Introduction
Combined antiretroviral therapy (cART) has dramatically reduced morbidity and mortality associated with HIV infection[1–4]. cART restores the immune response against opportunistic infections (OIs), but some patients experience an inflammatory reaction within weeks or months after cART initiation[5, 6]. This immune reconstitution inflammatory syndrome (IRIS), whose pathogenesis is not fully elucidated, can result in clinical worsening of existing opportunistic infections after commencing cART (paradoxical IRIS) or in the appearance soon after cART initiation of a new and previously unrecognised opportunistic infections (unmasking IRIS)[7]. IRIS may be associated with significant morbidity, is a diagnostic challenge and complicates clinical management[7].
IRIS has been described in patients with opportunistic infections and AIDS malignancies caused by infections [8–17] as well as in patients with non-infectious conditions such as rheumatoid arthritis and sarcoidosis, although with a different immunopathogenesis [18, 19]. Whereas there is a solid body of literature documenting reactions associated with immune restoration for mycobacterial infections both in the HIV-negative[20] and the HIV-positive population[21–24], our knowledge of IRIS for other conditions is mainly based upon case reports of patients on cART. Most of these describe cases of paradoxical phenomena and only few studies have reported on unmasking IRIS. Further, because case definitions have not been implemented in large observational databases, it has been problematic to estimate its magnitude.
The HIV-CAUSAL Collaboration has recently reported an increase in tuberculosis incidence shortly after cART initiation which was particularly marked in patients with CD4 counts below 50 cells/µL, a pattern strongly suggestive of unmasking IRIS[25]. This pattern was not seen for Pneumocystis jirovecii pneumonia. Here we update and extend our study to the effect of cART on AIDS-defining events suggested to be associated with IRIS in the literature: tuberculosis, mycobacterium avium complex (MAC), cytomegalovirus (CMV) retinitis, progressive multifocal leukoencephalopathy (PML), herpes simplex virus, Kaposi Sarcoma, Non Hodgkin Lymphoma, cryptococcosis and candidiasis. For each of these AIDS-defining events we explore whether changes in incidence after cART initiation are compatible with unmasking IRIS.
Methods
Study population
We used data from the HIV-CAUSAL collaboration, which includes HIV-positive individuals from prospective cohorts in 6 European countries and the United States[25]. All cohorts are based on routinely collected data in clinical practice within settings with universal access to care. Initiation of cART was defined as the date on which an individual initiated treatment with at least two nucleoside reverse transcriptase inhibitors plus either one or more protease inhibitors, one nonnucleoside reverse transcriptase inhibitors, one entry/fusion inhibitor, or one integrase inhibitor.
Analyses included individuals who were HIV-positive between 1996–2013 aged≥18 years, and had a CD4 count and an HIV-RNA measurement within 6 months of each other while ART naïve. Individuals’ follow-up started at baseline, defined as the date when all the inclusion criteria were met, and ended at outcome diagnosis, death, 12 months after the most recent laboratory measurement, or cohort-specific administrative censoring, whichever occurred earlier. To prevent the misclassification of undiagnosed prevalent opportunistic infections and AIDS malignancies as incident cases and thus minimise the inclusion of cases of paradoxical IRIS our analyses excluded HIV-positive individuals who were not AIDS-free during the baseline month.
Outcomes
We considered as primary outcomes all AIDS-events previously suggested to be associated with IRIS. We included tuberculosis, CMV retinitis, cryptococcosis, PML and Kaposi Sarcoma because these were the most common AIDS-defining events in a systematic review of IRIS in observational studies[11]. We included MAC because its association with IRIS was observed soon after antiretroviral therapy was introduced[21]. We included Non-Hodgkin Lymphoma because rare manifestations of IRIS have been reported[10, 26]. We included candidiasis and herpes simplex virus (often not considered in association with IRIS) because a large cohort study of cART initiators in the United States[12] reported them as the most common IRIS-related events.
The diagnostic criteria for the AIDS-defining events[27] were those routinely used in clinical practice in each of the participating countries. Information on use of prophylaxis drugs for these conditions is not collected by the HIV-CAUSAL Collaboration because prophylaxis for these conditions is not widely implemented in most of the participating cohorts.
For each outcome, our working definition for unmasking IRIS was a newly diagnosed and non-previously detected AIDS-defining event in the first three months after starting cART.
Statistical methods
All analyses were conducted separately for each outcome. We computed incidence rates as number of cases per 1000 person-years and estimated the hazard ratio of each outcome for i) cART versus no cART and ii) no cART versus <3 and ≥3 months since cART initiation. We then estimated the cumulative incidence up to 3 months after cART initiation[28].
To estimate the hazard ratios we used a pooled logistic model for risk of the outcome at month m+1 that included a time-varying indicator for ever use of cART through month m, month of follow-up m (restricted cubic splines with 5 knots) and the following baseline covariates: CD4 cell count (<50,50–99,100–199,200–349,350–499, or ≥500 cells/mm3), HIV-RNA level (<4,4–5 or >5 log10 copies/mL), sex, transmission group (heterosexual, men who have sex with men, injecting drug users, or other/unknown), calendar year (1996–1998,1999–2000,2001–2003, or 2004–2013), age (<35,35–50, or >50 years), geographical origin (Western countries, sub-Saharan Africa, other, or unknown), time since HIV infection diagnosis (<3 versus ≥3 months) and cohort.
Because cART is more likely to be initiated in individuals with a low CD4 count and a high HIV-RNA level, estimates from the previous models have to be adjusted for these time-dependent confounders. Because CD4 count and HIV-RNA are affected by prior treatment, adding them as time-dependent covariates in the logistic regression model may introduce bias[29]. Therefore we used inverse probability weighting to adjust for time-varying CD4 count and HIV-RNA. Formally, under the assumption that all time-varying predictors of both cART and AIDS were included in the analyses, the weighted model estimates the parameters of a marginal structural Cox model[30].
Each patient in the analysis received a time-dependent weight inversely proportional to the probability of having its own observed history of cART initiation, as described elsewhere[30]. To estimate each patient’s probability of cART initiation in each month, we fit a pooled logistic model that included the covariates listed above for the outcome model plus the most recent measurement of the following time-dependent covariates: CD4 cell count (restricted cubic splines with 5 knot), HIV-RNA level (<4, 4–5 or >5 log10 copies/mL), AIDS (yes or no) and time since last laboratory measurement. Inverse probability weights were also estimated to adjust for potential selection bias due to censoring by infrequent measurement. Both the cART initiation and censoring weights were stabilized and their product used to fit the weighted pooled logistic model. To avoid the influence of outliers on the variance of the estimates, we truncated the weights at a maximum of 10 which affected <1% of the individuals. The estimated weights used in the analyses had a mean of 1.01. Truncation did not materially change the hazard ratio estimates. We computed conservative 95% confidence intervals for the log hazard ratio by using a variance estimator that accounts for the estimation of the weights.
We performed several sensitivity analyses: i) we estimated the hazard ratio of no cART versus time since cART initiation categories <4 and ≥4 months, ii) in addition to censoring follow-up at 12 months without a laboratory measurement, we censored at 18 and 24 months after the last measurement, iii) the start of follow-up was delayed by 3 months to exclude prevalent cases, iv) we lagged CD4 count and HIV-RNA level 14 days or 21 days to ensure that cART initiation was predicted using prior measurements, v) we estimated inverse probability weights for censoring by death (so that estimates can be interpreted as if all deaths could be prevented), and vi) we included patients who started cART during the first month after baseline.
All analyses were conducted with SAS, version 9.3.
Results
Our analysis included 96,562 eligible individuals who contributed 377,324 person-years during a median [interquantile range (IQR)] follow-up of 31 [13, 65] months. Table 1 shows their baseline characteristics: 78% were men and 70% started follow-up after 2000. The median [IQR] CD4 cell count, HIV-RNA and age at baseline were 405 [263,570] cells/mm3, 4.4 [3.8,5.0] log10 copies/mL and 36 [30,43] years, respectively. Fifty-seven % of the included patients initiated cART during follow-up; the median [IQR] CD4 cell count, HIV-RNA and age at cART initiation were 279 [187,380] cells/mm3, 4.7 [4.0,5.2] log10 copies/mL and 38 (32,46) years, respectively.
Table 1.
Baseline characteristics of study participants, HIV-CAUSAL Collaboration 1996–2013
| Individuals | Median [IQR] follow-up months |
Person years |
cART Initiators (%) |
|
|---|---|---|---|---|
| CD4 count, cells/mm3 | ||||
| <50 | 3522 | 27 [11, 64] | 13088.33 | 2784 (79%) |
| 50 – 100 | 3061 | 33[12, 71] | 12360.83 | 2468 (81%) |
| 100 – 200 | 8641 | 34[14, 73] | 35951.17 | 7018 (81%) |
| 200 – 350 | 21807 | 33[14, 70] | 88392.08 | 15648 (72%) |
| 350 – 500 | 24553 | 31[14, 67] | 96822 | 13854 (56%) |
| >500 | 34978 | 30[13, 62] | 130709.67 | 13372 (38%) |
| HIV-RNA, log10 copies/mL | ||||
| <4 | 30596 | 29[13, 61] | 114836.42 | 12650 (41%) |
| 4–5 | 43419 | 32[14, 67] | 171358 | 25928 (60%) |
| >5 | 22547 | 34[14, 71] | 91129.67 | 16566 (73%) |
| Sex | ||||
| Male | 75049 | 32[14, 67] | 295646.25 | 43038 (57%) |
| Female | 21513 | 29[13, 65] | 81677.83 | 12106 (56%) |
| Age, years | ||||
| < 35 | 44698 | 30[13, 63] | 169760.17 | 23362 (52%) |
| 35 – 50 | 40154 | 33[14, 70] | 162430.83 | 23980 (60%) |
| ≥50 | 11710 | 32[12, 67] | 45133.08 | 7802 (67%) |
| Transmission group | ||||
| Heterosexual | 30060 | 31[14, 67] | 117387.58 | 17814 (59%) |
| Homo/bi-sexual | 39971 | 35[16, 71] | 166591.83 | 23014 (58%) |
| Injection drug-use | 8201 | 24[11, 56] | 29432.17 | 3817 (47%) |
| Other/unknown | 18330 | 26[11, 58] | 63912.5 | 10499 (57%) |
| Geographical origin | ||||
| Western Countries | 57041 | 31[13, 67] | 224174.75 | 32765 (57%) |
| Sub-Saharan Africa | 12497 | 29[13, 62] | 44686.75 | 7453 (60%) |
| Rest of World | 7577 | 27[13, 55] | 25211.42 | 4054 (54%) |
| Unknown country | 19447 | 34[15,74] | 83251.17 | 10872 (56%) |
| Calendar period | ||||
| 1996 – 1998 | 20317 | 38 [14, 118] | 112796.42 | 11251 (55%) |
| 1999 – 2000 | 8940 | 40[14, 110] | 44845.33 | 5276 (59%) |
| 2001 – 2003 | 16639 | 43[16, 98] | 77702 | 9742 (59%) |
| 2004–2013 | 50666 | 27 [12,50] | 141980.33 | 28875 (57%) |
| Overall | 96562 | 31 [13,65] | 377324.08 | 55144 (57%) |
cART. Combined antiretroviral therapy; IQR. Interquantile range.
