. 1982;23:1012. [Google Scholar]
- 35.Peppas NA, Meadows DL. J Membr. Sci. 1983;16:361. [Google Scholar]
- 36.Langer R, Vacanti J. Science. 1993;260:920. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 37.Shin H, Mikos AG. Biomaterials. 2003;24:4353. doi: 10.1016/s0142-9612(03)00339-9. [DOI] [PubMed] [Google Scholar]
- 38.Hersel U, Dahmen C, Kessler H. Biomaterials. 2003;24:4385. doi: 10.1016/s0142-9612(03)00343-0. [DOI] [PubMed] [Google Scholar]
- 39.Elisseeff J, Anseth K, Sims D, McIntosh W, Randolph M, Langer R. Proc. Natl. Acad. Sci. USA. 1999;96:3104. doi: 10.1073/pnas.96.6.3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elisseeff J, McIntosh W, Anseth K, Riley S, Ragan P, Langer R. J Biomed. Mater. Res. 2000;51:164. doi: 10.1002/(sici)1097-4636(200008)51:2<164::aid-jbm4>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 41.Suggs LJ, Mikos AG. Cell Transplant. 1999;8:345. doi: 10.1177/096368979900800402. [DOI] [PubMed] [Google Scholar]
- 42.Mann BK, Gobin AS, Tsai AT, Schmedlen RH, West JL. Biomaterials. 2001;22:3045. doi: 10.1016/s0142-9612(01)00051-5. [DOI] [PubMed] [Google Scholar]
- 43.Nguyen KT, West JL. Biomaterials. 2002;23:4307. doi: 10.1016/s0142-9612(02)00175-8. [DOI] [PubMed] [Google Scholar]
- 44.Hillwest JL, Chowdhury SM, Slepian MJ, Hubbell JA. Proc. Natl. Acad. Sci. USA. 1994;91:5967. doi: 10.1073/pnas.91.13.5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.West JL, Hubbell JA. Proc. Natl. Acad. Sci. USA. 1996;93:13188. doi: 10.1073/pnas.93.23.13188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.HillWest JL, Dunn RC, Hubbell JA. J Surg. Res. 1995;59:759. doi: 10.1006/jsre.1995.1236. [DOI] [PubMed] [Google Scholar]
- 47.Sawhney AS, Pathak CP, van Rensburg JJ, Dunn RC, Hubbell JA. J Biomed. Mater. Res. 1994;28:831. doi: 10.1002/jbm.820280710. [DOI] [PubMed] [Google Scholar]
- 48.An YJ, Hubbell JA. J Controlled Release. 2000;64:205. doi: 10.1016/s0168-3659(99)00143-1. [DOI] [PubMed] [Google Scholar]
- 49.Chowdhury SM, Hubbell JA. J Surg. Res. 1996;61:58. doi: 10.1006/jsre.1996.0081. [DOI] [PubMed] [Google Scholar]
- 50.Lu SX, Ramirez WF, Anseth KS. J Pharm. Sci. 2000;89:45. doi: 10.1002/(SICI)1520-6017(200001)89:1<45::AID-JPS5>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 51.Bergmann NM, Peppas NA. Prog. Polym. Sci. 2008;33:271. [Google Scholar]
- 52.Peppas NA, Kim B. J Drug Delivery Sci. Technol. 2006;16:11. [Google Scholar]
- 53.Peppas NA. MRS Bull. 2006;31:888. [Google Scholar]
- 54.Ladet S, David L, Domard A. Nature. 2008;452:76. doi: 10.1038/nature06619. [DOI] [PubMed] [Google Scholar]
- 55.Cruise GM, Hegre OD, Lamberti FV, Hager SR, Hill R, Scharp DS, Hubbell JA. Cell Transplant. 1999;8:293. doi: 10.1177/096368979900800310. [DOI] [PubMed] [Google Scholar]
- 56.Guyton A, Hall J. Textbook of Medical Physiology. 10th ed. Philadelphia, PA: Elsevier Saunders; 2000. p. 1064. [Google Scholar]
- 57.Williams DF. The Williams’ Dictionary of Biomaterials. Liverpool, UK: Liverpool University Press; 1999. [Google Scholar]
- 58.Bryant SJ, Nuttelman CR, Anseth KS. J Biomater. Sci. Polym. Ed. 2000;11:439. doi: 10.1163/156856200743805. [DOI] [PubMed] [Google Scholar]
- 59.Stevens MM, Marini RP, Schaefer D, Aronson J, Langer R, Shastri VP. Proc. Natl. Acad. Sci. USA. 2005;102:11450. doi: 10.1073/pnas.0504705102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ko HCH, Milthorpe BK, McFarland CD. Eur. Cells Mater. 2007;14:1. doi: 10.22203/ecm.v014a01. [DOI] [PubMed] [Google Scholar]
- 61.Lee KY, Peters MC, Mooney DJ. J Controlled Release. 2003;87:49. doi: 10.1016/s0168-3659(02)00349-8. [DOI] [PubMed] [Google Scholar]
- 62.Lee KY, Peters MC, Anderson KW, Mooney DJ. Nature. 2000;408:998. doi: 10.1038/35050141. [DOI] [PubMed] [Google Scholar]
- 63.Tabata Y, Hijikata S, Ikada Y. J Controlled Release. 1994;31:189. [Google Scholar]
- 64.Peattie RA, Nayate AP, Firpo MA, Shelby J, Fisher RJ, Prestwich GD. Biomaterials. 2004;25:2789. doi: 10.1016/j.biomaterials.2003.09.054. [DOI] [PubMed] [Google Scholar]
- 65.Peattie RA, Rieke ER, Hewett EM, Fisher RJ, Shu XZ, Prestwich GD. Biomaterials. 2006;27:1868. doi: 10.1016/j.biomaterials.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 66.Bakshi A, Fisher O, Dagci T, Himes BT, Fischer I, Lowman A. J Neurosurg. Spine. 2004;1:322. doi: 10.3171/spi.2004.1.3.0322. [DOI] [PubMed] [Google Scholar]
