Abstract
The concept of comorbidity between attention deficit/hyperactivity disorder (ADHD) and obsessive-compulsive disorder (OCD) has been discussed for two decades. No review, however, has examined this question in light of the stark contrast in disorder-specific phenomenology and neurobiology. We review reported prevalence rates and the methodological, phenomenological, and theoretical issues concerning concomitant ADHD-OCD. Reported co-occurrence rates are highly inconsistent in the literature. Studies aimed at examining the potential for comorbidity have suffered from various methodological problems, including the existence of very few community samples, highly variable exclusionary criteria, and possible clinical misinterpretation of symptoms. Despite numerous studies suggesting an ADHD-OCD comorbidity, thus far etiological (i.e., genetic) backing has been provided only for a pediatric comorbidity. Additionally, inflated rates of ADHD-OCD co-occurrence may be mediated by the presence of tic disorders, and evidence of impaired neuronal maturational processes in pediatric OCD may lead to possibly transient phenotypical expressions that resemble ADHD symptomatology. Thus, clinicians are encouraged to consider the possibility that ADHD-like symptoms resulting from OCD-specific symptomatology may be misdiagnosed as ADHD. This suggestion may account for the lower co-occurrence rates reported in adolescents and adults and for the lack of a theoretical account for comorbidity in these age groups. Existing literature is summarized and critically reviewed, and recommendations are made for future research.
Keywords: attention-deficit/hyperactivity disorder, comorbidity, diagnosis, obsessive-compulsive disorder, prevalence, tic disorders
INTRODUCTION
Attention deficit/hyperactivity disorder (ADHD) and obsessive-compulsive disorder (OCD) are common developmental neuropsychiatric disorders associated with significant distress and dysfunction. ADHD is characterized by inattention and by hyperactivity and impulsiveness present since childhood, whereas OCD is characterized by intrusive obsessions and by compulsions that are typically performed in response to those obsessions. In the 1990s, studies examining the prevalence and clinical manifestation of juvenile OCD began to report elevated rates of co-occurring ADHD (i.e., the simultaneous presentation of two diagnoses).1–5 Within a few years, numerous studies reported ADHD-OCD comorbidity in pediatric OCD samples.6–43 Fewer studies examined ADHD-OCD comorbidity in adults.24,29,35,37,38,44–52
As we elaborate below, the research on ADHD-OCD comorbidity faces empirical and conceptual difficulties that are likely interrelated. Empirically, reports of co-occurrence rates are highly inconsistent, ranging from 0% to 60%,53 with much lower co-occurrence rates reported in adults relative to youth samples. Conceptually, the notion of genuine comorbidity between the two disorders (i.e., simultaneous presentation of the two disorders; as opposed to false comorbidity, where symptoms of one disorder mimic those of the other) is not easily reconciled with the fundamental neurobiological, phenomenological, and behavioral differences between ADHD and OCD. For example, relative to controls, OCD patients exhibit frontostriatal hyperactivation, whereas ADHD patients exhibit frontostriatal hypoactivation. In addition, several potential confounding factors (e.g., the mediating effect of tic disorders, the presence of ADHD-like symptoms in OCD) may contribute to the variability of reported co-occurrence rates of the two disorders.53 In light of these difficulties, there is a need for a systematic and critical review of the studies reporting co-occurrence between ADHD and OCD. In this article we aim to (1) conduct a systematic review of reported ADHD-OCD co-occurrence rates, (2) outline methodological caveats that may have contributed to the variability seen across studies, (3) evaluate the theoretical and etiological accounts for ADHD-OCD comorbidity in light of fundamental neurobiological and clinical differences between the two disorders, (4) examine potential confounding factors that may inflate reported co-occurrence rates, and (5) offer directions for future research.
We begin our review by describing the differences between OCD and ADHD in terms of phenomenology, neurobiology, neuropsychology, and pharmacotherapy. We suggest that these extensive differences pose a challenge for the concept of genuine comorbidity between the two disorders, especially in adults. We continue with a systematic review of reported co-occurrence rates of ADHD and OCD. We then address several methodological issues that may contribute to the variability in reported co-occurrence rates, followed by examination of the available etiological accounts for ADHD-OCD co-occurrence (which are presently limited to pediatric populations). Thereafter, we outline potential confounding factors that may contribute to inflation of co-occurrence rates. Specifically, we suggest that the presence of tic disorders as well as OCD-related executive function impairments may result in ADHD symptoms in OCD that may further contribute to inflated reported rates of concomitance. In addition, we propose a neurodevelopmental perspective according to which deficient neuromaturational processes in pediatric OCD may transiently produce ADHD symptoms in children with OCD. Finally, we outline implications and future directions for research.
Phenomenology of OCD and ADHD
ADHD is considered a congenital disorder with a strong heritable component.54 OCD is also thought to have a hereditary component.55,56 Estimated heritability in OCD (40%) is much less prominent than in ADHD, however, in which 70%–80% of the phenotypic variance can be explained by genetic factors.57 In addition, whereas ADHD presents in childhood, only 25%–50% of adults with OCD experience onset before age 18.49
ADHD is an externalizing disorder characterized predominantly by inattention, behavioral impulsivity, and hyperactivity; it is further associated with risk-taking behavior, drug abuse, and novelty seeking.54,58,59 By contrast, OCD is an internalizing disorder characterized predominantly by obsessions and compulsions, and is associated with harm/risk avoidance and restrained behavior.58,60–62 In contrast to the prominent impulsiveness phenotype associated with ADHD, OCD is associated with generally inhibited temperament typified by behavioral restraint, withdrawal, and avoidance of novel stimuli.63,64 Moreover, studies indicate that individuals diagnosed with OCD are no more behaviorally impulsive than nonpsychiatric control participants, with several studies reporting lower levels of behavioral impulsivity in OCD than in nonpsychiatric controls.60,65–68
When addressing the concept of comorbidity between ADHD and OCD, it is useful to consider the notion of an impulsive-compulsive continuum, as suggested by Hollander.69 According to this hypothetical scheme, the compulsive end of this continuum—which is associated with OCD—is characterized by harm avoidant and risk-aversive behaviors. Conversely, the impulsive end of the continuum is characterized by behavioral impulsivity (i.e., behaviors lacking forethought) and risk taking.69 According to Hollander, the concept of polarity between the two constructs is further supported by neurobiological and neurochemical differences. For example, on the neurotransmitter level, impulsivity is mediated by dopamine, whereas compulsivity is mediated by serotonin.69 In sum, ADHD and OCD appear to be remarkably different in terms of their phenomenology.53,70
Given that the symptoms of behavioral impulsivity differ so dramatically between the two disorders, it may be reasonable to assume that among OCD patients, there would be a higher probability of identifying primary inattentive type of ADHD (ADHD-I), rather than the predominantly hyperactive subtype (ADHD-H) or the combined type (ADHD-C). Estimations of the prevalence of ADHD subtypes vary as a function of the assessment methods. A recent meta-analysis reported, however, that best-estimation diagnosis yielded 57% prevalence rates for ADHD-C, 30% for ADHD-I, and 13% for ADHD-H.71 A limited number of studies reported the breakdown of ADHD subtypes in individuals with ADHD-OCD comorbidity. Some studies report percentages similar to those in Willcutt’s meta-analysis. For example, Geller and colleagues14 reported 69% of ADHD-C and 24% of ADHD-I in a sample of youth with comorbid OCD and ADHD.14 Other reports, however, indicated a subtype breakdown favoring ADHD-I. For example, Anholt and colleagues (2010)35 found 48% ADHD-I and 36% ADHD-C in a sample of adults with comorbid ADHD-OCD. In sum, the data are currently too sparse to permit a persuasive inference regarding the speculative hypothesis of a higher probability of identifying ADHD-I in patients with comorbid ADHD-OCD.
Neurobiology of ADHD and OCD
As one might expect from the phenomenological dissimilarity, review of the ADHD and OCD neurobiological literature suggests that each disorder is associated with a distinct pattern of brain pathophysiology. A large body of research reveals an abnormal pattern of brain activity along the frontostriatal system and among selected regions of interest (see below) throughout this network in both ADHD72–74 and OCD.75–78 The frontostriatal functional abnormalities contrast sharply, however, between the two disorders. OCD is associated with abnormally increased activity (hypermetabolism) in frontal and striatal regions such as the orbitofrontal cortex, the basal ganglia, and thalamus.75,77,79,80 Furthermore, OCD patients exhibit hyperactivated frontostriatal functional connectivity.76,81,82 By contrast, decreased activity (hypometabolism) in similar prefrontal and striatal brain regions has repeatedly been found in ADHD,73,83,84 along with correspondingly reduced frontostriatal connectivity.85–87 To our knowledge, the only study that directly compared neuronal network abnormalities in ADHD and OCD found increased activation in OCD and decreased functional connectivity in ADHD.88
In light of the different clinical picture and the opposing patterns of frontostriatal pathophysiology, it is important to compare the associations between symptom severity and frontostriatal activation in ADHD and OCD. A review of the relevant literature shows that symptom severity and frontostriatal activation are negatively associated in ADHD but are positively associated in OCD. The first line of evidence supporting this distinct pattern arises from research examining the correlation between brain activity and disorder-specific symptom severity. In ADHD, symptom severity (predominantly impulsiveness) appears to be inversely correlated with brain activity in prefrontal89,90 and striatal85 regions. These findings are supported by recent research demonstrating an inverse correlation between frontostriatal functional connectivity and symptom severity in ADHD.84–86,91 By contrast, a positive association between brain activity and symptom severity has been repeatedly demonstrated in OCD.76,92–94 This interaction was demonstrated in a recent study that compared OCD and ADHD patients, reporting that the correlation of symptoms severity with frontostriatal brain activity was positive in the OCD sample but negative in the ADHD sample.88
Neuropsychology of ADHD and OCD
The opposing clinical and neurobiological features of ADHD and OCD support Hollander’s impulsive-compulsive continuum model69 and, as such, appear to challenge the notion of full-blown comorbidity between the two disorders. Interestingly, despite the opposing clinical and neurobiological profile of the two disorders, individuals with ADHD and OCD often present similar neuropsychological profiles. Both disorders tend to underperform relative to nonpsychiatric controls on tasks of executive functions, including working memory, planning, and response inhibition.95–99 Our review of the neuropsychological literature suggests, however, that the similarity in neuropsychological performance between the two disorders may be only apparent. Whereas neuropsychological findings in ADHD are highly consistent, neuropsychological findings in OCD are remarkably variable.95,100 For example, whereas one recent meta-analysis suggested significant heterogeneity across neuropsychological studies in adult OCD,95 a similar meta-analysis in adult ADHD reported homogeneity across studies.101 In addition, meta-analyses examining neuropsychological function in ADHD suggested larger effect sizes compared to those found in OCD.95,102,103 In fact, it has recently been suggested that the moderate effect sizes representing neuropsychological functioning in OCD may not be clinically significant.95
The contrasting frontostriatal abnormalities and clinical pictures of the two disorders suggest that the deficient performance of people with ADHD and those with OCD on neuropsychological tests may stem from different mechanisms. A number of studies have revealed a negative assciation between neuropsychological test performance and frontostriatal brain activity in OCD.92,104,105 By contrast, frontostriatal brain activity was positively correlated with neuropsychological test performance (predominantly executive functions) in ADHD.106–108 In sum, converging evidence from imaging and neuropsychological studies suggests that neuropsychological deficits and clinical manifestations are associated with differing brain pathophysiology in ADHD and OCD.
