Abstract
Although significant progress has been made using insect taxa as model organisms, non-tracheated terrestrial arthropods, such as Collembola, are underrepresented as model species. This underrepresentation reflects the difficulty in maintaining populations of specialist Collembola species in the laboratory. Until now, no species from the family Neanuridae have been successfully reared. Here we use controlled growth experiments to provide explicit evidence that the species Neanura muscorum can be raised under laboratory conditions when its diet is supplemented with slime mould. Significant gains in growth were observed in Collembola given slime mould rather than a standard diet of algae-covered bark. These benefits are further highlighted by the reproductive success of the experimental group and persistence of laboratory breeding stocks of this species and others in the family. The necessity for slime mould in the diet is attributed to the ‘suctorial’ mouthpart morphology characteristic of the Neanuridae. Maintaining laboratory populations of neanurid Collembola species will facilitate their use as model organisms, paving the way for studies that will broaden the current understanding of the environmental physiology of arthropods.
Much progress in understanding the evolution of environmental tolerances has been made by integrating the outcomes of work on model organisms with investigations of non-model taxa. Work on Drosophila, which includes the model D. melanogaster, provides a compelling example1,2. However, to determine how general the findings are from such studies, investigations across a range of other taxa are required. Significant progress has been made in understanding the diversity of environmental responses in other insect taxa3,4 and non-arthropod groups5,6,7, but for other terrestrial arthropods much remains unknown. This is especially true for non-tracheated arthropods, such as springtails and spiders, and for those that occupy important, but oft-underexplored habitats, such as soil and leaf litter.
The establishment of experimentally tractable populations, comparable to those of Drosophila, of novel taxa that occupy understudied habitats will help to address the challenges posed in generalising the findings of model organism studies. The broad geographic distribution of springtails8 (Collembola; Arthropoda: Hexapoda), combined with their high abundances (up to 105 m−2 in temperate regions9) and diversity10,11, make them ideal organisms to fill this role. At least three Collembola species have been widely established as model organisms (Folsomia candida, Hypogastrura tullbergi, and Orchesella cincta12,13,14,15,16), with a range of work also being done on field populations of these species17,18,19. A growing body of work likewise concerns the environmental tolerances of a range of springtail species, especially from temperate and polar environments20,21,22,23. Nonetheless, much remains to be known about this diverse group, particularly in the context of responses of these soil dwellers to rapidly changing environments24.
Successfully maintaining laboratory cultures requires knowledge of how to maintain healthy, disease-free laboratory colonies, and to avoid problems such as inbreeding and laboratory adaptation25,26. For some springtail species, including the three laboratory models, this knowledge is available given their long history of culture14,27. However, these species possess the more-typical ‘mandibulate’ mouthpart morphology8,28 associated with a generalist diet that may vary depending on resource availability29,30. In contrast, little is known of Collembola species that deviate from the typical ‘mandibulate’ mouthpart morphology, such as those of the family Neanuridae.
Neanuridae, with c. 1417 species in 161 genera31, comprises about 20% of Collembola species11. Unlike their more generalist counterparts, the Neanuridae have ‘suctorial’ mouthparts28,32,33, and have proven difficult to culture in the laboratory. Neanurids apparently incorporate slime moulds into their diet34,35,36,37, but whether slime moulds are their primary resource, ensuring growth and survival, or are incidental to their diet remains uncertain. Other arthropod taxa, such as the aptly named slime mould beetles (e.g. Agathidium spp.; Coleoptera, Leiodidae), rely almost exclusively on slime mould as a nutrient resource38. The rearing of any neanurid Collembola species on a diet of slime mould has not been reported and attempts to keep neanurids in the laboratory on generalist diets have proven unsuccessful, with no feeding or reproductive behaviour observed36. In consequence, a diverse family of Collembola, which offers the possibility of additional insights into the evolution and life history of this important class of soil organisms, has to date largely been unavailable for laboratory investigation. Here we directly examine the extent to which Neanuridae can survive, grow, and reproduce on slime moulds using Neanura muscorum (Templeton, 1835) as a representative organism with a view to extend this technique to rear other neanurid species. Specifically we establish a broadly applicable rearing method for this family, so improving its tractability as a source of model organisms to broaden current understanding of the environmental tolerances of soil invertebrates.
