Abstract
Purpose.
Cataract surgery involves removal of lens tissue, but is associated with a high complication rate due to regrowth of residual lens epithelial cells to produce posterior capsule opacification (PCO) and diminished visual acuity. As inhibitors of aldose reductase (AR) have been shown to suppress markers of PCO, our studies were designed to identify a role for AR in the pathogenesis of PCO.
Methods.
Sorbinil-mediated AR inhibition was determined by measuring sorbitol accumulation. Cell migration was measured using both transwell and scratch assays. Proteins in the SMAD signaling pathway were measured by Western blotting. The interactions of AR and SMADs were demonstrated by co-immunoprecipitation (Co-IP) and proximity ligation assay (PLA). Epithelial-to-mesenchymal transition (EMT) expression was measured by Western blot and quantitative PCR (q-PCR). Matrix metalloproteinase (MMP)-2 and MMP-9 activities were measured in conditioned medium by zymography.
Results.
We observed that either Sorbinil-mediated AR inhibition or siRNA-mediated AR gene knockdown prevented migration of lens epithelial cells following exposure to TGF-β2. AR inhibition or AR knockdown reduced SMAD and MMP activation triggered by TGF-β2. In addition, we demonstrated AR inhibition or AR knockdown decreased TGF-β2–induced expression of EMT markers. Co-IP studies and PLA were used to demonstrate that AR and SMAD2 interact either directly or in close concert with additional factor(s) in a nonenzymatic manner.
Conclusions.
This study demonstrates that AR participates in the response of lens epithelial cells to TGF-β2. Our studies raise the possibility that AR inhibition may be effective in preventing development of PCO by disrupting the TGF-β2/SMAD pathway.
Keywords: aldose reductase, PCO, TGF-β2, SMAD2, SMAD3, Sorbinil, EMT
Aldose reductase and SMAD2 interact either directly or in close concert with additional factor(s). Aldose reductase inhibitors suppress TGF-β–induced SMAD activation and expression of EMT markers and thus may be useful against posterior capsular opacification.
Posterior capsule opacification (PCO), also called secondary cataract, is the most common complication following cataract surgery.1 After surgery to extract the bulk of lens tissue associated with the cataract, some residual lens epithelial cells (LECs) can proliferate, transdifferentiate to a myofibroblastic phenotype, and migrate toward the central posterior capsule where they cause contraction and wrinkling of the normally smooth and uniform posterior capsule.2 These changes result in severely degraded visual acuity3 that must be remedied with an additional surgical procedure involving formation of a capsulotomy. Therefore, inhibition of LEC proliferation, migration, and transdifferentiation, as well as matrix contraction4 and matrix deposition, may be considered as possible points to intervene in the pathogenesis of PCO.
Investigators have probed a variety of structural and signaling proteins to identify druggable targets for prevention of PCO. These include matrix proteins, such as secreted protein acidic rich in cysteine (SPARC)5 and matrix metalloproteinase (MMP),6–8 involved in cell migration, as well as signaling proteins downstream from growth factor receptors. It has been reported that TGF-β influences epithelial-to-mesenchymal transition (EMT) of LECs, resulting in cells with a spindle-shaped myofibroblastic morphology.2 Lens epithelial cells that undergo EMT express extracellular components, such as α-smooth muscle actin (α-SMA), fibronectin, and type I collagen.9–13 In addition, transgenic mice designed for overexpression of TGF-β in lens develop morphological changes that closely mimic PCO in humans.12 Therefore, the TGF-β/SMAD signaling pathway is important for development of PCO.13 A variety of studies have shown that blockade of this pathway, such as through interference at the level of SMAD gene expression,14,15 or through downregulation of molecules, such as connective tissue growth factor and gremlin, that facilitate TGF-β/SMAD signaling,16 result in suppression of EMT markers associated with PCO.
Recent studies have implicated aldose reductase (AR; E.C. 1.1.1.21) in the pathogenesis of PCO. Although AR is best known for its role in catalyzing the conversion of glucose to sorbitol as the first step of the polyol pathway, recent work suggests that AR may also participate in the pathogenesis of PCO. Using a pig eye model, AR inhibitors (ARIs) were shown to reduce proliferation of LECs and expression of markers associated with PCO, such as α-SMA,17 but the mechanism linking AR to PCO pathogenesis is unknown. A downstream effect of ARIs is a reduction in reactive oxygen species (ROS),18–20 which has been suggested to cause PCO.21 In a corollary manner, AR overexpression is reported to induce EMT marker expression in LECs, which can contribute to PCO.22
Given the importance of TGF-β signaling in stimulating PCO, and the observation that AR inhibition suppresses LEC proliferation and expression of PCO biomarkers, we have carried out studies designed to test the hypothesis that ARIs prevent PCO by interfering with TGF-β signaling in LECs. Our results demonstrate that AR inhibition or genetic knockdown suppresses the TGF-β stimulation of SMAD2 and SMAD3 phosphorylation and thus interferes with the downstream signaling from the TGF-β receptor.
Materials and Methods
Materials and Cell Culture
The TGF-β2 was purchased from Humanzyme (Chicago, IL, USA). Sorbinil ([4S]-6-Fluoro-2,3-dihydro-spiro[4H-1-benzopyran-4,4′-imidazolidine]-2′,5′-dione) was generously provided by Pfizer Central Research (Groton, CT, USA). Control small-interfering RNA (siRNA) and AKR1B1 siRNA (siAR) were obtained from Qiagen (Valencia, CA, USA). Human lens epithelial (HLE B3) cells were obtained from Usha Andley (Washington University, St. Louis, MO, USA), and cultured in complete Dulbecco's modified Eagle's medium (DMEM) supplemented with 1 g/L glucose, 4 mM L-glutamine, 20% (vol/vol) fetal bovine serum, 100 units/mL penicillin, and 100 μg/mL streptomycin. Cells were maintained in a humidified incubator containing 5% carbon dioxide at 37°C.
Cultures of Lenses and Primary Lens Capsular Epithelial Cells
This research was conducted in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and following approval by the Institutional Animal Care and Use Committee at the University of Colorado Anschutz Medical Campus. Lenses were obtained from 8- to 10-week-old C57BL/6 mice (The Jackson Laboratory, Bar Harbor, ME, USA) by dissection under aseptic conditions. For isolation and growth of primary LECs, fiber cells were dissected away from the capsular bag, which was then incubated in Hank's balanced salt solution buffer containing 0.05% trypsin (ATCC, Manassas, VA, USA) at 37°C for 5 to 10 minutes. The dissociated cells were transferred into a 100-mm culture dish and incubated in DMEM (4 mM L-glutamine, 20% [vol/vol] fetal bovine serum, 100 units/mL penicillin, and 100 μg/mL streptomycin) for 3 to 4 weeks. Gene expression of Pax6 and Foxe3 was detected by quantitative PCR (q-PCR) to confirm these cultures as being derived from LECs.23,24 Culture conditions were the same for incubation of intact lenses from wild-type and AR knockout mice.25
Cloning of Human AR cDNA Into Expression Vector
Human AR sequences were excised from pMON5842 expression plasmid26 by complete digestion with Hind III and Xho I and ligated into the expression plasmid pcDNA3.1/V5-His C (Invitrogen, Carlsbad, CA, USA) that had been previously treated with Hind III and Xho I. A catalytically inactive mutant of AR (Y48F) was produced by PCR-mediated site-directed mutagenesis to produce ARY48F. 27 Plasmid sequences encoding AR or ARY48F were verified by DNA sequencing. To confirm the intended enzymatic activity of our AR constructs, we observed that HLE-B3 cells transfected with the wild-type AR plasmid accumulated substantial levels of sorbitol (see Supplementary Fig. S1). In contrast, cells transfected with the ARY48F plasmid were approximately equivalent to vector controls in their poor ability to accumulate sorbitol when cultured in the presence of high glucose (Supplementary Fig. S1). These results are consistent with previous kinetic studies showing that the Y48F mutant was essentially inactive as an aldo-keto reductase.27
Transfection of AR-V5 and SMAD-Flag Plasmids
Plasmids encoding AR fused to the V5 affinity epitope and SMADs (2 and 3) fused to the Flag affinity epitope (Addgene, Cambridge, MA, USA) were transfected into HLE B3 cells using Lipofectamine 2000 Reagent (Invitrogen) as a carrier and incubated for 72 hours. Western blotting of cell lysates confirmed expression of targeted proteins and their cognate affinity domains (see Supplementary Fig. S2)
Western Blotting
Cells were scraped and suspended in Laemmli sample buffer (Sigma-Aldrich Corp., St. Louis, MO, USA) and heated to 100°C for 10 minutes. Proteins were resolved by SDS-PAGE (Bio-Rad, Hercules, CA, USA) and transferred to nitrocellulose membranes (Amersham Pharmacia Biotech, Piscataway, NJ, USA), using a wet blotter (Bio-Rad). Membranes were blocked and then probed with primary antibodies: rabbit anti-p-SMAD2, SMAD2, p-SMAD3, SMAD3, SMAD4, α-SMA, vimentin (1:1000; Cell Signaling Technology, Inc., Danvers, MA, USA) or mouse anti-actin (1:4000; Sigma-Aldrich) or rabbit anti-AR (1:1000)28 overnight at 4°C. Membranes were washed and probed with secondary antibodies conjugated to horseradish peroxidase (Millipore, Bedford, MA, USA), and developed with the Western Blot Substrate kit (Bio-Rad) by detecting chemiluminescence using a Bio-Rad ChemiDoc XRS+ imaging system.