Pharmacological Treatment for ADHD and OCD
As noted above, impulsivity/disinhibition—the core symptoms of ADHD—are associated with hypoperfusion of prefrontal regions, whereas obsessiveness and compulsivity—the core symptoms of OCD—are associated with increased activation. For several decades, evidence-based expert guidelines have recommended stimulant medication such as methylphenidate as the first-line treatment for ADHD.109,110 Modulating dopamine reuptake, stimulant medication increases prefrontal activation and significantly improves both clinical symptomology and neurocognitive functioning in ADHD.109,111,112 First-line pharmacotherapy for OCD, by contrast, consists of serotonin reuptake inhibitors (SRIs), which target serotonergic pathways.113,114 These agents are thought to modulate hyperactivity along the frontostriatal network in OCD. Indeed, clinical improvement following pharmacological treatment or cognitive-behavioral therapy (CBT) is associated with a significant decrease in frontostriatal brain activity in OCD.75,115,116 Moreover, compulsive or repetitive behavior in rats may be induced by administering the dopamine agonist quinpirole117,118 and subsequently reduced by administering clomipramine,119 an effective SRI medication for OCD.120 Along the same lines, while stimulant medications alleviate symptoms in ADHD, they are thought to exacerbate OCD symptoms and may even induce obsessive-compulsive symptoms.121–124 Finally, whereas dopamine agonists have proven efficacious in the treatment of ADHD, dopamine blockers (i.e., neuroleptic/antipsychotic agents) have been found to be useful for SRI augmentation among OCD patients who only partially respond to SRIs.125
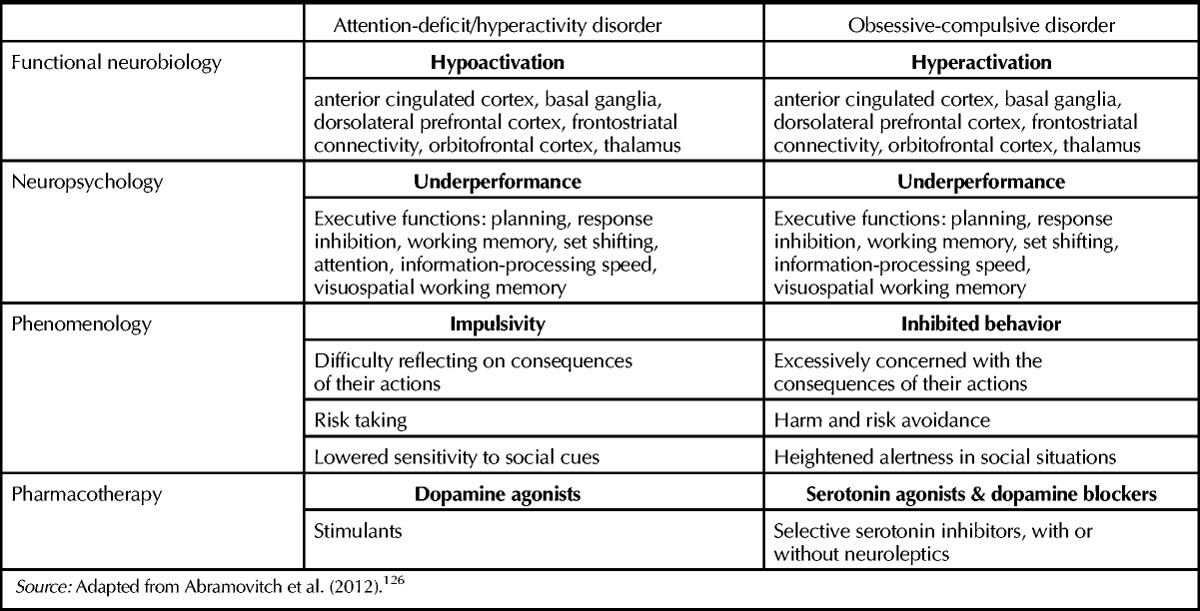
In sum, ADHD and OCD are two psychiatric syndromes that appear to lie on opposite ends of an impulsive-compulsive continuum (see Table 1). ADHD is characterized by reduced frontostriatal activity (associated with symptom severity), impulsive behavior, and positive response to stimulant therapy, whereas OCD is characterized by increased frontostriatal activity (associated with symptom severity), inhibited behavior, and harm- and risk-avoidance that responds to SRIs and dopamine blockers and not to stimulants. These profound differences seem to challenge the possibility of a genuine comorbidity between the two conditions, at least on the theoretical level.
Table 1.
Comparative Summary of Neurobiological, Neuropsychological, and Phenomenological Characteristics of Attention-Deficit/Hyperactivity Disorder and Obsessive-Compulsive Disordera
METHODS
We conducted a systematic literature search using PUBMED, ISI Web of Knowledge, and PsycInfo electronic databases, covering the period of 1980–2013. We also manually searched the work of relevant authors and the reference lists of identified articles. Search terms included obsessive-compulsive disorder, OCD, attention deficit/hyperactivity disorder, ADHD, comorbid, comorbidity, concomitant, and prevalence. A total of 206 abstracts was identified. We then excluded articles that were not in English, where diagnosis was not established using semistructured DSM-based measures, and where co-occurrence data were not presented and were unobtainable. Following the secondary screening process, 48 studies met inclusion criteria and were included in the present review, with publication dates ranging between 1990 and 2013. From these studies we examined 43 youth samples (<18 years of age) and 14 adult samples (≥18 years of age).
RESULTS
Summary of Findings
Our review of the literature reveals that the reported rates of ADHD-OCD co-occurrence are highly inconsistent, ranging from 0% to 60%.4,8,9,22,33,36 A detailed account of the samples reviewed for this manuscript is presented in Tables 2 and 3.
Table 2.
Co-occurrence Rates of Attention-Deficit/Hyperactivity Disorder and Obsessive-Compulsive Disorder in Children and Adolescents
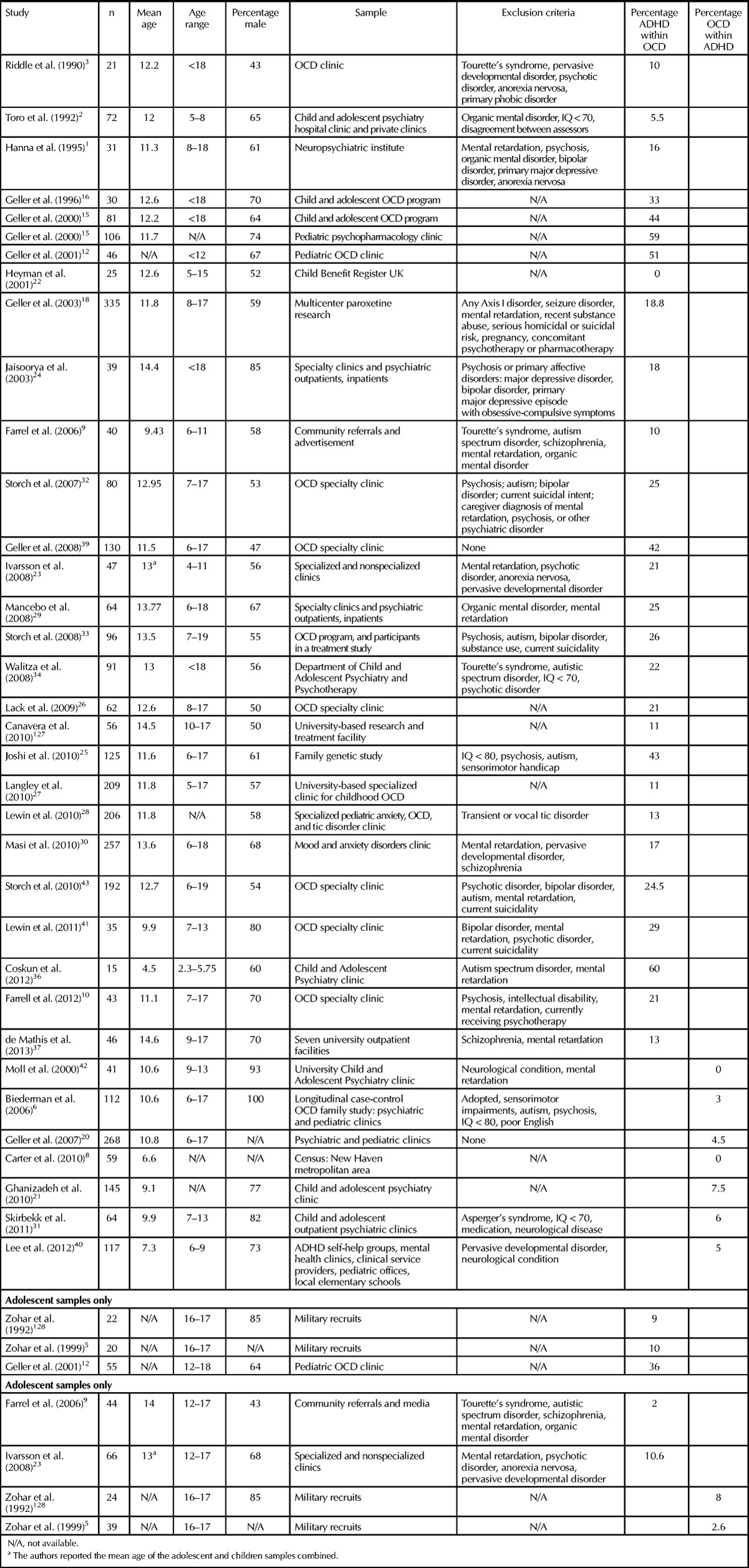
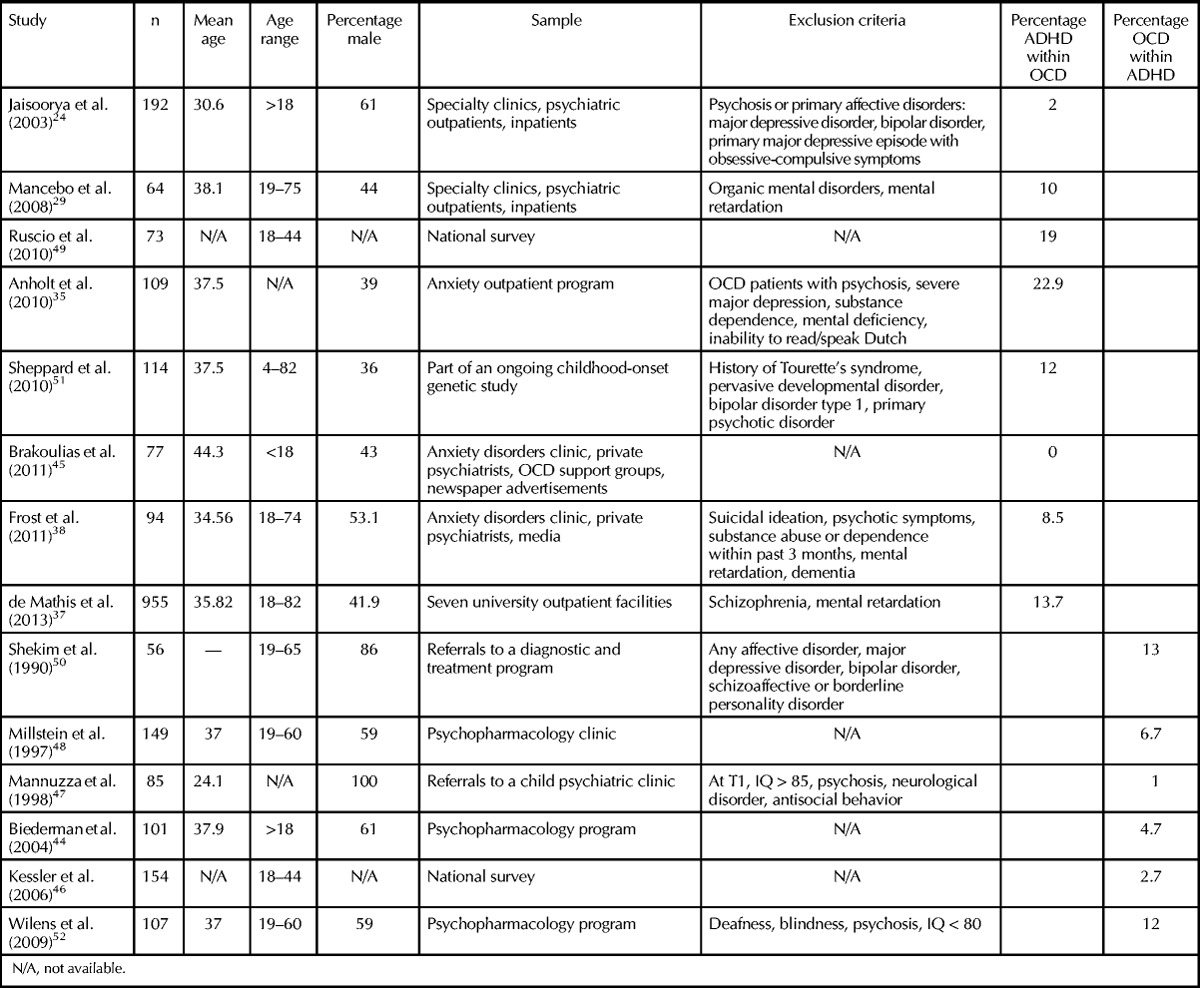
Table 3.
Co-occurrence Rates of Attention-Deficit/Hyperactivity Disorder and Obsessive-Compulsive Disorder in Adults
The vast majority of reports examined pediatric samples with a mean age ranging from 9 to 15 years. As shown in Tables 2 and 3, pediatric studies demonstrate higher variability in co-occurrence rates compared to adult and adolescent samples. For example, Heyman and colleagues22 analyzed data from the 1999 British Child Mental Health Survey, which encompassed 10,488 children ages 5 to 15. The authors reported that 40% of the 25 children diagnosed with OCD had an additional comorbid psychiatric condition, but none was diagnosed with ADHD. Similarly, Toro and colleagues2 reported that out of 72 children with OCD recruited from hospital and private clinics, only 5.5% had a concomitant diagnosis of ADHD. By contrast, Geller and colleagues15 examined 81 children from a specialty pediatric OCD clinic and 106 children from a general pediatric psychopharmacology clinic and reported that 59% of the children diagnosed with OCD in the general psychopharmacology clinic and 44% of the children in the OCD specialized clinic had a concomitant diagnosis of ADHD. As we discuss below, the extreme range of reported ADHD-OCD co-occurrence rates between samples recruited from specialized clinics and the general population (see Tables 2 and 3) may be partially due to referral biases.