The incidence rate (per 1000 person-years) ranged between 2.3 for tuberculosis and 0.3 for CMV retinitis and PML. For all outcomes, incidence rates were lower for higher CD4 cell count, younger age and lower HIV-RNA level at baseline (Figure 1). Appendix 1 shows the number of cases and incidence rates for each outcome by baseline characteristics. The hazard ratios (95% confidence intervals) for cART versus no cART were less than 1 for all outcomes, and ranged between 0.13 (0.05–0.38) for cryptococcosis and 0.76 (0.58–1.00) for herpes simplex infection. Appendix 2 shows the weighted and unweighted hazard ratio estimates.
Figure 1.
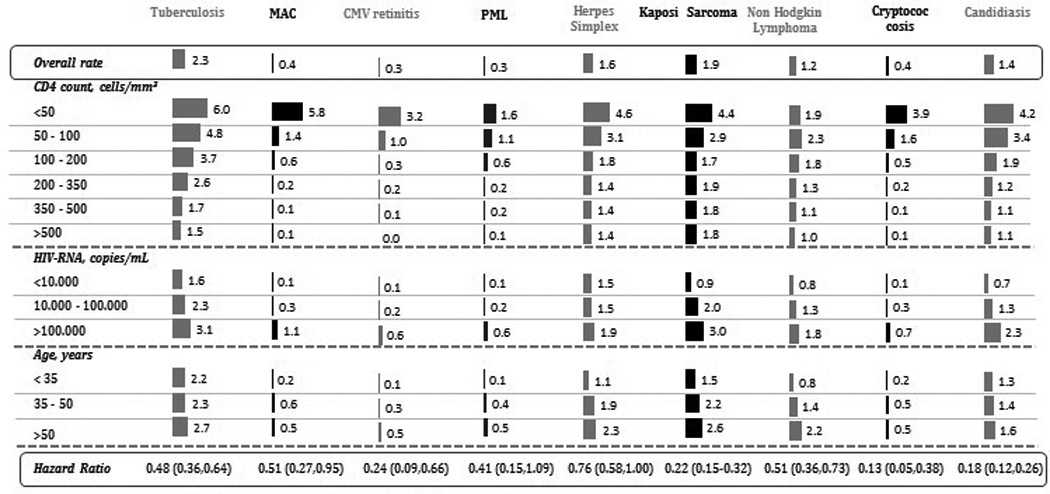
Incidence rates of AIDS-defining events per 1000 person-year of follow-up, HIV-CAUSAL Collaboration 1996–2013
cART Combined antiretroviral therapy; CMV cytomegalovirus; MAC Mycobacterium avium complex; PML Progressive multifocal leukoencephalopathy.
The median [IQR] CD4 cell count at event diagnosis was 291 [161,440] cells/mm3 for tuberculosis, 34 [10,189] cells/mm3 for MAC, 38 [10,189] cells/mm3 for CMV retinitis, 185 [72,310] cells/mm3 for PML, 360 [199,535] cells/mm3 for herpes simplex virus, 322 [186,457] cells/mm3 for Kaposi Sarcoma, 318 [192,466] cells/mm3 for Non Hodgkin Lymphoma, 55 [19,149] cells/mm3 for cryptococcosis and 241 [100,399] cells/mm3 for candidiasis.
Table 2 presents the hazard ratios of each outcome by time since initiation of cART. Compared with non-cART initiation, the hazard ratios up to 3 months after cART initiation were 1.21 (0.90–1.63) for tuberculosis, 2.61 (1.05–6.49) for MAC, 1.17 (0.34–4.08) for CMV retinitis, 1.18 (0.62–2.26) for PML, 1.21 (0.83–1.75) for herpes simplex virus, 1.18 (0.87–1.58) for Kaposi Sarcoma, 1.56 (0.82–2.95) for Non Hodgkin Lymphoma, 1.11 (0.56–2.18) for cryptococcosis and 0.77 (0.40–1.49) for candidiasis. The hazard ratios ≥3 months since cART initiation compared to non-cART initiation ranged between 0.06 (0.02–0.19) for cryptococcosis and 0.69 (0.51–0.92) for herpes simplex virus. The hazard ratio up to 3 months after cART initiation for any of the explored AIDS event compared to non-cART initiation was 1.25 (1.05,1.48).
Table 2.
Hazard ratios of AIDS-defining events by time since initiation of combined antiretroviral therapy, HIV-CAUSAL Collaboration 1996–2013
| Time since cART initiation |
N cases | Person-years | Incidence rates of events per 1000 person year |
Hazard Ratio (95% confidence intervals) |
|
|---|---|---|---|---|---|
| Tuberculosis | No cART | 422 | 143523.33 | 2.9 | 1 |
| <3 months | 97 | 9259.00 | 10.5 | 1.21 (0.90–1.63) | |
| ≥3 months | 379 | 236095.92 | 1.6 | 0.36 (0.26–0.49) | |
| Mycobacterium Avium Complex | No cART | 46 | 143936.50 | 0.3 | 1 |
| <3 months | 37 | 9306.83 | 4.0 | 2.61 (1.05–6.49) | |
| ≥3 months | 80 | 238799.67 | 0.3 | 0.31 (0.16–0.59) | |
| CMV Retinitis | No cART | 35 | 143938.83 | 0.2 | 1 |
| <3 months | 12 | 9308.42 | 1.3 | 1.17 (0.34,4.08) | |
| ≥3 months | 58 | 238917.67 | 0.2 | 0.13 (0.04–0.39) | |
| PML | No cART | 38 | 143944.00 | 0.3 | 1 |
| <3 months | 19 | 9307.42 | 2.0 | 1.18 (0.62–2.26) | |
| ≥3 months | 56 | 238960.75 | 0.2 | 0.21 (0.06–0.71) | |
| Herpes Simplex Virus | No cART | 254 | 143476.42 | 1.8 | 1 |
| <3 months | 42 | 9282.00 | 4.5 | 1.21 (0.83–1.75) | |
| ≥3 months | 324 | 236713.50 | 1.4 | 0.69 (0.51–0.92) | |
| Kaposi Sarcoma | No cART | 404 | 143755.17 | 2.8 | 1 |
| <3 months | 95 | 9250.67 | 10.3 | 1.18 (0.87–1.58) | |
| ≥3 months | 249 | 236065.50 | 1.1 | 0.14 (0.10–0.21) | |
| Non Hodgkin Lymphoma | No cART | 198 | 143875.92 | 1.4 | 1 |
| <3 months | 38 | 9288.17 | 4.1 | 1.56 (0.82–2.95) | |
| ≥3 months | 252 | 237871.50 | 1.1 | 0.40 (0.27–0.58) | |
| Cryptococcosis | No cART | 60 | 143924.67 | 0.4 | 1 |
| <3 months | 21 | 9305.67 | 2.3 | 1.11 (0.56,2.18) | |
| ≥3 months | 58 | 238860.92 | 0.2 | 0.06 (0.02–0.19) | |
| Candidiasis | No cART | 275 | 143745.33 | 1.9 | 1 |
| <3 months | 36 | 9275.67 | 3.9 | 0.77 (0.40–1.49) | |
| ≥3 months | 224 | 237213.75 | 0.9 | 0.13 (0.09–0.20) |
cART Combined antiretroviral therapy; CMV cytomegalovirus; MAC Mycobacterium avium complex; PML Progressive multifocal leukoencephalopathy.
The hazard ratio estimates by time since cART initiation stratified by CD4 cell count, HIV-RNA level, age and sex for events with more than 500 cases (tuberculosis, Kaposi Sarcoma, Non Hodgkin Lymphoma, candidiasis and herpes simplex virus) are presented in Appendices 3–7. The risk of tuberculosis up to 3 months after cART initiation was 1.77 (0.78,4.00) in patients with baseline CD4 count <50 cells/mm3, 2.10 (1.07,4.11) in patients with age>50 years and 1.21 (0.84,1.74) in males.
The hazard ratio estimates did not materially change in sensitivity analyses (Appendix 7). The cumulative incidence (95% confidence intervals) at 3 months following cART initiation ranged between 0.17% (0.14%- 0.20%) for tuberculosis and 0.02% (0.01%- 0.04%) for CMV retinitis. The cumulative incidence for any of the outcomes at 2 months following cART initiation was 0.67% (0.60–0.74%).
Discussion
Our study suggests that cART initiation reduces the overall incidence of tuberculosis, MAC, CMV retinitis, PML, Herpes Simplex virus, Kaposi Sarcoma, Non Hodgkin Lymphoma, cryptococcosis and candidiasis. In spite of this net overall reduction, there was evidence of an increased risk of MAC up to 3 months after cART initiation. The 3-month risk was also slightly elevated for tuberculosis, CMV retinitis, Herpes Simplex virus, Kaposi Sarcoma and Non Hodgkin Lymphoma, but the 95% confidence intervals were wide. The epidemiological patterns observed for MAC and TB are consistent with a relevant proportion of unmasking IRIS among the diagnosis; for the other conditions the evidence is less compelling. For candidiasis the evidence did not support unmasking IRIS.
Our results build on previous findings reported by the HIV-CAUSAL Collaboration with follow-up through 2007. We now report a lower incidence of TB (2.3 versus 3.2 cases per 1000 person-years) and a lower increase in TB incidence soon after cART initiation (21% versus 36%). Since median CD4 cell count at TB diagnosis has not increased over time (results not shown), these changes might be explained by a combination of random variability, temporal trends in TB incidence, and with enhanced pre-cART screening due to increased awareness of TB-related IRIS.
