Abstract
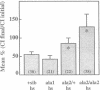
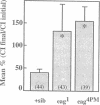
Similar defects in both synaptic transmission and associative learning are produced in Drosophila melanogaster by inhibition of calcium/calmodulin-dependent protein kinase II and mutations in the potassium channel subunit gene eag. These behavioral and synaptic defects are not simply additive in animals carrying both an eag mutation and a transgene for a protein kinase inhibitor, raising the possibility that the phenotypes share a common pathway. At the molecular level, a portion of the putative cytoplasmic domain of Eag is a substrate of calcium/calmodulin-dependent protein kinase II. These similarities in behavior and synaptic physiology, the genetic interaction, and the in vitro biochemical interaction of the two molecules suggest that an important component of neural and behavioral plasticity may be mediated by modulation of Eag function by calcium/calmodulin-dependent protein kinase II.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman S. L., Siegel R. W. Chemically reinforced conditioned courtship in Drosophila: responses of wild-type and the dunce, amnesiac and don giovanni mutants. J Neurogenet. 1986 Mar;3(2):111–123. doi: 10.3109/01677068609106898. [DOI] [PubMed] [Google Scholar]
- Bennett M. K., Erondu N. E., Kennedy M. B. Purification and characterization of a calmodulin-dependent protein kinase that is highly concentrated in brain. J Biol Chem. 1983 Oct 25;258(20):12735–12744. [PubMed] [Google Scholar]
- Brüggemann A., Pardo L. A., Stühmer W., Pongs O. Ether-à-go-go encodes a voltage-gated channel permeable to K+ and Ca2+ and modulated by cAMP. Nature. 1993 Sep 30;365(6445):445–448. doi: 10.1038/365445a0. [DOI] [PubMed] [Google Scholar]
- Cowan T. M., Siegel R. W. Drosophila mutations that alter ionic conduction disrupt acquisition and retention of a conditioned odor avoidance response. J Neurogenet. 1986 Jul;3(4):187–201. doi: 10.3109/01677068609106849. [DOI] [PubMed] [Google Scholar]
- Cowan T. M., Siegel R. W. Mutational and pharmacological alterations of neuronal membrane function disrupt conditioning in Drosophila. J Neurogenet. 1984 Dec;1(4):333–344. doi: 10.3109/01677068409107095. [DOI] [PubMed] [Google Scholar]
- Drysdale R., Warmke J., Kreber R., Ganetzky B. Molecular characterization of eag: a gene affecting potassium channels in Drosophila melanogaster. Genetics. 1991 Mar;127(3):497–505. doi: 10.1093/genetics/127.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganetzky B., Wu C. F. Drosophila mutants with opposing effects on nerve excitability: genetic and spatial interactions in repetitive firing. J Neurophysiol. 1982 Mar;47(3):501–514. doi: 10.1152/jn.1982.47.3.501. [DOI] [PubMed] [Google Scholar]
- Ganetzky B., Wu C. F. Neurogenetic analysis of potassium currents in Drosophila: synergistic effects on neuromuscular transmission in double mutants. J Neurogenet. 1983 Sep;1(1):17–28. doi: 10.3109/01677068309107069. [DOI] [PubMed] [Google Scholar]
- Griffith L. C., Greenspan R. J. The diversity of calcium/calmodulin-dependent protein kinase II isoforms in Drosophila is generated by alternative splicing of a single gene. J Neurochem. 1993 Oct;61(4):1534–1537. doi: 10.1111/j.1471-4159.1993.tb13650.x. [DOI] [PubMed] [Google Scholar]
- Griffith L. C., Verselis L. M., Aitken K. M., Kyriacou C. P., Danho W., Greenspan R. J. Inhibition of calcium/calmodulin-dependent protein kinase in Drosophila disrupts behavioral plasticity. Neuron. 1993 Mar;10(3):501–509. doi: 10.1016/0896-6273(93)90337-q. [DOI] [PubMed] [Google Scholar]