Small-Interfering RNA Transfection
Transient transfection of siRNA was performed using HiPerFect transfection reagent (Qiagen) according to the manufacturer's protocol. Human lens epithelial B3 cells (106 cells/dish) were seeded in a 100-mm culture dish. After 16 hours, cells were approximately 70% confluent and cells were transfected with control or AR siRNA (10 nM) and cultured for an additional 72 hours. Efficiency of AR knockdown was confirmed by Western blot.
In Vitro Migration Assay
Human lens epithelial B3 cells (2 × 104 cells) were cultured in modified Boyden chambers fitted with filter inserts (pore size 8 μm; Greiner Bio-one, Monroe, NC, USA) in 24-well plates. Cells were plated in the upper chambers. The TGF-β2 with/without AR inhibitor was added to the lower chambers. Twenty-four hours after exposure, the migrated cells in the upper chamber were fixed with cold methanol for 15 minutes, stained with 2% crystal violet for 30 minutes, and the total number of cells averaged from three randomly selected visual fields.
Zymography
The MMP-2 and MMP-9 gelatinase activities were measured in conditioned medium by zymography. Equal amounts of conditioned medium were subjected to electrophoresis on 10% zymography gels containing 0.1% gelatin (Bio-Rad). Gels were washed with renaturing buffer (Bio-Rad) for 30 minutes, incubated in developing buffer (Bio-Rad) overnight at 37°C, and stained with Coomassie blue. Gelatinase activity was identified by digestion of gelatin, which resulted in a band of transparency on the background of the otherwise opaque gel.
Immunoprecipitation Assay
Immunoprecipitation (IP) assays were performed as previously described.29 Briefly, antibodies including rabbit anti-AR, rabbit anti-Flag, rabbit anti-SMAD2 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), mouse anti-V5 (Santa Cruz Biotechnology), and A/G beads (Santa Cruz Biotechnology) were used in radioimmunoprecipitation assay buffer with 100 μM NADPH. After incubation for 16 hours at 4°C, beads were washed three times with wash buffer, and then were loaded onto SDS-PAGE. For loading controls, total lysates were probed with AR, SMAD2, V5, and Flag antibodies using Western blot protocol described above.
Proximity Ligation Assay
The Duolink PLA kit from Olink Bioscience (Uppsala, Sweden) was used to probe for the proximity of AR and SMAD2. Assays were performed as previously described30 and according to manufacturer's protocol. Proximity ligation assay (PLA) products were detected by using a Nikon Eclipse 80i light microscope fitted to a Nikon DS Qi1Mc camera (Nikon Instrument, Inc., Tokyo, Japan). Abundance of PLA product was quantified by counting fluorescent spots representing PLA signal. The number of spots was manually counted from at least three microscopic fields for each experimental condition (number of cells counted are 14∼24). During enumeration of PLA signals, personnel were masked to the sample identity.
Real-Time PCR
Total RNA was isolated (RNeasy Microarray Tissue Mini Kit; Qiagen) according to the manufacturer's protocol. Using the iScript cDNA Synthesis Kit (Bio-Rad), RNA (5 μg) was reverse transcribed according to the manufacturer's protocols. For real-time PCR, the iTaq Universal SYBR Green Supermix (Bio-Rad) was used according to the manufacturer's protocol on a CFX Connect Real-Time PCR Detection System (Bio-Rad). The primers were as follows: fibronectin forward, 5′-CTGAAGAATAATCAGAAGAGC-3′; fibronectin reverse, 5′-ACCATGTTCCTCAAAGATC-3′; α-SMA forward, 5′-GGCATCGTGCTGGACTC-3′; α-SMA reverse, 5′- TGGCTGGAACAGGGTCTC-3.′ The primers for human Snail and Slug human gene transcripts and murine α-SMA and fibronectin were directly designed by Integrated DNA Technologies (Coralville, IA, USA). The thermocycler parameters were 94°C for 5 minutes, followed by 40 cycles of 94°C for 30 seconds, 56°C for 30 seconds, and 72°C for 60 seconds. Glyceraldehyde 3-phosphate dehydrogenase and actin were used as internal control. All real-time PCR data were analyzed by 2ΔCt.
Monolayer Scratch Assay
Human lens epithelial B3 cells were grown to confluence in Culture-Insert dish (Ibidi, Munich, Germany). After 24 hours, the insert was gently removed by using sterile tweezers, generating a cell-free zone approximately 500 μm in width. After the indicated treatment, cells were fixed in paraformaldehyde for 30 minutes and stained with 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen) for enhanced visualization. Photographs were taken with Nikon Eclipse 80i light microscope fitted to a Nikon DS Qi1Mc camera (Nikon Instrument, Inc.).
Statistical Analysis
Results are shown as the means ± SEM of at least three experiments. Data were analyzed by ANOVA with Tukey's test. A P value less than 0.05 is considered significant.
Results
Pharmacological Inhibition or Genetic Knockdown of AR in HLE B3 Cells Prevents TGF-β2–Induced Cell Migration
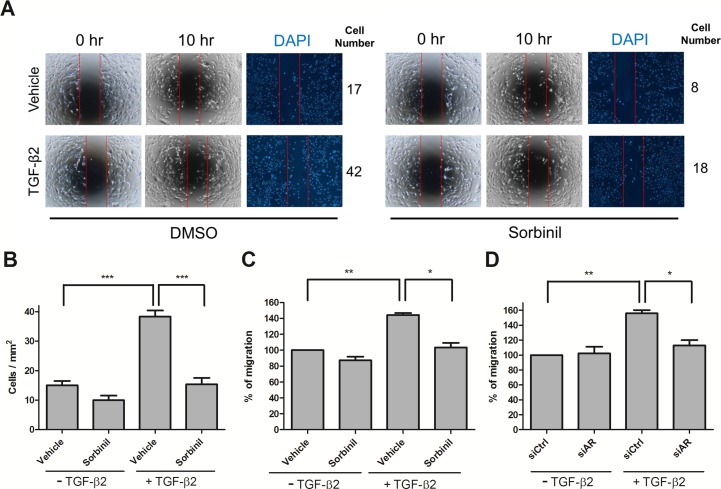
Transforming growth factor β2 has been reported to induce migration of LECs13,31; however, the possible involvement of AR in this process is not understood. To investigate the involvement of AR on cell migration, HLE B3 cells were treated with sorbinil, a well-established ARI. Although Sorbinil has been shown to inhibit growth of LECs,17 its effect on LEC migration has not been reported. In the scratch assay, we observed that sorbinil treatment attenuated the TGF-β2–induced cell migration of LECs (Figs. 1A, 1B). To confirm these observations, we measured the effect of AR inhibition on cell migration using a transwell assay. In the absence of TGF-β2, Sorbinil had virtually no effect on cell migration (Fig. 1C). However, the large increase in TGF-β2–induced cell migration was substantially prevented by Sorbinil (Fig. 1C). The activity of Sorbinil as an ARI was confirmed when we measured significantly reduced sorbitol accumulation in cells treated with Sorbinil but not vehicle control (Supplementary Fig. S3). To confirm that the ability of Sorbinil to suppress migration was due to AR inhibition, we probed the effect of AR through a siRNA-medicated genetic knockdown of AR expression. Transforming growth factor β2–induced HLE B3 cell migration was significantly reduced in the AR-knockdown cells but not in cells treated with a scrambled siRNA control (Fig. 1D). These studies demonstrate that pharmacological inhibition or genetic knockdown of AR in cells exposed to TGF-β2 results in reduced migration.