Methods
Leaf litter was collected in December 2013 from the Monash University, Clayton campus (Victoria, Australia; 37.9119 °S, 145.1317 °E). Invertebrates were extracted from litter using Berlese-Tullgren funnels39. Neanura muscorum individuals (n = 31) were identified using morphological characteristics and placed into closed 70 ml containers with a plaster of Paris and charcoal (9:1) substratum. This substratum was regularly saturated with reverse osmosis treated water to maintain humidity40. Containers were kept in temperature-controlled rooms set at 15 °C (mean/sd: 14.64 ± 0.61 °C; verified with iButton Hygrochron® temperature/humidity loggers, Maxim Integrated, San Jose, USA) on a 12:12 hour light:dark photoperiod.
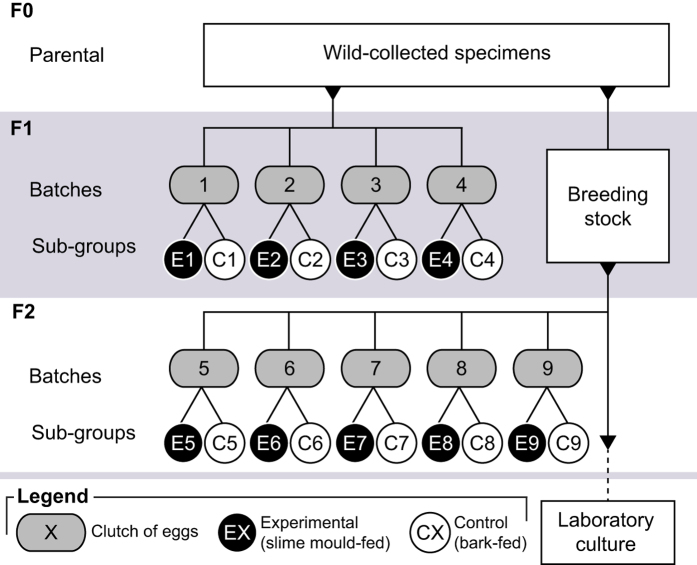
Parental lines (F0) were provided with a combined diet of slime mould (Physarum polycephalum; cultured on 1.5% agarose media at 25 °C on a diet of oats) and algae-covered plane tree (Platanus sp.) bark ad libitum. The latter is a standard diet used in rearing other Collembola species, which enables individuals to select amongst a range of algae, cyanobacteria, and fungi to reach their optimal nutrient target12,13,27. Collembola cultures were checked daily and eggs were removed to new containers and incubated in the temperature-controlled room until hatching. Four batches of first-generation (F1) eggs were used for experiments (Fig. 1). Subsequent clutches were combined and raised as a F1 breeding population under identical conditions to the parental line. Five batches of eggs laid by the F1 breeding population were then used to create F2 populations for a repetition of the experiments undertaken with the F1 generation (Fig. 1). Neanura muscorum F2 individuals that were not used for experimentation were pooled and kept as laboratory stocks. These stocks continue to be maintained on a combined slime mould and algae-covered plane tree bark diet.
Figure 1. Diagrammatic representation of the experimental design used in this study.
Experimental design
On hatching, individuals from each F1 and F2 batch were randomly assigned to either a control or an experimental group and raised in group and batch-specific containers. The control group were exclusively given algae-covered plane tree bark and the experimental group were given only slime mould (P. polycephalum). Individuals sharing a container, hereafter referred to as a sub-group (Fig. 1), were of a similar age (±2 days old). In total, four F1 batches and five F2 batches were divided into nine control and nine experimental sub-groups of N. muscorum in a pairwise design. Owing to the number of newly hatched individuals available at the founding stage, sub-group size was variable amongst batches (6–40 individuals, median 12.5). However, the pairwise design of this study meant the number of individuals in paired control and experimental sub-groups (e.g. E1 and C1, E2 and C2; Fig. 1) was approximately equal (±1 individual). Food was supplied ad libitum and containers were checked every second day to replenish food and water and check for eggs.