Whereas the above studies reported the occurrence of ADHD in children diagnosed with OCD, only a few studies documented the occurrence of OCD in children and adolescents diagnosed with ADHD. Interestingly, these studies report less variable and relatively low co-occurrence rates of 3%–7.5%6,31,129 compared to studies examining ADHD in OCD samples (0%–60%). A limited number of studies assessed ADHD-OCD co-occurrence exclusively in adolescent samples. While the variability in co-occurrence rates is somewhat similar to that seen in pediatric and adult samples, ranging from 2% to 36%, overall co-occurrence rates are generally lower than in pediatric samples. In a study conducted in Sweden, Ivarsson and colleagues23 examined 66 adolescents with OCD recruited from general and specialized psychiatric clinics and concluded that 10.6% of them exhibited concomitant ADHD. As in the case of pediatric OCD, we found only two studies reporting the occurrence of OCD in ADHD adolescent samples. Both studies were conducted in Israel and found concomitant OCD in 2.6% and 8% of participants with ADHD, respectively.5,128
The adult literature reveals a similar number of studies reporting the occurrence of ADHD in OCD samples and OCD in ADHD samples. While somewhat lower compared to those found in the pediatric literature, rates of the occurrence of ADHD in OCD samples are inconsistent, ranging from 0% to 23%. For example, in one of the largest samples included in our review, Jaisoorya and colleagues24 recruited 192 adults with OCD from specialized outpatient and inpatient clinics. The authors reported that 2% of their sample had a concomitant diagnosis of ADHD.
In contrast to the large number of co-occurrence reports in clinical settings, only one study reported prevalence of OCD in adult individuals with ADHD in a nationally representative sample. Kessler and colleagues46 used data from the US National Comorbidity Survey to examine comorbidities within a sample of 154 adults with ADHD. The authors reported that 2.7% of their ADHD sample had OCD, a finding that remarkably resembles the prevalence rate of OCD observed in the general population (2.3%).49 Interestingly, Ruscio and colleagues49 analyzed data collected from the same US National Comorbidity Survey and found that 19% of the OCD group (n = 73) had ADHD. Similar variability was found in studies reporting the occurrence of OCD in ADHD samples (1%–13%).
In sum, our review of the literature reveals notable variability in reported ADHD-OCD co-occurrence rates, as well as lower co-occurrence rates and less variability in adult as compared to youth samples. In addition, higher co-occurrence rates are reported for ADHD in OCD samples than for OCD in ADHD samples. Below, we suggest methodological factors that may account for this divergence in reported co-occurrence rates.
Methodological Issues
Sample characteristics
Most of the studies examining ADHD-OCD co-occurrence examined clinical samples in specialty clinics, whereas only few examined non-referred or general population samples. The latter are the only reliable source from which inferences regarding prevalence in the epidemiological sense can be drawn. Importantly, the range of ADHD-OCD co-occurrence rates in the three studies of large representative samples22,46,128 was between 0% and 10%, whereas most of the studies using samples from specialized clinics found co-occurrence rates of 20% to 59%. The discrepancy between the co-occurrence rates found in referred samples versus the general population is a well-known phenomenon130,131 that may be accounted for by two types of selection biases that are often underrecognized.132 The Berksonian bias133 expresses how every additional diagnosis increases the probability that a person will be referred to mental health services, whereas the related clinical selection bias134 refers to the fact that individuals with multiple diagnoses are usually more functionally impaired and thus are more likely to pursue treatment. Ultimately, these biases lead to a substantial overrepresentation of patients with comorbidities in specialized clinics relative to the general population. For example, McConaughy and Achenbach131 analyzed studies encompassing 2705 juvenile patients across four groups of disorders. They found that comorbidity rates in samples drawn from clinical settings were up to six times higher than those seen in non-referred or general population samples. Notably, several studies we reviewed recruited participants from different referral sources such as outpatient, inpatient, and private clinics,24,29,45 thereby potentially increasing the variability of reported co-occurrence rates.
Exclusion criteria
As portrayed in Tables 2 and 3, the studies we reviewed also differed considerably in terms of exclusion criteria. Some studies of adult, pediatric, or adolescent samples did not employ any exclusion criteria,5,12,15,26,27,44,48,49,128 whereas most studies excluded patients with mental retardation, psychosis, autism, or autistic spectrum disorders. Some studies also excluded major depressive and other Axis I disorders.1,18,24,32,50
Arguably, epidemiological examination of the prevalence rates of, and associations between, co-occurring conditions would benefit in terms of generalizability and ecological validity if no exclusion criteria were used. This is particularly important in the case of ADHD and OCD, both of which are characterized by a high prevalence of comorbidity with anxiety, tic, and depressive disorders. In this case, the exclusion of depressive or anxiety disorders may bias the reported prevalence rates and limit generalizability. One way to resolve this problem would be to provide a detailed account of the theoretical considerations underlying the selection of exclusion criteria determined for each study. Unfortunately, in the vast majority of the articles we reviewed, no explanations were offered for the choice of exclusion criteria.
In the case of ADHD-OCD co-occurrence, both excluding and failing to exclude particular diagnostic groups may have important consequences for the findings and their interpretation. For example, autistic spectrum disorders are associated with both impulsive and obsessive-compulsive symptoms and are highly comorbid with both ADHD and OCD.135,136 Therefore, including autistic spectrum disorders may inflate co-occurrence rates of ADHD and OCD.
