Abstract
The entomopathogenic fungus Beauveria bassiana and the predatory mite Neoseiulus barkeri are both potential biocontrol agents for their shared host/prey Frankliniella occidentalis. The combination of the two agents may enhance biological control of F. occidentalis if the fungus does not negatively affect N. barkeri. This study evaluated the indirect effects of B. bassiana strain SZ-26 on N. barkeri mediated by F. occidentalis using the age-stage, two-sex life table. When fed on the first instar larvae of F. occidentalis that had been exposed for 12 h to the SZ-26 suspension, the developmental time of preadult N. barkeri was significantly longer, and the longevity and fecundity were significantly lower than that of N. barkeri fed on untreated F. occidentalis. The mean generation time (T), net reproductive rate (R0), finite rate of increase (λ), intrinsic rate of natural increase (rm) and predation rates were correspondingly affected. The data showed that B. bassiana has indirect negative effects on N. barkeri population dynamics via influencing their prey F. occidentalis larvae, which indicates that there is a risk in combining B. bassiana with N. barkeri simultaneously for the biocontrol of F. occidentalis. The probable mechanism for the negative effects is discussed.
The western flower thrips, Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae), is a major insect pest of ornamental plants and vegetables that causes extensive economic losses in many crops worldwide1,2,3,4,5,6. The intensive use of conventional synthetic insecticides has led to widespread resistance7. Therefore, there has been an increasing interest in managing F. occidentalis populations using biological control agents. The predatory mite Neoseiulus barkeri (Amblyseius barkeri) Hughes (Acarina: Phytoseiidae) is a specialist predator of F. occidentalis that has been used as an alternative for F. occidentalis management on a variety of plants8,9,10,11. However, control efficiency is limited because the predatory mites prey primarily on only two larval stages of thrips, and because success in capturing prey depends largely on the size and feeding status of the predator12. Control with predatory mites alone is not generally effective when there is high density of F. occidentalis in a crop.
Entomopathogenic fungi have been successfully developed worldwide as biological control agents for many arthropod pests, including several species of thrips13,14,15. The entomopathogenic fungus Beauveria bassiana (Balsamo) Vuillemin (Hypocreales: Cordycipitaceae) has potential for managing F. occidentalis. While commercial development of thrips-active isolates of these fungi has been limited, recent studies in China screened several isolates of B. bassiana that are highly virulent against F. occidentalis and showed their potential as biological control agents16,17,18,19. Recently, in our bioassay experiments, strain SZ-26 was screened from 30 isolates of B. bassiana and identified as one of the most virulent strains, causing 97% mortality to F. occidentalis adults at 85 viable conidia per mm2, while it showed no virulence to N. barkeri when sprayed directly on the mites20. Although the fungus works rapidly and kills large numbers of adult F. occidentalis via infection in the short term, pathogenicity to immature stages of F. occidentalis is reduced because fungal conidia are shed with the exuvium following ecdysis14,21. Uninfected immature F. occidentalis continue to propagate and damage crops.
Recently, an integrated control approach, including the use of multiple biocontrol agents for pest management has been of growing interest to biological control practitioners22,23,24,25,26. The combination of N. barkeri with B. bassiana is a promising control measure for maintaining F. occidentalis populations below economically damaging levels, and solving problems of chemical resistance. However, the potential benefits and hazards of using fungus and predators simultaneously have not yet been widely evaluated. Multiple biological control species may act synergistically, additively or antagonistically27, and the potential for integrated pest management (IPM) may be compromised if the predators are susceptible to B. bassiana when the conidia are directly applied, or if the predators are indirectly affected via feeding on contaminated surfaces or infected prey.
The majority of previous studies evaluated the effects of pathogens on predators by exposing them directly to residues, or by short term topical application and then studying predator behavior, mortality and population abundance16,26,28,29,30,31. However, only a few studies were conducted to understand the effect of fungus infection on the physiology of the host and its indirect effect on predators by feeding behavior32. The quality and nutritional value of the prey may cause sub-lethal effects on predators33, such as changes in development time, consumption rate and life table parameters, which play an important role in determining the population growth rate. The objective of this study was to evaluate the effects of the B. bassiana-treated F. occidentalis on N. barkeri by studying the predator’s biological parameters.
Results
Life table
The development periods for each life stage, adult longevity, preoviposition period, and female fecundity of N. barkeri fed on B. bassiana-treated (treatment) and untreated (control) F. occidentalis larvae are shown in Table 1. Because the hatching of eggs and development of larvae are not affected by prey, the duration of the egg and larval stages of N. barkeri were not significantly different between the control and treatment. However, there was a significantly longer protonymph duration (P < 0.001), deutonymph duration (P < 0.001) and preadult period (P < 0.001) in the treatment than in the control. There were no differences between treatment and control groups in terms of adult pre-oviposition period (APOP). In contrast to the APOP, the total pre-oviposition period (TPOP) (from the beginning of the life table study to first egg production) was longer for the treatment than the control group (P < 0.001). Adult longevity (both female and male) was shorter (P < 0.001), and fewer eggs were produced (P < 0.001) in the treatment than the control group. The female adult sex ratio decreased and was less than 50% for the treatment.
Table 1. Developmental time, longevity and mean fecundity of N. barkeri in control and treatment.
| Stage | n | Control Developmental time (d) (mean ± SE) | n | TreatmentDevelopmental time (d) (mean ± SE) |
|---|---|---|---|---|
| Egg | 60 | 1.99 ± 0.06a | 60 | 2.12 ± 0.05a |
| Larva | 57 | 1.08 ± 0.05a | 57 | 1.15 ± 0.04a |
| Protonymph | 56 | 2.11 ± 0.05b | 49 | 2.46 ± 0.08a |
| Deutonymph | 48 | 1.84 ± 0.06b | 45 | 2.34 ± 0.06a |
| Preadult | 48 | 7.25 ± 0.10b | 45 | 8.39 ± 0.10a |
| Adult longevity | ||||
| Female | 27 | 29.46 ± 0.55a | 23 | 22.86 ± 0.38b |
| Male | 23 | 22.34 ± 0.32a | 24 | 17.39 ± 0.30b |
| Apop | 27 | 2.54 ± 0.12a | 23 | 2.50 ± 0.10a |
| Tpop | 27 | 10.13 ± 0.19b | 23 | 11.25 ± 0.13a |
| Mean fecundity | ||||
| Female | 27 | 52 ± 2a | 23 | 38 ± 1b |
Values followed by the different lowercase letters within a row are significantly different using t-tests (P < 0.05). APOP represents adult pre-oviposition period; TPOP represents total pre-oviposition period.