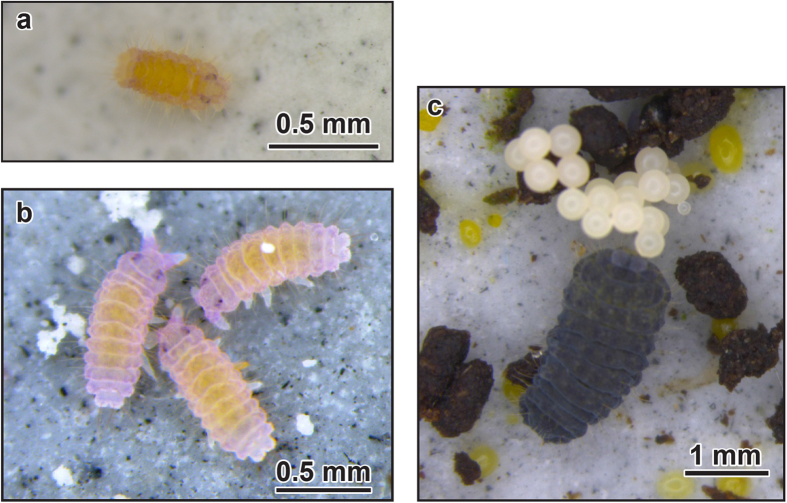
Multiple photographs of each sub-group (e.g. Fig. 2) were taken on a weekly basis using a calibrated microscope (M205C, Leica Microsystems, Wetzlar, Germany). Measurements of total body length (anterior of the head segment to the posterior of the ultimate abdominal segment) were made from these photographs using Leica Application Suite Software (Leica Microsystems). We aimed to photograph and measure at least five individuals from each sub-group. For large sub-groups, one photograph often captured more than five individuals so all specimens within the photograph were measured. Due to mortality, some of the smaller sub-groups contained fewer than five individuals toward the end of the study, in which case all individuals were photographed and measured. Measurements continued on a weekly basis until either the control or experimental sub-group was determined to have reached sexual maturity. The date of first egg-laying was noted for all sub-groups and all eggs were counted and removed to an incubator to determine their viability.
Figure 2. Photographs representing various Neanura muscorum growth-stages.
Neanura muscorum hatchling (a <1 day old), juvenile N. muscorum before first moult (b <5 days old), and adult N. muscorum laying eggs (c).
Statistical Analyses
Second order robust least-squares regression models41 were fitted to body-length measurements taken at regular time intervals to produce four separate growth curves for control and experimental F1 and F2 sub-groups. Robust statistics were used to account for data heteroscedasticity. Second order models were chosen to provide the best fit to sub-group growth curves, particularly to capture the expected asymptote in the curves as maturity is reached12,13. Robust F-tests were used to compare between fitted models and assess the effect of both treatment and generation. Cohen’s d was calculated as a measure of effect size. The effect of treatment as a factor was tested using within-generation comparisons, while generation effects were tested using within-treatment comparisons. Bonferroni correction was applied for all significance testing. All analyses were performed in R statistical software42.
Results
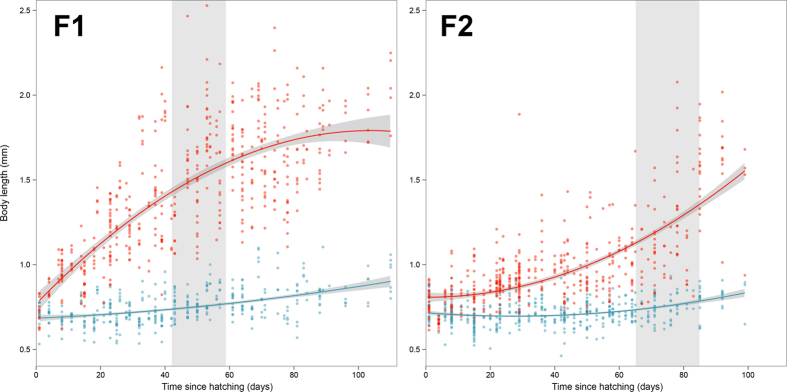
Only sub-groups of N. muscorum given the P. polycephalum slime mould diet reached sexual maturity and laid eggs. Six out of nine of these sub-groups laid eggs while the three remaining sub-groups, despite appearing to be of comparable maturity, did not. This may be attributed to the relatively small size of these sub-groups, which was dictated by the number of eggs available at the time of founding. F1 experimental sub-groups laid eggs at a younger age than their F2 counterparts (mean/sd age at first egg laying: 52.5 ± 5.20 and 75 ± 14.14 days respectively; Fig. 3), but individuals in F2 sub-groups were smaller than those in F1 sub-groups when viable eggs were laid.
Figure 3. Body length change over time for two generations (F1, F2) of Neanura muscorum.