Figure 1.
Aldose reductase inhibitors or AR knockdown decrease TGF-β2–induced migration. Human lens epithelial B3 cells were treated with dimethyl sulfoxide (DMSO) or Sorbinil for 30 minutes followed by treatment with TGF-β2 (5 ng/mL) for 10 hours for scratch migration assay (A). Staining with 4′,6-diamidino-2-phenylindole was used to visualize cell nuclei. Number of migrated cells in central zone was performed in statistic bar chart (B). Human lens epithelial B3 cells were further treated with vehicle (DMSO) or Sorbinil (10 μM) in the absence or presence of TGF-β2 (5 ng/mL) for 24 hours for transwell assay. Cells were further transfected with control or AR siRNA for 72 hours followed by treatment with TGF-β2. The cell migration ability (C, D) with ARI treatment or AR knockdown was determined after TGF-β2 exposures using transwell assay. The width of the cell-free gap is 500 ± 50 μm. Data shown are means ± SEM (n = 3). *P < 0.05; **P < 0.01; ***P < 0.001.
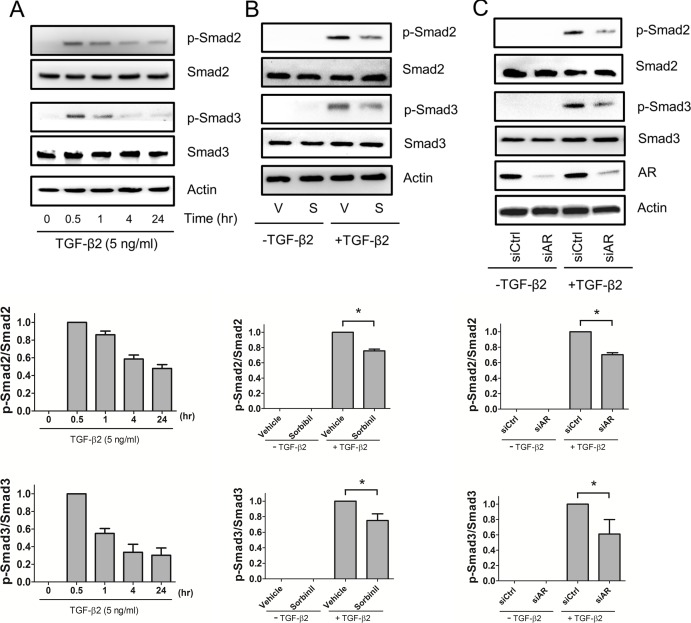
Aldose Reductase Inhibition or Knockdown Attenuates TGF-β2–Induced SMAD2 and SMAD3 Activation in HLE B3 Cells
It has been reported that SMAD2 is involved in TGF-β2–induced migration of HLE B3 cells.13 Given our results showing that blockade of AR by inhibitors or siRNA attenuates TGF-β2–induced cell migration, we next examined whether regulation of AR could impact SMAD2/3 signaling. Time-course studies showed that TGF-β2 induction of SMAD2 and SMAD3 activation was maximal following approximately 30 minutes of exposure (Fig. 2A). Pretreatment of HLE B3 cells with Sorbinil for 30 minutes decreased the TGF-β2–induced SMAD2 and SMAD3 activations (Fig. 2B). Similarly, siRNA-mediated knockdown of AR expression resulted in reduced levels of TGF-β2–induced SMAD2 and SMAD3 activations (Fig. 2C). Thus, pharmacological blockade of AR or downregulation of its gene expression results in reduced TGF-β2–induced SMAD activation.
Figure 2.
Aldose reductase inhibitors or AR knockdown decreases TGF-β2–dependent SMADs phosphorylation. Human lens epithelial B3 cells were incubated with TGF-β2 (5 ng/mL) for indicated times (A). Human lens epithelial B3 cells were pretreated with vehicle (V) or Sorbinil (S, 10 μM) for 30 minutes followed by TGF-β2 (5 ng/mL) for 30 minutes (B). Cells were also transfected with control or AR siRNA for 72 hours followed by treatment with TGF-β2 for 30 minutes (C). In all cases, the fold-activation of p-SMAD2 and p-SMAD3 were normalized to total SMAD2 and SMAD3, respectively. Fold change is compared with vehicle in the TGF-β2 group. The efficiency of AR knockdown was examined by probing with AR antibody. Changes in the level of proteins of interest were analyzed for statistical significance and are displayed in graphical form as mean values ± SEM (n = 3). *P < 0.05.
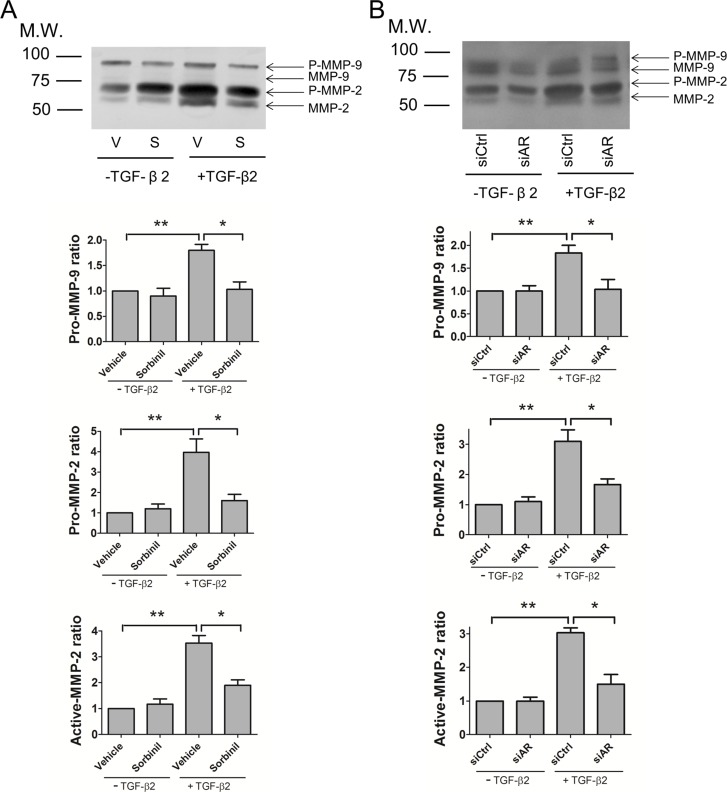
Aldose Reductase Inhibition or Knockdown Decreases TGF-β2–Induced MMP-2 and MMP-9 Activation in HLE B3 Cells
It is known that MMP-2 and MMP-9 can be induced by increased levels of TGF-β2 following the mechanical trauma of cataract surgery.4 To examine the effect of ARI on MMP-2 and MMP-9 activation, HLE B3 cells were pretreated with Sorbinil and co-cultured with TGF-β2. Culture medium was analyzed for gelatinase activity using a zymographic assay. Pro-forms of MMP-9 and MMP-2 were readily detected in cell culture supernatants, with levels of pro-MMP-2 being much higher than pro-MMP-9 (Fig. 3A). Of the active forms of the two proteinases, only MMP-2 was detected before or after treatment of cells with TGF-β2. Levels of pro-MMP-9 and pro-MMP-2 were increased approximately 1.8-fold and 3.9-fold, respectively, by TGF-β2 exposure, which also increased the active form of MMP-2 approximately 3.5-fold. Pretreatment of cells with Sorbinil substantially reduced but did not completely normalize the TGF-β2–induced increase in levels of the pro- and active forms of MMP-2 and pro-MMP-9 (Fig. 3A). We further confirmed the AR effect on TGF-β2–mediated pro-MMP-2 and pro-MMP-9 levels with transient knockdown experiments. Aldose reductase knockdown reduced TGF-β2–induced pro-MMP-2 and pro-MMP-9 levels as well as levels of active MMP-2 as measured with the zymography assay (Fig. 3B). These data are consistent with previous studies that showed that AR inhibition suppressed the production of MMPs.32–34 These results suggest that AR inhibition may lead to a reduction in TGF-β2–induced LEC migration by suppressing the production of MMP-2 and MMP-9.
Figure 3.