Most importantly, as observed by Sheppard and colleagues,51 few reports have assessed the co-occurrence of ADHD and OCD while excluding tic disorders (i.e., Tourette’s syndrome and chronic tic disorders). Comorbidity between tic disorders and OCD and between tic disorders and ADHD has been consistently found across numerous tic disorder studies, with co-occurrence rates ranging from 25% to 50%.137–140 In addition, very high rates of concomitant ADHD and OCD are regularly identified in tic disorder samples. For example, Lebowitz and colleagues138 examined a sample of 158 youth with chronic tic disorders and found that 24.1% had ADHD and OCD. Grados and Mathews137 found that 34% of a sample of 596 patients with tic disorders had OCD and ADHD. Elevated prevalence rates of ADHD-OCD comorbidity were also reported in first-degree relatives of patients with tic disorders.139
In our review, only four of the pediatric and adolescent samples (12%) excluded tic disorders. Given the high comorbidity of tic disorders with both ADHD and OCD,141,142 along with the elevated co-occurrence of ADHD and OCD in tic disorders,138 inclusion of tic disorders may lead to inflated rates of reported ADHD-OCD comorbidity. A few studies did not exclude tic disorders but did control for their presence when reporting results. For example, Geller and colleagues19 reported the co-occurrence of ADHD and OCD in unaffected relatives beyond that expected by chance, and found that co-occurrence was significant over and above the presence of tic disorders. The authors noted, however, that given the evidence suggesting that tic disorder, ADHD, and OCD often co-occur in families, it is plausible that some shared susceptibility genes account for some features of the three disorders. To our knowledge this study is the only one that specifically examined ADHD-OCD comorbidity while controlling for tic disorders, and more familial and epidemiological research is needed to replicate these findings and to further explore the mediating role of tic disorders in ADHD-OCD comorbidity. It is important to note that the majority of the studies reviewed in this article did not set out to examine ADHD-OCD co-occurrence in particular but typically aimed at examining a variety of general clinical variables or the prevalence of comorbid conditions in either ADHD or OCD.
Adult vs. pediatric populations
Our review of the literature reveals notable differences in ADHD-OCD co-occurrence rates reported in adult versus youth samples. As presented in Tables 2 and 3, considerably higher co-occurrence rates are found in youth samples (unweighted mean = 19%) relative to adult samples (unweighted mean = 9%).* In an attempt to account for this difference, it may be useful to consider neurodevelopmental aspects of pediatric OCD (for a review see Abramovitch et al. [2012]).143 Specifically, pediatric OCD may be neurobiologically distinct from OCD in adolescent and adult populations. As mentioned above, ample evidence suggests the presence of frontostriatal hypermetabolism in adults with OCD, but the same does not seem to hold for young children with OCD.
In a large-scale fMRI study, Fitzgerald and colleagues144 compared the resting-state frontostriatal connectivity of 60 individuals with OCD (age range, 8–40 years) and 61 matched healthy controls. The authors observed significantly increased connectivity in the OCD group relative to controls. In the youngest age group (mean age = 10.7), however, they found reduced functional connectivity that was correlated with OCD symptom severity. These findings may be attributed to abnormal maturational processes in pediatric OCD, which are hypothesized to encompass maturational reorganization, neuronal pruning, and myelination.145
Thus, in contrast to adolescent and adult OCD, pediatric (preadolescence) OCD may be associated with decreased frontostriatal activity, a recognized endophenotypic marker for ADHD that has been consistently identified across the lifespan. As reduced frontostriatal activation is associated with impulsive behavior,146 preadolescent OCD patients may display behaviors and symptoms that may potentially lead to misidentification of OCD as ADHD, consequently inflating the rate of reported comorbidity of the two disorders. This possibility is discussed in more detail below. At present, however, scant research has investigated the maturation and pruning processes in pediatric OCD. In view of the arguments supporting a distinct familial type of ADHD-OCD,19 and given the different developmental trajectories characterizing the two disorders, longitudinal investigation of the neurobiological changes and associated symptoms is much needed.
Gender ratios
The samples we reviewed were highly variable in terms of male:female ratios. Across samples, the percentages of males ranged between 36% and 100%, with a mean of 62% and a standard deviation of 15.5%. Considering the higher prevalence of ADHD in males relative to females, this level of variability may further confound research findings. Among adults and children with ADHD, the ratio of males to females is at least 2:1,147 with some estimates as high as 9:1, depending on ADHD subtype, age range, and sample characteristics.148,149 Whereas the average of 62% males in ADHD samples may be methodologically sound (as it reflects the conservative reports of 2:1 male:female ratio in ADHD), a higher proportion of males than females in OCD samples may artificially inflate the probability of finding cases of ADHD. For example, Farrell and colleagues10 reported that 19% of their sample of children and adolescents diagnosed with OCD met criteria for ADHD. The percentage of males in the OCD-only group was 23%, however, versus 77% in the OCD-comorbidity group, which included ADHD, pervasive developmental disorder, and depression. While matching groups in naturalistic studies is impossible, few of the studies we reviewed have acknowledged this potential bias.
ETIOLOGICAL ACCOUNTS OF ADHD-OCD COMORBIDITY
Concomitant ADHD-OCD as a Familial Subtype
Psychiatric comorbidity research is susceptible to several artifacts, including chance, sampling bias, unique samples that limit generalizability, and definitional overlap.150 For this reason, an etiological account is essential for assessing the legitimacy and validity of any psychiatric comorbidity. To our knowledge, the only etiological account of ADHD-OCD comorbidity was put forth by Geller and colleagues19,20 and is limited to youth.
In the first study of its kind, Geller and colleagues14 examined 121 school-age children and adolescents (67 with ADHD and 54 with comorbid ADHD-OCD). The authors compared the groups on several factors, including DSM-IV ADHD symptoms and clusters, frequency of ADHD subtypes, and several ADHD-related functional indices. The authors observed no differences between the groups on these variables and suggested that ADHD symptoms in OCD represent a genuine comorbidity between ADHD and OCD. In a later study,19 the same group examined a sample of 256 children with ADHD alone, 12 with OCD and ADHD, 235 controls, and 1540 first-degree relatives. They concluded that “(1) relatives of children with ADHD with and without OCD had similar and higher rates of ADHD than did relatives of non-ADHD comparison children, (2) the risk for OCD was elevated only among relatives of ADHD + OCD probands, (3) a strong and significant finding of co-segregation between ADHD and OCD emerged.” The authors also postulated that “in children, ADHD that is comorbid with OCD represents a distinct familial subtype.”19 In other words, according to Geller and colleagues, comorbid ADHD-OCD is a distinct condition in which the two disorders are genetically transmitted together. Notably, and in contrast to the co-occurrence suggested by Geller and colleagues19—the simultaneous presentation of two conditions that are not necessarily correlated in the general population—Lilienfeld and colleagues130 have argued that this type of comorbidity constitutes covariation, in which two conditions co-occur more often than expected by chance.
In sum, to date, only one etiological account has been offered for genuine ADHD-OCD comorbidity in children.19,20 This account postulates a unique and heritable condition consisting of a full-blown comorbidity of ADHD and OCD. To date, no familial studies on adults with ADHD-OCD comorbidity have been published.
The Executive Overload Model of OCD
A potential confounding factor that may contribute to both inflation and variability of reported co-occurrence of ADHD and OCD was recently suggested by Abramovitch and colleagues.126 As noted above, despite the phenomenological and neurobiological differences between ADHD and OCD, both disorders may be associated with some degree of reduced abilities in attention and executive function. Abramovitch and colleagues conducted a direct comparison of neuropsychological functioning of adults with ADHD and adults with OCD. As expected, the authors found that compared to nonpsychiatric controls, both patient groups underperformed on several neuropsychological tasks. At the same time, this study noted significant differences between the disorders in disorder-specific symptoms (e.g., ADHD subjects had higher impulsivity ratings compared to controls, whereas the OCD group did not differ from controls). Perhaps more importantly, neuropsychological underperformance was positively correlated with obsessive-compulsive symptoms in the OCD group but negatively correlated with obsessive-compulsive symptoms in the ADHD group.126 The authors proposed an Executive Overload Model, which focuses on the cognitive “cost” of obsessions in OCD. Specifically, they postulated that the overflow of obsessive thoughts in OCD may overload the executive system, subsequently resulting in neuropsychological deficits. Based on this rationale, the authors suggested that neuropsychological impairments in OCD should be conceived as an epiphenomenon in that they are associated with state-dependent symptom severity.
The Executive Overload Model may have important implications for the observed ADHD-OCD co-occurrence. It suggests that ADHD symptoms in OCD might actually be ADHD-like symptoms; that is, they may be behavioral manifestations of OCD-related neurocognitive impairment. Partial support for this possibility was provided in a another report by the same group,53 where the authors reasoned that if ADHD-like symptoms in OCD are actually a result of OCD-related symptoms, then childhood and adult ADHD symptoms would not be correlated within the OCD group. In support of this hypothesis, the authors found strong significant correlations between childhood and adult ADHD symptoms only in the ADHD and control groups.53
In sum, the Executive Overload Model126 suggests that the comorbidity between the two disorders in some cases may be only apparent and may actually reflect the presence of ADHD-like symptoms in OCD. Such ADHD-like symptoms may be especially pronounced in pediatric populations due to the hypothesized maturational abnormality in young children with OCD. Furthermore, the underlying OCD-related attentional symptoms may be incorrectly interpreted as indications of ADHD, especially in pediatric populations, in which diagnosis relies heavily on informants. Such misdiagnosis may be especially problematic if it leads to stimulant therapy, which may significantly exacerbate OCD symptoms.121,122
DSM Diagnostic Criteria
Criterion E for the diagnosis of ADHD in the DSM-IV requires that “the symptoms do not occur exclusively during the course of a Pervasive Developmental Disorder, Schizophrenia, or other Psychotic Disorder and are not better accounted for by another mental disorder (e.g., Mood Disorder, Anxiety Disorder, Dissociative Disorder or a Personality Disorder).”58 As suggested by Abramovitch and colleagues,126 it is possible that the overflow of obsessive thoughts in individuals with OCD overloads the executive system, leading to behavioral expressions similar to several DSM-IV ADHD criteria (e.g., difficulties in concentration, feeling of restlessness, avoidance of tasks that require cognitive effort, distraction by extraneous stimuli). It has already been noted that DSM-IV ADHD criteria pertaining to restlessness, increased speech, concentration difficulties, hyperactivity, and so on are also symptoms of other anxiety, affective, and personality disorders.151 Thus, behavioral and cognitive expression of OCD-related inattention symptoms may be misidentified as ADHD symptoms, potentially leading to a false DSM-IV diagnosis of ADHD.†
Moreover, the hallmark symptoms of ADHD (i.e., impulsivity) and OCD (i.e., compulsivity) are phenomenologically opposite. Compulsions are typically rituals that are carefully planned and repetitively performed according to rigid rules. In addition, clinical experience indicates that OCD patients are able to inhibit or postpone compulsive rituals (e.g., at work). Behavioral impulsivity, by contrast, is characterized by actions that are performed without forethought and with little ability to inhibit or postpone them. As noted above, several studies observed equal or decreased behavioral impulsivity in OCD patients relative to healthy controls. Indeed, as noted by Angold and colleagues,153 a paucity of research has investigated the differential characteristics of symptoms between comorbid disorders in general; that is, research examining disorder-specific versus shared symptoms between comorbid conditions is limited. For example, while patients with either ADHD or OCD appear to present symptoms of inattention, some aspects of that inattention that may be disorder-specific, and their identification would increase diagnostic accuracy. Future research into the differential characteristics of shared ADHD and OCD symptoms may aid in untangling this complex issue.