Although IRIS has been most often reported for opportunistic infections, IRIS associated with malignancies has also been described[9, 10]. Like previous studies[31, 32], we found small increases in risk of Non Hodgkin Lymphoma and Kaposi Sarcoma up to 3 months of cART initiation, but the 95% confidence intervals were wide. Given that the development of these cancers should be preceded by exposure to causative agents, the increased incidence for malignancies up to 3 months after cART initiation is consistent with unmasking IRIS leading to increased clinical symptoms and thus diagnostic steps in the case of prevalent subclinical cancers.
We also found that the risk at 3 months of cART initiation for any of the events was <0.7%. This risk is much lower than that reported in a meta-analysis (between 38% for CMV retinitis and 6% for Kaposi Sarcoma)[11] and in the HOPS cohort (between 23% for candidiasis and 0.5% for PML)[12]. Because these previous studies were not restricted to AIDS-free patients, their risk estimates encompass both paradoxical IRIS and unmasking IRIS. In fact, the risk of unmasking IRIS may be even lower because our study cannot distinguish cases of unmasking IRIS from new cases unrelated to IRIS. Further ascertainment bias may account for some of the cases recorded early after cART initiation, as these might have been previously undiagnosed cases due to more intensive clinical screening in newly treated patients. However, the fact that we found no significant initial increase in risk despite this potential bias strengthens our conclusions that unmasking IRIS for the explored events is not common after cART initiation in patients starting cART in recent times in the European and North American setting.
Our study had several limitations. First, like all observational studies the validity of our estimates relies on the assumption of no unmeasured confounding. Although we adjusted our models for CD4 count and HIV-RNA levels, the most important factors used by clinicians to decide whether to start cART, we cannot exclude the possibility that other unmeasured variables related to cART initiation could have also played a role. Second, we assumed that patients remained on therapy once it was initiated. If the diagnoses of the examined AIDS-defining events were largely occurring in individuals who had stopped cART or had poor adherence, then we might have underestimated the effect of cART initiation on the risk of the explored events. On the other hand, this bias is unlikely to have affected our conclusions on the trends in incidence up to 3 months after cART initiation. Finally, given the small number of events occurring during the first months of cART for some of the outcome events, we could not yet explore whether the effect of cART differed by patients characteristics that may be associated with development of IRIS. This is particularly important for baseline CD4 count[11, 25] because unmasking IRIS is mainly observed in patients with very low CD4 counts, who were a minority in our study population.
In summary, this study suggests that, with the exception of mycobacterial infections, unmasking IRIS is not common after cART initiation in AIDS-free patients in Europe and the United States. In order to make an early diagnosis and provide adequate treatment, clinicians should rule out MAC and TB meticulously in patients at risk before starting cART and monitor closely for these OIs during the early phases of treatment.
Acknowledgments
Funding sourceThis work was supported by the National Institute of Health (NIH) [R01 AI102634], a European Union Marie Curie Fellowship [Frame Program 7/2007-2013 274817] and of Juan de la Cierva Fellowship [JCI 2010-08151]. The funding organization had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Appendix
Appendix 1. Incidence rates (per 1000 person-years) of AIDS-defining events overall and by baseline characteristics, HIV-CAUSAL Collaboration 1996–2013
| Tuberculosis | MAC | CMV retinitis | PML | Herpes Simplex Virus |
Kaposi Sarcoma | Non Hodgkin Lymphoma |
Candidiasis | Cryptococcosis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable level | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate |
| Overall | 898 | 2.3 | 163 | 0.4 | 105 | 0.3 | 113 | 0.3 | 620 | 1.6 | 748 | 1.9 | 488 | 1.2 | 535 | 1.4 | 139 | 0.35 |
| CD4 count, cells/mm3 | ||||||||||||||||||
| <50 | 87 | 6 | 84 | 5.8 | 47 | 3.2 | 24 | 1.6 | 66 | 4.6 | 64 | 4.4 | 28 | 1.9 | 60 | 4.1 | 57 | 3.9 |
| 50 – 100 | 64 | 4.8 | 19 | 1.4 | 13 | 1 | 15 | 1.1 | 41 | 3.1 | 38 | 2.9 | 31 | 2.3 | 43 | 3.2 | 22 | 1.6 |
| 100 – 200 | 138 | 3.7 | 22 | 0.6 | 13 | 0.3 | 21 | 0.6 | 67 | 1.8 | 65 | 1.7 | 69 | 1.8 | 70 | 1.9 | 20 | 0.5 |
| 200 – 350 | 239 | 2.6 | 17 | 0.2 | 17 | 0.2 | 19 | 0.2 | 124 | 1.4 | 170 | 1.9 | 117 | 1.3 | 109 | 1.2 | 18 | 0.2 |
| 350 – 500 | 171 | 1.7 | 12 | 0.1 | 9 | 0.1 | 16 | 0.2 | 140 | 1.4 | 177 | 1.8 | 114 | 1.1 | 110 | 1.1 | 9 | 0.1 |
| >500 | 199 | 1.5 | 9 | 0.1 | 6 | 0 | 18 | 0.1 | 182 | 1.4 | 234 | 1.8 | 129 | 1 | 143 | 1.1 | 13 | 0.1 |
| HIV-RNA, copies/mL | ||||||||||||||||||
| <10,000 | 185 | 1.6 | 8 | 0.1 | 10 | 0.1 | 16 | 0.1 | 171 | 1.5 | 111 | 0.9 | 93 | 0.8 | 82 | 0.7 | 17 | 0.1 |
| 10,000 – 100,000 | 414 | 2.3 | 53 | 0.3 | 33 | 0.2 | 38 | 0.2 | 266 | 1.5 | 350 | 2 | 224 | 1.3 | 234 | 1.3 | 50 | 0.3 |
| ≥100000 | 299 | 3.1 | 102 | 1.1 | 62 | 0.6 | 59 | 0.6 | 183 | 1.9 | 287 | 3 | 171 | 1.8 | 219 | 2.3 | 72 | 0.7 |
| Sex | ||||||||||||||||||
| Male | 618 | 2 | 144 | 0.5 | 89 | 0.3 | 94 | 0.3 | 531 | 1.7 | 707 | 2.3 | 428 | 1.4 | 394 | 1.3 | 121 | 0.4 |
| Female | 280 | 3.4 | 19 | 0.2 | 16 | 0.2 | 19 | 0.2 | 89 | 1.1 | 41 | 0.5 | 60 | 0.7 | 141 | 1.7 | 18 | 0.2 |
| Age, years | ||||||||||||||||||
| < 35 | 383 | 2.2 | 39 | 0.2 | 26 | 0.1 | 26 | 0.1 | 191 | 1.1 | 262 | 1.5 | 140 | 0.8 | 220 | 1.3 | 31 | 0.2 |
| 35 – 50 | 389 | 2.3 | 99 | 0.6 | 55 | 0.3 | 61 | 0.4 | 322 | 1.9 | 364 | 2.2 | 242 | 1.4 | 241 | 1.4 | 83 | 0.5 |
| >50 | 126 | 2.7 | 25 | 0.5 | 24 | 0.5 | 26 | 0.5 | 107 | 2.3 | 122 | 2.6 | 106 | 2.2 | 74 | 1.6 | 25 | 0.5 |
| Transmission group | ||||||||||||||||||
| Heterosexual | 426 | 3.6 | 30 | 0.2 | 15 | 0.1 | 21 | 0.2 | 69 | 0.6 | 98 | 0.8 | 122 | 1 | 188 | 1.6 | 23 | 0.2 |
| Homo/bi-sexual | 147 | 0.9 | 34 | 0.2 | 18 | 0.1 | 40 | 0.2 | 104 | 0.6 | 482 | 2.8 | 235 | 1.4 | 178 | 1 | 19 | 0.1 |
| Injection drug-use | 86 | 2.8 | 14 | 0.5 | 5 | 0.2 | 13 | 0.4 | 18 | 0.6 | 9 | 0.3 | 43 | 1.4 | 124 | 4.1 | 9 | 0.3 |
| Other/unknown | 239 | 3.5 | 85 | 1.2 | 67 | 1 | 39 | 0.6 | 429 | 6.5 | 159 | 2.3 | 88 | 1.3 | 45 | 0.7 | 88 | 1.3 |
| Geographical origin | ||||||||||||||||||
| Western Countries | 251 | 1.4 | 50 | 0.3 | 25 | 0.1 | 51 | 0.3 | 88 | 0.5 | 337 | 1.8 | 253 | 1.4 | 292 | 1.6 | 25 | 0.1 |
| Sub-Saharan Africa | 298 | 6.6 | 6 | 0.1 | 4 | 0.1 | 9 | 0.2 | 32 | 0.7 | 57 | 1.2 | 49 | 1.1 | 53 | 1.1 | 10 | 0.2 |
| Rest of World | 82 | 3.2 | 4 | 0.2 | 2 | 0.1 | 6 | 0.2 | 22 | 0.8 | 35 | 1.4 | 24 | 0.9 | 50 | 1.9 | 7 | 0.3 |
| Unknown country | 267 | 2 | 103 | 0.8 | 74 | 0.5 | 47 | 0.3 | 478 | 3.6 | 319 | 2.4 | 162 | 1.2 | 140 | 1 | 97 | 0.7 |
| Calendar period | ||||||||||||||||||
| 1996 – 1998 | 240 | 2 | 55 | 0.5 | 48 | 0.4 | 33 | 0.3 | 230 | 2 | 211 | 1.8 | 143 | 1.2 | 223 | 1.9 | 42 | 0.4 |
| 1999 – 2000 | 117 | 2.5 | 26 | 0.6 | 15 | 0.3 | 16 | 0.3 | 89 | 1.9 | 87 | 1.9 | 62 | 1.3 | 74 | 1.6 | 31 | 0.7 |
| 2001–2003 | 239 | 3 | 35 | 0.4 | 23 | 0.3 | 23 | 0.3 | 150 | 1.9 | 164 | 2 | 108 | 1.3 | 109 | 1.3 | 32 | 0.4 |
| 2004 – 2013 | 302 | 2.1 | 47 | 0.3 | 19 | 0.1 | 41 | 0.3 | 151 | 1 | 286 | 2 | 175 | 1.2 | 129 | 0.9 | 34 | 0.2 |
CMV cytomegalovirus; MAC Mycobacterium avium complex; PML Progressive multifocal leukoencephalopathy;
Appendix 2. Hazard ratios of AIDS-defining events for cART initiation versus no cART from unweighted and inverse probability weighted models, HIV-CAUSAL Collaboration 1996–2013
| Unweighted models – baseline covariates |
Unweighted models – baseline covariates and time-varying covariates |
Weighted models – baseline covariates |
|
|---|---|---|---|
| Tuberculosis | 0.77 (0.65,0.91) | 0.95 (0.80,1.12) | 0.48 (0.36,0.64) |
| MAC | 1.12 (0.75,1.69) | 1.25 (0.84,1.86) | 0.51 (0.27,0.95) |
| CMV Retinitis | 0.86 (0.52,1.42) | 0.95 (0.58,1.54) | 0.24 (0.09,0.66) |
| PML | 1.04 (0.63,1.72) | 1.03 (0.63,1.67) | 0.41 (0.15,1.09) |
| Herpes Simplex Infection | 0.97 (0.78,1.19) | 1.09 (0.88,1.34) | 0.76 (0.58,1.00) |
| Kaposi Sarcoma | 0.53 (0.44,0.64) | 0.84 (0.70,1.02) | 0.22 (0.15,0.32) |
| Non Hodgkin Lymphoma | 0.88 (0.69,1.11) | 0.94 (0.74,1.20) | 0.51 (0.36,0.73) |
| Cryptococcosis | 0.46 (0.30,0.70) | 0.57 (0.38,0.87) | 0.15 (0.05,0.42) |
| Candidiasis | 0.43 (0.34,0.54) | 0.73 (0.58,0.90) | 0.18 (0.12,0.26) |
cART Combined antiretroviral therapy; CMV cytomegalovirus; MAC Mycobacterium avium complex; PML Progressive multifocal leukoencephalopathy.
