Abstract
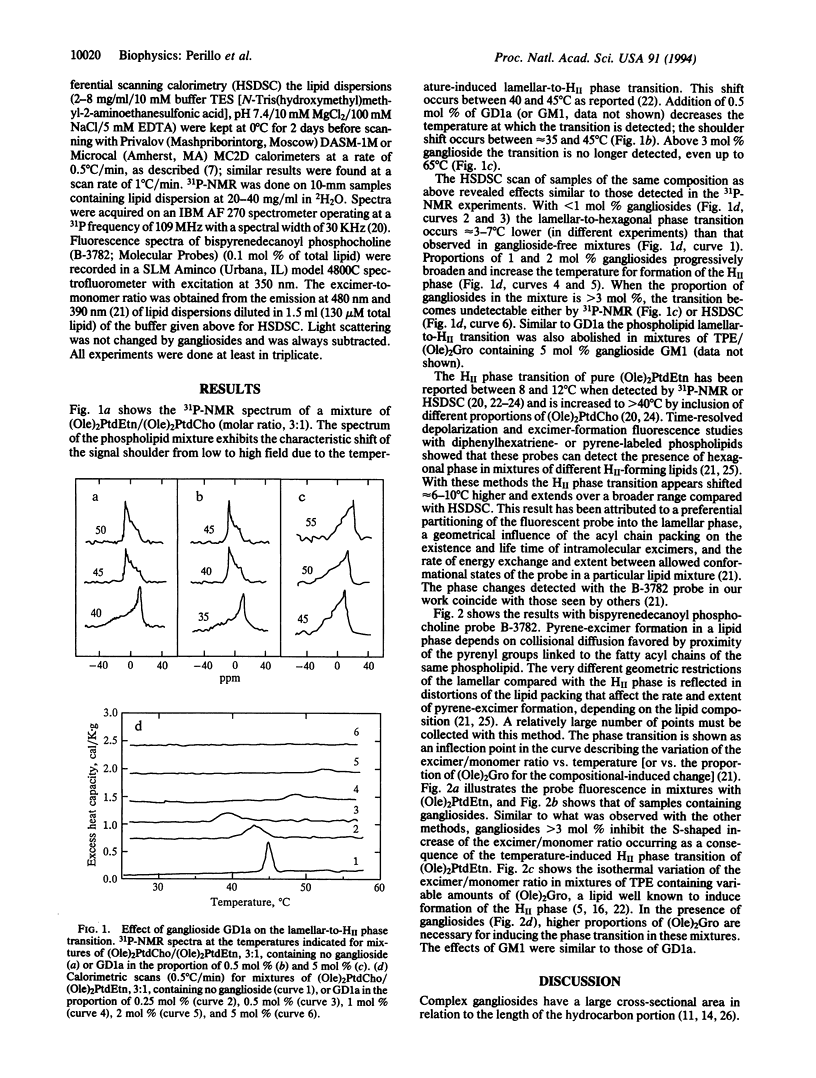
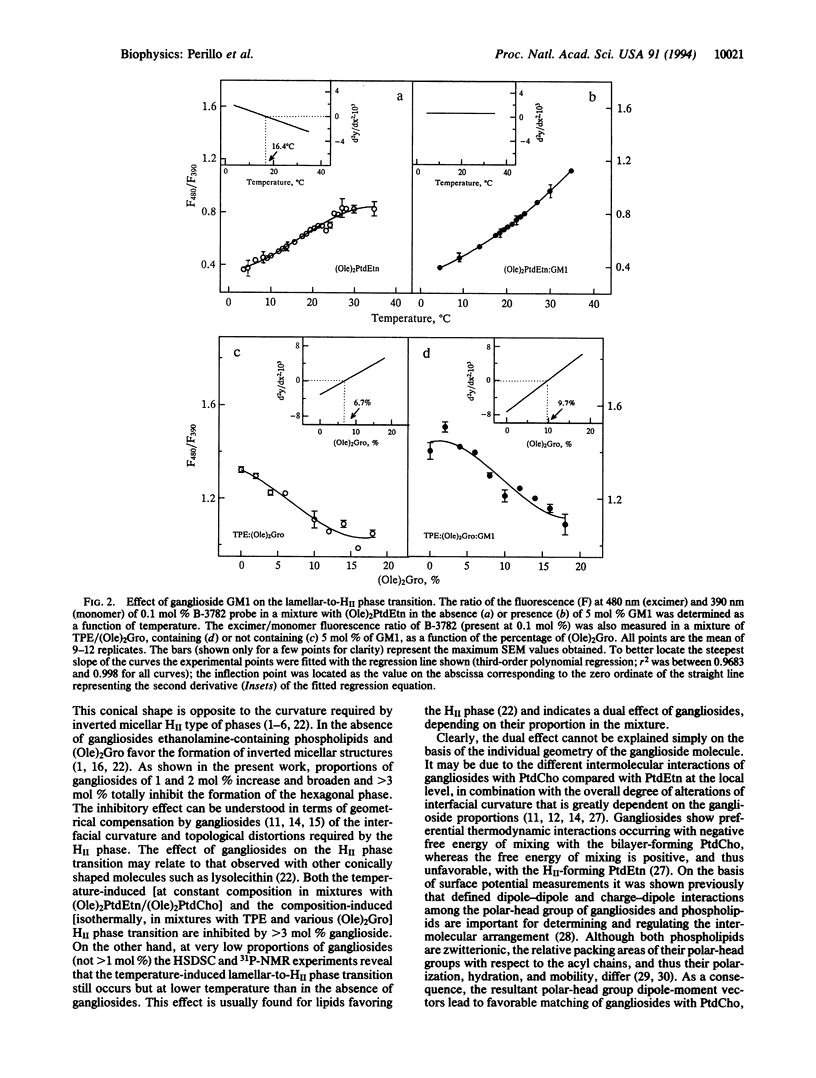
We studied the effect of gangliosides GD1a and GM1 on the lamellar-to-hexagonal II phase transition of mixtures of dioleoylphosphatidylethanolamine/dioleoylphosphatidyl choline, 3:1, and of transphosphatidylated phosphatidylethanolamine with dioleoylglycerol by high-sensitivity differential scanning calorimetry, 31P-NMR, and pyrene fluorescence of a phosphatidylcholine probe. Gangliosides had a dual effect. Below 1 mol % ganglioside the hexagonal II phase transition was affected but still occurred at lower temperature than in the absence of gangliosides. The presence of between 1 and 2 mol % gangliosides increased the temperature for formation of the hexagonal II phase and progressively decreased its cooperativity. Above 3 mol % gangliosides totally inhibited the formation of both the temperature-induced and composition-induced hexagonal phase, probably by opposing the geometric distortions necessary for the inverted micellar structures.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach D., Sela B., Miller I. R. Compositional aspects of lipid hydration. Chem Phys Lipids. 1982 Dec;31(4):381–394. doi: 10.1016/0009-3084(82)90073-1. [DOI] [PubMed] [Google Scholar]
- Batenburg A. M., van Esch J. H., de Kruijff B. Melittin-induced changes of the macroscopic structure of phosphatidylethanolamines. Biochemistry. 1988 Apr 5;27(7):2324–2331. doi: 10.1021/bi00407a013. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Bianco I. D., Fidelio G. D., Yu R. K., Maggio B. Degradation of dilauroylphosphatidylcholine by phospholipase A2 in monolayers containing glycosphingolipids. Biochemistry. 1991 Feb 12;30(6):1709–1714. doi: 10.1021/bi00220a037. [DOI] [PubMed] [Google Scholar]
- Cantù L., Corti M., Casellato R., Acquotti D., Sonnino S. Aggregation properties of GD1b, II3Neu5Ac2GgOse4Cer, and of GD1b-lactone, II3[alpha-Neu5Ac-(2----8, 1----9)-alpha-Neu5Ac]GgOse4Cer, in aqueous solution. Chem Phys Lipids. 1991 Dec;60(2):111–118. doi: 10.1016/0009-3084(91)90033-8. [DOI] [PubMed] [Google Scholar]
- Cheng K. H., Chen S. Y., Butko P., Van der Meer B. W., Somerharju P. Intramolecular excimer formation of pyrene-labeled lipids in lamellar and inverted hexagonal phases of lipid mixtures containing unsaturated phosphatidylethanolamine. Biophys Chem. 1991 Feb;39(2):137–144. [PubMed] [Google Scholar]