SUMMARY
OCD and ADHD are very different disorders in terms of pathophysiology, phenomenology, and treatment strategies (see Table 1). Numerous studies, however, have reported significant rates of ADHD-OCD co-occurrence. Increased co-occurrence rates were found in children and adolescents as compared to adults. In this review, we documented extreme variability across studies, with co-occurrence rates ranging from 0% to 60%. An attempt to provide an etiological account for ADHD-OCD comorbidity, primarily genetic in nature, was made only with regard to children.19 Few studies directly examined co-occurrence between ADHD and OCD in adults. Much more research is required to validate ADHD-OCD co-occurrence, let alone comorbidity, in this population. Specifically, more research is required to examine false comorbidity versus covariation of ADHD and OCD; such clarification would have important clinical implications.
Several methodological concerns have been identified in our review. First, we found gender proportions to vary significantly between samples, which may result in a bias in prevalence reports, as ADHD is more frequent in males. Second, most studies recruited patients from specialized clinics rather than from the community or representative samples. Given that few studies examined representative samples and that significantly higher rates of ADHD-OCD co-occurrence have been reported in samples recruited from specialized clinics, our understanding of the prevalence of co-occurring ADHD and OCD may be largely based on biased results. Third, we identified high variability in terms of exclusion criteria between samples. Most critically, the great majority of the studies did not exclude or control for tic disorders, a mediator and potential confound for ADHD-OCD co-occurrence.
As noted by Pennington and colleagues,150 a theoretical and etiological account is essential to validate a true comorbidity between disorders. Only one etiological account for ADHD-OCD comorbidity, which postulates a distinct heritable ADHD + OCD condition, is currently available.19 Finally, a potential confounding factor that received little attention is addressed by the Executive Overload Model offered by Abramovitch and colleagues.126 That model suggests that deficits in attention and executive function may be state dependent in OCD, resulting in a subsequent phenotypical expression that may resemble ADHD symptoms.
We suggest that future studies on ADHD-OCD comorbidity should strive to mitigate the biases and potential confounds elaborated above. Future studies should aim to address the potential bias associated with gender proportions and might be also advised to consider age stratification. Additionally, examination of ADHD-OCD in community samples is necessary to balance the majority of reports, which recruited participants via specialty clinics. Recruitment methodology ought to be more carefully considered in light of the known effects of the Berksonian and clinical-selection biases. If aggregation of various recruitment methods is necessary, future research ought to differentiate between cohorts recruited from different sources. Researchers are advised to carefully consider exclusion criteria when examining ADHD-OCD comorbidity, and should provide justifications for these criteria. Specifically, the control or exclusion of tic disorders ought to be carefully assessed due to their possible mediating effect on the estimation of ADHD-OCD co-occurrence. Moreover, according to the Executive Overload Model, ADHD-like symptoms may be present in OCD. Both clinicians and researchers ought to pay careful attention to this issue, especially in cases where comorbid tic disorders are identified.
The paucity of knowledge regarding ADHD-OCD comorbidity in adults needs to be addressed through further research. Our review underlines the need for more studies in this population, especially investigations into community samples. Additionally, prospective longitudinal studies are required that would examine the developmental trajectory of ADHD-OCD comorbidity. Such studies would be invaluable in elucidating factors differentiating ADHD-like symptoms in OCD from genuine comorbidity between the two disorders. Finally, the ultimate aim of research on psychiatric comorbidity is to further our understanding of complex conditions so as to advance treatment and prognosis.154 Along these lines, the first aim of the present investigation was to establish if ADHD and OCD are genuinely comorbid. The conclusion would have critical implications for treatment, especially in view of the well-known efficacy of treatment options for the two disorders (considered separately)—namely, stimulant medication (and possibly CBT) for ADHD,155,156 and CBT and SRIs for OCD.157 Several authors have suggested that individuals with comorbid ADHD-OCD are more functionally impaired. And if such comorbidity proves to be as common as some studies suggest, it is imperative to determine effective treatments for this complex condition.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgments
The authors would like to thank Drs. Janardhan Reddy, Adam Lewin, and Alice de Mathis for providing us with valuable information and clarifications.
* The nature of the reviewed samples does not allow for statistical analyses assessing differences between samples. Thus, when using terms such as higher or lower, we do not imply statistical significance.
† We have omitted discussion of DSM-5 in this review for two primary reasons. First, the review pertains to studies that employed DSM-IV or DSM-III. Second, DSM-V changes to ADHD criteria pertain to the hyperactivity/impulsivity symptom cluster and do not apply to the inattention cluster. We should also note that according to a recent analysis,152 these changes may potentially reduce diagnostic specificity and inflate diagnosis rates of ADHD, further complicating the problem presented here.
Original manuscript received 11 March 2014, accepted for publication subject to revision 19 May 2014; revised manuscript received 13 June 2014.
REFERENCES
- 1. Hanna GL. Demographic and clinical features of obsessive-compulsive disorder in children and adolescents. J Am Acad Child Adolesc Psychiatry 1995; 34: 19– 27. [DOI] [PubMed] [Google Scholar]
- 2. Toro J, Cervera M, Osejo E, Salamero M. Obsessive-compulsive disorder in childhood and adolescence: a clinical study. J Child Psychol Psychiatry 1992; 33: 1025– 37. [DOI] [PubMed] [Google Scholar]
- 3. Riddle MA, Scahill L, King R, et al. Obsessive compulsive disorder in children and adolescents: phenomenology and family history. J Am Acad Child Adolesc Psychiatry 1990; 29: 766– 72. [DOI] [PubMed] [Google Scholar]
- 4. Thomsen PH. Obsessive-compulsive disorder in children and adolescents: a review of the literature. Eur Child Adoles Psy 1994; 3: 138– 58. [DOI] [PubMed] [Google Scholar]
- 5. Zohar AH. The epidemiology of obsessive-compulsive disorder in children and adolescents. Child Adol Psych Cl 1999; 8: 445– 60. [PubMed] [Google Scholar]
- 6. Biederman J, Monuteaux MC, Mick E, et al. Young adult outcome of attention deficit hyperactivity disorder: a controlled 10-year follow-up study. Psychol Med 2006; 36: 167– 79. [DOI] [PubMed] [Google Scholar]
- 7. Meijer WM, Faber A, van den Ban E, Tobi H. Current issues around the pharmacotherapy of ADHD in children and adults. Pharm World Sci 2009; 31: 509– 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carter AS, Wagmiller RJ, Gray SA, McCarthy KJ, Horwitz SM, Briggs-Gowan MJ. Prevalence of DSM-IV disorder in a representative, healthy birth cohort at school entry: sociodemographic risks and social adaptation. J Am Acad Child Adolesc Psychiatry 2010; 49: 686– 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Farrell L, Barrett P. Obsessive-compulsive disorder across developmental trajectory: cognitive processing of threat in children, adolescents and adults. British J Psychol 2006; 97: 95– 114. [DOI] [PubMed] [Google Scholar]
- 10. Farrell L, Waters A, Milliner E, Ollendick TH. Comorbidity and treatment response in pediatric OCD: a pilot study of group cognitive-behavioral treatment. Psychiatry Res 2012. [DOI] [PubMed] [Google Scholar]
- 11. Garcia AM, Freeman JB, Himle MB, et al. Phenomenology of early childhood onset obsessive compulsive disorder. J Psychopathol Behav Assess 2009; 31: 104– 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Geller DA, Biederman J, Faraone SV, et al. Developmental aspects of obsessive compulsive disorder: findings in children, adolescents, and adults. J Nerv Ment Dis 2001; 189: 471– 7. [DOI] [PubMed] [Google Scholar]
- 13. Geller DA, Biederman J, Faraone SV, et al. Re-examining comorbidity of obsessive compulsive and attention-deficit hyperactivity disorder using an empirically derived taxonomy. Eur Child Adoles Psy 2004; 13: 83– 91. [DOI] [PubMed] [Google Scholar]
- 14. Geller DA, Biederman J, Faraone SV, et al. Attention-deficit/hyperactivity disorder in children and adolescents with obsessive-compulsive disorder: fact or artifact? J Am Acad Child Adolesc Psychiatry 2002; 41: 52– 8. [DOI] [PubMed] [Google Scholar]
- 15. Geller DA, Biederman J, Faraone SV, et al. Clinical correlates of obsessive compulsive disorder in children and adolescents referred to specialized and non-specialized clinical settings. Depress Anxiety 2000; 11: 163– 8. [DOI] [PubMed] [Google Scholar]
- 16. Geller DA, Biederman J, Griffin S, Jones J, Lefkowitz TR. Comorbidity of juvenile obsessive-compulsive disorder with disruptive behavior disorders. J Am Acad Child Adolesc Psychiatry 1996; 35: 1637– 46. [DOI] [PubMed] [Google Scholar]
- 17. Geller DA, Biederman J, Reed ED, Spencer T, Wilens TE. Similarities in response to fluoxetine in the treatment of children and adolescents with obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry 1995; 34: 36– 44. [DOI] [PubMed] [Google Scholar]
- 18. Geller DA, Coffey B, Faraone SV, et al. Does comorbid attention-deficit/hyperactivity disorder impact the clinical expression of pediatric obsessive-compulsive disorder? CNS Spectrums 2003; 8: 259– 64. [DOI] [PubMed] [Google Scholar]