Aldose reductase inhibitors or AR knockdown limit MMP-2 and MMP-9 activation by TGF-β2. Human lens epithelial B3 cells were pretreated with vehicle (V) or Sorbinil (S, 10 μM) for 30 minutes followed by TGF-β2 (5 ng/mL) exposure for 24 hours (A). Cells were further transfected with control (siCtrl) or AR siRNA (siAR) for 72 hours and followed by TGF-β2 exposure (B). Pro-MMP-2, -9 and active MMP-2, -9 secreted in medium were detected by gelatin zymography (A, B). Fold change of pro-MMPs is compared with vehicle or siCtrl in no TGF-β group. Changes in the level of proteins of interest were analyzed for statistical significance and are displayed in graphical form as mean values ± SEM (n = 3). *P < 0.05; **P < 0.01.
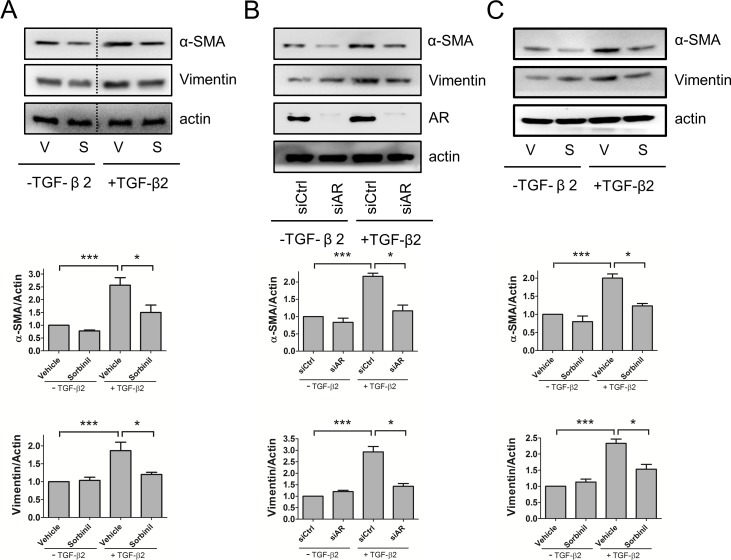
Inhibition or Knockdown of AR Reduces TGF-β2–Induced EMT Markers in HLE B3 Cells
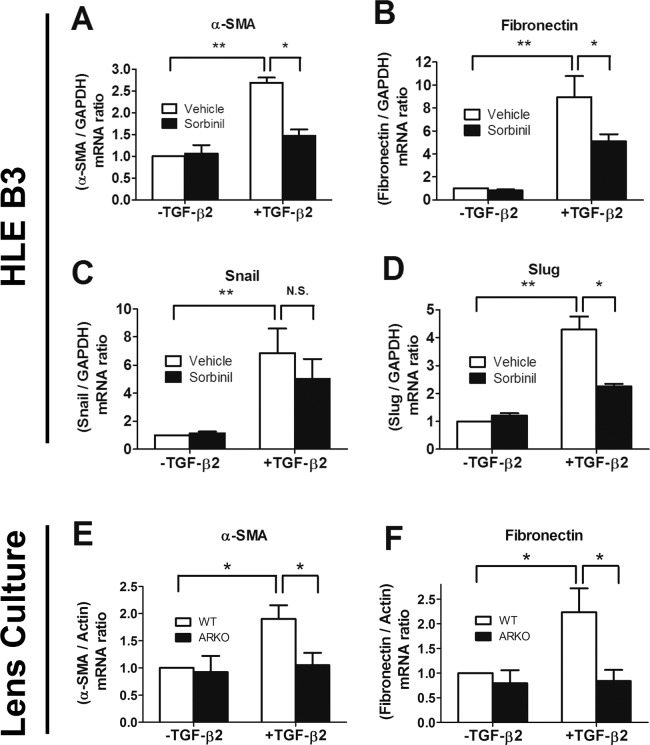
Because EMT plays an important role in development of PCO,2 inhibition of EMT is considered a therapeutic goal for prevention of PCO. Vimentin and α-SMA are key protein markers associated with EMT,4,13 and it has been shown that ARIs such as Sorbinil and Fidarestat suppress expression of α-SMA.17 In this study, we observed that α-SMA and vimentin were induced by TGF-β2 treatment, and Sorbinil reduced the TGF-β2–induced expression of these markers in human LEC line (Fig. 4A) or primary mouse lens capsular epithelial cells (Fig. 4C). To confirm the genetic role of AR, we used siRNA-mediated AR knockdown to investigate the TGF-β2 effect on HLE B3 cells. Aldose reductase knockdown resulted in reduced levels of α-SMA and vimentin compared with control cells treated with a scrambled siRNA (Fig. 4B). We further confirmed other EMT markers by measuring their mRNA changes by q-PCR. Sorbinil significantly reduced the TGF-β2–induced increases in transcripts for α-SMA (Fig. 5A), fibronectin (Fig. 5B), and Slug (Fig. 5D). Although the decrease by Sorbinil in Snail gene transcript levels followed this trend, the magnitude of reduction did not reach statistical significance (Fig. 5C).
Figure 4.
Aldose reductase inhibition or AR knockdown reduce TGF-β2–induced expression of EMT markers. Human lens epithelial B3 cells (A) or primary murine lens capsular epithelial cells (C) were pretreated with vehicle (V) or Sorbinil (S, 10 μM) for 30 minutes followed by TGF-β2 (5 ng/mL) for 48 hours. Cells were also transfected with control or AR siRNA for 72 hours followed by treatment with TGF-β2 (B). Values for relative band intensity are given below the relevant sets of blots. The fold-activation of α-SMA and vimentin were normalized to actin. Fold change is compared with vehicle or siCtrl in no TGF-β2 group. The efficiency of AR knockdown was examined by probing with AR antibody. Changes in the level of proteins of interest were analyzed for statistical significance and are displayed in graphical form as mean values ± SEM (n = 3). *P < 0.05; ***P < 0.001.
Figure 5.
Aldose reductase inhibition or ablation reduces levels of gene transcripts corresponding to TGF-β2–induced EMT markers. Messenger RNA was collected from either HLE B3 cells (A–D) or murine lens culture (E, F). Human lens epithelial B3 cells were treated with the vehicle or Sorbinil in the absence or presence of TGF-β2 (5ng/mL) for 24 hours. Lenses from wild-type or AR-null mice were treated with or without TGF-β2 (5 ng/mL) for 24 hours. Real-time analysis of mRNA levels of α-SMA (A, E), fibronectin (B, F), Snail (C), and Slug (D) was performed in HLE B3 cells or murine lens culture. Data shown are means ± SEM (n = 3). *P < 0.05; **P < 0.01.
To confirm that our observations using the HLE B3 cell were consistent with nonimmortalized LECs, we examined the role of AR in whole-lens organ cultures produced from wild-type and AR-null mice. We observed that TGF-β2–induced transcripts for α-SMA (Fig. 5E) and fibronectin (Fig. 5F) were lower in lens cultures from AR-null mice as compared with wild-type mice. Taken together, these data suggest that AR plays an important role in regulation of TGF-β2–induced EMT marker expression in human-derived HLE B3 cells and mouse LECs.