Appendix 3. Hazard ratios of tuberculosis by time since initiation of combined antiretroviral therapy and baseline characteristics, HIV-CAUSAL Collaboration 1996–2013
| No cART initiation |
Time since cART initiation, <3 months |
Time since cART initiation, ≥3 months | ||||
|---|---|---|---|---|---|---|
| Baseline characteristics | Cases, N |
HR | Cases, N | HR | Cases, N | HR |
| CD4 count, cells/mm3 | ||||||
| <50 | 16 | 1 | 24 | 1.77 (0.78–4.00) | 47 | 0.61 (0.24–1.56) |
| 50 – 100 | 21 | 1 | 14 | 0.94 (0.42–2.12) | 29 | 0.38 (0.21–0.63) |
| 100 – 200 | 44 | 1 | 17 | 0.77 (0.41–1.43) | 77 | 0.37 (0.21–0.63) |
| 200 – 350 | 107 | 1 | 26 | 0.92 (0.51–1.67) | 106 | 0.28 (0.15–0.53) |
| ≥350 | 234 | 1 | 16 | 1.06 (0.49–2.28) | 120 | 0.43 (0.29–0.63) |
| HIV-RNA, log10 copies/mL | ||||||
| <4 | 113 | 1 | 14 | 1.56 (0.73–3.33) | 58 | 0.45 (0.25–0.81) |
| 4–5 | 203 | 1 | 34 | 1.25 (0.78–2.01) | 177 | 0.40 (0.27–0.58) |
| >5 | 106 | 1 | 49 | 0.96 (0.63–1.47) | 144 | 0.29 (0.16–0.55) |
| Age, years | ||||||
| < 35 | 188 | 1 | 38 | 1.2 (0.73–1.98) | 157 | 0.36 (0.24–0.55) |
| 35 – 50 | 180 | 1 | 37 | 0.9 (0.58–1.40) | 172 | 0.35 (0.21–0.57) |
| ≥50 | 54 | 1 | 22 | 2.1 (1.07–4.11) | 50 | 0.38 (0.16–0.90) |
| Sex | ||||||
| Male | 288 | 1 | 70 | 1.21 (0.84–1.74) | 259 | 0.31 (0.21–0.47) |
| Female | 133 | 1 | 27 | 1.12 (0.67–1.88) | 120 | 0.44 (0.29–0.67) |
Appendix 4. Hazard ratios of Kaposi Sarcoma by time since initiation of combined antiretroviral therapy and baseline characteristics, HIV-CAUSAL Collaboration 1996–2013
| No cART initiation |
Time since cART initiation, <3 months |
Time since cART initiation, ≥3 months | ||||
|---|---|---|---|---|---|---|
| Baseline characteristics | Cases, N |
HR | Cases, N | HR | Cases, N | HR |
| CD4 count, cells/mm3 | ||||||
| <50 | 21 | 1 | 15 | 0.92 (0.46–1.82) | 28 | 0.24 (0.09–0.65) |
| 50 – 100 | 13 | 1 | 8 | 0.67 (0.24–1.87) | 17 | 0.12 (0.04–0.39) |
| 100 – 200 | 30 | 1 | 12 | 0.62 (0.31–1.22) | 23 | 0.05 (0.01–0.19) |
| 200 – 350 | 81 | 1 | 16 | 0.73 (0.41–1.32) | 73 | 0.16 (0.09–0.29) |
| ≥350 | 259 | 1 | 44 | 1.76 (1.13–2.74) | 108 | 0.19 (0.12–0.29) |
| HIV-RNA, log10 copies/mL | ||||||
| <4 | 59 | 1 | 13 | 1.56 (0.73–3.33) | 39 | 0.31 (0.15–0.64) |
| 4–5 | 192 | 1 | 43 | 1.25 (0.78–2.01) | 115 | 0.13 (0.07–0.24) |
| >5 | 153 | 1 | 39 | 0.96 (0.63–1.47) | 95 | 0.15 (0.09–0.24) |
| Age, years | ||||||
| < 35 | 151 | 1 | 34 | 1.2 (0.73–1.98) | 77 | 0.06 (0.03–0.1.2) |
| 35 – 50 | 198 | 1 | 49 | 0.9 (0.58–1.40) | 117 | 0.19 (0.11–0.33) |
| ≥50 | 55 | 1 | 12 | 2.1 (1.07–4.11) | 55 | 0.32 (0.15–0.69) |
| Sex | ||||||
| Male | 392 | 1 | 89 | 1.21 (0.84–1.74) | 226 | 0.14 (0.10–0.21) |
| Female | 12 | 1 | 6 | 1.12 (0.67–1.88) | 23 | 0.12 (0.02–0.71) |
Appendix 5. Hazard ratios of Non Hodgkin Lymphoma by time since initiation of combined antiretroviral therapy and baseline characteristics, HIV-CAUSAL Collaboration 1996–2013
| No cART initiation | Time since cART initiation, <3 months |
Time since cART initiation, ≥3 months | ||||
|---|---|---|---|---|---|---|
| Baseline characteristics | Cases, N | HR | Cases, N | HR | Cases, N | HR |
| CD4 count, cells/mm3 | ||||||
| <50 | 9 | 1 | 3 | 0.59 (0.25–1.40) | 16 | 0.18 (0.09–0.35) |
| 50 – 100 | 9 | 1 | 2 | 0.33 (0.06–1.71) | 20 | 0.08 (0.02–0.29) |
| 100 – 200 | 15 | 1 | 6 | 0.16 (0.05–0.55) | 48 | 0.15 (0.05–0.44) |
| 200 – 350 | 40 | 1 | 12 | 1.33 (0.34–5.13) | 65 | 0.11 (0.05–0.25) |
| ≥350 | 125 | 1 | 15 | 1.52 (0.79–2.93) | 103 | 0.37 (0.23–0.59) |
| HIV-RNA, log10 copies/mL | ||||||
| <4 | 54 | 1 | 4 | 0.63 (0.16–22.43) | 35 | 0.49 (0.24–0.99) |
| 4–5 | 98 | 1 | 18 | 1.20 (0.4–3.52) | 108 | 0.11 (0.06–0.19) |
| >5 | 46 | 1 | 16 | 0.44 (0.26–0.74) | 109 | 0.14 (0.09–0.22) |
| Age, years | ||||||
| < 35 | 68 | 1 | 9 | 0.26 (0.12–0.58) | 63 | 0.12 (0.06–0.23) |
| 35 – 50 | 100 | 1 | 26 | 1.24 (0.52–2.95) | 116 | 0.12 (0.07–0.20) |
| ≥50 | 30 | 1 | 3 | 0.44 (0.15–1.32) | 73 | 0.22 (0.09–0.56) |
| Sex | ||||||
| Male | 163 | 1 | 35 | 0.89 (0.40–1.98) | 229 | 0.13 (0.08–0.21) |
| Female | 35 | 1 | 2 | 0.46 (0.18–1.17) | 3 | 0.13 (0.06–0.26) |
Appendix 6. Hazard ratios of Candidiasis by time since initiation of combined antiretroviral therapy and baseline characteristics, HIV-CAUSAL Collaboration 1996–2013
| No cART initiation | Time since cART initiation, <3 months |
Time since cART initiation, ≥3 months | ||||
|---|---|---|---|---|---|---|
| Baseline characteristics | Cases, N |
HR | Cases, N | HR | Cases, N | HR |
| CD4 count, cells/mm3 | ||||||
| <50 | 20 | 1 | 11 | 0.59 (0.25–1.40) | 29 | 0.18 (0.09–0.35) |
| 50 – 100 | 20 | 1 | 3 | 0.33 (0.06–1.71) | 20 | 0.08 (0.02–0.29) |
| 100 – 200 | 26 | 1 | 3 | 0.16 (0.05–0.55) | 41 | 0.15 (0.05–0.04) |
| 200 – 350 | 53 | 1 | 9 | 1.33 (0.34–5.13) | 47 | 0.11 (0.05–0.25) |
| ≥350 | 156 | 1 | 10 | 0.62 (0.22–1.73) | 87 | 0.17 (0.11–0.29) |
| HIV-RNA, log10 copies/mL | ||||||
| <4 | 47 | 1 | 3 | 0.63 (0.16–2.43) | 32 | 0.49 (0.24–0.99) |
| 4–5 | 129 | 1 | 12 | 1.20 (0.41–3.52) | 93 | 0.11 (0.06–0.19) |
| >5 | 99 | 1 | 21 | 0.44 (0.26–0.74) | 99 | 0.14 (0.09–0.22) |
| Age, years | ||||||
| < 35 | 111 | 1 | 8 | 0.26 (0.12–0.58) | 101 | 0.12 (0.06–0.23) |
| 35 – 50 | 137 | 1 | 24 | 1.24 (0.52–2.95) | 80 | 0.12 (0.07–0.20) |
| ≥50 | 27 | 1 | 4 | 0.44 (0.15–1.32) | 43 | 0.22 (0.09–0.56) |
| Sex | ||||||
| Male | 205 | 1 | 27 | 0.89 (0.40–1.98) | 162 | 0.13 (0.08–0.21) |