- Cheng K. H. Fluorescence depolarization study of lamellar liquid crystalline to inverted cylindrical micellar phase transition of phosphatidylethanolamine. Biophys J. 1989 Jun;55(6):1025–1031. doi: 10.1016/S0006-3495(89)82901-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullis P. R., de Kruijff B. Lipid polymorphism and the functional roles of lipids in biological membranes. Biochim Biophys Acta. 1979 Dec 20;559(4):399–420. doi: 10.1016/0304-4157(79)90012-1. [DOI] [PubMed] [Google Scholar]
- Curatolo W. The physical properties of glycolipids. Biochim Biophys Acta. 1987 Jun 24;906(2):111–136. doi: 10.1016/0304-4157(87)90008-6. [DOI] [PubMed] [Google Scholar]
- Das S., Rand R. P. Modification by diacylglycerol of the structure and interaction of various phospholipid bilayer membranes. Biochemistry. 1986 May 20;25(10):2882–2889. doi: 10.1021/bi00358a022. [DOI] [PubMed] [Google Scholar]
- Dawson R. M., Irvine R. F., Bray J., Quinn P. J. Long-chain unsaturated diacylglycerols cause a perturbation in the structure of phospholipid bilayers rendering them susceptible to phospholipase attack. Biochem Biophys Res Commun. 1984 Dec 14;125(2):836–842. doi: 10.1016/0006-291x(84)90615-6. [DOI] [PubMed] [Google Scholar]
- Ellens H., Siegel D. P., Alford D., Yeagle P. L., Boni L., Lis L. J., Quinn P. J., Bentz J. Membrane fusion and inverted phases. Biochemistry. 1989 May 2;28(9):3692–3703. doi: 10.1021/bi00435a011. [DOI] [PubMed] [Google Scholar]
- Epand R. M. Diacylglycerols, lysolecithin, or hydrocarbons markedly alter the bilayer to hexagonal phase transition temperature of phosphatidylethanolamines. Biochemistry. 1985 Dec 3;24(25):7092–7095. doi: 10.1021/bi00346a011. [DOI] [PubMed] [Google Scholar]
- Fidelio G. D., Ariga T., Maggio B. Molecular parameters of gangliosides in monolayers: comparative evaluation of suitable purification procedures. J Biochem. 1991 Jul;110(1):12–16. doi: 10.1093/oxfordjournals.jbchem.a123529. [DOI] [PubMed] [Google Scholar]
- Gagné J., Stamatatos L., Diacovo T., Hui S. W., Yeagle P. L., Silvius J. R. Physical properties and surface interactions of bilayer membranes containing N-methylated phosphatidylethanolamines. Biochemistry. 1985 Jul 30;24(16):4400–4408. doi: 10.1021/bi00337a022. [DOI] [PubMed] [Google Scholar]
- Gruner S. M., Tate M. W., Kirk G. L., So P. T., Turner D. C., Keane D. T., Tilcock C. P., Cullis P. R. X-ray diffraction study of the polymorphic behavior of N-methylated dioleoylphosphatidylethanolamine. Biochemistry. 1988 Apr 19;27(8):2853–2866. doi: 10.1021/bi00408a029. [DOI] [PubMed] [Google Scholar]