Red points represent the experimental group provided with slime mould diet, blue points represent the control group on algae-covered plane tree bark. Fitted models shown with 95% confidence intervals (F1: experimental, y = 1.4205 + 6.1211 x − 1.5328 x2, weighted-R2 = 0.6906; control, y = 0.7486 + 0.9638 x + 0.1662 x2, wR2 = 0.6315. F2: experimental, y = 0.9771 + 3.8062 x + 1.0992 x2, wR2 = 0.6976; control, y = 0.7181 + 0.4565 x + 0.3624 x2, wR2 = 0.7575. Shaded area represents respective F1 or F2 sub-group age range at first egg laying event.
At the conclusion of the experiment, sub-groups ranged from 78 to 110 days old. The mean body length of individuals from the experimental (slime mould-fed) F1 sub-groups was 1.66 mm (sd ± 0.29 mm), while the control (bark-fed) group had a mean body size of 0.83 mm (sd ± 0.11 mm) at the end of the experimental period. This contrast in final body size of experimental and control sub-groups was mirrored in the F2 generation (1.18 ± 0.27 mm and 0.796 ± 0.09 mm, respectively). Fitted models demonstrated differences in growth rate between treatment groups and generations (Fig. 3). Both the F1 and F2 sub-groups feeding on slime mould showed significantly faster growth rates than their control counterparts (interaction between time since hatching and treatment; F1, F3, 854 = 793.62, p < 0.0001, Cohen’s d = 1.9372; F2, F3, 904 = 363.51, p < 0.0001, d = 1.2657). Generation effects were also observed with F1 individuals growing faster than those in the F2 sub-groups (interaction between time since hatching and generation; slime, F3, 941 = 628.33, p < 0.0001, d = 1.631; bark, F3, 817 = 75.44, p < 0.0001, d = 0.6082). As of February 2015 N. muscorum laboratory stocks had produced fourth generation (F4) individuals.
Discussion
The importance of slime moulds in the diet of the Neanuridae has been suggested previously, but clear evidence for them as a primary food source has been lacking. Owing to the similarities in the habitats occupied by neanurid Collembola and slime moulds, both are frequently found in in damp litter, soil, or logs8,43, it is perhaps unsurprising that Neanuridae can utilise slime mould as a nutritional resource. Anecdotal observations34,35,36,37 and stable isotope studies44,45 have lent support to the idea that they might be important, but here we present explicit evidence that at least one species of the family, Neanura muscorum, is able grow and reproduce when given a diet of slime mould. When given slime mould, significant gains in growth rate over bark-fed counterparts were observed. The benefits of a slime mould diet are further highlighted by the reproductive success of the experimental group. Six out of nine experimental sub-groups given slime mould produced viable eggs and healthy, reproductively active sub-groups of N. muscorum continue to persist in the laboratory on a diet including a slime mould. In contrast, growth is inhibited and no reproduction occurs when the slime mould resource is absent.
Based on the shared mouthpart morphology across the Neanuridae it is likely that the dependence on slime mould holds true for other members of the family. Previous studies on mouthpart morphology32,33 and feeding behaviour34,36,37 of other members of this family further suggest that this is the case. Members of the Neanuridae have ‘suctorial’ mouthparts (sensu Macnamara28) and, unlike the majority of Collembola families, lack a molar plate, which is necessary to process hard food items46. The molar plate enables non-neanurid Collembola to subsist on a generalist diet47,48, and hence be reared on standard algae-covered bark and yeast diets12,13,37. By contrast, Neanuridae mouthparts form a tube-like structure49,50 that is better suited for piercing and sucking8. Neanurids also lack the necessary enzymes to digest fungal cell walls47 and stable-nitrogen isotope analyses indicate that Neanuridae species have higher protein content in their diets than other springtail taxa44,45. Therefore, neanurid springtails require protein-rich fluid-based diets or small particles in suspension10,47,51. Slime moulds clearly fit this description.
As evidenced here, P. polycephalum is a viable food source for lab-rearing N. muscorum. Neanura muscorum is one of the most common and widespread Collembola in Europe and is invasive in many parts of the world52, including Australia. It is also the only member of the subfamily Neanurinae that is known to be parthenogenetic53,54. It can therefore be argued that N. muscorum is easier to keep and breed than other species of Neanuridae owing to the broad environmental tolerance associated with being invasive, and the reproductive flexibility afforded by parthenogenesis54. However, when used to supplement the standard algae-covered bark diet, slime mould has also been used to rear six other species across different tribes in the Neanuridae (Table 1; Supplementary Information online). The successful rearing of other, indigenous and non-parthenogenetic, Neanuridae species demonstrates that N. muscorum represents the rule rather than the exception.