| Female | 70 | 1 | 9 | 0.46 (0.18–1.17) | 62 | 0.13 (0.06–0.26) |
Appendix 7. Hazard ratios of Herpes Simplex Virus by time since initiation of combined antiretroviral therapy and baseline characteristics, HIV-CAUSAL Collaboration 1996–2013
| No cART initiation | Time since cART initiation, <3 months |
Time since cART initiation, ≥3 months | ||||
|---|---|---|---|---|---|---|
| Baseline characteristics | Cases, N |
HR | Cases, N | HR | Cases, N | HR |
| CD4 count, cells/mm3 | ||||||
| <100 | 23 | 1 | 13 | 1.02 (0.49–2.13) | 71 | 0.38 (0.19–0.78) |
| 100 – 200 | 15 | 1 | 4 | 0.58 (0.19–1.77) | 48 | 1.02 (0.39–2.64) |
| 200 – 350 | 41 | 1 | 6 | 0.90 (0.35–2.31) | 77 | 0.98 (0.56–1.71) |
| ≥350 | 175 | 1 | 19 | 1.43 (0.84–2.43) | 128 | 0.67 (0.46–0.98) |
| HIV-RNA, log10 copies/mL | ||||||
| <4 | 92 | 1 | 10 | 1.60 (0.75–3.40) | 69 | 0.74 (0.44–1.24) |
| 4–5 | 109 | 1 | 15 | 1.11 (0.60–2.04) | 142 | 0.74 (0.49–1.11) |
| >5 | 53 | 1 | 17 | 0.97 (0.55–1.73) | 113 | 0.59 (0.29–1.18) |
| Age, years | ||||||
| < 35 | 91 | 1 | 13 | 1.04 (0.53–2.01) | 87 | 0.56 (0.33–0.95) |
| 35 – 50 | 119 | 1 | 22 | 1.58 (0.96–2.59) | 181 | 0.82 (0.55–1.23) |
| ≥50 | 44 | 1 | 7 | 0.65 (0.26–1.66) | 56 | 0.56 (0.27–1.16) |
| Sex | ||||||
| Male | 216 | 1 | 36 | 1.28 (0.85–1.92) | 276 | 0.68 (0.49–0.93) |
| Female | 38 | 1 | 6 | 0.79 (0.31–2.00) | 45 | 0.70 (0.32–1.57) |
Appendix 8. Sensitivity analyses. Hazard ratios (95% confidence intervals) for combined antiretroviral therapy initiation versus time since cART initiation <3 months, HIV-CAUSAL Collaboration 1996–2013
| Main analysis (time since cART initiation <3 months) |
Time since cART initiation <4 months |
Not excluding patients who initiated cART in the first month after baseline |
Start of follow up delayed by 3 months |
Inverse probability weights for censoring by death |
CD4 count and HIV RNA lagged by 14 days |
CD4 count and HIV RNA lagged by 21 days |
Censoring at 18 months |
Censoring at 24 months |
|
|---|---|---|---|---|---|---|---|---|---|
| Tuberculosis | 1.21 (0.90–1.63) | 0.99 (0.75–1.30) | 1.38 (1.04–1.83) | 1.61 (1.00–2.59) | 1.21 (0.90–1.64) | 1.27 (0.95–1.70) | 1.26 (0.93–1.69) | 1.28 (0.95–1.71) | 1.34 (1.00–1.79) |
| MAC | 2.61 (1.05–6.49) | 2.07 (0.91–4.70) | 2.25 (1.10–4.62) | 2.87 (1.15–7.16) | 2.87 (1.15–7.16) | 2.03 (1.19–3.47) | 2.25 (1.31–3.86) | 2.56 (1.07–6.12) | 2.98 (1.39–6.42) |
| CMV Retinitis | 1.17 (0.34–4.08) | 0.84 (0.26–2.70) | 1.18 (0.40–3.44) | 1.52 (0.23–10.8) | 1.20 (0.35–4.11) | 1.26 (0.33–4.77) | 1.27 (0.30–5.33) | 1.38 (0.42–4.51) | 1.46 (0.46–4.66) |
| PML | 1.18 (0.62–2.26) | 1.41 (0.78–2.55) | 1.56 (0.78–3.13) | 1.02 (0.38–2.74) | 1.19 (0.63–2.27) | 1.17 (0.60–2.28) | 1.19 (0.59–2.38) | 1.23 (0.67–2.24) | 1.46 (0.82–2.59) |
| Herpes Simplex Infection | 1.21 (0.83–1.75) | 1.22 (0.88–1.69) | 0.81 (0.62–1.05) | 0.92 (0.53–1.59) | 1.18 (0.81–1.72) | 1.19 (0.82–1.73) | 1.27 (0.87–1.85) | 1.66 (1.00–2.74) | 1.72 (1.04–2.84) |
| Kaposi Sarcoma | 1.18 (0.87–1.58) | 1.02 (0.71–1.45) | 1.12 (0.84–1.48) | 1.02 (0.71–1.45) | 1.18 (0.88–1.58) | 1.34 (1.00–1.78) | 1.34 (1.00–1.78) | 1.24 (0.93,1.64) | 1.31 (0.99–1.73) |
| Non Hodgkin Lymphoma | 1.56 (0.82–2.95) | 1.55(0.86–2.77) | 1.52 (0.96–2.42) | 1.88 (0.82–4.27) | 1.54 (0.82–2.92) | 1.72 (0.89–3.32) | 1.72 (0.89–3.32) | 1.56 (0.86–2.85) | 0.79 (0.42–1.49) |
| Cryptococcosis | 1.11 (0.56–2.18) | 0.77 (0.40–1.48) | 1.21 (0.60–2.46) | 1.07 (0.35–3.27) | 1.48 (0.57–3.80) | 1.05 (0.53–2.10) | 1.06 (0.53–2.11) | 1.28 (0.67–2.46) | 0.99 (0.56–1.75) |
| Candidiasis | 0.77 (0.40–1.49) | 0.53 (0.28–1.02) | 0.78 (0.49–1.25) | 0.98 (0.38–2.54) | 0.76 (0.39–1.48) | 0.83 (0.41–1.66) | 0.82 (0.40–1.69) | 0.75 (0.39–1.43) | 0.75 (0.39–1.43) |
CMV cytomegalovirus; MAC Mycobacterium avium complex; PML Progressive multifocal leukoencephalopathy.
Contributors to the HIV-CAUSAL Collaboration
UK CHIC: Steering committee: J Ainsworth, J Anderson, A Babiker, V Delpech, D Dunn, P Easterbrook, M Fisher, B Gazzard, R Gilson, M Gompels, T Hill, M Johnson, C Leen, C Orkin, A Phillips, D Pillay, K Porter, C Sabin (PI), A Schwenk, J Walsh. Central co-ordination: UCL Medical School, London (L Bansi, T Hill, A Phillips, C Sabin); Medical Research Council Clinical Trials Unit, London (D Dunn, K Porter, A Glabay). Participating centres: Barts and The London NHS Trust, London (C Orkin, R Thomas, K Jones); Brighton and Sussex University Hospitals NHS Trust (M Fisher, N Perry, A Pullin, D Churchill); Chelsea and Westminster NHS Trust, London (B Gazzard, M Nelson, D Asboe, S Bulbeck, S Mandalia, J Clarke); Health Protection Agency –Centre for Infections, London (V Delpech); Homerton University Hospital NHS Trust, London (J Anderson, S Munshi); King’s College Hospital, London (F Post, P Easterbrook, Y Khan, P Patel, F Karim, S Duffell); UCL Medical School and The Mortimer Market Centre, London (R Gilson, S-L Man, I Williams); North Bristol NHS Trust (M Gompels, D Dooley); North Middlesex University Hospital NHS Trust, London (A Schwenk, J Ainsworth); Royal Free NHS Trust and Department of Infection & Population Health, UCL Medical School, London (M Johnson, M Youle, F Lampe, C Smith, H Grabowska, C Chaloner, D Ismajani Puradiredja, L Bansi, T Hill, A Phillips, C Sabin); Imperial College Healthcare NHS Trust, London (J Walsh, J Weber, C Kemble, N Mackie, A Winston); The Lothian University Hospitals NHS Trust, Edinburgh (C Leen, A Wilson).