The curves of the age-stage survival rate (sxj) show the probability that an N. barkeri egg will survive to age x and stage j (Fig. 1). The overlap of the stage-specific survivorship curves is the result of the variable developmental rates among individuals34. The probability that a newly hatched larva survives to the adult stage was 78.3% in the treatment group, which was lower than the control (83.3%). The curve of the age-stage survival rate (lx) includes all individuals of the cohort and is a simplified version of the sxj curves (Fig. 2). The age-specific fecundity of the total population (mx) was also plotted in Fig. 2. The curve of mx shows approximate periodic peaks in reproduction. The highest age-specific reproductive peak occurred at age 12.5 days, with the highest fecundity of 1.5 hatched eggs within 12 h in the control. The reproductive peak occurred at age 16 days, with the highest fecundity of 0.8 hatched eggs within 12 h for the treatment group.
Figure 1. Age-stage survival rate (sxj) of N. barkeri in control and treatment.
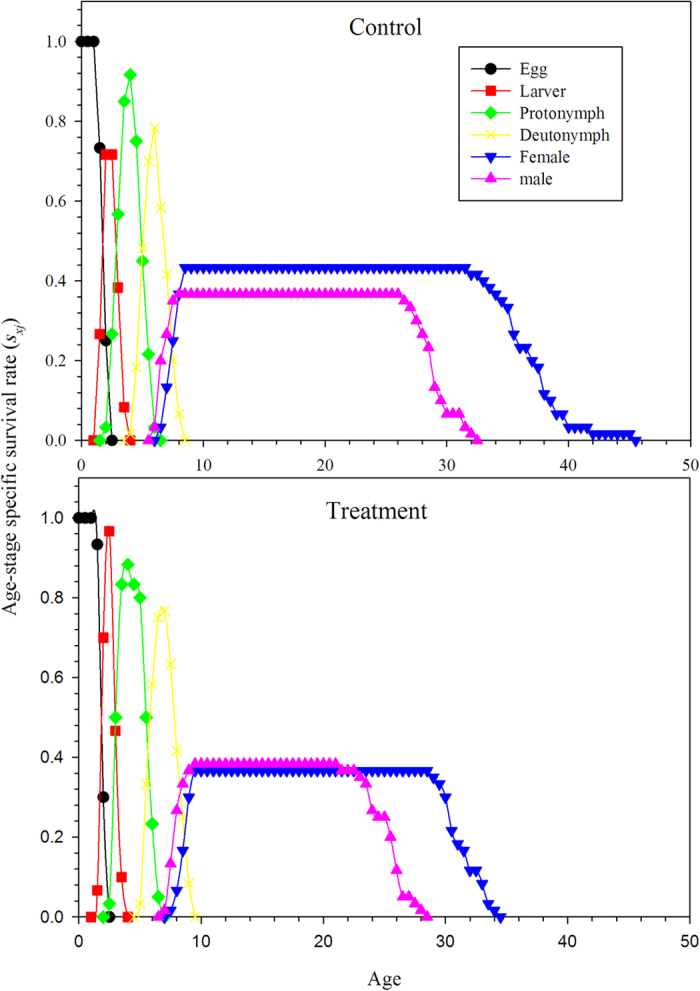
Control means feeding on untreated F. occidentalis; Treatment means feeding on B. bassiana-treated F. occidentalis. Notation is the same as for Figs 2, 3, 4, 5, 6.
Figure 2. Age-specific survival rate (lx) and fecundities (mx) of N. barkeri in control and treatment.
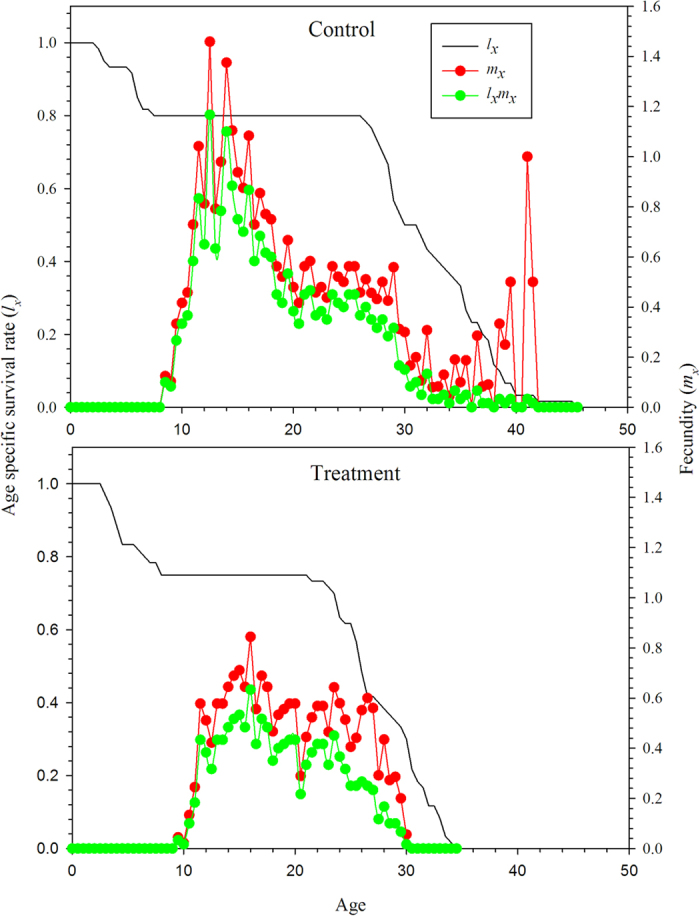
Population parameters were calculated based on data from the entire cohort35. The means and standard errors of the intrinsic rate of increase (r), finite rate of increase (λ), net reproductive rate (R0) and mean generation time (T) are listed in Table 2. Statistical analysis showed that r, λ and R0 were lower in the treatment group than the control. Because of the longer developmental time of the pre-adults in the treatment group, T was significantly longer than in the control.
Table 2. Population parameters of N. barkeri in control and treatment.
| Parameter | Control (mean ± SE) | Treatment (mean ± SE) |
|---|---|---|
| Intrinsic rate of increase, r (d−1) | 0.1896 ± 0.0108a | 0.1461 ± 0.0109b |
| Finite rate of increase, λ (d−1) | 1.2088 ± 0.0130a | 1.1572 ± 0.0170b |
| Net reproductive rate, R0 (offspring) | 22.4833 ± 3.3773a | 13.4634 ± 2.3000b |
| Mean generation time, T (d) | 16.3684 ± 0.2580b | 17.7014 ± 0.2660a |
Values followed by the different lowercase letters within a row are significantly different using t-tests (P < 0.05).