Table 1. Species of Neanuridae (Collembola) that have been successfully reared on a diet of slime mould and algae-covered bark using the methods recommended in this study.
| Subfamily | Tribe | Genus | Species | Generation |
|---|---|---|---|---|
| Neanurinae | Neanurini | Neanura | muscorum | F4 |
| Neanurinae | Paleonurini | ?Australonura | sp. | F3 |
| Neanurinae | Paleonurini | Unknown | sp. 1 | F2 |
| Neanurinae | Anuridini | Anurida | c.f. granaria | F2 |
| Pseudachorutinae | Pseudochorutini | ?Pseudachorutes | sp. | F2 |
| Neanurinae | Lobellini | Unknown | sp. 2 | F2 |
| Neanurinae | Lobellini | Unknown | sp. 3 | F2 |
Latest generations as of February 2015. All lines are ongoing. See Supplementary Table 1 online for sample collection data.
The intergenerational differences in growth-rate and onset of reproduction found for N. muscorum may be due to differences in maternal and grandmaternal investment, although other factors may also be important. F1 individuals that were fed the slime mould P. polycephalum grew significantly faster than their F2 counterparts and reproduced at a much younger age. However, when F2 individuals ultimately reached reproductive maturity they were substantially smaller than reproductively active F1 individuals. Although less pronounced, differences in growth rate were mirrored in the control, bark-fed, group. Folsomia candida exhibits remarkable flexibility in its reproductive investment in response to food supply and crowding55, and food availability influences age at maturity and reproductive output through maternal and grandmaternal effects56. Comparable transgenerational effects arising from nutrient variability have been found to span at least two generations in Drosophila melanogaster57,58. The parents of F1 and F2 study-groups (F0 and F1 respectively) were raised on identical diets of slime mould and algae-covered plane tree bark in plentiful supply. While we know grandparents of F2 groups (F0) were also given this diet, grandparents of F1 groups were wild and their diet and nutritional intake is unknown. It is therefore possible that maternal and grandmaternal effects, comparable to those reported for F. candida56, are contributing toward the generational differences reported in this study. A test of this hypothesis was not possible, however, unpublished results indicate a growth trajectory in the F3 generation similar to that of the F2 generation.
On initial assessment the inter-generation differences observed in this study suggest that slime mould is a sub-optimal resource for N. muscorum as the F2 generation mature later and at a smaller size than their F1 counterparts, which is characteristic of depreciating diet quality in F. candida56. However, two important factors must be considered that strongly support slime mould as a food source. First, we have reared N. muscorum to four generations on this diet and, although viability of offspring was highly variable for all generations, no obvious decline in viability was observed amongst generations. Successful rearing and maintenance would not be possible if insufficient nutrition was provided. The bark-fed control group demonstrate that, when nutritional resources are poor, little growth and no reproduction occurs. Second, the control group also show a decrease in growth rate and body-length. This second point in particular suggests that the inter-generation differences are due to the aforementioned parental effects or to unknown laboratory-effects rather than resource limitation of slime mould. Various laboratory effects have been documented in cultures of Drosophila26,59,60 and, while it is suggested that caution is needed when extrapolating from model organism studies, these effects are generally believed to have negligible impacts.
Our success in creating a laboratory culture of N. muscorum using a slime mould diet provides us with a methodology that can be used to culture other members of the Neanuridae family in a laboratory setting. Alternative nutritional resources, or resource combinations, may provide further gains over a slime mould diet, but have not been tested. While algae-covered bark is clearly not a suitable food source for neanurid springtails it may have value beyond that of a nutrient resource in providing shelter or an egg-laying surface. Therefore, we suggest that providing bark, or an inert alternative, alongside the slime mould diet will better replicate the natural habitat of leaf litter or decaying wood in which Collembola are found8. We have utilised this feeding regime to raise an additional six Neanuridae species (Table 1; Supplementary Information online), and it may also be used to rear other Collembola species with suctorial mouthparts. This new method will facilitate the future use of neanurid Collembola as model taxa, so providing further opportunities to broaden current understanding of the environmental physiology of arthropods by integrating investigations of model and non-model species.