ATHENA: Director: P Reiss, Stichting HIV Monitoring, Amsterdam. Data analysis group: DO Bezemer, LAJ Gras, AM Kesselring, AI van Sighem, C Smit. Data collection: S Zaheri. Participating centres (*Site coordinating physicians): Medisch Centrum Alkmaar, Alkmaar: G van Twillert*, W Kortmann*. Flevoziekenhuis, Almere: J Branger*. Academic Medical Center of the University of Amsterdam, Amsterdam: JM Prins*, TW Kuijpers, HJ Scherpbier, JTM van der Meer, FWMN Wit, MH Godfried, P Reiss, T van der Poll, FJB Nellen, JMA Lange, SE Geerlings, M van Vugt, D Pajkrt, JC Bos, M van der Valk, ML Grijsen, WJ Wiersinga. Onze Lieve Vrouwe Gasthuis, Amsterdam: K Brinkman*, WL Blok, PHJ Frissen, WEM Schouten, GEL van den Berk. Sint Lucas Andreas Ziekenhuis, Amsterdam: J Veenstra*, KD Lettinga. Slotervaartziekenhuis, Amsterdam: JW Mulder*, SME Vrouenraets, FN Lauw. Stichting Medisch Centrum Jan van Goyen, Amsterdam: A van Eeden*, DWM Verhagen. VU Medisch Centrum, Amsterdam: MA van Agtmael*, RM Perenboom, FAP Claessen, M Bomers, EJG Peters. Rijnstate, Arnhem: C Richter*, JP van der Berg, EH Gisolf. HagaZiekenhuis, Den Haag: EF Schippers*, C van Nieuwkoop, EP van Elzakker. Medisch Centrum Haaglanden, Den Haag: EMS Leyten*, LBS Gelinck. Catharina Ziekenhuis, Eindhoven: MJH Pronk*, B Bravenboer. Medisch Spectrum Twente, Enschede: GJ Kootstra*, CE Delsing. Universitair Medisch Centrum Groningen, Goningen: HG Sprenger*, R Doedens (until June, 2012), EH Scholvinck, S van Assen, WFW Bierman. Kennemer Gasthuis, Haarlem: R Soetekouw*, RW ten Kate. Medisch Centrum Leeuwarden, Leeuwarden: MGA van Vonderen*, DPF van Houte. Leids Universitair Medisch Centrum, Leiden: FP Kroon*, JT van Dissel, SM Arend, MGJ de Boer, H Jolink, HJM ter Vollaard, MP Bauer. MC Zuiderzee, Lelystad: S Weijer*, R el Moussaoui. Academisch Ziekenhuis Maastricht, Maastricht: S Lowe*, G Schreij, A Oude Lashof, D Posthouwer. Universitair Medisch Centrum Sint Radboud, Nijmegen: PP Koopmans*, M Keuter, AJAM van der Ven, HJM ter Hofstede, ASM Dofferhoff, A Warris, R van Crevel. Erasmus Medisch Centrum, Rotterdam: ME van der Ende*, TEMS de Vries-Sluijs, CAM Schurink, JL Nouwen, MH Nispen tot Pannerden, A Verbon, BJA Rijnders, ECM van Gorp, RJ Hassing, AWM Smeulders. Erasmus Medisch Centrum–Sophia, Rotterdam: NG Hartwig, GJA Driessen. Maasstad Ziekenhuis, Rotterdam: JG den Hollander*, K Pogany. Sint Elisabeth Ziekenhuis, Tilburg: JR Juttmann*, MEE van Kasteren. Universitair Medisch Centrum Utrecht, Utrecht: AIM Hoepelman*, T Mudrikova, MME Schneider, CAJJ Jaspers, PM Ellerbroek, JJ Oosterheert, JE Arends, MWM Wassenberg, RE Barth. Wilhelmina Kinderziekenhuis, Utrecht: SPM Geelen, TFW Wolfs, LJ Bont. Admiraal De Ruyter Ziekenhuis, Vlissingen: M van den Berge*, A Stegeman. Isala Klinieken, Zwolle: PHP Groeneveld*, MA Alleman, JW Bouwhuis.
FHDH-ANRS CO4: Scientific committee: S Abgrall, F Barin, M Bentata, E Billaud, F Boué, C Burty, A Cabié, D Costagliola, L Cotte, P De Truchis, X Duval, C Duvivier, P Enel, L Fredouille-Heripret, J Gasnault, C Gaud, J Gilquin, S Grabar, C. Katlama, MA Khuong, JM Lang, AS Lascaux, O Launay, A Mahamat, M Mary-Krause, S Matheron, JL Meynard, J Pavie, G Pialoux, F Pilorgé, I Poizot-Martin, C Pradier, J Reynes, E Rouveix, A Simon, P Tattevin, H Tissot-Dupont, JP Viard, N Viget. DMI2 coordinating center: French Ministry of Health (Valérie Salomon), Technical Hospitalization Information Agency, ATIH (N Jacquemet). Statistical analysis center: U943 INSERM et UPMC (S Abgrall, D Costagliola, S Grabar, M Guiguet, E Lanoy, L Lièvre, M Mary-Krause, H Selinger-Leneman), INSERM Transfert (JM Lacombe, V Potard). COREVIH: Paris area: Corevih Ile de France Centre (GH Pitié-Salpétrière: F Bricaire, S Herson, C Katlama, A Simon; Hôpital Saint-Antoine: N Desplanque, PM Girard, JL Meynard, MC Meyohas, O Picard; Hôpital Tenon: J Cadranel, C Mayaud, G Pialoux), Corevih Ile de France Est (Hôpital Saint-Louis: JP Clauvel, JM Decazes, L Gerard, JM Molina; GH Lariboisière-Fernand Widal: M Diemer, P Sellier; Hôpital Avicenne: M Bentata, P Honoré; Hôpital Jean Verdier: V Jeantils, S Tassi; Hôpital Delafontaine: D Mechali, B Taverne), Corevih Ile de France Nord (Hôpital Bichat-Claude Bernard: E Bouvet, B Crickx, JL Ecobichon, S Matheron, C Picard-Dahan, P Yeni), Corevih Ile de France Ouest (Hôpital Ambroise Paré: H Berthé, C Dupont; Hôpital Louis Mourier: C Chandemerle, E Mortier; Hôpital Raymond Poincaré: P de Truchis), Corevih Ile de France Sud (Hôpital Européen Georges Pompidou: D Tisne-Dessus, L Weiss; GH Tarnier-Cochin: D Salmon; Hôpital Saint-Joseph: I Auperin, J Gilquin; Hôpital Necker adultes: L Roudière, JP Viard; Hôpital Antoine Béclère: F Boué, R Fior; Hôpital de Bicêtre: JF Delfraissy, C Goujard; Hôpital Henri Mondor: C Jung, Ph Lesprit; Hôpital Paul Brousse: D Vittecoq). Outside Paris area: Corevih Alsace (CHRU de Strasbourg: P Fraisse, JM Lang, D Rey; CH de Mulhouse: G Beck-Wirth), Corevih de l’Arc Alpin (CHU de Grenoble: JP Stahl, P Lecercq), Corevih Auvergne-Loire (CHU de Clermont-Ferrand: F Gourdon, H Laurichesse; CHRU de Saint-Etienne: A Fresard, F Lucht); Corevih Basse-Normandie (CHRU de Caen: C Bazin, R Verdon), Corevih Bourgogne (CHRU de Dijon: P Chavanet), Corevih Bretagne (CHU de Rennes: C Arvieux, C Michelet), Corevih Centre (CHRU de Tours: P Choutet, A Goudeau, MF Maître), Corevih Franche-Comté (CHRU de Besançon: B Hoen; CH de Belfort: P Eglinger, JP Faller); Corevih Haute-Normandie (CHRU de Rouen: F Borsa-Lebas, F Caron), Corevih Languedoc-Roussillon (CHU de Montpellier: J Reynes; CHG de Nîmes: JP Daures), Corevih Lorraine (Nancy Hôpital de Brabois: T May, C Rabaud; CHRU de Reims: JL Berger, G Rémy), Corevih de Midi-Pyrénées (Toulouse CHU Purpan: E Arlet-Suau, L Cuzin, P Massip, MF Thiercelin Legrand; Toulouse Hôpital la Grave: G Pontonnier; Toulouse CHU Rangueil), Corevih Nord-Pas de Calais (CH de Tourcoing: N Viget, Y Yasdanpanah), Corevih PACA Est (Nice Hôpital Archet 1: P Dellamonica, C Pradier, P Pugliese; CHG Antibes-Juan les Pins: K Aleksandrowicz, D Quinsat), Corevih PACA Ouest (Marseille Hôpital de la Conception: I Ravaux, H Tissot-Dupont; Marseille Hôpital Nord: JP Delmont, J Moreau; Marseille Institut Paoli Calmettes: JA Gastaut; Marseille Hôpital Sainte-Marguerite: I Poizot-Martin, F Retornaz, J Soubeyrand; Marseille Centre pénitentiaire des Baumettes: A Galinier, JM Ruiz; CHG d’Aix-En-Provence: T Allegre, PA Blanc; CH d’Arles: D Bonnet-Montchardon; CH d’Avignon: G Lepeu; CH de Digne Les Bains: P Granet-Brunello; CH de Gap: JP Esterni, L Pelissier; CH de Martigues: R |Cohen-Valensi, M Nezri; CHI de Toulon: S Chadapaud, A Laffeuillade), Corevih Pays de la Loire (CHRU de Nantes: E Billaud, F Raffi), Corevih de la Vallée du Rhône (Lyon Hôpital de la Croix-Rousse: A Boibieux, D Peyramond; Lyon Hôpital Edouard Herriot: JM Livrozet, JL Touraine; Lyon Hôtel-Dieu: L Cotte, C Trepo). Overseas: Corevih Guadeloupe (CHRU de Pointe-à-Pitre: M Strobel; CH Saint-Martin: F Bissuel), Corevih Guyane (CHG de Cayenne: R Pradinaud, M Sobesky), Corevih Martinique (CHRU de Fort-de-France: A Cabié), Corevih de La Réunion (CHD Félix Guyon: C Gaud, M Contant).
Swiss HIV Cohort Study (SHCS): Aubert V, Barth J, Battegay M, Bernasconi E, Böni J, Bucher HC, Burton-Jeangros C, Calmy A, Cavassini M, Egger M, Elzi L, Fehr J, Fellay J, Furrer H (Chairman of the Clinical and Laboratory Committee), Fux CA, Gorgievski M, Günthard H (President of the SHCS), Haerry D (deputy of "Positive Council"), Hasse B, Hirsch HH, Hösli I, Kahlert C, Kaiser L, Keiser O, Klimkait T, Kovari H, Ledergerber B, Martinetti G, Martinez de Tejada B, Metzner K, Müller N, Nadal D, Pantaleo G, Rauch A (Chairman of the Scientific Board), Regenass S, Rickenbach M (Head of Data Center), Rudin C (Chairman of the Mother & Child Substudy), Schmid P, Schultze D, Schöni-Affolter F, Schüpbach J, Speck R, Taffé P, Tarr P, Telenti A, Trkola A, Vernazza P, Weber R, Yerly S.