- 67.Zisch AH, Lutolf MP, Ehrbar M, Raeber GP, Rizzi SC, Davies N, Schmokel H, Bezuidenhout D, Djonov V, Zilla P, Hubbell JA. FASEB J. 2003;17:2260. doi: 10.1096/fj.02-1041fje. [DOI] [PubMed] [Google Scholar]
- 68.Golden AP, Tien J. Lab Chip. 2007;7:720. doi: 10.1039/b618409j. [DOI] [PubMed] [Google Scholar]
- 69.Ferreira LS, Gerecht S, Fuller J, Shieh HF, Vunjak-Novakovic G, Langer R. Biomaterials. 2007;28:2706. doi: 10.1016/j.biomaterials.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gerecht S, Burdick JA, Ferreira LS, Townsend SA, Langer R, Vunjak-Novakovic G. Proc. Natl. Acad. Sci. USA. 2007;104:11298. doi: 10.1073/pnas.0703723104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Toole BP. Semin. Cell Dev. Biol. 2001;12:79. doi: 10.1006/scdb.2000.0244. [DOI] [PubMed] [Google Scholar]
- 72.Feinberg RN, Beebe DC. Science. 1983;220:1177. doi: 10.1126/science.6857242. [DOI] [PubMed] [Google Scholar]
- 73.Bullen EC, Longaker MT, Updike DL, Benton R, Ladin D, Hou ZZ, Howard EW. J Invest. Dermatol. 1995;104:236. doi: 10.1111/1523-1747.ep12612786. [DOI] [PubMed] [Google Scholar]
- 74.Ratner BD. Biomaterials Science: An Introduction to Materials in Medicine. 2nd ed. Amsterdam: Elsevier Academic Press; 2004. p. 851. [Google Scholar]
- 75.Bryant SJ, Anseth KS. J Biomed. Mater. Res, Part A. 2003;64:70. doi: 10.1002/jbm.a.10319. [DOI] [PubMed] [Google Scholar]
- 76.Lutolf MP, Lauer-Fields JL, Schmoekel HG, Metters AT, Weber FE, Fields GB, Hubbell JA. Proc. Natl. Acad. Sci. USA. 2003;100:5413. doi: 10.1073/pnas.0737381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kraehenbuehl TP, Zammaretti P, Van der Vlies AJ, Schoenmakers RG, Lutolf MP, Jaconi ME, Hubbell JA. Biomaterials. 2008;29:2757. doi: 10.1016/j.biomaterials.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 78.Adelow C, Segura T, Hubbell JA, Frey P. Biomaterials. 2008;29:314. doi: 10.1016/j.biomaterials.2007.09.036. [DOI] [PubMed] [Google Scholar]
- 79.Park Y, Lutolf MP, Hubbell JA, Hunziker EB, Wong M. Tissue Eng. 2004;10:515. doi: 10.1089/107632704323061870. [DOI] [PubMed] [Google Scholar]
- 80.Seliktar D, Zisch AH, Lutolf MP, Wrana JL, Hubbell JA. J Biomed. Mater. Res, Part A. 2004;68:704. doi: 10.1002/jbm.a.20091. [DOI] [PubMed] [Google Scholar]
- 81.Ingber DE. FASEB J. 2006;20:811. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- 82.Engler A, Bacakova L, Newman C, Hategan A, Griffin M, Discher D. Biophys. J. 2004;86:617. doi: 10.1016/S0006-3495(04)74140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Engler AJ, Sen S, Sweeney HL, Discher DE. Cell. 2006;126:677. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 84.Bryant SJ, Anseth KS, Lee DA, Bader DL. J Orthop. Res. 2004;22:1143. doi: 10.1016/j.orthres.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 85.Bryant SJ, Chowdhury TT, Lee DA, Bader DL, Anseth KS. Ann. Biomed. Eng. 2004;32:407. doi: 10.1023/b:abme.0000017535.00602.ca. [DOI] [PubMed] [Google Scholar]
- 86.Bryant SJ, Durand KL, Anseth KS. J Biomed. Mater. Res, Part A. 2003;67:1430. doi: 10.1002/jbm.a.20003. [DOI] [PubMed] [Google Scholar]
- 87.Bryant SJ, Anseth KS. J Biomed. Mater. Res. 2002;59:63. doi: 10.1002/jbm.1217. [DOI] [PubMed] [Google Scholar]
- 88.Ratner BD. J Biomed. Mater. Res. 1993;27:837. doi: 10.1002/jbm.820270702. [DOI] [PubMed] [Google Scholar]
- 89.Brauker JH, Carr-Brendel VE, Martinson LA, Crudele J, Johnston WD, Johnson RC. J Biomed. Mater. Res. 1995;29:1517. doi: 10.1002/jbm.820291208. [DOI] [PubMed] [Google Scholar]
- 90.Chirila TV. Biomaterials. 2001;22:3311. doi: 10.1016/s0142-9612(01)00168-5. [DOI] [PubMed] [Google Scholar]
- 91.Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT. Nature. 2003;425:841. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 92.Fawcett JW, Asher RA. Brain Res. Bull. 1999;49:377. doi: 10.1016/s0361-9230(99)00072-6. [DOI] [PubMed] [Google Scholar]
- 93.Freund P, Schmidlin E, Wannier T, Bloch J, Mir A, Schwab ME, Rouiller EM. Nat. Med. 2006;12:790. doi: 10.1038/nm1436. [DOI] [PubMed] [Google Scholar]
- 94.Khademhosseini A, Langer R, Borenstein J, Vacanti JP. Proc. Natl. Acad. Sci. USA. 2006;103:2480. doi: 10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lutton C, Goss B. Nat. Biotechnol. 2008;26:613. doi: 10.1038/nbt0608-613. [DOI] [PubMed] [Google Scholar]