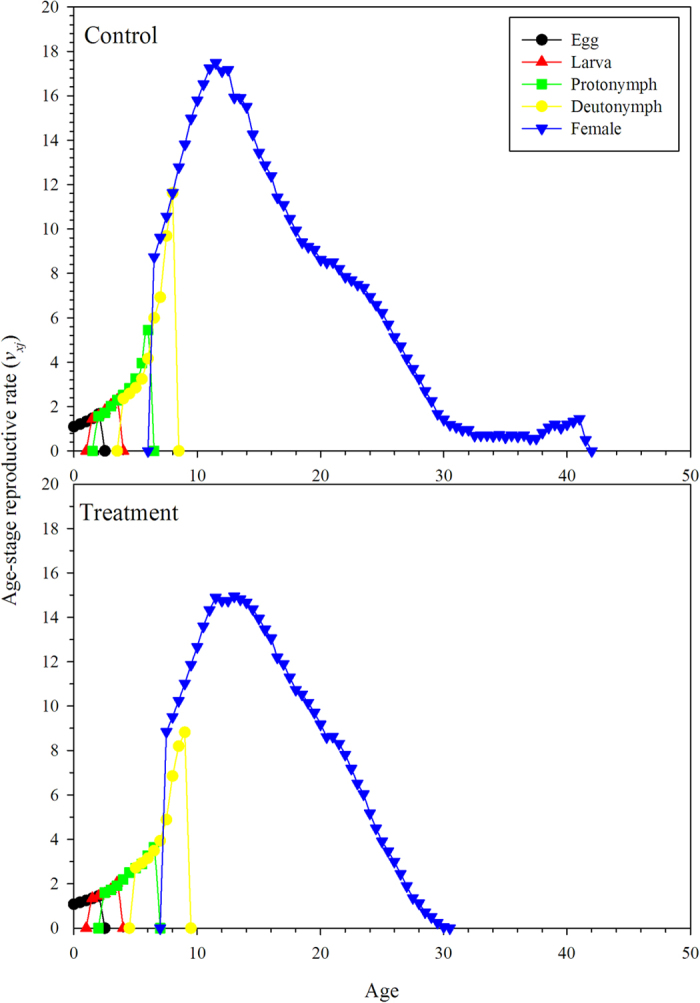
The age-stage life expectancy (exj) is the total time that an individual of age x and stage j is expected to live after age x (Fig. 3). The life expectancy of the N. barkeri treatment cohort was shorter than the control. The life expectancy decreased gradually with age. The age-stage-specific reproductive values (vxj) of N. barkeri represent the contribution of an individual at age x and stage j to the future population. The reproductive value increased significantly when N. barkeri began to produce hatchable eggs. In the control, the reproductive value increased at age 11.5 days and reached a peak of 17.5. Despite the low fecundity in the treatment group, the peak reproductive value also occurred at age 11.5 days and remained high for 2 days (Fig. 4).
Figure 3. Age-stage-specific life expectancy (exy) of N. barkeri in control and treatment.
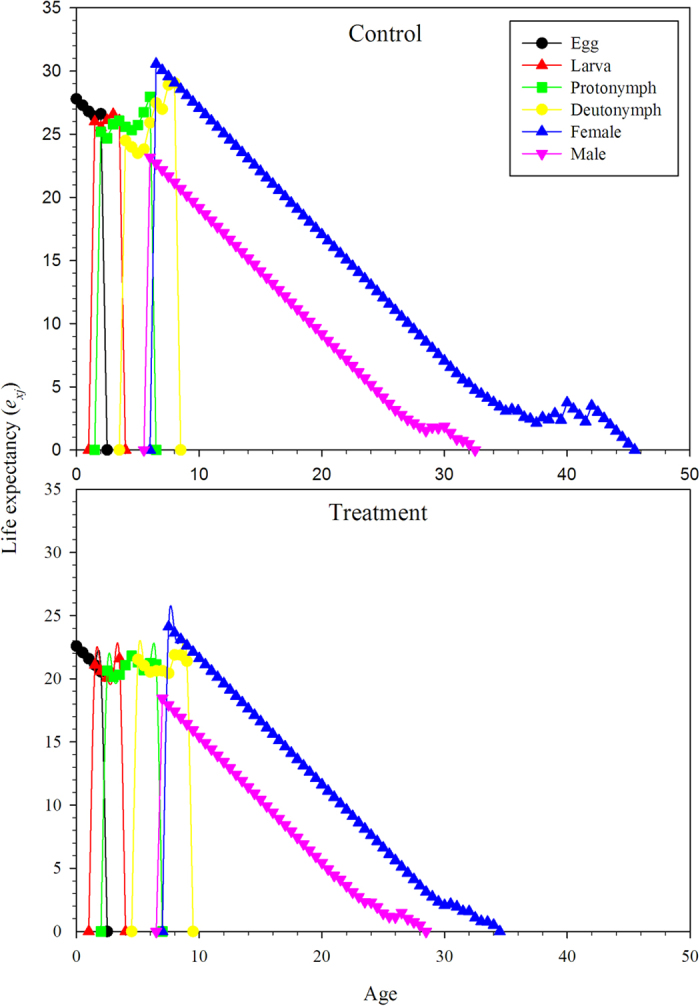
Figure 4. Age-stage-specific reproductive value (vxy) of N. barkeri in control and treatment.
Predation rate
The age-stage predation rate (cxj) of N. barkeri fed on B. bassiana-treated (treatment) and untreated F. occidentalis larvae (control) are shown in Fig. 5. This shows the trend in the age-stage specific mean number of F. occidentalis larvae consumed by a predator at age x and stage j. Using equations (9) and (10), we calculated the age-specific predation rate (kx) and age-specific net predation rate (qx). Both kx and qx were calculated by considering the sex and stage differentiation (Fig. 6). When offered B. bassiana-treated F. occidentalis larvae, the protonymphs and deutonymphs of N. barkeri consumed more prey, while both female and male adults lived longer when fed on untreated F. occidentalis larvae, and consumed more total prey as adults. By taking survival rates and longevities into account, the net predation rate, C0, was 86.0320 when fed on untreated thrips larvae and 70.1384 when fed on B. bassiana-treated thrips larvae. However, the finite predation rate of N. barkeri was 1.0459 when fed on treated prey, higher than that of N. barkeri fed on untreated prey (Table 3).
Figure 5. Age-stage, two-sex predation rate (cxj) of N. barkeri in control and treatment.