- 19. Geller DA, Petty C, Vivas F, Johnson J, Pauls D, Biederman J. Examining the relationship between obsessive-compulsive disorder and attention-deficit/hyperactivity disorder in children and adolescents: a familial risk analysis. Biol Psychiatry 2007; 61: 316– 21. [DOI] [PubMed] [Google Scholar]
- 20. Geller DA, Petty C, Vivas F, Johnson J, Pauls D, Biederman J. Further evidence for co-segregation between pediatric obsessive compulsive disorder and attention deficit hyperactivity disorder: a familial risk analysis. Biol Psychiatry 2007; 61: 1388– 94. [DOI] [PubMed] [Google Scholar]
- 21. Ghanizadeh A. Comorbidity of enuresis in children with attention-deficit/hyperactivity disorder. J Atten Disord 2010; 13: 464– 7. [DOI] [PubMed] [Google Scholar]
- 22. Heyman I, Fombonne E, Simmons H, Ford T, Meltzer H, Goodman R. Prevalence of obsessive-compulsive disorder in the British nationwide survey of child mental health. Br J Psychiatry 2001; 179: 324– 9. [DOI] [PubMed] [Google Scholar]
- 23. Ivarsson T, Melin K, Wallin L. Categorical and dimensional aspects of co-morbidity in obsessive-compulsive disorder (OCD). Eur Child Adolesc Psychiatry 2008; 17: 20– 31. [DOI] [PubMed] [Google Scholar]
- 24. Jaisoorya TS, Janardhan Reddy YC, Srinath S. Is juvenile obsessive-compulsive disorder a developmental subtype of the disorder?–Findings from an Indian study. Eur Child Adolesc Psychiatry 2003; 12: 290– 7. [DOI] [PubMed] [Google Scholar]
- 25. Joshi G, Wozniak J, Petty C, et al. Clinical characteristics of comorbid obsessive-compulsive disorder and bipolar disorder in children and adolescents. Bipolar Disord 2010; 12: 185– 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lack CW, Storch EA, Keeley ML, et al. Quality of life in children and adolescents with obsessive-compulsive disorder: base rates, parent-child agreement, and clinical correlates. Soc Psychiatry Psychiatr Epidemiol 2009; 44: 935– 42. [DOI] [PubMed] [Google Scholar]
- 27. Langley AK, Lewin AB, Bergman RL, Lee JC, Piacentini J. Correlates of comorbid anxiety and externalizing disorders in childhood obsessive compulsive disorder. Eur Child Adolesc Psychiatry 2010; 19: 637– 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lewin AB, Chang S, McCracken J, McQueen M, Piacentini J. Comparison of clinical features among youth with tic disorders, obsessive-compulsive disorder (OCD), and both conditions. Psychiatry Res 2010; 178: 317– 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mancebo MC, Garcia AM, Pinto A, et al. Juvenile-onset OCD: clinical features in children, adolescents and adults. Acta Psychiatr Scand 2008; 118: 149– 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Masi G, Millepiedi S, Mucci M, Bertini N, Pfanner C, Arcangeli F. Comorbidity of obsessive-compulsive disorder and attention-deficit/hyperactivity disorder in referred children and adolescents. Compr Psychiatry 2006; 47: 42– 7. [DOI] [PubMed] [Google Scholar]
- 31. Skirbekk B, Hansen BH, Oerbeck B, Kristensen H. The relationship between sluggish cognitive tempo, subtypes of attention-deficit/hyperactivity disorder, and anxiety disorders. J Abnorm Child Psychol 2011; 39: 513– 25. [DOI] [PubMed] [Google Scholar]
- 32. Storch EA, Lack CW, Merlo LJ, et al. Clinical features of children and adolescents with obsessive-compulsive disorder and hoarding symptoms. Compr Psychiatry 2007; 48: 313– 8. [DOI] [PubMed] [Google Scholar]
- 33. Storch EA, Merlo LJ, Larson MJ, et al. Impact of comorbidity on cognitive-behavioral therapy response in pediatric obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry 2008; 47: 583– 92. [DOI] [PubMed] [Google Scholar]
- 34. Walitza S, Zellmann H, Irblich B, et al. Children and adolescents with obsessive-compulsive disorder and comorbid attention-deficit/hyperactivity disorder: preliminary results of a prospective follow-up study. J Neural Transm 2008; 115: 187– 90. [DOI] [PubMed] [Google Scholar]
- 35. Anholt GE, Cath DC, van Oppen P, et al. Autism and ADHD symptoms in patients with OCD: are they associated with specific OC symptom dimensions or OC symptom severity? J Autism Dev Disord 2010; 40: 580– 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Coskun M, Zoroglu S, Ozturk M. Phenomenology, psychiatric comorbidity and family history in referred preschool children with obsessive-compulsive disorder. Child Adolesc Psychiatry Ment Health 2012; 6: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. de Mathis MA, Diniz J, Hounie A, et al. Trajectory in obsessive-compulsive disorder comorbidities. Eur Neuropsychopharmacol 2013; 23: 594– 601. [DOI] [PubMed] [Google Scholar]
- 38. Frost RO, Steketee G, Tolin DF. Comorbidity in hoarding disorder. Depress Anxiety 2011; 28: 876– 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Geller D, Wieland N, Carey K, et al. Perinatal factors affecting expression of obsessive compulsive disorder in children and adolescents. J Child Adolesc Psychopharmacol 2008; 18: 373– 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee SS, Falk AE, Aguirre VP. Association of comorbid anxiety with social functioning in school-age children with and without attention-deficit/hyperactivity disorder (ADHD). Psychiatry Res 2012; 197: 90– 6. [DOI] [PubMed] [Google Scholar]
- 41. Lewin A, Wood J, Gunderson S, Murphy T, Storch E. Phenomenology of comorbid autism spectrum and obsessive-compulsive disorders among children. J Dev Phys Disabil 2011; 23: 543– 53. [Google Scholar]
- 42. Moll GH, Eysenbach K, Woerner W, Banaschewski T, Schmidt MH, Rothenberger A. Quantitative and qualitative aspects of obsessive-compulsive behaviour in children with attention-deficit hyperactivity disorder compared with tic disorder. Acta Psychiatr Scand 2000; 101: 389– 94. [DOI] [PubMed] [Google Scholar]
- 43. Storch EA, Lewin AB, Geffken GR, Morgan JR, Murphy TK. The role of comorbid disruptive behavior in the clinical expression of pediatric obsessive-compulsive disorder. Behav Res Ther 2010; 48: 1204– 10. [DOI] [PubMed] [Google Scholar]
- 44. Biederman J, Faraone SV, Monuteaux MC, Bober M, Cadogen E. Gender effects on attention-deficit/hyperactivity disorder in adults, revisited. Biol Psychiatry 2004; 55: 692– 700. [DOI] [PubMed] [Google Scholar]
- 45. Brakoulias V, Starcevic V, Sammut P, et al. Obsessive-compulsive spectrum disorders: a comorbidity and family history perspective. Australas Psychiatry 2011; 19: 151– 5. [DOI] [PubMed] [Google Scholar]
- 46. Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry 2006; 163: 716– 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mannuzza S, Klein RG, Bessler A, Malloy P, LaPadula M. Adult psychiatric status of hyperactive boys grown up. Am J Psychiatry 1998; 155: 493– 8. [DOI] [PubMed] [Google Scholar]
- 48. Millstein RB, Wilens TE, Biederman J, Spencer T. Presenting ADHD symptoms and subtypes in clinically referred adults with ADHD. J Atten Disord 1997; 2: 159– 66. [Google Scholar]
- 49. Ruscio A, Stein D, Chiu W, Kessler R. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry 2010; 15: 53– 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shekim WO, Asarnow RF, Hess E, Zaucha K, Wheeler N. A Clinical and demographic profile of a sample of adults with attention-deficit hyperactivity disorder, residual state. Compr Psychiatry 1990; 31: 416– 25. [DOI] [PubMed] [Google Scholar]
- 51. Sheppard B, Chavira D, Azzam A, et al. ADHD prevalence and association with hoarding behaviors in childhood-onset OCD. Depress Anxiety 2010; 27: 667– 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wilens TE, Biederman J, Faraone SV, Martelon M, Westerberg D, Spencer TJ. Presenting ADHD symptoms, subtypes, and comorbid disorders in clinically referred adults with ADHD. J Clin Psychiatry 2009; 70: 1557– 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Abramovitch A, Dar R, Mittelman A, Schweiger A. Don’t judge a book by its cover: ADHD-like symptoms in obsessive-compulsive disorder. J Obsessive Compuls Relat Disord 2013; 2: 53– 61. [Google Scholar]
- 54. Biederman J. Attention-deficit/hyperactivity disorder: a selective overview. Biol Psychiatry 2005; 57: 1215– 20. [DOI] [PubMed] [Google Scholar]
- 55. Nicolini H, Arnold P, Nestadt G, Lanzagorta N, Kennedy JL. Overview of genetics and obsessive-compulsive disorder. Psychiatry Res 2009; 170: 7– 14. [DOI] [PubMed] [Google Scholar]
- 56. Pauls DL. The genetics of obsessive compulsive disorder: a review of the evidence. Am J Med Genet C Semin Med Genet 2008; 148C: 133– 9. [DOI] [PubMed] [Google Scholar]
- 57. Franke B, Faraone SV, Asherson P, et al. The genetics of attention deficit/hyperactivity disorder in adults, a review. Mol Psychiatry 2012; 17: 960– 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed, text rev. Washington, DC: APA, 2000. [Google Scholar]
- 59. Barkley RA. International consensus statement on ADHD. January 2002. Clin Child Fam Psychol Rev 2002; 5: 89– 111. [DOI] [PubMed] [Google Scholar]
- 60. Alonso P, Menchon JM, Jimenez S, et al. Personality dimensions in obsessive-compulsive disorder: relation to clinical variables. Psychiatry Res 2008; 157: 159– 68. [DOI] [PubMed] [Google Scholar]
- 61. Salkovskis PM. Obsessional-compulsive problems—a cognitive-behavioral analysis. Behav Res Ther 1985; 23: 571– 83. [DOI] [PubMed] [Google Scholar]
- 62. Szechtman H, Woody E. Obsessive-compulsive disorder as a disturbance of security motivation. Psychol Rev 2004; 111: 111– 27. [DOI] [PubMed] [Google Scholar]
- 63. Coll CG, Kagan J, Reznick JS. Behavioral-inhibition in young-children. Child Dev 1984; 55: 1005– 19. [Google Scholar]
- 64. Vaidya CJ, Austin G, Kirkorian G, et al. Selective effects of methylphenidate in attention deficit hyperactivity disorder: a functional magnetic resonance study. Proc Natl Acad Sci U S A 1998; 95: 14494– 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fullana MA, Mataix-Cols D, Caseras X, et al. High sensitivity to punishment and low impulsivity in obsessive-compulsive patients with hoarding symptoms. Psychiatry Res 2004; 129: 21– 7. [DOI] [PubMed] [Google Scholar]