- 96.Lutolf MP, Hubbell JA. Nat. Biotechnol. 2005;23:47. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 97.Ingber DE, Levin M. Development. 2007;134:2541. doi: 10.1242/dev.003707. [DOI] [PubMed] [Google Scholar]
- 98.Suh KY, Seong J, Khademhosseini A, Laibinis PE, Langer R. Biomaterials. 2004;25:557. doi: 10.1016/s0142-9612(03)00543-x. [DOI] [PubMed] [Google Scholar]
- 99.Karp JM, Yeo Y, Geng WL, Cannizarro C, Yan K, Kohane DS, Vunjak-Novakovic G, Langer RS, Radisic M. Biomaterials. 2006;27:4755. doi: 10.1016/j.biomaterials.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 100.Burdick JA, Khademhosseini A, Langer R. Langmuir. 2004;20:5153. doi: 10.1021/la049298n. [DOI] [PubMed] [Google Scholar]
- 101.Burdick JA, Anseth KS. Biomaterials. 2002;23:4315. doi: 10.1016/s0142-9612(02)00176-x. [DOI] [PubMed] [Google Scholar]
- 102.Burdick JA, Mason MN, Hinman AD, Thorne K, Anseth KS. J Controlled Release. 2002;83:53. doi: 10.1016/s0168-3659(02)00181-5. [DOI] [PubMed] [Google Scholar]
- 103.Sargeant TD, Guler MO, Oppenheimer SM, Mata A, Satcher RL, Dunand DC, Stupp SI. Biomaterials. 2008;29:161. doi: 10.1016/j.biomaterials.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 104.Ryu JH, Kim IK, Cho MC, Hwang KK, Piao H, Piao S, Lim SH, Hong YS, Choi CY, Yoo KJ, Kim BS. Biomaterials. 2005;26:319. doi: 10.1016/j.biomaterials.2004.02.058. [DOI] [PubMed] [Google Scholar]
- 105.Davis ME, Motion JPM, Narmoneva DA, Takahashi T, Hakuno D, Kamm RD, Zhang SG, Lee RT. Circulation. 2005;111:442. doi: 10.1161/01.CIR.0000153847.47301.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Magnani A, Rappuoli R, Lamponi S, Barbucci R. Polym. Adv. Technol. 2000;11:488. [Google Scholar]
- 107.Park SH, Park SR, Chung SI, Pai KS, Min BH. Artif. Organs. 2005;29:838. doi: 10.1111/j.1525-1594.2005.00137.x. [DOI] [PubMed] [Google Scholar]
- 108.Elisseeff J, McIntosh W, Fu K, Blunk T, Langer R. J Orthop. Res. 2001;19:1098. doi: 10.1016/S0736-0266(01)00054-7. [DOI] [PubMed] [Google Scholar]
- 109.Hwang NS, Varghese S, Theprungsirikul P, Canver A, Elisseeff J. Biomaterials. 2006;27:6015. doi: 10.1016/j.biomaterials.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 110.Lee HJ, Lee JS, Chansakul T, Yu C, Elisseeff JH, Yu SM. Biomaterials. 2006;27:5268. doi: 10.1016/j.biomaterials.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 111.Yang F, Williams CG, Wang DA, Lee H, Manson PN, Elisseeff J. Biomaterials. 2005;26:5991. doi: 10.1016/j.biomaterials.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 112.Varghese S, Hwang NS, Canver AC, Theprungsirikul P, Lin DW, Elisseeff J. Matrix Biol. 2008;27:12. doi: 10.1016/j.matbio.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 113.Martens PJ, Bryant SJ, Anseth KS. Biomacromolecules. 2003;4:283. doi: 10.1021/bm025666v. [DOI] [PubMed] [Google Scholar]
- 114.Stevens MM, Qanadilo HF, Langer R, Shastri VP. Biomaterials. 2004;25:887. doi: 10.1016/j.biomaterials.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 115.Willers C, Chen J, Wood D, Zheng MH. Tissue Eng. 2005;11:1065. doi: 10.1089/ten.2005.11.1065. [DOI] [PubMed] [Google Scholar]
- 116.Liao E, Yaszemski M, Krebsbach P, Hollister S. Tissue Eng. 2007;13:537. doi: 10.1089/ten.2006.0117. [DOI] [PubMed] [Google Scholar]
- 117.Williams CG, Kim TK, Taboas A, Malik A, Manson P, Elisseeff J. Tissue Eng. 2003;9:679. doi: 10.1089/107632703768247377. [DOI] [PubMed] [Google Scholar]
- 118.Elisseeff J, Anseth K, Sims D, McIntosh W, Randolph M, Yaremchuk M, Langer R. Plast. Reconstr. Surg. 1999;104:1014. doi: 10.1097/00006534-199909040-00017. [DOI] [PubMed] [Google Scholar]
- 119.Sharma B, Williams CG, Khan M, Manson P, Elisseeff JH. Plast. Reconstr. Surg. 2007;119:112. doi: 10.1097/01.prs.0000236896.22479.52. [DOI] [PubMed] [Google Scholar]
- 120.Gong YH, He LJ, Li J, Zhou QL, Ma ZW, Gao CY, Shen JC. J Biomed. Mater. Res, Part B. 2007;82:192. doi: 10.1002/jbm.b.30721. [DOI] [PubMed] [Google Scholar]
- 121.Tang SQ, Vickers SM, Hsu HP, Spector M. J Biomed. Mater. Res, Part A. 2007;82:323. doi: 10.1002/jbm.a.30974. [DOI] [PubMed] [Google Scholar]
- 122.Dare EV, Vascotto SG, Carlsson DJ, Hincke MT, Griffith M. Int. J. Artif. Organs. 2007;30:619. doi: 10.1177/039139880703000710. [DOI] [PubMed] [Google Scholar]