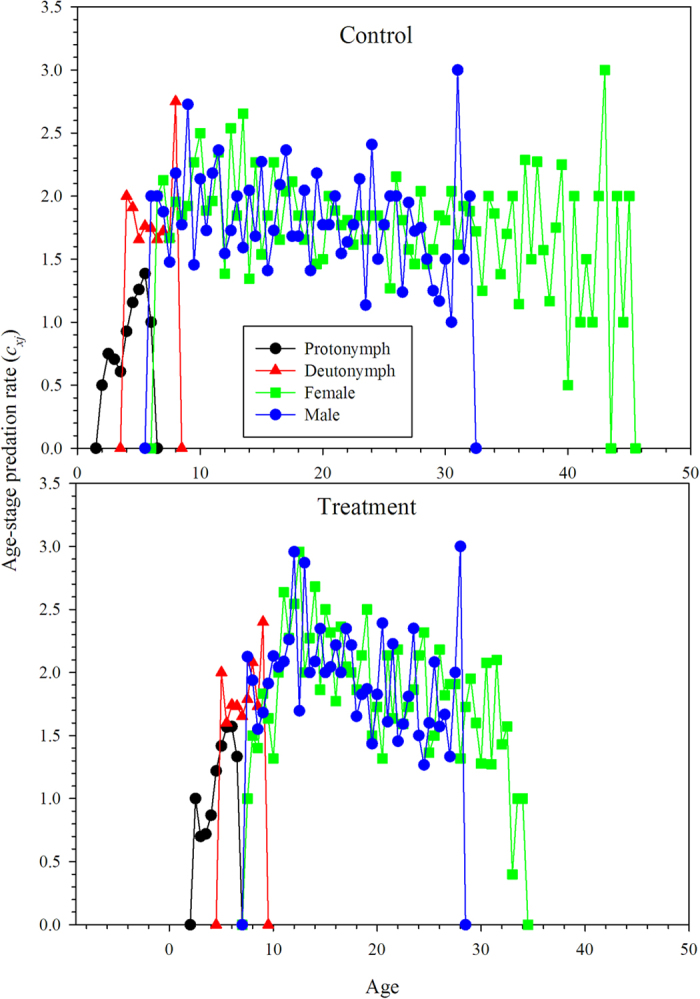
Figure 6. Age-specific survival rate (lx), predation rate (kx), and net predation rate (qx) of N. barkeri in control and treatment.
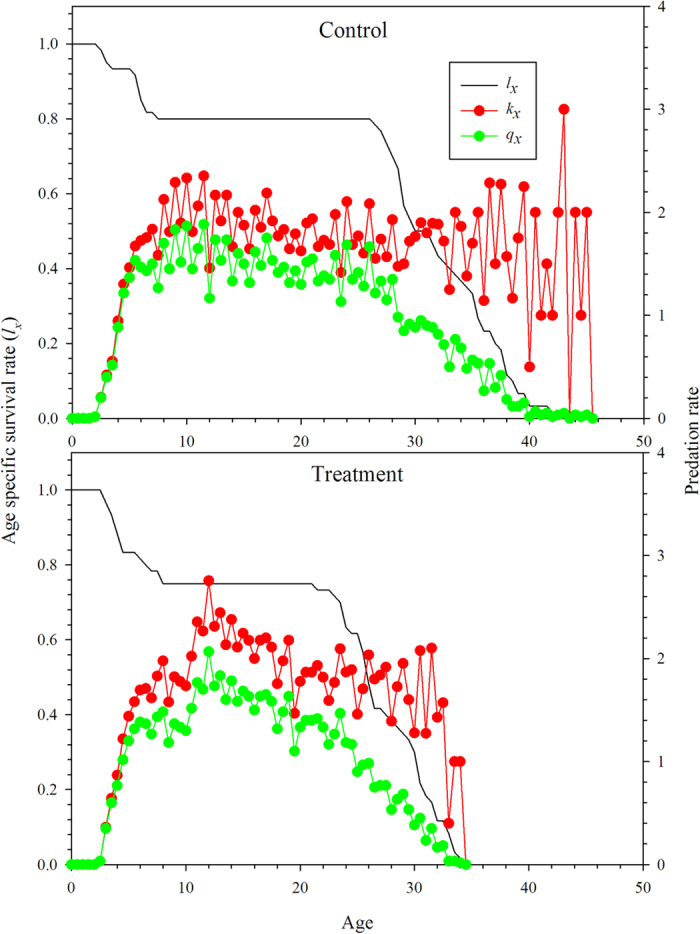
Table 3. Predation rates of N. barkeri in control and treatment.
| Stage | Predation rate (preys/predator) |
|
|---|---|---|
| Control(mean ± SE) | Treatment (mean ± SE) | |
| Larva | 0 | 0 |
| Protonymph | 3.93 ± 0.17b | 5.88 ± 0.22a |
| Deutonymph | 6.71 ± 0.26b | 8.49 ± 0.26a |
| Preadult | 10.71 ± 0.35b | 14.31 ± 0.4a |
| Adult | 96.02 ± 2.74a | 78.36 ± 1.94b |
| Net predation rate, C0 | 86.0320 ± 5.8587a | 70.1384 ± 5.1868b |
| Stable predation rate, ψ | 0.7862 ± 0.0250b | 0.9038 ± 0.0288a |
| Finite predation rate, ω | 0.9503 ± 0.0328b | 1.0459 ± 0.0381a |
Values followed by the different lowercase letters within a row are significantly different using t-tests (P < 0.05).
Discussion
Fungal biological agents are used to suppress many species of pests; however, they may also negatively affect natural insect enemies through direct infection or indirectly by decreasing the prey population27 or affecting the quality of prey32. Our previous studies demonstrated that entomopathogenic fungus B. bassiana strain SZ-26 showed high toxicity against F. occidentalis, but no direct pathogenicity to the predatory mite N. barkeri20. In the present study, the indirect effects of SZ-26 on N. barkeri fed on B. bassiana-treated F. occidentalis larvae were examined, and the results indicated that biological parameters of the predators were affected. Similar results by Simelane et al.36, demonstrated that when Coccinella septempunctata L. (Coleoptera: Coccinellidae) fed on aphids infected with Neozygites fresenii (Nowakowski) (Entomophthorales: Neozygitaceae), the developmental time was significantly longer and the fecundity was lower than C. septempunctata fed on uninfected aphids. Seiedy et al.32 also demonstrated that the longevity and fecundity of the predatory mite Phytoseiulus persimilis Athias-Henriot (Acari: Phytoseiidae) were adversely affected when fed on Tetranychus urticae Koch (Acari: Tetranychidae) treated with B. bassiana at 24, 48 and 72 h post-inoculation.