Additional Information
How to cite this article: Hoskins, J. L. et al. Growth and reproduction of laboratory-reared neanurid Collembola using a novel slime mould diet. Sci. Rep. 5, 11957; doi: 10.1038/srep11957 (2015).
Supplementary Material
Acknowledgments
This work was funded by Australian Research Council grant DP140102815. We thank Carla Sgrò and Penny Greenslade for valuable discussion of this work, Robert Bryson-Richardson for microscopy assistance, and all members of the ChownLab for laboratory support and helpful comments on the manuscript.
Footnotes
The authors declare no competing financial interests.
Author Contributions J.L.H., C.J.-S., S.L.C. and G.A.D. wrote the main manuscript text and designed the experiment. J.L.H., C.J.-S. and G.A.D. collected specimens. J.L.H. and G.A.D. performed all laboratory work. G.A.D. analysed data and prepared the figures. All authors reviewed the manuscript.
References
- Hoffmann A. A. Physiological climatic limits in Drosophila: patterns and implications. J. Exp. Biol. 213, 870–880 (2010). [DOI] [PubMed] [Google Scholar]
- Van Heerwaarden B. & Sgrò C. M. Is adaptation to climate change really constrained in niche specialists? Proc. Roy. Soc. B-Biol. Sci. 281, 1790 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chown S. L. & Terblanche J. S. Physiological diversity in insects: ecological and evolutionary contexts. Adv. Insect Physiol. 33, 50–152 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colinet H., Sinclair B. J., Vernon P. & Renault D. Insects in fluctuating thermal environments. Annu. Rev. Entomol. 60, 123–140 (2015). [DOI] [PubMed] [Google Scholar]
- Sunday J. M., Bates A. E. & Dulvy N. K. Thermal tolerance and the global redistribution of animals. Nature Clim. Change. 2, 686–690 (2012). [Google Scholar]
- Araújo M. B. et al. Heat freezes niche evolution. Ecol. Lett. 16, 1206–1219 (2013). [DOI] [PubMed] [Google Scholar]
- Khaliq I., Hof C., Prinzinger R., Böhning-Gaese K. & Pfenninger M. Global variation in thermal tolerances and vulnerability of endotherms to climate change. Proc. Roy. Soc. B-Biol. Sci. 281, 1789 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkin S. P. Biology of the Springtails (Insecta: Collembola) (Oxford University Press, 1997). [Google Scholar]
- Petersen H. & Luxton M. A comparative analysis of soil fauna populations and their role in decomposition processes. Oikos. 39, 288–388 (1982). [Google Scholar]
- Rusek J. Biodiversity of Collembola and their functional role in the ecosystem. Biodivers. Conserv. 7, 1207–1219 (1998). [Google Scholar]
- Deharveng L. Recent advances in Collembola systematics. Pedobiologia. 48, 415–433 (2004). [Google Scholar]
- Birkemoe T. & Leinaas H. P. Effects of temperature on the development of an arctic Collembola (Hypogastrura tullbergi). Funct. Ecol. 14, 693–700 (2000). [Google Scholar]
- Birkemoe T. & Leinaas H. P. Growth and development in a high arctic Collembola: adaptive variation in local populations living in contrasting thermal environments. Ecol. Entomol. 26, 100–105 (2001). [Google Scholar]
- Fountain M. T. & Hopkin S. P. Folsomia candida (Collembola): A ‘standard’ soil arthropod. Annu. Rev. Entomol. 50, 201–222 (2005). [DOI] [PubMed] [Google Scholar]
- Ellers J., Mariën J., Driessen G. & Van Straalen N. M. Temperature-induced gene expression associated with different thermal reaction norms for growth rate. J. Exp. Zool. Part B. 310, 137–147 (2008). [DOI] [PubMed] [Google Scholar]
- Sørensen J. G. & Holmstrup M. Cryoprotective dehydration is widespread in Arctic springtails. J. Insect. Physiol. 57, 1147–1153 (2011). [DOI] [PubMed] [Google Scholar]
- Coulson S. et al. Simulated climate change: the interaction between vegetation type and microhabitat temperatures at Ny Ålesund, Svalbard. Polar Biol. 13, 67–70 (1993). [Google Scholar]