- 123.Kisiday J, Jin M, Kurz B, Hung H, Semino C, Zhang S, Grodzinsky AJ. Proc. Natl. Acad. Sci. USA. 2002;99:9996. doi: 10.1073/pnas.142309999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shu XZ, Ghosh K, Liu YC, Palumbo FS, Luo Y, Clark RA, Prestwich GD. J Biomed. Mater. Res, Part A. 2004;68:365. doi: 10.1002/jbm.a.20002. [DOI] [PubMed] [Google Scholar]
- 125.Shu XZ, Ahmad S, Liu YC, Prestwich GD. J Biomed. Mater. Res, Part A. 2006;79:902. doi: 10.1002/jbm.a.30831. [DOI] [PubMed] [Google Scholar]
- 126.Pape LG, Balazs EA. Ophthalmology. 1980;87:699. doi: 10.1016/s0161-6420(80)35185-3. [DOI] [PubMed] [Google Scholar]
- 127.Vijayasekaran S, Chirila TV, Robertson TA, Lou X, Fitton JH, Hicks CR, Constable IJ. J Biomater. Sci. Polym. Ed. 2000;11:599. doi: 10.1163/156856200743896. [DOI] [PubMed] [Google Scholar]
- 128.Chang SCN, Rowley JA, Tobias G, Genes NG, Roy AK, Mooney DJ, Vacanti CA, Bonassar LJ. J Biomed. Mater. Res. 2001;55:503. doi: 10.1002/1097-4636(20010615)55:4<503::aid-jbm1043>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 129.Duranti F, Salti G, Bovani B, Calandra M, Rosati ML. Dermatol. Surg. 1998;24:1317. doi: 10.1111/j.1524-4725.1998.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 130.Burns JM, Skinner K, Colt J, Sheidlin A, Bronson R, Yaacobi Y, Goldberg EP. J Surg. Res. 1995;59:644. doi: 10.1006/jsre.1995.1218. [DOI] [PubMed] [Google Scholar]
- 131.Itoh S, Takakuda K, Kawabata S, Aso Y, Kasai K, Itoh H, Shinomiya K. Biomaterials. 2002;23:4475. doi: 10.1016/s0142-9612(02)00166-7. [DOI] [PubMed] [Google Scholar]
- 132.Semino CE, Kasahara J, Hayashi Y, Zhang S. Tissue Eng. 2004;10:643. doi: 10.1089/107632704323061997. [DOI] [PubMed] [Google Scholar]
- 133.Belkas JS, Munro CA, Shoichet MS, Johnston M, Midha R. Biomaterials. 2005;26:1741. doi: 10.1016/j.biomaterials.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 134.Cruise GM, Hegre OD, Scharp DS, Hubbell JA. Biotechnol. Bioeng. 1998;57:655. doi: 10.1002/(sici)1097-0290(19980320)57:6<655::aid-bit3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 135.Bryant SJ, Cuy JL, Hauch KD, Ratner BD. Biomaterials. 2007;28:2978. doi: 10.1016/j.biomaterials.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kirker KR, Luo Y, Nielson JH, Shelby J, Prestwich GD. Biomaterials. 2002;23:3661. doi: 10.1016/s0142-9612(02)00100-x. [DOI] [PubMed] [Google Scholar]
- 137.Thacharodi D, Rao KP. Biomaterials. 1996;17:1307. [PubMed] [Google Scholar]
- 138.Currie LJ, Sharpe JR, Martin R. Plast. Reconstr. Surg. 2001;108:1713. doi: 10.1097/00006534-200111000-00045. [DOI] [PubMed] [Google Scholar]
- 139.Ghosh K, Ren XD, Shu XZ, Prestwich GD, Clark RAF. Tissue Eng. 2006;12:601. doi: 10.1089/ten.2006.12.601. [DOI] [PubMed] [Google Scholar]
- 140.Joosten EAJ, Veldhuis WB, Hamers FPT. J Neurosci. Res. 2004;77:127. doi: 10.1002/jnr.20088. [DOI] [PubMed] [Google Scholar]
- 141.Duflo S, Thibeault SL, Li WH, Shu XZ, Prestwich GD. Tissue Eng. 2006;12:2171. doi: 10.1089/ten.2006.12.2171. [DOI] [PubMed] [Google Scholar]
- 142.Hahn MS, Teply BA, Stevens MM, Zeitels SM, Langer R. Biomaterials. 2006;27:1104. doi: 10.1016/j.biomaterials.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 143.Drury JL, Mooney DJ. Biomaterials. 2003;24:4337. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 144.Kopecek J. Biomaterials. 2007;28:5185. doi: 10.1016/j.biomaterials.2007.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Varghese S, Elisseeff JH. Polymers for Regenerative Medicine. In: Werner C, editor. Advances in Polymer Science. Vol. 203. Berlin: Springer; 2006. p. 95. [Google Scholar]
- 146.Ward JH, Peppas NA. Macromolecules. 2000;33:5137. [Google Scholar]
- 147.Lutolf MP, Hubbell JA. Biomacromolecules. 2003;4:713. doi: 10.1021/bm025744e. [DOI] [PubMed] [Google Scholar]
- 148.Lutolf MP, Tirelli N, Cerritelli S, Cavalli L, Hubbell JA. Bioconjugate Chem. 2001;12:1051. doi: 10.1021/bc015519e. [DOI] [PubMed] [Google Scholar]
- 149.Vanderhooft JL, Mann BK, Prestwich GD. Biomacromolecules. 2007;8:2883. doi: 10.1021/bm0703564. [DOI] [PubMed] [Google Scholar]
- 150.Shu XZ, Liu YC, Palumbo FS, Lu Y, Prestwich GD. Biomaterials. 2004;25:1339. doi: 10.1016/j.biomaterials.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 151.Anderson DG, Tweedie CA, Hossain N, Navarro SM, Brey DM, Van Vliet KJ, Langer R, Burdick JA. Adv. Mater. 2006;18:2614. [Google Scholar]