- 66. Fullana MA, Mataix-Cols D, Trujillo Jl, et al. Personality characteristics in obsessive-compulsive disorder and individuals with subclinical obsessive-compulsive problems. Br J Clin Psychol 2004; 43: 387– 98. [DOI] [PubMed] [Google Scholar]
- 67. Shoval G, Zalsman G, Sher L, Apter A, Weizman A. Clinical characteristics of inpatient adolescents with severe obsessive-compulsive disorder. Depress Anxiety 2006; 23: 62– 70. [DOI] [PubMed] [Google Scholar]
- 68. Wu KD, Clark LA, Watson D. Relations between obsessive-compulsive disorder and personality: beyond Axis I–Axis II comorbidity. J Anxiety Disord 2006; 20: 695– 717. [DOI] [PubMed] [Google Scholar]
- 69. Hollander E. Obsessive-compulsive disorder and spectrum across the life span. Int J Psychiatry Clin Pract 2005; 9: 79– 86. [DOI] [PubMed] [Google Scholar]
- 70. Carlsson ML. On the role of prefrontal cortex glutamate for the antithetical phenomenology of obsessive compulsive disorder and attention deficit hyperactivity disorder. Prog Neuropsychopharmacol Biol Psychiatry 2001; 25: 5– 26. [DOI] [PubMed] [Google Scholar]
- 71. Willcutt EG. The prevalence of DSM-IV attention-deficit/hyperactivity disorder: a meta-analytic review. Neurotherapeutics 2012; 9: 490– 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Pujol J, Soriano-Mas C, Alonso P, et al. Mapping structural brain alterations in obsessive-compulsive disorder. Arch Gen Psychiatry 2004; 61: 720– 30. [DOI] [PubMed] [Google Scholar]
- 73. Bush G, Valera EM, Seidman LJ. Functional neuroimaging of attention-deficit/hyperactivity disorder: a review and suggested future directions. Biol Psychiatry 2005; 57: 1273– 84. [DOI] [PubMed] [Google Scholar]
- 74. Dickstein SG, Bannon K, Xavier Castellanos F, Milham MP. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. J Child Psychol Psyc 2006; 47: 1051– 62. [DOI] [PubMed] [Google Scholar]
- 75. Baxter LR. Neuroimaging studies of obsessive compulsive disorder. Psychiatr Clin North Am 1992; 15: 871– 84. [PubMed] [Google Scholar]
- 76. Harrison BJ, Soriano-Mas C, Pujol J, et al. Altered corticostriatal functional connectivity in obsessive-compulsive disorder. Arch Gen Psychiatry 2009; 66: 1189– 200. [DOI] [PubMed] [Google Scholar]
- 77. Saxena S, Rauch SL. Functional neuroimaging and the neuroanatomy of obsessive-compulsive disorder. Psychiatr Clin North Am 2000; 23: 563– 86. [DOI] [PubMed] [Google Scholar]
- 78. van den Heuvel OA, Veltman DJ, Groenewegen HJ, et al. Frontal-striatal dysfunction during planning in obsessive-compulsive disorder. Arch Gen Psychiatry 2005; 62: 301– 9. [DOI] [PubMed] [Google Scholar]
- 79. Mataix-Cols D, Cullen S, Lange K, et al. Neural correlates of anxiety associated with obsessive-compulsive symptom dimensions in normal volunteers. Biol Psychiatry 2003; 53: 482– 93. [DOI] [PubMed] [Google Scholar]
- 80. Whiteside SP, Port JD, Abramowitz JS. A meta-analysis of functional neuroimaging in obsessive-compulsive disorder. Psychiatry Res 2004; 132: 69– 79. [DOI] [PubMed] [Google Scholar]
- 81. Sakai Y, Narumoto J, Nishida S, et al. Corticostriatal functional connectivity in non-medicated patients with obsessive-compulsive disorder. Eur Psychiatry 2011; 26: 463– 9. [DOI] [PubMed] [Google Scholar]
- 82. Zhang TJ, Wang JH, Yang YC, et al. Abnormal small-world architecture of top-down control networks in obsessive-compulsive disorder. J Psychiatry Neurosci 2011; 36: 23– 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Cubillo A, Rubia K. Structural and functional brain imaging in adult attention-deficit/hyperactivity disorder. Expert Rev Neurother 2010; 10: 603– 20. [DOI] [PubMed] [Google Scholar]
- 84. Wolf RC, Plichta MM, Sambataro F, et al. Regional brain activation changes and abnormal functional connectivity of the ventrolateral prefrontal cortex during working memory processing in adults with attention-deficit/hyperactivity disorder. Hum Brain Mapp 2009; 30: 2252– 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Cubillo A, Halari R, Ecker C, Giampietro V, Taylor E, Rubia K. Reduced activation and inter-regional functional connectivity of fronto-striatal networks in adults with childhood attention-deficit hyperactivity disorder (ADHD) and persisting symptoms during tasks of motor inhibition and cognitive switching. J Psychiatr Res 2010; 44: 629– 39. [DOI] [PubMed] [Google Scholar]
- 86. Konrad K, Eickhoff SB. Is the ADHD brain wired differently? A review on structural and functional connectivity in attention deficit hyperactivity disorder. Hum Brain Mapp 2010; 31: 904– 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Rubia K, Smith AB, Halari R, et al. Disorder-specific dissociation of orbitofrontal dysfunction in boys with pure conduct disorder during reward and ventrolateral prefrontal dysfunction in boys with pure ADHD during sustained attention. Am J Psychiatry 2009; 166: 83– 94. [DOI] [PubMed] [Google Scholar]
- 88. Rubia K, Cubillo A, Woolley J, Brammer MJ, Smith A. Disorder-specific dysfunctions in patients with attention-deficit/hyperactivity disorder compared to patients with obsessive-compulsive disorder during interference inhibition and attention allocation. Hum Brain Mapp 2011; 32: 601– 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zametkin AJ, Liebenauer LL, Fitzgerald GA, et al. Brain metabolism in teenagers with attention-deficit hyperactivity disorder. Arch Gen Psychiatry 1993; 50: 333– 40. [DOI] [PubMed] [Google Scholar]
- 90. Zametkin AJ, Nordahl TE, Gross M, et al. Cerebral glucose metabolism in adults with hyperactivity of childhood onset. N Engl J Med 1990; 323: 1361– 6. [DOI] [PubMed] [Google Scholar]
- 91. Bush G. Cingulate, frontal, and parietal cortical dysfunction in attention-deficit/hyperactivity disorder. Biol Psychiatry 2011; 69: 1160– 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lacerda AL, Dalgalarrondo P, Caetano D, Haas GL, Camargo EE, Keshavan MS. Neuropsychological performance and regional cerebral blood flow in obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry 2003; 27: 657– 65. [DOI] [PubMed] [Google Scholar]
- 93. Maltby N, Tolin DF, Worhunsky P, O’Keefe TM, Kiehl KA. Dysfunctional action monitoring hyperactivates frontal-striatal circuits in obsessive-compulsive disorder: an event-related fMRI study. Neuroimage 2005; 24: 495– 503. [DOI] [PubMed] [Google Scholar]
- 94. Mataix-Cols D, Wooderson S, Lawrence N, Brammer MJ, Speckens A, Phillips ML. Distinct neural correlates of washing, checking, and hoarding symptom dimensions in obsessive-compulsive disorder. Arch Gen Psychiatry 2004; 61: 564– 76. [DOI] [PubMed] [Google Scholar]
- 95. Abramovitch A, Abramowitz JS, Mittelman A. The neuropsychology of adult obsessive-compulsive disorder: a meta-analysis. Clin Psychol Rev 2013; 33: 1163– 71. [DOI] [PubMed] [Google Scholar]
- 96. Abramovitch A, Dar R, Schweiger A, Hermesh H. Neuropsychological impairments and their association with obsessive-compulsive symptom severity in obsessive-compulsive disorder. Arch Clin Neuropsychol 2011; 26: 364– 76. [DOI] [PubMed] [Google Scholar]
- 97. Chamberlain SR, Blackwell AD, Fineberg NA, Robbins TW, Sahakian BJ. The neuropsychology of obsessive compulsive disorder: the importance of failures in cognitive and behavioural inhibition as candidate endophenotypic markers. Neurosci Biobehav R 2005; 29: 399– 419. [DOI] [PubMed] [Google Scholar]
- 98. Chamberlain SR, Fineberg NA, Blackwell AD, Robbins TW, Sahakian BJ. Motor inhibition and cognitive flexibility in obsessive-compulsive disorder and trichotillomania. Am J Psychiatry 2006; 163: 1282– 4. [DOI] [PubMed] [Google Scholar]
- 99. Penades R, Catalan R, Rubia K, Andres S, Salamero M, Gasto C. Impaired response inhibition in obsessive compulsive disorder. Eur Psychiatry 2007; 22: 404– 10. [DOI] [PubMed] [Google Scholar]
- 100. Kuelz AK, Hohagen F, Voderholzer U. Neuropsychological performance in obsessive-compulsive disorder: a critical review. Biol Psychol 2004; 65: 185– 236. [DOI] [PubMed] [Google Scholar]
- 101. Schoechlin C, Engel RR. Neuropsychological performance in adult attention-deficit hyperactivity disorder: Meta-analysis of empirical data. Arch Clin Neuropsychol 2005; 20: 727– 44. [DOI] [PubMed] [Google Scholar]
- 102. Frazier TW, Demaree HA, Youngstrom EA. Meta-analysis of intellectual and neuropsychological test performance in attention-deficit/hyperactivity disorder. Neuropsychology 2004; 18: 543– 55. [DOI] [PubMed] [Google Scholar]
- 103. Huang-Pollack C, Karalunas S, Tam H, Moore A. Evaluating vigilance deficits in ADHD: a meta-analysis of CPT performance. J Abnorm Psychol 2012; 121: 360– 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Penades R, Catalan R, Andres S, Salamero M, Gasto C. Executive function and nonverbal memory in obsessive-compulsive disorder. Psychiatry Res 2005; 133: 81– 90. [DOI] [PubMed] [Google Scholar]
- 105. Segalas C, Alonso P, Labad J, et al. Verbal and nonverbal memory processing in patients with obsessive-compulsive disorder: Its relationship to clinical variables. Neuropsychology 2008; 22: 262– 72. [DOI] [PubMed] [Google Scholar]
- 106. Cherkasova MV, Hechtman L. Neuroimaging in attention-deficit hyperactivity disorder: beyond the frontostriatal circuitry. Can J Psychiatry 2009; 54: 651– 64. [DOI] [PubMed] [Google Scholar]
- 107. Seidman LJ, Doyle A, Fried R, Valera E, Crum K, Matthews L. Neuropsychological function in adults with attention-deficit/hyperactivity disorder. Psychiatr Clin North Am 2004; 27: 261– 82. [DOI] [PubMed] [Google Scholar]
- 108. Tamm L, Menon V, Reiss AL. Maturation of brain function associated with response inhibition. J Am Acad Child Adolesc Psychiatry 2002; 41: 1231– 8. [DOI] [PubMed] [Google Scholar]
- 109. Faraone SV, Spencer T, Aleardi M, Pagano C, Biederman J. Meta-analysis of the efficacy of methylphenidate for treating adult attention-deficit/hyperactivity disorder. J Clin Psychopharmacol 2004; 24: 24– 9. [DOI] [PubMed] [Google Scholar]
- 110. Gibbins C, Weiss M. Clinical recommendations in current practice guidelines for diagnosis and treatment of ADHD in adults. Curr Psychiatry Rep 2007; 9: 420– 6. [DOI] [PubMed] [Google Scholar]