- Hakomori S. Bifunctional role of glycosphingolipids. Modulators for transmembrane signaling and mediators for cellular interactions. J Biol Chem. 1990 Nov 5;265(31):18713–18716. [PubMed] [Google Scholar]
- Hauser H., Pascher I., Pearson R. H., Sundell S. Preferred conformation and molecular packing of phosphatidylethanolamine and phosphatidylcholine. Biochim Biophys Acta. 1981 Jun 16;650(1):21–51. doi: 10.1016/0304-4157(81)90007-1. [DOI] [PubMed] [Google Scholar]
- Hoekstra D. Glycolipids, glycoproteins and membrane fusion. Indian J Biochem Biophys. 1988 Feb-Apr;25(1-2):76–84. [PubMed] [Google Scholar]
- Huang C., Mason J. T. Geometric packing constraints in egg phosphatidylcholine vesicles. Proc Natl Acad Sci U S A. 1978 Jan;75(1):308–310. doi: 10.1073/pnas.75.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israelachvili J. N., Marcelja S., Horn R. G. Physical principles of membrane organization. Q Rev Biophys. 1980 May;13(2):121–200. doi: 10.1017/s0033583500001645. [DOI] [PubMed] [Google Scholar]
- Macala L. J., Yu R. K., Ando S. Analysis of brain lipids by high performance thin-layer chromatography and densitometry. J Lipid Res. 1983 Sep;24(9):1243–1250. [PubMed] [Google Scholar]
- Maggio B., Albert J., Yu R. K. Thermodynamic-geometric correlations for the morphology of self-assembled structures of glycosphingolipids and their mixtures with dipalmitoylphosphatidylcholine. Biochim Biophys Acta. 1988 Nov 22;945(2):145–160. doi: 10.1016/0005-2736(88)90477-4. [DOI] [PubMed] [Google Scholar]
- Maggio B., Ariga T., Sturtevant J. M., Yu R. K. Thermotropic behavior of binary mixtures of dipalmitoylphosphatidylcholine and glycosphingolipids in aqueous dispersions. Biochim Biophys Acta. 1985 Aug 8;818(1):1–12. doi: 10.1016/0005-2736(85)90131-2. [DOI] [PubMed] [Google Scholar]
- Maggio B., Bianco I. D., Montich G. G., Fidelio G. D., Yu R. K. Regulation by gangliosides and sulfatides of phospholipase A2 activity against dipalmitoyl- and dilauroylphosphatidylcholine in small unilamellar bilayer vesicles and mixed monolayers. Biochim Biophys Acta. 1994 Feb 23;1190(1):137–148. doi: 10.1016/0005-2736(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Maggio B., Cumar F. A., Caputto R. Configuration and interaction of the polar head group in gangliosides. Biochem J. 1980 Sep 1;189(3):435–440. doi: 10.1042/bj1890435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio B., Cumar F. A., Caputto R. Interactions of gangliosides with phospholipids and glycosphingolipids in mixed monolayers. Biochem J. 1978 Dec 1;175(3):1113–1118. doi: 10.1042/bj1751113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio B., Cumar F. A., Caputto R. Molecular behaviour of glycosphingolipids in interfaces. Possible participation in some properties of nerve membranes. Biochim Biophys Acta. 1981 Dec;650(2-3):69–87. doi: 10.1016/0304-4157(81)90001-0. [DOI] [PubMed] [Google Scholar]