- 152.Lynn DM, Anderson DG, Putnam D, Langer R. J Am. Chem. Soc. 2001;123:8155. doi: 10.1021/ja016288p. [DOI] [PubMed] [Google Scholar]
- 153.Anderson DG, Levenberg S, Langer R. Nat. Biotechnol. 2004;22:863. doi: 10.1038/nbt981. [DOI] [PubMed] [Google Scholar]
- 154.Anderson DG, Putnam D, Lavik EB, Mahmood TA, Langer R. Biomaterials. 2005;26:4892. doi: 10.1016/j.biomaterials.2004.11.052. [DOI] [PubMed] [Google Scholar]
- 155.Lutz JF, Zarafshani Z. Adv. Drug Delivery Rev. 2008;60:958. doi: 10.1016/j.addr.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 156.Malkoch M, Vestberg R, Gupta N, Mespouille L, Dubois P, Mason AF, Hedrick JL, Liao Q, Frank CW, Kingsbury K, Hawker CJ. Chem. Commun. 2006:2774. doi: 10.1039/b603438a. [DOI] [PubMed] [Google Scholar]
- 157.Crescenzi V, Cornelio L, Di Meo C, Nardecchia S, Lamanna R. Biomacromolecules. 2007;8:1844. doi: 10.1021/bm0700800. [DOI] [PubMed] [Google Scholar]
- 158.Wichterle O, Lim D. Nature. 1960;185:117. [Google Scholar]
- 159.Crawford GJ, Hicks CR, Lou X, Vijayasekaran S, Tan D, Mulholland B, Chirila TV, Constable IJ. Ophthalmology. 2002;109:883. doi: 10.1016/s0161-6420(02)00958-2. [DOI] [PubMed] [Google Scholar]
- 160.Madsen F, Peppas NA. Biomaterials. 1999;20:1701. doi: 10.1016/s0142-9612(99)00071-x. [DOI] [PubMed] [Google Scholar]
- 161.Eichenbaum GM, Kiser PF, Shah D, Meuer WP, Needham D, Simon SA. Macromolecules. 2000;33:4087. [Google Scholar]
- 162.Li Y, Gong YK, Nakashima K. Langmuir. 2002;18:6727. [Google Scholar]
- 163.Schmedlen KH, Masters KS, West JL. Biomaterials. 2002;23:4325. doi: 10.1016/s0142-9612(02)00177-1. [DOI] [PubMed] [Google Scholar]
- 164.Pan YS, Xiong DS, Ma RY. Wear. 2007;262:1021. [Google Scholar]
- 165.Noguchi T, Yamamuro T, Oka M, Kumar P, Kotoura Y, Hyon S, Ikada Y. J Appl. Biomater. 1991;2:101. doi: 10.1002/jab.770020205. [DOI] [PubMed] [Google Scholar]
- 166.Oka M, Noguchi T, Kumar P, Ikeuchi K, Yamamuro T, Hyon SH, Ikada Y. Clin. Mater. 1990;6:361. doi: 10.1016/0267-6605(90)90053-x. [DOI] [PubMed] [Google Scholar]
- 167.Kobayashi M, Chang YS, Oka M. Biomaterials. 2005;26:3243. doi: 10.1016/j.biomaterials.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 168.Alcantar NA, Aydil ES, Israelachvili JN. J Biomed. Mater. Res. 2000;51:343. doi: 10.1002/1097-4636(20000905)51:3<343::aid-jbm7>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 169.Merrill EW, Salzman EW. ASAIO J. 1983;6:60. [Google Scholar]
- 170.Gombotz WR, Wang GH, Horbett TA, Hoffman AS. J Biomed. Mater. Res. 1991;25:1547. doi: 10.1002/jbm.820251211. [DOI] [PubMed] [Google Scholar]
- 171.Ostuni E, Chapman RG, Holmlin RE, Takayama S, Whitesides GM. Langmuir. 2001;17:5605. doi: 10.1021/la0015258. [DOI] [PubMed] [Google Scholar]
- 172.Lee JH, Lee HB, Andrade JD. Prog. Polym. Sci. 1995;20:1043. [Google Scholar]
- 173.Massia SP, Hubbell J. J Cell Biol. 1991;114:1089. doi: 10.1083/jcb.114.5.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kohane DS, Langer R. Pediatr. Res. 2008;63:487. doi: 10.1203/01.pdr.0000305937.26105.e7. [DOI] [PubMed] [Google Scholar]
- 175.Lapcik L, Lapcik L, De Smedt S, Demeester J, Chabrecek P. Chem. Rev. 1998;98:2663. doi: 10.1021/cr941199z. [DOI] [PubMed] [Google Scholar]
- 176.Noble PW. Matrix Biol. 2002;21:25. doi: 10.1016/s0945-053x(01)00184-6. [DOI] [PubMed] [Google Scholar]
- 177.Goa KL, Benfield P. Drugs. 1994;47:536. doi: 10.2165/00003495-199447030-00009. [DOI] [PubMed] [Google Scholar]
- 178.Khademhosseini A, Eng G, Yeh J, Fukuda J, Blumling J, III, Langer R, Burdick JA. J Biomed. Mater. Res, Part A. 2006;79:522. doi: 10.1002/jbm.a.30821. [DOI] [PubMed] [Google Scholar]
- 179.Yeh J, Ling YB, Karp JM, Gantz J, Chandawarkar A, Eng G, Blumling J, Langer R, Khademhosseini A. Biomaterials. 2006;27:5391. doi: 10.1016/j.biomaterials.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 180.Leach JB, Bivens KA, Patrick CW, Schmidt CE. Biotechnol. Bioeng. 2003;82:578. doi: 10.1002/bit.10605. [DOI] [PubMed] [Google Scholar]
- 181.Lu PL, Lai JY, Ma DHK, Hsiue GH. J Biomater. Sci. Polym. Ed. 2008;19:1. doi: 10.1163/156856208783227695. [DOI] [PubMed] [Google Scholar]
- 182.Pitarresi G, Palumbo FS, Calabrese R, Craparo EF, Giammona G. J Biomed. Mater. Res, Part A. 2008;84:413. doi: 10.1002/jbm.a.31316. [DOI] [PubMed] [Google Scholar]