- 111. Bush G, Spencer T, Holmes J, et al. Functional magnetic resonance imaging of methylphenidate and placebo in attention-deficit/hyperactivity disorder during the multi-source interference task. Arch Gen Psychiatry 2008; 65: 102– 14. [DOI] [PubMed] [Google Scholar]
- 112. Volkow ND, Wang GJ, Fowler JS, Ding YS. Imaging the effect of methylphenidate on brain dopamine: new model on its therapeutic actions for attention-deficit/hyperactivity disorder. Biol Psychiatry 2005; 57: 1410– 5. [DOI] [PubMed] [Google Scholar]
- 113. Bandelow B, Zohar J, Hollander E, Kasper S, Moller H-J. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the pharmacological treatment of anxiety, obsessive-compulsive and posttraumatic stress disorders. World J Biol Psychia 2002; 3: 171– 99. [DOI] [PubMed] [Google Scholar]
- 114. March JS, Frances A, Kahn DA, Carpenter D, eds. The Expert Consensus Guideline Series: treatment of obsessive-compulsive disorder. J Clin Psychiatry 1997; 58. [Google Scholar]
- 115. Freyer T, Kloppel S, Tuscher O, et al. Frontostriatal activation in patients with obsessive-compulsive disorder before and after cognitive behavioral therapy. Psychol Med 2011; 41: 207– 16. [DOI] [PubMed] [Google Scholar]
- 116. Nakao T, Nakagawa A, Yoshiura T, et al. Brain activation of patients with obsessive-compulsive disorder during neuropsychological and symptom provocation tasks before and after symptom improvement: a functional magnetic resonance imaging study. Biol Psychiatry 2005; 57: 901– 10. [DOI] [PubMed] [Google Scholar]
- 117. Szechtman H, Eckert MJ, Tse WS, et al. Compulsive checking behavior of quinpirole-sensitized rats as an animal model of obsessive-compulsive disorder (OCD): form and control. BMC Neurosci 2001; 2: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Zadicario P, Ronen S, Eilam D. Modulation of quinpirole-induced compulsive-like behavior in rats by environmental changes: implications for OCD rituals and for exploration and navigation. BMC Neurosci 2007; 8: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Szechtman H, Sulis W, Eilam D. Quinpirole induces compulsive checking behavior in rats: a potential animal model of obsessive-compulsive disorder (OCD). Behav Neurosci 1998; 112: 1475– 85. [DOI] [PubMed] [Google Scholar]
- 120. Fineberg NA, Brown A, Reghunandanan S, Pampaloni I. Evidence-based pharmacotherapy of obsessive-compulsive disorder. Int J Neuropsychopharmacol 2012; 1–19. [DOI] [PubMed] [Google Scholar]
- 121. Koizumi HM. Obsessive-compulsive symptoms following stimulants. Biol Psychiatry 1985; 20: 1332– 3. [DOI] [PubMed] [Google Scholar]
- 122. Kouris S. Methylphenidate-induced obsessive-compulsiveness. J Am Acad Child Adolesc Psychiatry 1998; 37: 135. [DOI] [PubMed] [Google Scholar]
- 123. Serby M. Methylphenidate-induced obsessive-compulsive symptoms in an elderly man. CNS Spectr 2003; 8: 612– 3. [DOI] [PubMed] [Google Scholar]
- 124. Woolley JB, Heyman I. Dexamphetamine for obsessive-compulsive disorder. Am J Psychiatry 2003; 160: 183. [DOI] [PubMed] [Google Scholar]
- 125. Bloch MH, Landeros-Weisenberger A, Kelmendi B, Coric V, Bracken MB, Leckman JF. A systematic review: antipsychotic augmentation with treatment refractory obsessive-compulsive disorder. Mol Psychiatry 2006; 11: 622– 32. [DOI] [PubMed] [Google Scholar]
- 126. Abramovitch A, Dar R, Hermesh H, Schweiger A. Comparative neuropsychology of adult obsessive-compulsive disorder and attention deficit/hyperactivity disorder: implications for a novel executive overload model of OCD. J Neuropsychol 2012; 6: 161– 91. [DOI] [PubMed] [Google Scholar]
- 127. Canavera KE, Ollendick TH, May JT, Pincus DB. Clinical correlates of comorbid obsessive-compulsive disorder and depression in youth. Child Psychiatry Hum Dev 2010; 41: 583– 94. [DOI] [PubMed] [Google Scholar]
- 128. Zohar AH, Ratzoni G, Pauls DL, et al. An epidemiological study of obsessive-compulsive disorder and related disorders in Israeli adolescents. J Am Acad Child Adolesc Psychiatry 1992; 31: 1057– 61. [DOI] [PubMed] [Google Scholar]
- 129. Ghanizadeh A. Predictors of different types of developmental coordination problems in ADHD: the effect of age, gender, ADHD symptom severity and comorbidities. Neuropediatrics 2010; 41: 176– 81. [DOI] [PubMed] [Google Scholar]
- 130. Lilienfeld SO, Waldman ID, Israel AC. A critical examination of the use of the term and concept of comorbidity in psychopathology research. Clin Psychol 1994; 1: 71– 83. [Google Scholar]
- 131. McConaughy SH, Achenbach TM. Comorbidity of empirically based syndromes in matched general population and clinical samples. J Child Psychol Psychiatry 1994; 35: 1141– 57. [DOI] [PubMed] [Google Scholar]
- 132. Lilienfeld SO. Comorbidity between and within childhood externalizing and internalizing disorders: reflections and directions. J Abnorm Child Psychol 2003; 31: 285– 91. [DOI] [PubMed] [Google Scholar]
- 133. Berkson J. Limitations of the application of fourfold table analysis to hospital data. Biometrics 1946; 2: 47– 53. [PubMed] [Google Scholar]
- 134. Galbaud du Fort G, Newman SC, Bland RC. Psychiatric comorbidity and treatment seeking. Sources of selection bias in the study of clinical populations. J Nerv Ment Dis 1993; 181: 467– 74. [PubMed] [Google Scholar]
- 135. Rommelse NN, Franke B, Geurts HM, Hartman CA, Buitelaar JK. Shared heritability of attention-deficit/hyperactivity disorder and autism spectrum disorder. Eur Child Adolesc Psychiatry 2010; 19: 281– 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. van Steensel FJ, Bogels SM, Perrin S. Anxiety disorders in children and adolescents with autistic spectrum disorders: a meta-analysis. Clin Child Fam Psychol Rev 2011; 14: 302– 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Grados MA, Mathews CA. Latent class analysis of Gilles de la Tourette syndrome using comorbidities: clinical and genetic implications. Biol Psychiatry 2008; 64: 219– 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Lebowitz ER, Motlagh MG, Katsovich L, et al. Tourette syndrome in youth with and without obsessive compulsive disorder and attention deficit hyperactivity disorder. Eur Child Adolesc Psychiatry 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Mathews CA, Grados MA. Familiality of Tourette syndrome, obsessive-compulsive disorder, and attention-deficit/hyperactivity disorder: heritability analysis in a large sib-pair sample. J Am Acad Child Adolesc Psychiatry 2011; 50: 46– 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Termine C, Balottin U, Rossi G, et al. Psychopathology in children and adolescents with Tourette’s syndrome: a controlled study. Brain Dev 2006; 28: 69– 75. [DOI] [PubMed] [Google Scholar]
- 141. de Alvarenga PG, de Mathis MA, Dominguez Alves AC, et al. Clinical features of tic-related obsessive-compulsive disorder: results from a large multicenter study. CNS Spectr 2012; 17: 87– 93. [DOI] [PubMed] [Google Scholar]
- 142. Yoshimasu K, Barbaresi WJ, Colligan RC, et al. Childhood ADHD is strongly associated with a broad range of psychiatric disorders during adolescence: a population-based birth cohort study. J Child Psychol Psychiatry 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Abramovitch A, Mittelman A, Henin A, Geller DA. Neuroimaging and neuropsychological findings in pediatric obsessive-compulsive disorder: a review and developmental considerations. Neuropsychiatry 2012; 2: 313– 29. [Google Scholar]
- 144. Fitzgerald KD, Welsh RC, Stern ER, et al. Developmental alterations of frontal-striatal-thalamic connectivity in obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry 2011; 50: 938– 48 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Rosenberg DR, Keshavan MS. A.E. Bennett Research Award. Toward a neurodevelopmental model of obsessive-compulsive disorder. Biol Psychiatry 1998; 43: 623– 40. [DOI] [PubMed] [Google Scholar]
- 146. Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nat Rev Neurosci 2002; 3: 617– 28. [DOI] [PubMed] [Google Scholar]
- 147. Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry 2007; 164: 942– 8. [DOI] [PubMed] [Google Scholar]
- 148. Gaub M, Carlson CL. Behavioral characteristics of DSM-IV ADHD subtypes in a school-based population. J Abnorm Child Psychol 1997; 25: 103– 11. [DOI] [PubMed] [Google Scholar]
- 149. Gershon J. A meta-analytic review of gender differences in ADHD. J Atten Disord 2002; 5: 143– 54. [DOI] [PubMed] [Google Scholar]
- 150. Pennington BF, Willcutt EG, Rhee SH. Analyzing comorbidity. In: Robert VK, ed. Advances in child development and behavior. Oxford: Elsevier, 2005: 263– 304. [DOI] [PubMed] [Google Scholar]
- 151. McGough JJ, Barkley RA. Diagnostic controversies in adult attention deficit hyperactivity disorder. Am J Psychiatry 2004; 161: 1948– 56. [DOI] [PubMed] [Google Scholar]
- 152. Ghanizadeh A. Agreement between Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, and the proposed DSM-V attention deficit hyperactivity disorder diagnostic criteria: an exploratory study. Compr Psychiatry 2012; 54: 7– 10. [DOI] [PubMed] [Google Scholar]
- 153. Angold A, Costello EJ, Erkanli A. Comorbidity. J Child Psychol Psychiatry 1999; 40: 57– 87. [PubMed] [Google Scholar]
- 154. Rachman S. A psychological approach to the study of comorbidity. Clin Psychol Rev 1991; 11: 461– 4. [Google Scholar]
- 155. Kupfer DJ, Baltimore RS, Berry DA, et al. National Institutes of Health Consensus Development Conference Statement: diagnosis and treatment of attention-deficit/hyperactivity disorder (ADHD). J Am Acad Child Adolesc Psychiatry 2000; 39: 182– 93. [DOI] [PubMed] [Google Scholar]
- 156. Van der Oord S, Prins PJM, Oosterlaan J, Emmelkamp PMG. Efficacy of methylphenidate, psychosocial treatments and their combination in school-aged children with ADHD: a meta-analysis. Clin Psychol Rev 2008; 28: 783– 800. [DOI] [PubMed] [Google Scholar]
- 157. Koran LM, Hanna GL, Hollander E, Nestadt G, Simpson HB. Practice guideline for the treatment of patients with obsessive-compulsive disorder. Am J Psychiatry 2007; 164: 5– 53. [PubMed] [Google Scholar]