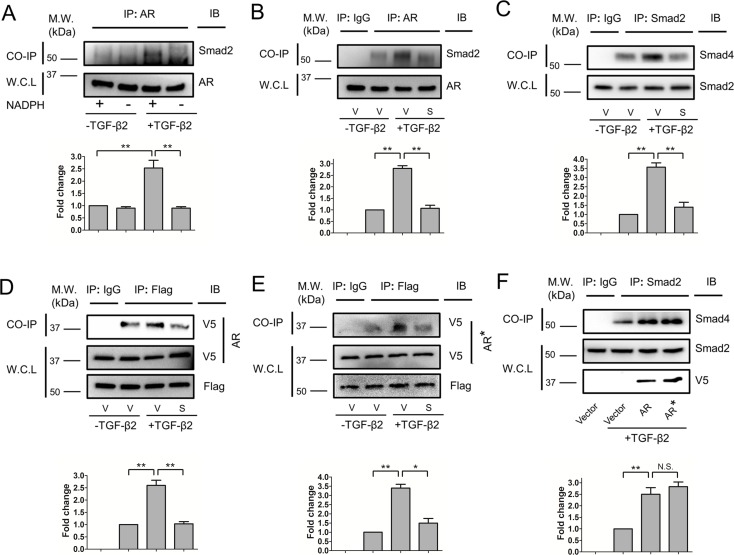
Sorbinil Interrupts AR-SMAD2 and SMAD2-SMAD4 Interaction
Our foregoing studies demonstrated that pharmacological blockade of AR or downregulation of AR gene expression resulted in a marked reduction of TGF-β2–induced SMAD2 phosphorylation. To establish a possible mechanism, we constructed the hypothesis that AR interacts directly with SMAD2 to effect downstream signaling from the TGF-β2 receptor. To test this hypothesis, we used co-immunoprecipitation experiments to probe for direct interactions between AR and SMAD2, as well as SMAD2 and SMAD4. Western blotting of complexes pulled down using our antibody to AR revealed elevated SMAD2 signal from lysates produced from TGF-β2–treated cells as compared with cells without previous exposure to TGF-β2 (Fig. 6A). Apparent AR-SMAD2 interactions were dependent on NADPH, a nucleotide cofactor that is most likely bound to AR in vivo.35 AR-SMAD2 (Fig. 6B) and SMAD2-SMAD4 (Fig. 6C) interactions were substantially reduced by treatment of cells with Sorbinil. To test whether AR catalytic activity is involved with its role in SMAD activation, we examined AR-SMAD interactions in cells transfected with a gene construct encoding AR with a Phe substitution at the catalytic Tyr-48 (ARY48F). Overexpression of AR or ARY48F does not lead to significantly enhanced SMAD2 activation in the absence of TGF-β2 (Supplementary Fig. S4). Immunoprecipitates of SMAD2 demonstrated strong positivity for AR in TGF-β2–stimulated cells co-transfected with either wild-type AR (Fig. 6D) or ARY48F (Fig. 6E), demonstrating that catalytic activity is not required for interaction of AR with SMAD2. Both AR and ARY48F overexpression enhanced TGF-β2–induced SMAD2-SMAD4 interactions, indicating that the interactions between SMAD2 and SMAD4 mediated by AR are not influenced by the protein's catalytic competence (Fig. 6F).
Figure 6.
Aldose reductase inhibitors inhibit AR-SMAD2 and SMAD2-SMAD4 interactions. The HLE B3 cells were treated with or without TGF-β2 (5 ng/mL) in the presence or absence of NADPH during co-immunoprecipitations (A).The HLE B3 cells were pretreated with vehicle (V) or Sorbinil (S, 10 μM) for 1 hour followed by TGF-β2 (5 ng/mL) for 1 hour (B–E). Co-immunoprecipitation was performed by using AR or SMAD2 antibody for pull down and SMAD2 (B) or SMAD4 (C) was detected by Western blot after pull-down assay. Immunoglobulin G was used for negative control. The SMAD2 and AR in whole-cell lysate (WCL) were used for loading control. Aldose reductase catalysis is irrelevant for SMAD2 interactions (D, E). Human lens epithelial B3 cells were co-transfected with either AR-V5 (D) or ARY48F-V5 (E) and Flag-SMAD2 plasmids followed by co-immunoprecipitation using Flag antibody for pull down. Western blot detected V5 after pull-down assay. Immunoglobulin G was used for negative control. Flag and V5 in WCL was used for loading control. Values for relative band intensity are given below the relevant sets of blots. Overexpression of AR or ARY48F (AR*) enhances TGF-β2–induced SMAD2-SMAD4 interactions (F). Changes in the level of proteins of interest were analyzed for statistical significance and are displayed in graphical form as mean values ± SEM (n = 3). *P < 0.05; **P < 0.01.
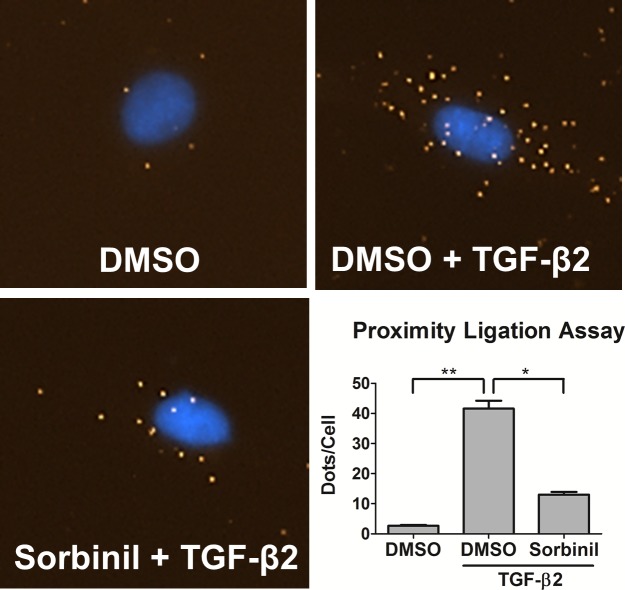
Because most of our pull-down assays demonstrating interactions involving AR and SMADs were carried out with cell lysates, we wanted to test for corroborating evidence in intact cells. For this we turned to the PLA. In this method, a signal for close interaction is observed only when protein domains recognized by different antibodies are separated by 40 nm or less. Using a proximity strategy reliant on antibodies to SMAD2 and AR made in mouse and rabbit, respectively, we observed a TGF-β2–dependent increase from approximately 3 to 42 fluorescent dots per cell. Sorbinil pretreatment significantly reduced the number of fluorescent spots down to a range of approximately 13 per cell (Fig. 7). These results are consistent with our co-immunoprecipitation studies and provide additional evidence that TGF-β2 triggers AR-SMAD2 interaction and that Sorbinil interrupts this interaction.
Figure 7.
Proximity ligation assay of AR-SMAD2 interaction. The HLE B3 cells were pretreated with DMSO or Sorbinil for 1 hour in the presence or absence of TGF-β2 (5 ng/mL) for another 1 hour. Proximity ligation assay was done by using AR and SMAD2 primary antibodies and oligonucleotide-linked PLA secondary antibodies. A spot is formed when AR and SMAD2 are separated by no more than an approximately 40-nm distance. Data shown are number of PLA spots per cell. Data shown are means ± SEM (n = 3). *P < 0.05; **P < 0.01.
Discussion
Vision loss due to cataracts is the world's leading cause of blindness.36 Given the large increase in the incidence of cataract, there is considerable need to better understand the molecular mechanisms leading to cataract and complications from cataract surgery. Surgical removal of the cataractous lens tissue is required to restore light transmission to the retinal photoreceptors. Although this process clears the visual axis, up to 30% of patients require a subsequent procedure to correct visual defects caused by regrowth of LECs left from the surgical procedure. As a wound repair response mediated in part by TGF-β, the residual epithelial cells can replicate and undergo EMT10,12 and associated migration and spread over the posterior surface of the capsular bag to produce the condition of PCO. Over time, these cells can induce contraction of the capsule, which again disrupts the visual axis and leads to impaired vision. Creation of a capsulotomy using an Nd:YAG laser can be used to restore vision, but this procedure adds considerable cost to the course of treatment for cataracts and approximately doubles the risk for additional complications, such as retinal detachment and retinal edema.37 It has been shown that PCO is more severe and frequent in young than elderly because of better wound-healing response.38 Epithelial-to-mesenchymal transition has been shown as an important event in transformation of LECs during wound healing after cataract surgery,10,12 which leads to PCO.
Previous studies demonstrated that blockade of AR protected against LEC growth and expression of EMT markers in a pig eye capsular bag model of PCO.17 Our previous studies also showed that overexpression of AR in the lens of transgenic mice resulted in lens defects and activated expression of α-SMA, an EMT marker.22 Hallmarks of LEC changes associated with PCO include increased cell migration, increased expression of αSMA, and increased secretion of MMP-2 and MMP-9.7,13,14,31 Lower expression of these markers was observed when cells were treated with Sorbinil or when gene expression was transiently suppressed using siRNA targeted to AR.
The transition of LECs after cataract has been shown to be mediated by TGF-β.39 Previous studies have identified the SMAD signaling pathway as playing a critical downstream role in TGF-β–mediated signaling.40 Activation of the TGF-β receptor results in recruitment of receptor-regulated SMADs (R-SMADs: SMAD2 and SMAD3) to the receptor to bind with the adaptor protein SARA (SMAD anchor for receptor activation). After phosphorylation by the receptor kinase activity, the activated R-SMAD is released from the receptor complex and subsequently forms a heterodimeric complex with SMAD4 and accumulates in the nucleus. Previous work has demonstrated that TGF-β signaling in LECs relies heavily on SMAD2 and SMAD4.13,14 Phosphorylation of SMAD2 has been shown to trigger EMT processes.41 Therefore, we consider that inhibition of SMAD2 activation may be an effective strategy for preventing EMT associated with PCO.