- 183.Park YD, Tirelli N, Hubbell JA. Biomaterials. 2003;24:893. doi: 10.1016/s0142-9612(02)00420-9. [DOI] [PubMed] [Google Scholar]
- 184.Brigham M, Bick A, Lo E, Bendali A, Burdick J, Khademhosseini A. Tissue Eng. 2009;15:1. doi: 10.1089/ten.tea.2008.0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhao HG, Ma L, Zhou J, Mao ZW, Gao CY, Shen J. Biomed. Mater. 2008;3:1. doi: 10.1088/1748-6041/3/1/015001. [DOI] [PubMed] [Google Scholar]
- 186.Thompson DF, Letassy NA, Thompson GD. Drug Intell. Clin. Pharm. 1998;22:946. doi: 10.1177/106002808802201203. [DOI] [PubMed] [Google Scholar]
- 187.Smidsrod O, Skjakbraek G. Trends Biotechnol. 1990;8:71. doi: 10.1016/0167-7799(90)90139-o. [DOI] [PubMed] [Google Scholar]
- 188.Kong HJ, Lee KY, Mooney DJ. Polymer. 2002;43:6239. [Google Scholar]
- 189.Klein J, Stock J, Vorlop KD. Eur. J. Appl. Microbiol. Biotechnol. 1983;18:86. [Google Scholar]
- 190.Stewart WW, Swaisgood HE. Enzyme Microb. Technol. 1993;15:922. [Google Scholar]
- 191.Kuo CK, Ma PX. J Biomed. Mater. Res, Part A. 2008;84:899. doi: 10.1002/jbm.a.31375. [DOI] [PubMed] [Google Scholar]
- 192.Drury JL, Dennis RG, Mooney DJ. Biomaterials. 2004;25:3187. doi: 10.1016/j.biomaterials.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 193.Kuo CK, Ma PX. Biomaterials. 2001;22:511. doi: 10.1016/s0142-9612(00)00201-5. [DOI] [PubMed] [Google Scholar]
- 194.Kulseng B, Skjak-Braek G, Ryan L, Andersson A, King A, Faxvaag A, Espevik T. Transplantation. 1999;67:978. doi: 10.1097/00007890-199904150-00008. [DOI] [PubMed] [Google Scholar]
- 195.Rowley JA, Madlambayan G, Mooney DJ. Biomaterials. 1999;20:45. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 196.Rowley JA, Mooney DJ. J Biomed. Mater. Res. 2002;60:217. doi: 10.1002/jbm.1287. [DOI] [PubMed] [Google Scholar]
- 197.Drury JL, Boontheeku T, Mooney DJ. J Biomech. Eng. Trans. ASME. 2005;127:220. doi: 10.1115/1.1865194. [DOI] [PubMed] [Google Scholar]
- 198.Lee DW, Choi WS, Byun MW, Park HJ, Yu YM, Lee CM. J Agric. Food Chem. 2003;51:4819. doi: 10.1021/jf021053y. [DOI] [PubMed] [Google Scholar]
- 199.Bouhadir KH, Lee KY, Alsberg E, Damm KL, Anderson KW, Mooney DJ. Biotechnol. Prog. 2001;17:945. doi: 10.1021/bp010070p. [DOI] [PubMed] [Google Scholar]
- 200.Bouhadir KH, Hausman DS, Mooney DJ. Polymer. 1999;40:3575. [Google Scholar]
- 201.Suzuki Y, Tanihara M, Nishimura Y, Suzuki K, Yamawaki Y, Kudo H, Kakimaru Y, Shimizu Y. J Biomed. Mater. Res. 1999;48:522. doi: 10.1002/(sici)1097-4636(1999)48:4<522::aid-jbm18>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 202.Waldrone D, Paul TM, Skoryna SC. Nature. 1965;205:1117. doi: 10.1038/2051117a0. [DOI] [PubMed] [Google Scholar]
- 203.Buehler MJ. Nanotechnology. 2007;18:295102. [Google Scholar]
- 204.Riesle J, Hollander AP, Langer R, Freed LE, Vunjak-Novakovic G. J Cell. Biochem. 1998;71:313. doi: 10.1002/(sici)1097-4644(19981201)71:3<313::aid-jcb1>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 205.Mullerglauser W, Humbel B, Glatt M, Strauli P, Winterhalter KH, Bruckner P. J Cell Biol. 1986;102:1931. doi: 10.1083/jcb.102.5.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wu JJ, Woods PE, Eyre DR. J Biol. Chem. 1992;267:23007. [PubMed] [Google Scholar]
- 207.Zeltinger J, Sherwood JK, Graham DA, Mueller R, Griffith LG. Tissue Eng. 2001;7:557. doi: 10.1089/107632701753213183. [DOI] [PubMed] [Google Scholar]
- 208.Lu JX, Flautre B, Anselme K, Hardouin P, Gallur A, Descamps M, Thierry B. J Mater. Sci. Mater. Med. 1999;10:111. doi: 10.1023/a:1008973120918. [DOI] [PubMed] [Google Scholar]
- 209.Yeong WY, Chua CK, Leong KF, Chandrasekaran M, Lee MW. J Biomed. Mater. Res, Part B. 2007;82:260. doi: 10.1002/jbm.b.30729. [DOI] [PubMed] [Google Scholar]
- 210.Kaufman HE, Steinemann TL, Lehman E, Thompson HW, Varnell ED, Jacoblabarre JT, Gebhardt BM. J Ocul. Pharmacol. 1994;10:17. doi: 10.1089/jop.1994.10.17. [DOI] [PubMed] [Google Scholar]
- 211.Rossler B, Kreuter J, Scherer D. J Microencapsulation. 1995;12:49. doi: 10.3109/02652049509051126. [DOI] [PubMed] [Google Scholar]
- 212.Lucas PA, Syftestad GT, Goldberg VM, Caplan AI. J Biomed. Mater. Res, Part B. 1989;23:23. doi: 10.1002/jbm.820231306. [DOI] [PubMed] [Google Scholar]
- 213.Lee CH, Singla A, Lee Y. Int. J. Pharm. 2001;221:1. doi: 10.1016/s0378-5173(01)00691-3. [DOI] [PubMed] [Google Scholar]
- 214.McGuigan AP, Leung B, Sefton MV. Nat. Protoc. 2006;1:2963. doi: 10.1038/nprot.2006.443. [DOI] [PubMed] [Google Scholar]