Predatory mites prefer to prey on the first instar of thrips12, the first instars develop to second instars within 24–48 h under normal laboratory conditions37, so the first instars were supplied to N. barkeri as prey every 12 h, and the life table raw data of individual N. barkeri were recorded. Using the age-stage, two-sex life table, we accurately and precisely described the survival rate and stage structure, thereby evaluating the sub-lethal effects of B. bassiana on N. barkeri. From our statistical analysis, the protonymphs and deutonymphs of N. barkeri readily fed more on infected F. occidentalis larvae than on untreated larvae. Also, the finite predation rate of N. barkeri fed on infected prey was higher than that of N. barkeri fed on untreated prey. However, the longevity of N. barkeri fed on infected prey became shorter, consequently the net predation rate of N. barkeri was lower than the N. barkeri fed untreated prey.
Our data show that B. bassiana has indirect negative effects on N. barkeri mediated by their shared host/prey F. occidentalis. However, when considering the probable mechanism, it is complicated due to the complex predator-fungus-pest interactions. After having been treated with B. bassiana suspension for 12 h, a large number of conidia adhered to the cuticles of the first instars. The majority of conidia germinated and the germ tubes oriented towards the cuticle of the F. occidentalis larvae, and some germ tubes penetrated the cuticle of F. occidentalis larvae under fluorescence and scanning electron microscopy (SEM) (see Supplementary Fig. S1). Although these infected larvae remained live at this time, their vitality had declined due to fungal penetration and thus they were more vulnerable to predation38. Therefore, N. barkeri consumed more infected prey than normal prey, perhaps because of the greater catch efficiency of N. barkeri on infected F. occidentalis larvae, rather than a preference for infected larvae. While they consumed more infected thrips, the two-sex life table parameters of N. barkeri were negatively affected. We put forward two possible reasons to account for the results: (1) the poorer nutritional quality of B. bassiana-infected thrips could not meet the needs of normal development and reproduction by N. barkeri. Huang et al.39 reported that proteins or saccharides are the essential nutrients for mass production of N. barkeri. When B. bassiana invades the insect body, it absorbs amino acids from the blood40. From our SEM observations, germ tubes of conidia would absorb nutrition from thrips once its cuticle was penetrated. The fungal hyphae would grow further and colonize the host, and the host nutrients were absorbed gradually41. Moreover, when offered B. bassiana-infected thrips, nearly 50% of the observed dead thrips were partially consumed by N. barkeri, further indicating that the quality of the prey was affected by the fungus. When fed on infected thrips, the body size of female N. barkeri was smaller than conspecifics fed a diet of untreated thrips. The length and width of female N. barkeri adults fed on infected thrips for 15 d were 361.5 and 238.9 μm, which were significantly shorter than those fed on untreated thrips (length: 403.2 μm; width: 296.9 μm) (see Supplementary Table S1); (2) the second possible reason could be because metabolites42 or toxins43 were produced when the tissues of F. occidentalis larvae were invaded by fungal hyphae, which may have harmed the predators. This hypothesis was supported by Agboton et al.25 and Seiedy et al.32
Agboton et al.25 reported that the predatory mite Typhlodromalus aripo could consume significant amounts of the entomopathogenic fungus Neozygites tanajoa-infected cassava green mite (CGM), Mononychellus tanajoa, under laboratory conditions and that the oviposition and survival rate of T. aripo were reduced. Onto et al.26 further showed in screenhouse experiments that the simultaneous presence of T. aripo and N. tanajoa improved the biological control of the CGM, even though the two agents on the same cassava plants had a negative effect on their respective population growth, such that the T. aripo population increased while the N. tanajoa population decreased. Our data indicate it is risky for N. barkeri when feeding on the B. bassiana -infected F. occidentalis larvae. This study will help to enhance our understanding about predator-fungus-pest interactions. However, field studies of B. bassiana applications in conjunction with N. barkeri for biological control of F. occidentalis should be conducted, as it remains unknown whether N. barkeri behavior, such as search activity, may be reduced under field conditions; our results indicate that N. barkeri prefer to prey on relatively less mobile infected thrips. Moreover, if the fungus-infected prey is detrimental to the predator, it might be expected that the latter should have evolved the ability to avoid areas where the fungus is present30. Therefore, whether N. barkeri will display behavioral avoidance of infected F. occidentalis when co-existing with B. bassiana and F. occidentalis requires further exploration.
Methods
Ethics Statement
No specific permissions were required for these locations/activities.
None of the species used in this study are endangered or protected.
Fungal preparations
The isolate of B. bassiana sensu lato SZ-26 was derived from Ostrinia nubilalis (Lepidoptera: Pyralidae) collected in Suizhong, Liaoning (2010). Fresh cultures, initiated using conidia inoculum, were maintained, and conidia were produced on Sabouraud dextrose agar (SDA) at 26 ± 1 °C with continuous darkness. The conidial concentrations were determined using a hemocytometer and adjusted with sterile 0.05% Tween-80. Viability of the conidia was confirmed according to the method of Wen et al.44 and was >90%.
Predatory mite colony
A colony of N. barkeri was obtained from the laboratory of insect natural enemies, Institute of Plant Protection, Chinese Academy of Agricultural Sciences. The stock colony was then cultured on excised kidney bean leaves and supplied Tyrophagus putrescentiae (Schrank) (Acari: Acaridae) as prey in plastic boxes (15 cm × 15 cm × 10 cm) with rims. Circular moist sponges (10-cm diameter) were placed at the edges of the boxes to prevent escape. A hole (12-cm diameter) was punched on the lid and covered with a fine mesh to allow for ventilation. The culture boxes were maintained at 25 ± 1 °C, 65 ± 5% RH and L16:D8 h photoperiod in climatic chambers. Cotton silk was placed on the leaf surfaces as an oviposition substrate. The newly laid eggs were collected and transferred into new boxes using a fine brush after 6 hours to allow the emergent larvae to develop in synchrony.
Western flower thrips colony
A colony of western flower thrips, F. occidentalis, was maintained according to the methods described by Liang et al.45. Briefly, colonies were continuously reared on kidney beans (Phaseolus vulgaris L. var. Gonggeizhe) in tube-shaped jars (0.5 L) with snap-on lids. A hole (10-cm diameter) was punched on the lid and covered with a fine mesh to allow for ventilation. The rearing jars were maintained at 26 ± 2 °C, 65 ± 5% RH and L13:D11 h photoperiod in climatic chambers. The thrips at similar development stages were obtained by incubating the adults on fresh plants for oviposition. After 3 days, thrips were removed and we allowed the different stages of F. occidentalis to develop in synchrony. The first instar larvae were collected for experimental use.