PISCIS: Coordinators: J. Casabona, Centre d'Estudis Epidemiològics les Infeccions de Transmissió Sexual i Sida de Catalunya (CEEISCAT), Jose M. Miró (Hospital Clínic de Barcelona-Idibaps, Universitat de Barcelona). Field coordinator: A. Gallois (CEEISCAT). Steering committee: J. Casabona, A. Gallois, A. Esteve (CEEISCAT), Jose M. Miró (Hospital Clínic de Barcelona-Idibaps, Universitat de Barcelona), D. Podzamczer (Hospital de Bellvitge de Barcelona),J. Murillas (Hospital Son Espases). Scientific committee: JM Gatell, C. Manzardo (Hospital Clínic-Idibaps, Universitat de Barcelona), C. Tural, B. Clotet (Hospital Universitari Germans Trias i Pujol, Universitat Autónoma de Barcelona), E. Ferrer (Hospital de Bellvitge), M. Riera (Hospital Son Espases), F. Segura, G. Navarro (Corporación Sanitaria Universitaria Parc Taulí, Universitad Autónoma de Barcelona), L. Force (Hospital de Mataró), J. Vilaró (Hospital General de Vic), A. Masabeu (Hospital de Palamós), I. García (Hospital General d’Hospitalet), M.Guadarrama (Hospital Alt Penedès de Vilafranca), C. Cifuentes (Hospital Son Llàtzer), D. Dalmau, À. Jaen (Hospital Universitari Mútua de Terrassa), C. Agustí (CEEISCAT). Data Management and statistical analysis: A. Esteve, A. Montoliu (CEEISCAT), I. Pérez (Hospital Clínic- Idibaps, Universitat de Barcelona). Technical support: I. Pérez (Hospital Clínic de Barcelona- Idibaps, Universitat de Barcelona), Freyra Gargoulas (Hospital Son Espases and Hospital Son Llàtzer). Clinicians involved: JL Blanco, F. Garcia-Alcaide, E. Martínez, J. Mallolas, M. López-Dieguez, JF García-Goez, (Hospital Clínic- Idibaps, Universitat de Barcelona), G. Sirera, J. Romeu, A. Jou. E. Negredo, C. Miranda, MC Capitan (Hospital Universitari Germans Trias i Pujol, Universitat Autónoma de Barcelona), M. Saumoy, A. Imaz, JM Tiraboschi, O. Murillo, F. Bolao, C. Peña, C. Cabellos, M Masó, A. Vila (Hospital Universitari de Bellvitge), M. Sala, M. Cervantes, Mª Jose Amengual, M. Navarro, E Penelo (Corporación Sanitaria Universitaria Parc Taulí, Universitad Autónoma de Barcelona), P. Barrufet, G. Bejarano ( Hospital de Mataró, Barcelona ), J. Molina, M. Guadarrama, M. Alvaro, J. Mercadal (Hospital Alt Penedès de Vilafranca). Civil society representatives: Juanse Fernández (Comitè 1er de Desembre), Jesús E. Ospina (RedVIH).
CoRIS/CoRIS-MD: Steering committee: J Berenguer, J del Amo, F García, F Gutiérrez, P Labarga, S Moreno, MA Muñoz. Field work, data management, and statistical analyses: AM Caro-Murillo, P Sobrino, I Jarrín. Participating centres: Hospital Universitario de Canarias, Santa Cruz de Tenerife (JL Gómez Sirvent, P Rodríguez, MR Alemán, MM Alonso, AM López, MI Hernández), Hospital Carlos III, Madrid (V Soriano, P Labarga, P Barreiro, J Medrano, P Rivas, D Herrero, F Blanco, ME Vispo, L Martín, G Ramírez, M de Diego), Hospital Doce de Octubre, Madrid (R Rubio, F Pulido, V Moreno, C Cepeda, Rl Hervás), Hospital Donostia, San Sebastián (JA Iribarren, J Arrizabalaga, MJ Aramburu, X Camino, F Rodríguez-Arrondo, MA von Wichmann, L Pascual, MA Goenaga), Hospital General Universitario de Elche (F Gutiérrez, M Masiá, JM Ramos, S Padilla, V Sánchez-Hellín, E Bernal, C Escolano, F Montolio, Y Peral), Hospital Gregorio Marañón, Madrid (J Berenguer, JC López, P Miralles, J Cosín, M Sánchez, I Gutiérrez, M Ramírez, B Padilla), Hospital Universitari de Tarragona Joan XXIII (F Vidal, M Sanjuan, J Peraire, S Veloso, C Viladés, M López-Dupla, M Olona, M Vargas), Hospital La Fe, Valencia (JL Aldeguer, M Blanes, J Lacruz, M Salavert, M Montero, S Cuéllar), Hospital de la Princesa, Madrid (I de los Santos, J Sanz), Hospital San Pedro, Logroño (JA Oteo, JR Blanco, V Ibarra, L Metola, M Sanz, L Pérez-Martínez), Hospital de Navarra, Pamplona (J Sola, J Uriz, J Castiello, J Reparaz, MJ Arriaza, C Irigoyen), Hospital Ramón y Cajal, Madrid (S Moreno, A Antela, JL Casado, F Dronda, A Moreno, MJ Pérez, D López, C Gutiérrez, B Hernández, M Pumares, P Martí, L García, C Page), Hospital San Cecilio, Granada (F García, J Hernández, A Peña, L Muñoz, J Parra), Hospital Universitario Virgen del Rocío, Sevilla (P Viciana, M Leal, LF López-Cortés, M Trastoy, R Mata).
Veterans Aging Cohort Study-Virtual Cohort: Principal investigator and co-principal investigator: AC Justice, DA Fiellin. Participating VA centers: Atlanta, GA (D. Rimland, C Jones-Taylor), Baltimore, MD (KA Oursler, R Titanji), Bronx, NY (S Brown, S Garrison), Houston, TX (M Rodriguez-Barradas, N Masozera), Los Angeles, CA (M Goetz, D Leaf), Manhattan-Brooklyn, NY (M Simberkoff, D Blumenthal, J Leung), Pittsburgh, PA (A Butt, E Hoffman), and Washington, DC (C Gibert, R Peck). Core Faculty: K Mattocks (Deputy Director), S Braithwaite, C Brandt, K Bryant, R Cook, J Conigliaro, K Crothers, J Chang, S Crystal, N Day, J Erdos, M Freiberg, M Kozal, N Gandhi, M Gaziano, M Gerschenson, B Good, A Gordon, JL Goulet, MA Hernán, K Kraemer, J Lim, S Maisto, P Miller, L Mole, P O’Connor, R Papas, JM Robins, C Rinaldo, M Roberts, J Samet, B Tierney, J Whittle.
UK Register of HIV Seroconverters: Steering Committee: A Phillips (Chair), University College London (UCL), London; A Babiker, MRC CTU, London; R Brettle, The Lothian University Hospitals NHS Trust, Edinburgh; J Darbyshire, MRC CTU, London; V Delpech, Health Protection Agency, London; P Easterbrook, King’s College Hospital, London; S Fidler, St Mary’s Hospital, London; M Fisher, Brighton & Sussex University Hospitals NHS Trust, Brighton; R Gilson, West London Centre for Sexual Health, London; D Goldberg, Health Protection Scotland, Glasgow; D Hawkins, Chelsea & Westminster NHS Trust, London; H Jaffe, University of Oxford, Oxford; A Johnson, UCL, London; M Johnson, UCL and Royal Free NHS Trust, London; K McLean, West London Centre for Sexual Health, London; D Pillay, UCL, London. Central co-ordination: Kholoud Porter (PI), Adam Cursley, Fiona Ewings, Keith Fairbrother, Louisa Gnatiuc, Sara Lodi, Brendan Murphy. Clinical centres and collaborators: G Douglas, Aberdeen City Hospital, Aberdeen; N Kennedy, Monklands Hospital, Airdrie; J Pritchard, Ashford Hospital, Ashford; U Andrady, Ysbyty Gwynedd, Bangor; N Rajda, North Hampshire Hospital, Basingstoke; R Maw, S McKernan, Royal Victoria Hospital, Belfast; S Drake, G Gilleran, D White, Birmingham Heartlands Hospital, Birmingham; J Ross, Whittall Street Clinic, Birmingham; S Toomer, Blackpool Victoria Hospital, Blackpool; R Hewart, Royal Bolton Hospital, Bolton; H Wilding, R Woodward, Royal Bournemouth Hospital, Bournemouth; G Dean, L Heald, Royal Sussex County Hospital, Brighton; P Horner, Bristol Royal Infirmary, Bristol; S Glover, Southmead Hospital, Bristol; D Bansaal, Queens Hospital, Burton-upon-Trent; S Eduards, West Suffolk Hospital, Bury St Edmunds; C Carne, Addenbrooke's Hospital, Cambridge; M Browing, R Das, Cardiff Royal Infirmary, Cardiff; B Stanley, North Cumbria Acute Hospitals NHS Trust, Carlisle; S Estreich, A Magdy, St Helier Hospital, Carshalton; C O’Mahony, Countess of Chester Hospital, Chester; P Fraser, Chesterfield & North Derbyshire Royal Hospital, Chesterfield; B Hayman, St Richard's Hospital, Chichester; SPR Jebakumar, Essex County Hospital, Colchester; U Joshi, Castle Hill Hospital, Cottingham; S Ralph, Bishop Auckland General Hospital, County Durham; A Wade, Coventry & Warwickshire Hospital, Coventry; R Mette, Mayday University Hospital, Croydon; J Lalik, Doncaster Royal Infirmary, Doncaster; H Summerfield, Weymouth Community Hospital, Dorset; A El-Dalil, Guest Hospital, Dudley; A J France, Dundee Royal Infirmary, Dundee; C White, University Hospital of North Durham, Durham; R Robertson, Muirhouse Medical Group, Edinburgh; S Gordon, S McMillan, S Morris, Royal Infirmary of Edinburgh, Edinburgh; C Lean, S Morris, Western General Hospital, Edinburgh; K Vithayathil, Leatherhead Hospital, Epsom; L McLean, A Winter, Gartnavel General Hospital & Glasgow Royal Infirmary, Glasgow; D Gale, S Jacobs, Gloucestershire Royal Hospital, Gloucester; Salford Hope Hospital, Greater Manchester; Farnham Road Hospital, Guildford; S