- 215.McGuigan AP, Sefton MV. Proc. Natl. Acad. Sci. USA. 2006;103:11461. doi: 10.1073/pnas.0602740103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.McGuigan AP, Sefton MV. Tissue Eng. 2007;13:1079. doi: 10.1089/ten.2006.0245. [DOI] [PubMed] [Google Scholar]
- 217.McGuigan AP, Sefton MV. Tissue Eng. 2007;13:1069. doi: 10.1089/ten.2006.0253. [DOI] [PubMed] [Google Scholar]
- 218.McGuigan AP, Sefton MV. Biomaterials. 2008;29:2453. doi: 10.1016/j.biomaterials.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Mahoney MJ, Krewson C, Miller WM, Saltzman J. Tissue Eng. 2006;12:1915. doi: 10.1089/ten.2006.12.1915. [DOI] [PubMed] [Google Scholar]
- 220.Mahoney MJ, Anseth KS. J Biomed. Mater. Res, Part A. 2007;81:269. doi: 10.1002/jbm.a.30970. [DOI] [PubMed] [Google Scholar]
- 221.Adams DJ, Holtzmann K, Schneider C, Butler MF. Langmuir. 2007;23:12729. doi: 10.1021/la7011183. [DOI] [PubMed] [Google Scholar]
- 222.Guler MO, Stupp SI. J Am. Chem. Soc. 2007;129:12082. doi: 10.1021/ja075044n. [DOI] [PubMed] [Google Scholar]
- 223.Williams BAR, Lund K, Liu Y, Yan H, Chaput JC. Angew. Chem. Int. Ed. 2007;46:3051. doi: 10.1002/anie.200603919. [DOI] [PubMed] [Google Scholar]
- 224.Guler MO, Soukasene S, Hulvat JF, Stupp SI. Nano Lett. 2005;5:249. doi: 10.1021/nl048238z. [DOI] [PubMed] [Google Scholar]
- 225.Hung AM, Stupp SI. Nano Lett. 2007;7:1165. doi: 10.1021/nl062835z. [DOI] [PubMed] [Google Scholar]
- 226.Bull SR, Guler MO, Bras RE, Meade TJ, Stupp SI. Nano Lett. 2005;5:1. doi: 10.1021/nl0484898. [DOI] [PubMed] [Google Scholar]
- 227.Niece KL, Hartgerink JD, Donners J, Stupp SI. J Am. Chem. Soc. 2003;125:7146. doi: 10.1021/ja028215r. [DOI] [PubMed] [Google Scholar]
- 228.Hartgerink JD, Beniash E, Stupp SI. Proc. Natl. Acad. Sci. USA. 2002;99:5133. doi: 10.1073/pnas.072699999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Hartgerink JD, Beniash E, Stupp SI. Science. 2001;294:1684. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- 230.Hwang JJ, Lyer SN, Li LS, Claussen R, Harrington DA, Stupp SI. Proc. Natl. Acad. Sci. USA. 2002;99:9662. doi: 10.1073/pnas.152667399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Zhang S. Nat. Biotechnol. 2003;21:1171. doi: 10.1038/nbt874. [DOI] [PubMed] [Google Scholar]
- 232.Santoso S, Hwang W, Hartman H, Zhang SG. Nano Lett. 2002;2:687. [Google Scholar]
- 233.Mikos AG, Herring SW, Ochareon P, Elisseeff J, Lu HH, Kandel R, Schoen FJ, Toner M, Mooney D, Atala A, Van Dyke ME, Kaplan D, Vunjak-Novakovic G. Tissue Eng. 2006;12:3307. doi: 10.1089/ten.2006.12.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Khademhosseini A, Langer R. Biomaterials. 2007;28:5087. doi: 10.1016/j.biomaterials.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 235.Moon J, West J. Curr. Top. Med. Chem. 2008;8:300. doi: 10.2174/156802608783790983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Hahn MS, Miller JS, West JL. Adv. Mater. 2006;18:2679. [Google Scholar]
- 237.Koh WG, Revzin A, Pishko MV. Langmuir. 2002;18:2459. doi: 10.1021/la0115740. [DOI] [PubMed] [Google Scholar]
- 238.Mironov V, Boland T, Trusk T, Forgacs G, Markwald RR. Trends Biotechnol. 2003;21:157. doi: 10.1016/S0167-7799(03)00033-7. [DOI] [PubMed] [Google Scholar]
- 239.Ngai T, Behrens SH, Auweter H. Chem. Commun. 2005:331. doi: 10.1039/b412330a. [DOI] [PubMed] [Google Scholar]
- 240.Zhang H, Tumarkin E, Peerani R, Nie Z, Sullan RMA, Walker GC, Kumacheva E. J Am. Chem. Soc. 2006;128:12205. doi: 10.1021/ja0635682. [DOI] [PubMed] [Google Scholar]
- 241.Demirci U, Montesano G. Lab Chip. 2007;7:1139. doi: 10.1039/b704965j. [DOI] [PubMed] [Google Scholar]
- 242.Du Y, Lo E, Ali S, Khademhosseini A. Proc. Natl. Acad. Sci. USA. 2008;105:9522. doi: 10.1073/pnas.0801866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Mironov V, Kasyanov V, Drake C, Markwald RR. Regen. Med. 2008;3:93. doi: 10.2217/17460751.3.1.93. [DOI] [PubMed] [Google Scholar]
- 244.Ling Y, Rubin J, Deng Y, Huang C, Demirci U, Karp JM, Khademhosseini A. Lab Chip. 2007;7:756. doi: 10.1039/b615486g. [DOI] [PubMed] [Google Scholar]
- 245.King KR, Wang CCJ, Kaazempur-Mofrad MR, Vacanti JP, Borenstein JT. Adv. Mater. 2004;16:2007. [Google Scholar]
- 246.Cabodi M, Choi NW, Gleghorn JP, Lee CS, Bonassar LJ, Stroock AD. J Am. Chem. Soc. 2005;127:13788. doi: 10.1021/ja054820t. [DOI] [PubMed] [Google Scholar]