Life table study
The experimental units contained two uniform Plexiglas (6 cm × 5 cm × 4 mm) containers. Water-saturated filter paper was placed on the bottom of one piece, with a freshly excised kidney bean leaf added upside down on the filter paper surface. A hole (2.5-cm diameter) was punched on another piece of glass and pressed onto the leaf. A chamber was formed between two pieces of organic-glass as an experimental platform. Sixty eggs of N. barkeri were randomly collected from the colony within 6 h. Each egg was transferred carefully into an individual chamber using a fine paintbrush. The experimental units were placed on Petri dishes (20-cm diameter) with moist sponges at the bottom and then placed in a climatic chamber at 25 ± 1 °C, 65 ± 5% RH and L16:D8 h photoperiod. The first instar larvae of F. occidentalis were sprayed with a conidia suspension (108 conidia mL−1) with a small hand pressure sprayer (2 ml)38. After 12 h, the treated thrips were supplied with N. barkeri as prey. The units were observed every 12 h until the eggs hatched into larvae. In the preliminary test, each larva developed into a protonymph normally without feeding. Therefore, in our life table study, when the larvae developed into a protonymph, each protonymph was provided 5 B. bassiana-treated F. occidentalis larvae as prey every 12 h, and each deutonymph was provided 10 B. bassiana-treated F. occidentalis larvae as prey every 12 h. The survival and development time of each stage were recorded every 12 h. Because the female deutonymphs generally develop females slower than males, the newly emerged males continue to be provided with 10 treated F. occidentalis larvae as prey every 12 h. The newly emerged females were paired with young male adults recruited from the colony for mating in individual chambers and provided 20 treated F. occidentalis larvae every 12 h. The number of F. occidentalis larvae was based on the preliminary feeding test. Survivorship, fecundity, and predation were recorded every 12 h for N. barkeri. Because each protonymph, deutonymph and male was housed in an individual chamber, predation was recorded every 12 h for each individual. However, because the adult females and recruited males were housed as pairs in an individual chamber, we ignored the differences between the sexes, and one-half of the daily predation of a pair was assigned to each female as long as both remained alive. If the male died, the total daily predation rate was attributed to the female, and if the female died, the experiment was terminated. The control was the untreated-F. occidentalis larvae and was performed as described above.
Age-stage, two-sex life table data analysis
The life history data of all N. barkeri individuals were analyzed based on the age-stage, two-sex life table46 with the computer program of TWO-SEX-MSChart47. Following the method of Chi and Liu35, the survival rate (sxj) (x = age, j = stage) and fecundity fxj were calculated. The age-specific survival rate (lx) was then calculated as follows:
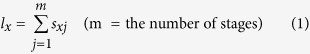 |
The age-specific fecundity (mx) was calculated as follows:
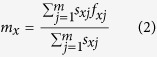 |
The net reproductive rate (R0) was calculated as follows:
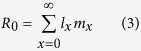 |
The intrinsic rate of increase (r) was estimated according to the Euler–Lotka formula with the age indexed from 048 as follows:
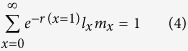 |
The finite rate (λ) was calculated as follows:
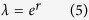 |
The mean generation time (T) was calculated as follows:
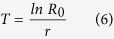 |
The age-stage-specific life expectancy (exy) was calculated by using the method of Chi and Su49 as follows:
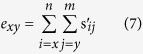 |
where s'ij represents the probability that an individual at age x and stage y will survive to age i and stage j.
The reproductive value (vxy) is defined as the contribution of individuals at age x and stage y to the future population50:
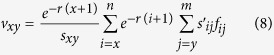 |
The bootstrap technique was used to estimate the means and standard errors of the population parameters51,52. Differences between treatment and control were compared using t-tests.
Predation rate analysis
The predation rate data were analyzed based on the computer program of CONSUME-MSChart53. The daily predation of all individuals, including males, females, and those dying before adulthood, was used to calculate the age-stage specific consumption rate cxj. This is the mean predation rate of an individual at age x and stage j. Following the method of Chi and Yang34 and Yu et al.54, the age-specific predation rate (kx) represents the mean predation rate of an individual at age x and was calculated as follows:
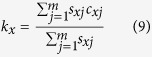 |
When the age-specific survival rate was taken into consideration, the age-specific net predation rate (qx) was calculated as follows:
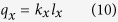 |
Net predation rate (C0) represents the total number of prey that an average individual predator can kill during its life span and was calculated as follows:
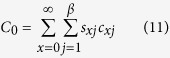 |
In order to compare the predation potential of different individuals consume the same prey or the same individual consume different preys, Chi et al.55 suggested using the finite predation rate (ω) and was calculated as follows:
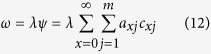 |
where λ represents the finite rate of an individual with a stable age-stage distribution, ω represents the stable predation rate (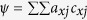 ), and axj represents the proportion that individuals belong to age x and stage j with a stable age-stage distribution.
), and axj represents the proportion that individuals belong to age x and stage j with a stable age-stage distribution.
The bootstrap technique was used to estimate the means and standard errors of the population parameters52. Differences between treatment and control were compared using t-tests.
Additional Information
How to cite this article: Wu, S. et al. Feeding on Beauveria bassiana-treated Frankliniella occidentalis causes negative effects on the predatory mite Neoseiulus barkeri. Sci. Rep. 5, 12033; doi: 10.1038/srep12033 (2015).
Supplementary Material
Acknowledgments
We thank Prof. Hsin Chi for assistant in data processing. We thank Prof. Mark Goettel for helpful comments on the manuscript. We thank the International Organization for Biological Control (IOBC) writing partnership program for help with English language editing. This research was supported by China Agriculture Research System, CARS-25-B-07 and National Modern Agricultural Science and Technology City Industry of Beijing (Z121100001212006).
Footnotes
Author Contributions Conceived and designed the experiments: S.W., X.X., Z.L. Performed the experiments: S.W., Y.G., D.W. and J.L. Analyzed the data: S.W., D.W. and H.W. Contributed materials: X.X., Y.G., E.W., X.X. and Z.L. Wrote the paper: S.W., Y.G. and Z.L.