Given that EMT of LECs is associated with development of PCO, and these changes are dependent on TGF-β stimulation and can be suppressed with AR inhibitors, we reasoned that AR inhibitors may prevent PCO by interfering with TGF-β signaling in LECs. However, very little was previously known about a possible mechanism to link AR to SMAD2 signaling. Our experiments demonstrated that interactions involving AR and SMAD2 are substantially increased following TGF-β exposure. As TGF-β causes R-SMADs to be recruited to the TGF-β receptor, this observation is consistent with the idea that AR initially interacts with them in the context of the TGF-β receptor complex. An alternate explanation, which cannot be ruled out at this time, is that AR preferentially binds the phosphorylated R-SMADs, and TGF-β treatment simply increases the abundance of the phosphorylated form. In either event, interaction between AR and SMAD2, either directly or in complex with additional components, was confirmed by pull-down assays from LEC lysates using antibodies directed against AR and the individual R-SMADs, respectively. These results were confirmed by pull-down assays using cells transfected with AR and SMAD2 constructs, each fused to unique affinity epitopes for selective immunoprecipitation. It is apparent that AR functions noncatalytically in this capacity, as wild-type AR and its catalytically inactive mutant (ARY48F) interact to a quantitatively similar degree with SMAD2 after TGF-β2 exposure. We also confirmed the interaction between AR and SMAD3 using overexpressed proteins in the presence of TGF-β2 with or without Sorbinil (see Supplementary Fig. S5). Data indicated that SMAD3 acts in a similar role as observed in SMAD2.
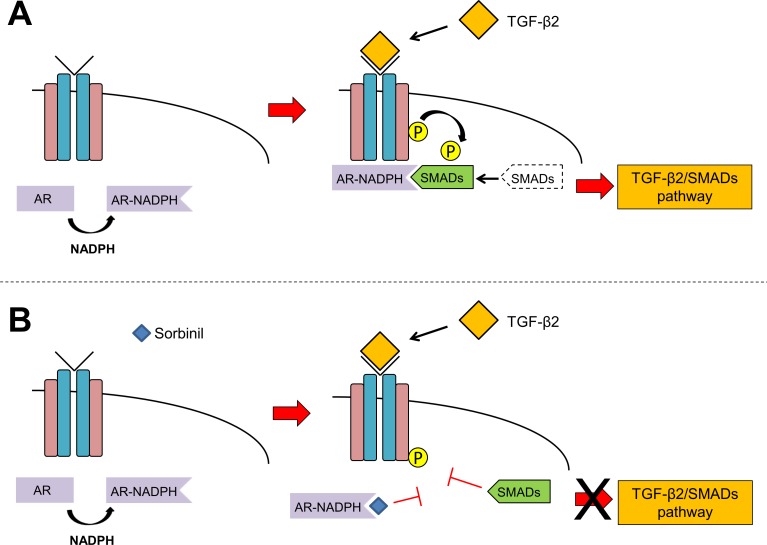
Pull-down assays demonstrated an approximate 3-fold increase in AR-SMAD interaction following LEC exposure to TGF-β if the binding extracts were supplemented with NADPH, a nicotinamide cofactor that binds AR with high affinity (Fig. 6A). In light of the substantial conformational change that occurs when AR binds nicotinamide cofactors,42 we favor the hypothesis that cofactor binding by AR exposes a SMAD-binding domain to facilitate interaction between these proteins. Suppression of this interaction by Sorbinil suggests that the SMAD-binding domain may well overlap the inhibitor binding site.43 Based on these results, we propose a model in which the binary AR-NADPH complex binds to SMAD2 following TGF-β2 binding to the receptor (Fig. 8A). Binding of Sorbinil to AR blocks interaction of AR and SMAD2 and prevents SMAD2 phosphorylation, which results in the attenuation of TGF-β2/SMAD downstream signaling (Fig. 8B). There is ample precedent for competition among interacting proteins for a discrete binding domain on SMAD2 to regulate its activity or subcellular distribution.40,44,45
Figure 8.
Model of AR-SMAD interaction. Aldose reductase binding to NADPH induces a conformational change that exposes a SMAD binding domain. Under TGF-β2 treatment, AR-NADPH binary complex binds to SMADs (A). However, Sorbinil binding to AR blocks the SMADs binding domain of AR, preventing their interaction at the TGF-β2 receptor (B).
A further confirmation of the close physical association between AR and SMAD2 was provided by a PLA. Positive PLA signals result from proteins that must be within approximately 30 to 40 nm distance of each other.30 The number of positive PLA signals was increased approximately 15-fold following exposure of cells of TGF-β; this increase was reduced 5-fold by Sorbinil. The PLA results, which showed a pattern of increased interaction between SMAD2 and AR following TGF-β stimulation, and suppression of this interaction by Sorbinil, are fully concordant with results from our pull-down assays.
Previous studies have demonstrated a key role for MMPs in the pathogenesis of PCO as well as anterior subcapsular cataracts, both of which are lens disorders brought about by fibrotic changes taken on by LECs.4,46-48 In the case of PCO, tissue trauma associated with cataract surgery is thought to stimulate the production of growth factors such as TGF-β. The MMPs expressed in response to TGF-β stimulation play an important role in cell-matrix interactions and facilitate contraction of the extracellular matrix and associated wrinkling of the lens capsular bag. Thus, TGF-β–induced MMP expression is a key driver of PCO. Evidence suggests that MMP-9 is induced by TGF-β via SMAD signaling49 and relies on actin cytoskeleton remodeling to facilitate cell invasion and migration.50 Our current studies in HLE B3 cells demonstrate that AR inhibition prevents TGF-β–induced increases in pro-MMP-9 and pro- and active MMP-2 expression, similar to previous observations involving the lipopolysaccharide-induced activation of MMP-9 in LECs.31 Although the exact mechanism linking AR to TGF-β–induced activation of these MMPs is yet to be resolved, our results are consistent with previous studies, which demonstrated that reduction of MMP-2 and MMP-9 resulted in decreased cell migration.31,51 Therefore, our results point to AR as a druggable target for prevention of TGF-β–induced LEC migration.
Some of our studies were carried out using the HLE B3 immortalized cell line derived from human lenses. Because of the possibility that the cell line may not fully represent normal LECs, we carried out confirmatory experiments using primary LEC cultures and whole-lens organ cultures from mice, taking advantage of mutant strains that were engineered for either over- or underexpression of the AR gene. Our observations regarding the involvement of AR in mediating TGF-β/SMAD signaling were similar in HLE B3 cells and primary/organ cultures derived from mouse lenses. Treatment with Sorbinil diminished the TGF-β2–induced expression of EMT markers in HLE B3 cells as well as in primary lens epithelium cultures or whole-lens organ cultures, demonstrating the similarities of human and mouse lens cells in this regard.
Interest in AR has traditionally centered on its role in catalyzing the conversion of glucose to sorbitol as the first step in the polyol pathway linked to diabetic complications.52–54 However, the nonenzymatic function of AR has not been extensively explored. Here, we report that AR plays an important noncatalytic role in TGF-β2/SMAD2 pathway. Our studies have uncovered a novel mechanism by which AR participates in downstream signaling from the TGF-β receptor and point to a new mechanism by which AR inhibitors may block key steps in EMT and therefore provide protection against EMT-related diseases ranging from cataracts to cancer.
Although the SMAD pathway plays a crucial role in facilitating TGF-β–mediated signaling in the pathogenesis of PCO, it is likely that TGF-β2 is also capable of inducing cellular responses independent of SMAD signaling.55 For example, Dawes and colleagues55 have observed that SMAD4, which is considered a key player in the canonical pathway of TGF-β–stimulated induction of EMT-related genes, can be substantially downregulated without significantly affecting the TGF-β–induced nuclear translocation of SMAD2/3 or matrix contraction in the FHL 124 LEC line. Given that TGF-β can induce changes in the activation state of MAP signaling kinases (RAS/MEK/ERK) in lens-derived cell models55 as well as in other cell types,56 it is possible that some of the beneficial effects of AR inhibitors could arise from their ability to influence MAP kinase signaling. Indeed, we have shown that upregulation of AR is associated with elevated levels of pERK1/2,57 and others have shown that ARIs can normalize levels of pERK1/2 in models of diabetes.58 Therefore, additional work will be required to more fully understand whether the ability of ARIs to suppress TGF-β2 signaling extends beyond the effects on SMAD activation we observed in the current work.