- 247.Stachowiak AN, Bershteyn A, Tzatzalos E, Irvine DJ. Adv. Mater. 2005;17:399. [Google Scholar]
- 248.Hilt JZ, Gupta AK, Bashir R, Peppas NA. Biomed. Microdev. 2003;5:177. [Google Scholar]
- 249.Grayson ACR, Shawgo RS, Johnson AM, Flynn NT, Li Y, Cima MJ, Langer R. Proc. IEEE. 2004;92:6. [Google Scholar]
- 250.Zekorn T, Siebers U, Horcher A, Schnettler R, Zimmermann U, Bretzel RG, Federlin K. Acta Diabetol. 1992;29:41. doi: 10.1007/BF00572829. [DOI] [PubMed] [Google Scholar]
- 251.Uludag H, De Vos P, Tresco PA. Adv. Drug Delivery Rev. 2000;42:29. doi: 10.1016/s0169-409x(00)00053-3. [DOI] [PubMed] [Google Scholar]
- 252.Magyar JP, Nemir M, Ehler E, Suter N, Perriard JC, Eppenberger HM. Ann. N. Y. Acad. Sci. 2001;944:135. doi: 10.1111/j.1749-6632.2001.tb03828.x. [DOI] [PubMed] [Google Scholar]
- 253.Dang SM, Kyba M, Perlingeiro R, Daley GQ, Zandstra PW. Biotechnol. Bioeng. 2002;78:442. doi: 10.1002/bit.10220. [DOI] [PubMed] [Google Scholar]
- 254.Fukuda J, Khademhosseini A, Yeo Y, Yang X, Yeh J, Eng G, Blumling J, Wang CF, Kohane DS, Langer R. Biomaterials. 2006;27:5259. doi: 10.1016/j.biomaterials.2006.05.044. [DOI] [PubMed] [Google Scholar]
- 255.Kaihara S, Borenstein J, Koka R, Lalan S, Ochoa ER, Ravens M, Pien H, Cunningham B, Vacanti JP. Tissue Eng. 2000;6:105. doi: 10.1089/107632700320739. [DOI] [PubMed] [Google Scholar]
- 256.Tang MD, Golden AP, Tien J. J Am. Chem. Soc. 2003;125:12988. doi: 10.1021/ja037677h. [DOI] [PubMed] [Google Scholar]
- 257.Paguirigan A, Beebe DJ. Lab Chip. 2006;6:407. doi: 10.1039/b517524k. [DOI] [PubMed] [Google Scholar]
- 258.Leach JB, Bivens KA, Patrick CW, Jr, Schmidt CE. Biotechnol. Bioeng. 2003;82:578. doi: 10.1002/bit.10605. [DOI] [PubMed] [Google Scholar]
- 259.Franzesi GT, Ni B, Ling Y, Khademhosseini A. J Am. Chem. Soc. 2006;128:15064. doi: 10.1021/ja065867x. [DOI] [PubMed] [Google Scholar]
- 260.Rolland JP, Maynor BW, Euliss LE, Exner AE, Denison GM, DeSimone JM. J Am. Chem. Soc. 2005;127:10096. doi: 10.1021/ja051977c. [DOI] [PubMed] [Google Scholar]
- 261.Gratton SEA, Pohlhaus PD, Lee J, Guo J, Cho MJ, DeSimone JM. J Controlled Release. 2007;121:10. doi: 10.1016/j.jconrel.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Geng Y, Dalhaimer P, Cai S, Tsai R, Tewari M, Minko T, Discher DE. Nat. Nanotechnol. 2008;2:249. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Zhan W, Seong GH, Crooks RM. Anal. Chem. 2002;74:4647. doi: 10.1021/ac020340y. [DOI] [PubMed] [Google Scholar]
- 264.Ward JH, Bashir R, Peppas NA. J Biomed. Mater. Res. 2001;56:351. doi: 10.1002/1097-4636(20010905)56:3<351::aid-jbm1103>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 265.Revzin A, Russell RJ, Yadavalli VK, Koh WG, Deister C, Hile DD, Mellott MB, Pishko MV. Langmuir. 2001;17:5440. doi: 10.1021/la010075w. [DOI] [PubMed] [Google Scholar]
- 266.Liu VA, Bhatia SN. Biomed. Microdev. 2002;4:257. [Google Scholar]
- 267.Mapili G, Lu Y, Chen S, Roy K. J Biomed. Mater. Res, Part B. 2005;75B:414. doi: 10.1002/jbm.b.30325. [DOI] [PubMed] [Google Scholar]
- 268.Luo Y, Shoichet MS. Nat. Mater. 2004;3:249. doi: 10.1038/nmat1092. [DOI] [PubMed] [Google Scholar]
- 269.Tan W, Desai TA. Biomaterials. 2004;25:1355. doi: 10.1016/j.biomaterials.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 270.Tan W, Desai TA. Tissue Eng. 2003;9:255. doi: 10.1089/107632703764664729. [DOI] [PubMed] [Google Scholar]
- 271.Xu S, Nie Z, Seo M, Lewis P, Kumacheva E, Stone HA, Garstecki P, Weibel DB, Gitlin I, Whitesides GM. Angew. Chem. Int. Ed. 2005;44:3799. doi: 10.1002/anie.200462226. [DOI] [PubMed] [Google Scholar]
- 272.Zhang H, Tumarkin E, Sullan RMA, Walker GC, Kumacheva E. Macromol. Rapid Commun. 2007;28:527. [Google Scholar]
- 273.Zaari N, Rajagopalan SK, Kim SK, Engler AJ, Wong JY. Adv. Mater. 2004;16:2133. [Google Scholar]
- 274.Dendukuri D, Pregibon DC, Collins J, Hatton TA, Doyle PS. Nat. Mater. 2006;5:365. doi: 10.1038/nmat1617. [DOI] [PubMed] [Google Scholar]
- 275.Panda P, Shamsher A, Lo E, Chung BG, Hatton TA, Khademhosseini A, Doyle PS. Lab Chip. 2008;8:1056. doi: 10.1039/b804234a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Johnson PC, Mikos AG, Fisher JP, Jansen JA. Tissue Eng. 2007;13:2827. doi: 10.1089/ten.2007.0335. [DOI] [PubMed] [Google Scholar]