References
- Kirk W. D. J. & Terry L. I. The spread of the western flower thrips Frankliniella occidentalis (Pergande). Agr. Forest Entomol. 5, 301–310 (2003). [Google Scholar]
- Rhainds M. & Shipp J. L. Dispersal of adult western flower thrips (Thysanoptera: Thripidae) on chrysanthemum plants: impact of feeding-induced senescence of inflorescences. Environ. Entomol. 32, 1056–1065 (2003). [Google Scholar]
- Morse J. G. & Hoddle M. S. Invasion biology of thrips. Ann. Rev. Entomol. 51, 67–89 (2006). [DOI] [PubMed] [Google Scholar]
- Frantz G. & Mellinger H. C. Shifts in western flower thrips, Frankliniella occidentalis (Thysanoptera: Thripidae), population abundance and crop damage. Fla. Entomol. 92, 29–34 (2009). [Google Scholar]
- Reitz S. R., Gao Y. L. & Lei Z. R. Thrips: pests of concern to China and the United States. Agri. Sci. in China 10, 867–892 (2011). [Google Scholar]
- Gao Y. L., Lei Z. R. & Reitz S. R. Western flower thrips resistance to insecticides: detection, mechanisms and management strategies. Pest Manag. Sci. 68, 1111–1121 (2012). [DOI] [PubMed] [Google Scholar]
- Bielza P. Insecticide resistance management strategies against the western flower thrips, Frankliniella occidentalis. Pest Manag. Sci. 64, 1131–1138 (2008). [DOI] [PubMed] [Google Scholar]
- Glockemann. Biological control of Frankliniella occidentalis on ornamental plants using predatory mites. EPPO Bull. 22, 397–404 (1992). [Google Scholar]
- Van Houten Y. M., Van Stratum P., Bruin J. & Veerman A. Selection for non-diapause in Amblyseius cucumeris and Amblyseius barkeri and exploration of the effectiveness of selected strains for thrips control. Entomol. Exp. Appl. 77, 289–295 (1995). [Google Scholar]
- Zhang J. P., Fan Q. H. & Zhang F. Evaluation of the potential biocontrol capability of Neoseiulus barkeri (Acari:Phytoseiidae) on Frankliniella occidentalis (Thysanoptera,Thripidae) based on life table. J. Environ. Entomol. 30, 229–232 (2008). [Google Scholar]
- Wang E. D., Xu X. N. & Wu S. Y. Control effects of Amblyseius barkeri on Frankliniella occidentalis on the eggplants and their natural enemy Orius sauteri in the greenhouse. Chin. Plant Prot. 36, 101–104 (2010). [Google Scholar]
- Van der Hoeven W. A. D. & van Rijn P. C. J. Factors affecting the attack success of predatory mites on thrips larvae. P. Exp. Appl. Entomol. Neth. Entomol. Soc. (NEV Amsterdam) 1, 25–30 (1990). [Google Scholar]
- Wu S. Y. et al. Laboratory and greenhouse evaluation of a new entomopathogenic strain of Beauveria bassiana for control of the onion thrips Thrips tabaci. Biocontrol Sci. Techn . 23, 794–802 (2013). [Google Scholar]
- Maniania N. K., Ekesi S., Löhr B. & Mwangi F. Prospects for biological control of the western flower thrips, Frankliniella occidentalis, with the entomopathogenic fungus, Metarhizium anisopliae, on chrysanthemum. Mycopathologia 155, 229–235 (2012). [DOI] [PubMed] [Google Scholar]
- Down R. E., Cuthbertson A. G. S., Mathers J. J. & Walters K. F. A. Dissemination of the Entomopathogenic Fungi, Lecanicillium longisporum and L. muscarium, by the Predatory Bug, Orius laevigatus, to Provide Concurrent Control of Myzus persicae, Frankliniella occidentalis and Bemisia tabaci. Biol. Control 50, 172–178 (2009). [Google Scholar]
- Wang J., Lei Z. R., Xu H. F., Gao Y. L. & Wang H. H. Virulence of Beauveria bassiana isolates against the first instar nymphs of Frankliniella occidentalis and effects on natural enemy Amblyseius barkeri. Chin. J. Biol. Control 27, 479–484 (2011). [Google Scholar]
- Yuan S. Y. et al. Detection of pathogenicity of Beauveryia bassiana MZ060812 against Frankliniella occidentalis. J. Northwest A & F Univ-Nat. Sci. Edit. 38, 145–149 (2010). [Google Scholar]
- Gao Y. L., Reitz S. R., Wang J. & Lei Z. R. Potential of a strain of the entomopathogenic fungus Beauveria bassiana (Hypocreales: Cordycipitaceae) as a biological control agent against western flower thrips, Frankliniella occidentalis (Thysanoptera: Thripidae). Biocontrol Sci. Techn. 22, 491–495 (2012). [Google Scholar]
- Wang J. P. & Zheng C. Y. Characterization of a newly discovered Beauveria bassiana isolate to Franklimiella occidentalis Perganda, a non-native invasive species in China. Microbiol. Res. 167, 116–120 (2012). [DOI] [PubMed] [Google Scholar]
- Wu S. Y. et al. An entomopathogenic strain of Beauveria bassiana against Frankliniella occidentalis with no detrimental effect on the predatory mite Neoseiulus barkeri: evidence from laboratory bioassay and scanning electron microscopic observation. PLoS ONE 9, e84732 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestergaard S. Pathogenicity of the hyphomycete fungi Verticillium lecanii and Metarhizium anisopliae to the western flower thrips, Frankliniella occidentalis. Biocontrol Sci. Techn. 5, 185–192 (1995). [Google Scholar]
- Reddy G. V. P. Comparative effectiveness of an integrated pest management system and other control tactics for managing spider mite Tetranychus ludeni (Acari: Tetranychidae) on eggplant. Exp. Appl. Acarol. 25, 985–992 (2001). [DOI] [PubMed] [Google Scholar]
- Onto A., Hanna R., Janssen A. & Sabelis M. W. Interactions between two neotropical phytoseiid predators on cassava plants and consequences for biological control of a shared spider mite prey: a screenhouse evaluation. Biocontrol Sci. Techn. 14, 63–76 (2004). [Google Scholar]
- Alma C. R., Goettel M. S., Roitberg B. D. & Gillespie D. R. Combined effects of the entomopathogenic fungus, Paecilomyces fumosoroseus Apopka-97, and the generalist predator, Dicyphus hesperus, on whitefly populations. BioControl 52, 669–681 (2007). [Google Scholar]
- Agboton B. V., Hanna R., Onzo A., Vidal S. & von Tiedemann A. Interactions between the predatory mite Typhlodromalus aripo and the entomopathogenic fungus Neozygites tanajoae and consequences for the suppression of their shared prey/host Mononychellus tanajoa. Exp. Appl. Acarol. 60, 205–217 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onzo A., Bello I. A. & Hanna R. Effects of the entomopathogenic fungus Neozygites tanajoae and the predatory mite Typhlodromalus aripo on cassava green mite densities: screenhouse experiments. BioControl 58, 397–405 (2013). [Google Scholar]