In considering the therapeutic possibilities that arise from these studies, it seems reasonable to propose that ARI formulations could be placed directly in the capsular bag at the time of cataract surgery as a means to prevent the immediate tissue wound response mediated by TGF-β2. A variety of drug-delivery options are readily available for this purpose, including drug-eluting materials incorporated into portions of the intraocular lens implant59 as well as nanoparticle-based drug-delivery systems that could be introduced at the time of surgery. Recent studies have shown that topical formulations of an ARI were effective against cataract onset and progression in dogs with diabetes mellitus,60 opening the possibility that topical therapy with an ARI-based PCO inhibitor could extend treatment well beyond the initial phase of wound healing immediately following cataract surgery.
Supplementary Material
Acknowledgments
We thank James Friedman, PhD, for providing pcDNA3.1 vector and Niklaus H. Mueller, PhD, for help in generating plasmid constructs.
Supported by National Institutes of Health Grant EY005856 (JMP). The authors alone are responsible for the content and writing of the paper.
Disclosure: K.-C. Chang, None; J.M. Petrash, None
References
- 1. Dewey S. Posterior capsule opacification. Curr Opin Ophthalmol. 2006; 17: 45–53. [DOI] [PubMed] [Google Scholar]
- 2. de Iongh RU,, Wederell E,, Lovicu FJ,, McAvoy JW. Transforming growth factor-beta-induced epithelial-mesenchymal transition in the lens: a model for cataract formation. Cells Tissues Organs. 2005; 179: 43–55. [DOI] [PubMed] [Google Scholar]
- 3. Wormstone IM. Posterior capsule opacification: a cell biological perspective. Exp Eye Res. 2002; 74: 337–347. [DOI] [PubMed] [Google Scholar]
- 4. Wormstone IM,, Tamiya S,, Anderson I,, Duncan G. TGF-beta2–induced matrix modification and cell transdifferentiation in the human lens capsular bag. Invest Ophthalmol Vis Sci. 2002; 43: 2301–2308. [PubMed] [Google Scholar]
- 5. Gotoh N,, Perdue NR,, Matsushima H,, Sage EH,, Yan Q,, Clark JI. An in vitro model of posterior capsular opacity: SPARC and TGF-beta2 minimize epithelial-to-mesenchymal transition in lens epithelium. Invest Ophthalmol Vis Sci. 2007; 48: 4679–4687. [DOI] [PubMed] [Google Scholar]
- 6. Shimada A,, Miyata Y,, Kosano H. Type I collagen accelerates the spreading of lens epithelial cells through the expression and activation of matrix metalloproteinases. Curr Eye Res. 2014; 39: 460–471. [DOI] [PubMed] [Google Scholar]
- 7. Wormstone IM,, Wang L,, Liu CS. Posterior capsule opacification. Exp Eye Res. 2009; 88: 257–269. [DOI] [PubMed] [Google Scholar]
- 8. Zelenka PS,, Arpitha P. Coordinating cell proliferation and migration in the lens and cornea. Semin Cell Dev Biol. 2008; 19: 113–124. [DOI] [PubMed] [Google Scholar]
- 9. Liu J,, Hales AM,, Chamberlain CG,, McAvoy JW. Induction of cataract-like changes in rat lens epithelial explants by transforming growth factor beta. Invest Ophthalmol Vis Sci. 1994; 35: 388–401. [PubMed] [Google Scholar]
- 10. Saika S,, Miyamoto T,, Ishida I,, et al. TGFbeta-Smad signalling in postoperative human lens epithelial cells. Br J Ophthalmol. 2002; 86: 1428–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ignotz RA,, Massague J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986; 261: 4337–4345. [PubMed] [Google Scholar]
- 12. Hales AM,, Schulz MW,, Chamberlain CG,, McAvoy JW. TGF-beta 1 induces lens cells to accumulate alpha-smooth muscle actin, a marker for subcapsular cataracts. Curr Eye Res. 1994; 13: 885–890. [DOI] [PubMed] [Google Scholar]
- 13. Li J,, Tang X,, Chen X. Comparative effects of TGF-beta2/Smad2 and TGF-beta2/Smad3 signaling pathways on proliferation migration, and extracellular matrix production in a human lens cell line. Exp Eye Res. 2011; 92: 173–179. [DOI] [PubMed] [Google Scholar]
- 14. Wang Y,, Li W,, Zang X,, et al. MicroRNA-204-5p regulates epithelial-to-mesenchymal transition during human posterior capsule opacification by targeting SMAD4. Invest Ophthalmol Vis Sci. 2013; 54: 323–332. [DOI] [PubMed] [Google Scholar]
- 15. Saika S,, Ikeda K,, Yamanaka O,, et al. Transient adenoviral gene transfer of Smad7 prevents injury-induced epithelial-mesenchymal transition of lens epithelium in mice. Lab Invest. 2004; 84: 1259–1270. [DOI] [PubMed] [Google Scholar]
- 16. Ma B,, Kang Q,, Qin L,, Cui L,, Pei C. TGF-beta2 induces transdifferentiation and fibrosis in human lens epithelial cells via regulating gremlin and CTGF. Biochem Biophys Res Commun. 2014; 447: 689–695. [DOI] [PubMed] [Google Scholar]
- 17. Yadav UC,, Ighani-Hosseinabad F,, van Kuijk FJ,, Srivastava SK,, Ramana KV. Prevention of posterior capsular opacification through aldose reductase inhibition. Invest Ophthalmol Vis Sci. 2009; 50: 752–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chang KC,, Laffin B,, Ponder J,, et al. Beta-glucogallin reduces the expression of lipopolysaccharide-induced inflammatory markers by inhibition of aldose reductase in murine macrophages and ocular tissues. Chem Biol Interact. 2013; 202: 283–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chang KC,, Snow A,, LaBarbera DV,, Petrash JM. Aldose reductase inhibition alleviates hyperglycemic effects on human retinal pigment epithelial cells. Chem Biol Interact. 2015; 234: 254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ramana KV,, Fadl AA,, Tammali R,, Reddy AB,, Chopra AK,, Srivastava SK. Aldose reductase mediates the lipopolysaccharide-induced release of inflammatory mediators in RAW264.7 murine macrophages. J Biol Chem. 2006; 281: 33019–33029. [DOI] [PubMed] [Google Scholar]
- 21. Chen KC,, Zhou Y,, Zhang W,, Lou MF. Control of PDGF-induced reactive oxygen species (ROS) generation and signal transduction in human lens epithelial cells. Mol Vis. 2007; 13: 374–387. [PMC free article] [PubMed] [Google Scholar]
- 22. Zablocki GJ,, Ruzycki PA,, Overturf MA,, Palla S,, Reddy GB,, Petrash JM. Aldose reductase-mediated induction of epithelium-to-mesenchymal transition (EMT) in lens. Chem Biol Interact. 2011; 191: 351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wormstone IM,, Tamiya S,, Eldred JA,, et al. Characterisation of TGF-beta2 signalling and function in a human lens cell line. Exp Eye Res. 2004; 78: 705–714. [DOI] [PubMed] [Google Scholar]
- 24. Terrell AM,, Anand D,, Smith SF,, et al. Molecular characterization of mouse lens epithelial cell lines and their suitability to study RNA granules and cataract associated genes. Exp Eye Res. 2015; 131: 42–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ho HT,, Chung SK,, Law JW,, et al. Aldose reductase-deficient mice develop nephrogenic diabetes insipidus. Mol Cell Biol. 2000; 20: 5840–5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Petrash JM,, Harter TM,, Devine CS,, et al. Involvement of cysteine residues in catalysis and inhibition of human aldose reductase. Site-directed mutagenesis of Cys-80, -298, and -303. J Biol Chem. 1992; 267: 24833–24840. [PubMed] [Google Scholar]
- 27. Tarle I,, Borhani DW,, Wilson DK,, Quiocho FA,, Petrash JM. Probing the active site of human aldose reductase. Site-directed mutagenesis of Asp-43 Tyr-48, Lys-77, and His-110. J Biol Chem. 1993; 268: 25687–25693. [PubMed] [Google Scholar]