- Coulson S. J., Coulson J., Leinaas H. P., Ims R. A. & Søvik G. Experimental manipulation of the winter surface ice layer: the effects on a High Arctic soil microarthropod community. Ecography. 23, 299–306 (2000). [Google Scholar]
- Fountain M. T. & Hopkin S. P. A comparative study of the effects of metal contamination on Collembola in the field and in the laboratory. Ecotoxicology. 13, 573–587 (2004). [DOI] [PubMed] [Google Scholar]
- Block W. & Convey P. Seasonal and long-term variation in body-water content of an Antarctic springtail–a response to climate change? Polar Biol. 24, 764–770 (2001). [Google Scholar]
- Slabber S., Worland M. R., Leinaas H. P. & Chown S. L. Acclimation effects on thermal tolerances of springtails from sub-Antarctic Marion Island: indigenous and invasive species. J. Insect. Physiol. 53, 113–125 (2007). [DOI] [PubMed] [Google Scholar]
- Hawes T. C., Bale J. S., Worland M. R. & Convey P. Trade-offs between microhabitat selection and physiological plasticity in the Antarctic springtail, Cryptopygus antarcticus (Willem). Polar Biol. 31, 681–689 (2008). [Google Scholar]
- Teets N. & Denlinger D. L. Surviving in a frozen desert: environmental stress physiology of terrestrial Antarctic arthropods. J. Exp. Biol. 217, 84–93 (2014). [DOI] [PubMed] [Google Scholar]
- Van Dooremalen C., Berg M. P. & Ellers J. Acclimation responses to temperature vary with vertical stratification: implications for vulnerability of soil-dwelling species to extreme temperature events. Glob. Change Biol. 19, 975–984 (2013). [DOI] [PubMed] [Google Scholar]
- Cacoyianni Z., Kovacs I. V. & Hoffmann A. A. Laboratory adaptation and inbreeding in Helicoverpa punctigera (Lepidoptera: Noctuidae). Aust. J. Zool. 43, 83–90 (1995). [Google Scholar]
- Sgrò C. M. & Partridge L. Evolutionary responses of the life history of wild caught Drosophila melanogaster to two standard methods of laboratory culture. Am. Nat. 156, 341–353 (2000). [Google Scholar]
- Janion C., Leinaas H. P., Terblanche J. S. & Chown S. L. Trait means and reaction norms: the consequences of climate change/invasion interactions at the organism level. Evol. Ecol. 24, 1365–1380 (2010). [Google Scholar]
- Macnamara C. The food of Collembola. Can. Entomol. 56, 99–105 (1924). [Google Scholar]
- Anderson J. & Healey I. Seasonal and inter-specific variation in major components of the gut contents of some woodland Collembola. J. Anim. Ecol. 41, 359–368 (1972). [Google Scholar]
- Saur É. & Ponge J.-F. Alimentary studies on the Collembolan Paratullbergia callipygos using transmission electron microscopy. Pedobiologia. 31, 355–379 (1988). [Google Scholar]
- Janssens F. & Christiansen K. A. Class Collembola Lubbock, 1870. Animal biodiversity: An outline of higher-level classification and survey of taxonomic richness. Zootaxa. 3148, 192–194 (2011). [Google Scholar]
- Cassagnau P. Sur un essai de classification des Neanuridae holarctiques et sur quelques especes de ce groupe. Rev. Fr. Entomol. 22, 134–163 (1955). [Google Scholar]
- Cassagnau P. L’évolution des pieces buccales et la polytenie chez les collemboles. C. R. Acad. Sci. Paris Ser. D. 267, 106–109 (1968). [Google Scholar]
- Ing B. Myxomycetes as food for other organisms. Proc. S. London Ent. Nat. Hist. Soc. 1967, 18–23 (1967). [Google Scholar]
- Chassain M. Capture d’un insecte collembole par deux myxomycetes. Doc. Mycol. 2, 37–38 (1973). [Google Scholar]
- Greenslade P., Moore S. & Farrow R. Observations on the feeding behaviour of Uchidanurinae (Collembola: Neanuridae) in Australia. Victorian Nat. 119, 221–223 (2002). [Google Scholar]
- Smolis A. Redescription and lectotype designation of Thaumanura carolii (Stach, 1920) (Collembola, Neanuridae), with remarks on its biology. Deut. Entomol. Z. 56, 73–83 (2009). [Google Scholar]
- Wheeler Q. D. & Miller K. B. Slime-mold beetles of the genus Agathidium Panzer in North and Central America, Part I. Coleoptera: Leiodidae. Bull. Am. Mus. Nat. Hist. 290, 1–95 (2005). [Google Scholar]