- Maggio B. Geometric and thermodynamic restrictions for the self-assembly of glycosphingolipid-phospholipid systems. Biochim Biophys Acta. 1985 May 14;815(2):245–258. doi: 10.1016/0005-2736(85)90295-0. [DOI] [PubMed] [Google Scholar]
- Maggio B., Lucy J. A. Polar-group behaviour in mixed monolayers of phospholipids and fusogenic lipids. Biochem J. 1976 May 1;155(2):353–364. doi: 10.1042/bj1550353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio B., Yu R. K. Modulation by glycosphingolipids of membrane-membrane interactions induced by myelin basic protein and melittin. Biochim Biophys Acta. 1992 Nov 23;1112(1):105–114. doi: 10.1016/0005-2736(92)90260-s. [DOI] [PubMed] [Google Scholar]
- Nieva J. L., Goñi F. M., Alonso A. Phospholipase C-promoted membrane fusion. Retroinhibition by the end-product diacylglycerol. Biochemistry. 1993 Feb 2;32(4):1054–1058. doi: 10.1021/bi00055a009. [DOI] [PubMed] [Google Scholar]
- Perillo M. A., Guidotti A., Costa E., Yu R. K., Maggio B. Modulation of phospholipases A2 and C activities against dilauroylphosphorylcholine in mixed monolayers with semisynthetic derivatives of ganglioside and sphingosine. Mol Membr Biol. 1994 Apr-Jun;11(2):119–126. doi: 10.3109/09687689409162229. [DOI] [PubMed] [Google Scholar]
- Seddon J. M. Structure of the inverted hexagonal (HII) phase, and non-lamellar phase transitions of lipids. Biochim Biophys Acta. 1990 Feb 28;1031(1):1–69. doi: 10.1016/0304-4157(90)90002-t. [DOI] [PubMed] [Google Scholar]
- Seelig J., Seelig A. Lipid conformation in model membranes and biological membranes. Q Rev Biophys. 1980 Feb;13(1):19–61. doi: 10.1017/s0033583500000305. [DOI] [PubMed] [Google Scholar]
- Smith R., Cornell B. A. Myelin basic protein induces hexagonal phase formation in dispersions of diacylphosphatidic acid. Biochim Biophys Acta. 1985 Aug 27;818(2):275–279. doi: 10.1016/0005-2736(85)90569-3. [DOI] [PubMed] [Google Scholar]
- Tilcock C. P., Bally M. B., Farren S. B., Cullis P. R. Influence of cholesterol on the structural preferences of dioleoylphosphatidylethanolamine-dioleoylphosphatidylcholine systems: a phosphorus-31 and deuterium nuclear magnetic resonance study. Biochemistry. 1982 Sep 14;21(19):4596–4601. doi: 10.1021/bi00262a013. [DOI] [PubMed] [Google Scholar]