- 28. Huang SP,, Palla S,, Ruzycki P,, et al. Aldo-keto reductases in the eye. J Ophthalmol. 2010; 2010: 521204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sasaki-Suzuki N,, Arai K,, Ogata T,, et al. Growth hormone inhibition of glucose uptake in adipocytes occurs without affecting GLUT4 translocation through an insulin receptor substrate-2-phosphatidylinositol 3-kinase-dependent pathway. J Biol Chem. 2009; 284: 6061–6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schedin-Weiss S,, Inoue M,, Teranishi Y,, et al. Visualizing active enzyme complexes using a photoreactive inhibitor for proximity ligation—application on gamma-secretase. PLoS One. 2013; 8: e63962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yao J,, Yang W,, Liu Y,, Sun YX,, Jiang Q. Dexamethasone inhibits TGF-beta2–induced migration of human lens epithelial cells: implications for posterior capsule opacification prevention. Mol Med Report. 2012; 5: 1509–1513. [DOI] [PubMed] [Google Scholar]
- 32. Pladzyk A,, Reddy AB,, Yadav UC,, Tammali R,, Ramana KV,, Srivastava SK. Inhibition of aldose reductase prevents lipopolysaccharide-induced inflammatory response in human lens epithelial cells. Invest Ophthalmol Vis Sci. 2006; 47: 5395–5403. [DOI] [PubMed] [Google Scholar]
- 33. Reddy AB,, Ramana KV,, Srivastava S,, Bhatnagar A,, Srivastava SK. Aldose reductase regulates high glucose-induced ectodomain shedding of tumor necrosis factor (TNF)-alpha via protein kinase C-delta and TNF-alpha converting enzyme in vascular smooth muscle cells. Endocrinology. 2009; 150: 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chang KC,, Ponder J,, Labarbera DV,, Petrash JM. Aldose reductase inhibition prevents endotoxin-induced inflammatory responses in retinal microglia. Invest Ophthalmol Vis Sci. 2014; 55: 2853–2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wilson DK,, Bohren KM,, Gabbay KH,, Quiocho FA. An unlikely sugar substrate site in the 1.65 A structure of the human aldose reductase holoenzyme implicated in diabetic complications. Science. 1992; 257: 81–84. [DOI] [PubMed] [Google Scholar]
- 36. Thylefors B,, Negrel AD,, Pararajasegaram R,, Dadzie KY. Global data on blindness. Bull World Health Organ. 1995; 73: 115–121. [PMC free article] [PubMed] [Google Scholar]
- 37. Petrash JM. Aging and age-related diseases of the ocular lens and vitreous body. Invest Ophthalmol Vis Sci. 2013; 54: ORSF 54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dawes LJ,, Duncan G,, Wormstone IM. Age-related differences in signaling efficiency of human lens cells underpin differential wound healing response rates following cataract surgery. Invest Ophthalmol Vis Sci. 2013; 54: 333–342. [DOI] [PubMed] [Google Scholar]
- 39. Xu H,, Chen M,, Forrester JV,, Lois N. Cataract surgery induces retinal pro-inflammatory gene expression and protein secretion. Invest Ophthalmol Vis Sci. 2011; 52: 249–255. [DOI] [PubMed] [Google Scholar]
- 40. Shi Y,, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003; 113: 685–700. [DOI] [PubMed] [Google Scholar]
- 41. Derynck R,, Akhurst RJ,, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet. 2001; 29: 117–129. [DOI] [PubMed] [Google Scholar]
- 42. Ye Q,, Hyndman D,, Green NC,, Li L,, Jia Z,, Flynn TG. The crystal structure of an aldehyde reductase Y50F mutant-NADP complex and its implications for substrate binding. Chem Biol Interact. 2001; 130-132: 651–658. [DOI] [PubMed] [Google Scholar]
- 43. Urzhumtsev A,, Tete-Favier F,, Mitschler A,, et al. A ‘specificity' pocket inferred from the crystal structures of the complexes of aldose reductase with the pharmaceutically important inhibitors tolrestat and sorbinil. Structure. 1997; 5: 601–612. [DOI] [PubMed] [Google Scholar]
- 44. ten Dijke P,, Hill CS. New insights into TGF-beta-Smad signalling. Trends Biochem Sci. 2004; 29: 265–273. [DOI] [PubMed] [Google Scholar]
- 45. Wu G,, Chen YG,, Ozdamar B,, et al. Structural basis of Smad2 recognition by the Smad anchor for receptor activation. Science. 2000; 287: 92–97. [DOI] [PubMed] [Google Scholar]
- 46. Dwivedi DJ,, Pino G,, Banh A,, et al. Matrix metalloproteinase inhibitors suppress transforming growth factor-beta-induced subcapsular cataract formation. Am J Pathol. 2006; 168: 69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Eldred JA,, Hodgkinson LM,, Dawes LJ,, Reddan JR,, Edwards DR,, Wormstone IM. MMP2 activity is critical for TGFbeta2–induced matrix contraction—implications for fibrosis. Invest Ophthalmol Vis Sci. 2012; 53: 4085–4098. [DOI] [PubMed] [Google Scholar]
- 48. Nathu Z,, Dwivedi DJ,, Reddan JR,, Sheardown H,, Margetts PJ,, West-Mays JA. Temporal changes in MMP mRNA expression in the lens epithelium during anterior subcapsular cataract formation. Exp Eye Res. 2009; 88: 323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sinpitaksakul SN,, Pimkhaokham A,, Sanchavanakit N,, Pavasant P. TGF-beta1 induced MMP-9 expression in HNSCC cell lines via Smad/MLCK pathway. Biochem Biophys Res Commun. 2008; 371: 713–718. [DOI] [PubMed] [Google Scholar]
- 50. Zhang K,, Chen D,, Jiao X,, et al. Slug enhances invasion ability of pancreatic cancer cells through upregulation of matrix metalloproteinase-9 and actin cytoskeleton remodeling. Lab Invest. 2011; 91: 426–438. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51. Awasthi N,, Wang-Su ST,, Wagner BJ. Downregulation of MMP-2 and -9 by proteasome inhibition: a possible mechanism to decrease LEC migration and prevent posterior capsular opacification. Invest Ophthalmol Vis Sci. 2008; 49: 1998–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Obrosova IG,, Kador PF. Aldose reductase/polyol inhibitors for diabetic retinopathy. Curr Pharm Biotechnol. 2011; 12: 373–385. [DOI] [PubMed] [Google Scholar]
- 53. Reddy AB,, Ramana KV. Aldose reductase inhibition: emerging drug target for the treatment of cardiovascular complications. Recent Pat Cardiovasc Drug Discov. 2010; 5: 25–32. [DOI] [PubMed] [Google Scholar]
- 54. Oates PJ. Aldose reductase inhibitors and diabetic kidney disease. Curr Opin Investig Drugs. 2010; 11: 402–417. [PubMed] [Google Scholar]
- 55. Dawes LJ,, Sleeman MA,, Anderson IK,, Reddan JR,, Wormstone IM. TGFbeta/Smad4-dependent and -independent regulation of human lens epithelial cells. Invest Ophthalmol Vis Sci. 2009; 50: 5318–5327. [DOI] [PubMed] [Google Scholar]
- 56. Derynck R,, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003; 425: 577–584. [DOI] [PubMed] [Google Scholar]
- 57. Snow A,, Shieh B,, Chang KC,, et al. Aldose reductase expression as a risk factor for cataract. Chem Biol Interact. 2015; 234: 247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zatechka DS,, Jr,, Kador PF,, Garcia-Castineiras S,, Lou MF. Diabetes can alter the signal transduction pathways in the lens of rats. Diabetes. 2003; 52: 1014–1022. [DOI] [PubMed] [Google Scholar]
- 59. Kugelberg M,, Shafiei K,, van der Ploeg I,, Zetterstrom C. Intraocular lens as a drug delivery system for dexamethasone. Acta Ophthalmol. 2010; 88: 241–244. [DOI] [PubMed] [Google Scholar]
- 60. Kador PF,, Webb TR,, Bras D,, Ketring K,, Wyman M. Topical KINOSTAT ameliorates the clinical development and progression of cataracts in dogs with diabetes mellitus. Vet Ophthalmol. 2010; 13: 363–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.