- Southwood T. R. E. & Henderson P. A. Ecological Methods 3rd Edition. 462–502. Wiley-Blackwell, 2000). [Google Scholar]
- Janion C., Worland. M. R. & Chown S. L. Assemblage level variation in springtail lower lethal temperature: the role of invasive species on sub-Antarctic Marion Island. Physiol. Entomol. 34, 284–291 (2009). [Google Scholar]
- Huber P. J. Robust Statistics (Wiley, 1981). [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2014). [Google Scholar]
- Blanton R. L. Slime Moulds (Wiley, 2001). [Google Scholar]
- Ruess L., Häggblom M. M., Langel R. & Scheu S. Nitrogen isotope ratios and fatty acid composition as indicators of animal diets in below ground systems. Oecologia. 139, 336–346 (2004). [DOI] [PubMed] [Google Scholar]
- Chahartaghi M., Langel R., Scheu S. & Ruess L. Feeding guilds in Collembola based on nitrogen stable isotope ratios. Soil Biol. Biochem. 37, 1718–1725 (2005). [Google Scholar]
- Greenslade P., Simpson J. A. & Grgurinovic C. A. Collembola associated with fungal fruit-bodies in Australia: Proceedings of the Xth international Colloquium on Apterygota, České Budějovice 2000: Apterygota at the Beginning of the Third Millennium. Pedobiologia. 46, 345–352 (2002). [Google Scholar]
- Berg M. P., Stoffer M. & van den Heuvel H. H. Feeding guilds in Collembola based on digestive enzymes. Pedobiologia. 48, 589–601 (2004). [Google Scholar]
- Castaño-Meneses G., Palacios-Vargas J. G. & Cutz-Pool L. Q. Feeding habits of Collembola and their ecological niche. An. Inst. Biol. Univ. Nac. Auton. Mex. Ser. Zool. 75, 135–142 (2004). [Google Scholar]
- Richards O. & Davies R. Imms’ General Textbook of Entomology. (Springer, 1977). [Google Scholar]
- Deharveng L. Morphologie évolutive des Collemboles Neanurinae en particulier de la lignée Neanurienne. Travaux du Laboratoire d’Ecobiologie des Arthropodes Edaphiques, Toulouse. 4, 1–63 (1983). [Google Scholar]
- Singh S. Preliminary observations on the food preference of certain Collembola (Insecta). Rev. Ecol. Biol. Sol. 6, 461–467 (1969). [Google Scholar]
- Bellinger P. F., Christiansen K. A. & Janssens F. Checklist of the Collembola of the World (2015). Available at: www.collembola.org. (Accessed: 20th March 2015).
- Cassagnau P. Parthenogenese geographique et polyploidie chez Neanura muscorum. C. R. Acad. Sc. Paris 274, 1846–1848 (1972). [Google Scholar]
- Chernova N. M., Potapov M. B., Savenkova Y. Y. & Bokova A. I. Ecological significance of parthenogenesis in collembola. Entomol. Rev. 90, 23–38 (2010). [Google Scholar]
- Tully T. & Ferrière R. Reproductive flexibility: genetic variation, genetic costs and long-term evolution in a Collembola. PLoS one 3, e3207 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafer N., Ebil S., Uller T. & Pike N. Transgenerational effects of food availability on age at maturity and reproductive output in an asexual collembolan species. Biol. Lett. 7, 755–758 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtonen T. M., Kangassalo K., Pölkki M. & Rantala M. J. Transgenerational effects of parental larval diet on offspring development time, adult body size and pathogen resistance in Drosophila melanogaster. PLoS one. 7, e31611 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buescher J. L. et al. Evidence for transgenerational metabolic programming in Drosophila. Dis. Model. Mech. 6, 1123–1132 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann A. A., Hallas R., Sinclair C. & Partridge L. Rapid loss of stress resistance in Drosophila melanogaster under adaptation to laboratory culture. Evolution. 55, 436–438 (2001). [DOI] [PubMed] [Google Scholar]
- Griffiths J. A., Schiffer M. & Hoffmann A. A. Clinal variation and laboratory adaptation in the rainforest species Drosophila birchii for stress resistance, wing size, wing shape and development time. J. Evolution. Biol. 18, 213–222 (2005). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.