- Roy H. E. & Pell J. K. Interactions between entomopathogenic fungi and other natural enemies: implications for biological control. Biocontrol Sci. Techn. 10, 737–752 (2000). [Google Scholar]
- Poprawski T. J., Legaspi J. C. & Parker P. E. Influence of entomopathogenic fungi on Serangiumparcesetosum (Coleoptera: Coccinellidae), an important predator of whiteflies (Homoptera: Aleyrodidae). Environ. Entomol. 27, 785–795 (1998). [Google Scholar]
- Jacobson R. J., Chandler D., Fenlon J. & Russell K. M. Compatibility of Beauveria bassiana (Balsamo) Vuillemin with Amblyseius cucumeris Oudemans (Acarina: Phytotseiidae) to control Frankliniella occidentalis Pergande (Thysanoptera: Thripidae) on cucumber plants. Biocontrol Sci. Techn. 11, 391–400 (2001). [Google Scholar]
- Wekesa V. W., Moraes G. J., Knapp M. Jr Delalibera I. Interactions of two natural enemies of Tetranychus evansi, the fungal pathogen Neozygites floridana (Zygomycetes: Entomophthorales) and the predatory mite, Phytoseiulus longipes (Acari: Phytoseiidae). Biol. Control 41, 408–414 (2007). [Google Scholar]
- Donka A., Sermann H. & Buttner C. Effect of the entomopathogenic fungus Lecanicillinm muscarium on the predatory mite Phytoseiulus persimilis as a non-target organism. Commun. Agri. Appl. Biol. Sci. 73, 395–404 (2008). [PubMed] [Google Scholar]
- Seiedy M., Saboori A. & Allahyari H. Interactions of two natural enemies of Tetranychus urticae, the fungal entomopathogen Beauveria bassiana and the predatory mite, Phytoseiulus persimilis. Biocontrol Sci. Techn. 22, 873–882 (2012). [Google Scholar]
- Nagata R. T., Wilksion L. M. & Nuessly G. S. Longevity fecundity, and leaf stippling of Liriomyza trifolii (Diptera: Agromyzidae) as affected by lettuce cultivar and supplemental feeding. J. Econ. Entomol. 91, 999–1004 (1998). [Google Scholar]
- Chi H. & Yang T. C. Two-sex life table and predation rate of Propylaea japonica Thunberg (Coleoptera: Coccinellidae) fed on Myzus persicae (Sulzer) (Homoptera: Aphididae). Environ. Entomol. 32, 327–333 (2003). [Google Scholar]
- Chi H. & Liu H. Two new methods for study of insect population ecology. B. Inst. Zool. Acad. Sinica 24, 225–240 (1985). [Google Scholar]
- Simelane D. O., Steinkraus D. C. & Kring T. J. Predation rate and development of Coccinella septempunctata L. influenced by Neozygites fresenii-infected cotton aphid prey. Biol. Control 44, 128–135 (2008). [Google Scholar]
- Zhang Z. J. et al. Life history of western flower thrips, Frankliniella occidentalis (Thysan., Thripae), on five different vegetable leaves. J. Appl. Entomol. 131, 347–354 (2007). [Google Scholar]
- Wu S. Y., Gao Y. L., Xu X. N., Goettel M. S. & Lei Z. R. Compatibility of Beauveria bassiana with Neoseiulus barkeri for control of Frankliniella occidentalis. J. Integr. Agri . 14, 98–105 (2015). [Google Scholar]
- Huang H. et al. Impact of proteins and saccharides on mass production of Tyrophagus putrescentiae (Acari: Acaridae) and its predator Neoseiulus barkeri (Acari: Phytoseiidae). Biocontrol Sci. Techn. 23, 1231–1244 (2013). [Google Scholar]
- Li H. P., Huang D. Z. & Tang X. G. Changes in the Contents of Proteins and Amino Acids of Apriona germari Larvae Infected by Beauveria bassiana. Sci. Silvae Sinicae 48, 159–163 (2012). [Google Scholar]
- LV D. D., Li Z. Z. & Wang C. S. Advances in molecular pathogenesis and genetic engineering of entomopathogenic fungi. Microbiology 35, 443–449 (2008). [Google Scholar]
- Strasser H., Vey A. & Butt T. M. Are there any risks in using entomopathogenic fungi for pest control, with particular reference to the bioactive metabolites of Metarhizium, Tolypocladium and Beauveria species? Biocontrol Sci. Techn. 10, 717–735 (2000). [Google Scholar]
- Gillespie J. P., Bailey A. M., Cobb B. & Vilcinskas A. Fungi as elicitor of insect immune responses. Arch. Insect Biochem. Physiol. 44, 49–68 (2000). [DOI] [PubMed] [Google Scholar]
- Wen J. Z. et al. Pathogenicity of five Beauveria bassiana strains against Locusta migratoria. Chin. Plant Protec. 29, 50–52 (2003). [Google Scholar]
- Liang X. H., Lei Z. R., Wen J. Z. & Zhu M. L. The diurnal flight activity and influential factors of Frankliniella occidentalis in the greenhouse. Insect Sci. 17, 535–541 (2010). [Google Scholar]
- Chi H. Life-table analysis incorporating both sex and variable development rate among individuals. Environ. Entomol. 17, 26–31 (1988). [Google Scholar]
- Chi H. TWOSEX-MSChart: a computer program for the age stage, two-sex life table analysis (2012). Available from: http://140.120.197.173/Ecology/. (Accessed: 18th January 2013).
- Goodman D. Optimal life histories, optimal notation, and the value of reproductive value. Am. Nat. 119, 803–823 (1982). [Google Scholar]
- Chi H., Su H. Y. Age-stge, two-sex life tables of Aphidius gifuensis (Ashmead) (Hymenotera: Braconidae) and its host Myzus persicae (Sulzer) (Homoptera: Aphididae) with mathematical proof of the relationship between female fecundity and the net reproductive rate. Environ. Entomol. 35, 10–21 (2006). [Google Scholar]
- Fisher R. A. The Genetical Theory of Natural Selection. Clarendon Press, Oxford (1930).
- Huang Y. B. & Chi H. Age-stage, two-sex life tables of Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae) with a discussion on the problem of applying female age-specific life tables to insect populations. Insect Sci. 19, 263–273 (2012). [Google Scholar]
- Efron B. & Tibshirani R. J. An Introduction to the Bootstrap. Chapman & Hall, New York (1993).
- Chi H. CONSUME-MSChart: a computer program for consumption rate analysis based on the age stage, two-sex life table (2012). Available from: Available from: http://140.120.197.173/Ecology/. (Accessed: 18th January 2013).
- Yu J. Z., Chi H. & Chen B. H. Life table and predation of Lemnia biplagiata (Coleoptera: Coccinellidae) fed on Aphis gossypii (Homoptera: Aphididae) with a proof on relationship among gross reproduction rate, net reproduction rate, and preadult survivorship. Ann. Entomol. Soc. Am. 98, 475–482 (2005). [Google Scholar]
- Chi H. et al. Finite predation rate: a novel parameter for the quantitative measurement of predation potential of predator at population level. In: Nature Proceedings (2011). Available from: http://precedings.nature.com/documents/6651/version/1 (Accessed: 18th January 2013).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