- Guy H. R., Durell S. R., Warmke J., Drysdale R., Ganetzky B. Similarities in amino acid sequences of Drosophila eag and cyclic nucleotide-gated channels. Science. 1991 Nov 1;254(5032):730–730. doi: 10.1126/science.1658932. [DOI] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. Properties of the larval neuromuscular junction in Drosophila melanogaster. J Physiol. 1976 Oct;262(1):189–214. doi: 10.1113/jphysiol.1976.sp011592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow R., Schulman H., Tsien R. W. Inhibition of postsynaptic PKC or CaMKII blocks induction but not expression of LTP. Science. 1989 Aug 25;245(4920):862–866. doi: 10.1126/science.2549638. [DOI] [PubMed] [Google Scholar]
- Schwartz J. H., Greenberg S. M. Molecular mechanisms for memory: second-messenger induced modifications of protein kinases in nerve cells. Annu Rev Neurosci. 1987;10:459–476. doi: 10.1146/annurev.ne.10.030187.002331. [DOI] [PubMed] [Google Scholar]
- Shuster M. J., Camardo J. S., Siegelbaum S. A., Kandel E. R. Cyclic AMP-dependent protein kinase closes the serotonin-sensitive K+ channels of Aplysia sensory neurones in cell-free membrane patches. 1985 Jan 31-Feb 6Nature. 313(6001):392–395. doi: 10.1038/313392a0. [DOI] [PubMed] [Google Scholar]
- Siegel R. W., Hall J. C. Conditioned responses in courtship behavior of normal and mutant Drosophila. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3430–3434. doi: 10.1073/pnas.76.7.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva A. J., Paylor R., Wehner J. M., Tonegawa S. Impaired spatial learning in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992 Jul 10;257(5067):206–211. doi: 10.1126/science.1321493. [DOI] [PubMed] [Google Scholar]
- Silva A. J., Stevens C. F., Tonegawa S., Wang Y. Deficient hippocampal long-term potentiation in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992 Jul 10;257(5067):201–206. doi: 10.1126/science.1378648. [DOI] [PubMed] [Google Scholar]
- Tompkins L., Siegel R. W., Gailey D. A., Hall J. C. Conditioned courtship in Drosophila and its mediation by association of chemical cues. Behav Genet. 1983 Nov;13(6):565–578. doi: 10.1007/BF01076402. [DOI] [PubMed] [Google Scholar]
- Wakamatsu Y., Watanabe Y., Shimono A., Kondoh H. Transition of localization of the N-Myc protein from nucleus to cytoplasm in differentiating neurons. Neuron. 1993 Jan;10(1):1–9. doi: 10.1016/0896-6273(93)90236-k. [DOI] [PubMed] [Google Scholar]
- Warmke J., Drysdale R., Ganetzky B. A distinct potassium channel polypeptide encoded by the Drosophila eag locus. Science. 1991 Jun 14;252(5012):1560–1562. doi: 10.1126/science.1840699. [DOI] [PubMed] [Google Scholar]
- Wu C. F., Ganetzky B., Haugland F. N., Liu A. X. Potassium currents in Drosophila: different components affected by mutations of two genes. Science. 1983 Jun 3;220(4601):1076–1078. doi: 10.1126/science.6302847. [DOI] [PubMed] [Google Scholar]
- Zhong Y., Wu C. F. Alteration of four identified K+ currents in Drosophila muscle by mutations in eag. Science. 1991 Jun 14;252(5012):1562–1564. doi: 10.1126/science.2047864. [DOI] [PubMed] [Google Scholar]
- Zhong Y., Wu C. F. Altered synaptic plasticity in Drosophila memory mutants with a defective cyclic AMP cascade. Science. 1991 Jan 11;251(4990):198–201. doi: 10.1126/science.1670967. [DOI] [PubMed] [Google Scholar]
- Zhong Y., Wu C. F. Modulation of different K+ currents in Drosophila: a hypothetical role for the Eag subunit in multimeric K+ channels. J Neurosci. 1993 Nov;13(11):4669–4679. doi: 10.1523/JNEUROSCI.13-11-04669.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]