Tayal, Hartlepool University Hospital, Hartlepool; L Short, Huddersfield Royal Infirmary, Huddersfield; Ayrshire Central Hospital, Irvine; M Roberts, S Green, Kidderminster General Hospital, Kidderminster; G Williams, Crosshouse Hospital, Kilmarnock; K Sivakumar, The Queen Elizabeth Hospital, King's Lynn; D N Bhattacharyya, Victoria Hospital, Kirkaldy; E Monteiro, Leeds General Infirmary, Leeds; J Minton, St James Hospital, Leeds; J Dhar, Leicester Royal Infirmary, Leicester; F Nye, Royal Liverpool University Hospital, Liverpool; CB DeSouza, A Isaksen, Barts & The London NHS Trust, London; L McDonald, Central Middlesex Hospital, London; K McLean, Charing Cross Hospital, London; A Franca, D Hawkins, Chelsea & Westminster Hospital, London; L William, Ealing Hospital, London; I Jendrulek, B Peters, Guy’s & St Thomas NHS Trust, London; S Shaunak, Hammersmith Hospital, London; S El-Gadi, Homerton Hospital, London; PJ Easterbrook, King's College Hospital, London; C Mazhude, Lewisham University Hospital, London; R Gilson, R Johnstone, Mortimer Market Centre, London; A Fakoya, Newham General Hospital, London; J Mchale, A Waters, North Middlesex Hospital, London; S Kegg, S Mitchell, Queen Elizabeth Hospital Woolwich, London; P Byrne, M Johnson, Royal Free Hospital, London; P Rice, St George’s Hospital, London; S Fidler, SA Mullaney, St Mary's Hospital, London; S McCormack, Victoria Sexual Health Clinic, London; D David, West Middlesex University Hospital, London; R Melville, Whipps Cross Hospital, London; K Phillip, Whittington Hospital, London; T Balachandran, Luton & Dunstable Hospital, Luton; S Mabey-Puttock, A Sukthankar, Manchester Royal Infirmary, Manchester; C Murphy, E Wilkins, North Manchester General Hospital, Manchester; S Ahmad, Withington Hospital, Manchester; S Tayal, James Cook Hospital, Middlesbrough; J Haynes, Milton Keynes General Hospital, Milton Keynes; E Evans, E Ong, Newcastle General Hospital, Newcastle; R Das, Royal Gwent Hospital, Newport; R Grey, J Meaden, Norfolk & Norwich University Hospital, Norwich; C Bignell, City Hospital, Nottingham; D Loay, K Peacock, George Eliot Hospital, Nunneaton; MR Girgis, Royal Oldham Hospital, Oldham; B Morgan, Radcliffe Infirmary, Oxford; A Palfreeman, Peterborough District Hospital, Peterborough; J Wilcox, Freedom Fields Hospital, Plymouth; J Tobin, L Tucker, St Mary's Hospital, Portsmouth; AM Saeed, Royal Preston Hospital, Preston; F Chen, Royal Berkshire Hospital, Reading; A Deheragada, East Surrey Hospital, Redhill; O Williams, Glan Clwyd District General, Rhyl; H Lacey, Baillie Street Health Centre, Rochdale; S Herman, D Kinghorn, Royal Hallamshire Hospital, Sheffield; S V Devendra, J Wither, Royal Shrewsbury Hospital, Shrewsbury; S Dawson, Upton Hospital, Slough; D Rowen, Royal South Hampshire Hospital, Southampton; J Harvey, Stirling Royal Infirmary, Stirling; E Wilkins, Stepping Hill Hospital, Stockport; A Bridgwood, G Singh, North Staffordshire Hospital, Stoke-on-Trent; M Chauhan, Sunderland Royal Hospital, Sunderland; D Kellock, S Young, King’s Mill Centre, Sutton-in-Ashfield; S Dannino, Y Kathir, Singleton Hospital, Swansea; G Rooney, The Great Western Hospital, Swindon; J Currie, M. Fitzgerald, Taunton & Somerset Hospital, Taunton; S Devendra, Princess Royal Hospital, Telford; F Keane, Royal Cornwall Hospital, Truro; G Booth, T Green, Clayton Hospital, Wakefield; J Arumainayyagam, S Chandramani, Manor Hospital, Walsall; S Rajamanoharan, T Robinson, Watford General Hospital, Watford; E Curless, Royal Albert Edward Infirmary, Wigan; R Gokhale, Arrowe Park Hospital, Wirral; A Tariq, New Cross Hospital, Wolverhampton; M Roberts, Worcester Royal Infirmary, Worcester; O Williams, Maelor Hospital, Wrexham; G Luzzi, Wycombe General Hospital, Wycombe; M FitzGerald, Yeovil District Hospital, Yeovil; I Fairley, F Wallis, Monkgate Health Centre, York Hospital NHS Trust, York. Laboratories: E Smit, HPA Birmingham; F Ward, St Bartholomew's and the Royal London NHS Trust
PRIMO: JM Molina, B Loze (St Louis - Paris), P Morlat, M Bonarek, F Bonnet, C Nouts, I Louis (St André - Bordeaux), F Raffi, V Reliquet, F Sauser, C Biron, O Mounoury, H Hue, D Brosseau (Hotel Dieu - Nantes), JF Delfraissy, C Goujard, J Ghosn, MT Rannou (Bicêtre – Le Kremlin Bicêtre), JF Bergmann, E Badsi, A Rami, M Diemer, MParrinello (Lariboisière - Paris), PM Girard, D Samanon-Bollens, P Campa, M Tourneur, N Desplanques (St Antoine - Paris), JM Livrozet, F Jeanblanc, P Chiarello, D Makhloufi (E Herriot - Lyon), AP Blanc, T Allègre (CHG - Aix en Provence), J Reynes, V Baillat, V Lemoing, C Merle de Boever, C Tramoni (Gui de Chauliac - Montpellier), A Cabié, G Sobesky, S Abel, V Beaujolais (CHU - Fort de France), G Pialoux, L Slama, C Chakvetadze, V Berrebi (Tenon - Paris), P Yeni, E Bouvet, I Fournier, J Gerbe (Bichat - Paris), C Trepo, K Koffi, C Augustin-Normand, P Miailhes, V Thoirain, C Brochier (Hotel Dieu - Lyon), R Thomas, F Souala, M Ratajczak (Pontchaillou - Rennes), J Beytoux, C Jacomet, F Gourdon (G Montpied - Clermont-Ferrand), E Rouveix, S Morelon, C Dupont, C Olivier (A Paré - Boulogne), O Lortholary, B Dupont, JP Viard, A Maignan (Necker - Paris), JM Ragnaud, I Raymond (Pellegrin - Bordeaux), C Leport, C Jadand, C Jestin, P Longuet, S Boucherit (Bichat - Paris), D Sereni, C Lascoux, F Prevoteau (St Louis - Paris), A Sobel, Y Levy, JD Lelièvre, AS Lascaux, S Dominguez, C Dumont (H Mondor - Créteil), H Aumaître, B Delmas, M Saada, M Medus (St Jean - Perpignan), L Guillevin, D Salmon, T Tahi (Cochin - Paris), Y Yazdanpanah, S Pavel, MC Marien (CH Dron - Tourcoing), B Drenou, G Beck-Wirth, C Beck, M Benomar (E Muller - Mulhouse), C Katlama, R Tubiana, H Ait Mohand, A Chermak, S Ben Abdallah (Pitié-Salpétrière - Paris), M Bentata, F Touam, (Avicenne - Bobigny), B Hoen, C Drobacheff, A Folzer (St Jacques - Besançon), P Massip, M Obadia, L Prudhomme, E Bonnet, F Balzarin (Purpan - Toulouse), E Pichard, JM Chennebault, P Fialaire, J Loison (CHR - Angers), P Galanaud, F Boué, D Bornarel (Béclère - Clamart), R Verdon, C Bazin, M Six, P Ferret (CHR Côte de Nacre - Caen), L Weiss, D Batisse, G Gonzales-Canali, D Tisne-Dessus (HEGP - Paris), A Devidas, P Chevojon, I Turpault (Corbeil Essonnes), A Lafeuillade, A Cheret, G Philip (Chalucet - Toulon), P Morel, J Timsit (St Louis - Paris), S Herson, N Amirat, A Simon, C Brancion (Pitié-Salpétrière - Paris), J Cabane, O Picard, J Tredup, N Desplanques (St Antoine - Paris), A Stein, I Ravault (La Conception - Marseille), C Chavanet, M Buisson, S Treuvetot (Bocage - Dijon), P Choutet, P Nau, F Bastides (Bretonneau - Tours), T May, L Boyer, S Wassoumbou (CHU - Nancy), E Oksenhendeler, L Gérard (St Louis - Paris), L Bernard, P De Truchis, H Berthé (R Poincaré - Garches), Y Domart, D Merrien (CH - Compiègne), A Greder Belan, (A Mignot - Le Chesnay), M Gayraud, L Bodard, A Meudec (IMM Jourdan - Paris), C Beuscart, C Daniel, E Pape (La Beauchée - St Brieuc), P Vinceneux, AM Simonpoli, A Zeng (L Mourier - Colombes), L Fournier (M Jacquet - Melun), JG Fuzibet, C Sohn, E Rosenthal, M Quaranta (L’Archet - Nice), P Dellamonica, S Chaillou, M Sabah (L’Archet - Nice), B Audhuy, A Schieber (L Pasteur - Colmar), P Moreau, M Niault, O Vaillant (Bretagne Sud - Lorient), G Huchon, A Compagnucci (Hotel-Dieu - Paris), I De Lacroix Szmania, L Richier (Intercommunal - Créteil), I Lamaury (Abymes - Pointe à Pitre), F Saint-Dizier, D Garipuy (Ducuing – Toulouse), JA Gastaut, MP Drogoul, I Poizot Martin, G Fabre (St Marguerite – Marseille), G Lambert de Cursay, B Abraham, C Perino (CH - Brives), P Lagarde, F David (CH - Lagny), J Roche-Sicot, JL Saraux, A Leprêtre (S Veil - Eaubonne), B Fampin, A Uludag, AS Morin (Beaujon – Clichy), O Bletry, D Zucman (Foch - Suresnes), A Regnier (CH - Vichy), JJ Girard (CH - Loches), DT Quinsat, L Heripret (CH - Antibes), F Grihon (Haute Vallée de l’Oise - Noyon), D Houlbert (CH - Alençon), M Ruel, K Chemlal (CH - Nanterre), F Caron, Y Debab (C Nicolle - Rouen), F Tremollieres, V Perronne (F Quesnay - Mantes La Jolie), G Lepeu, B Slama (H Duffaut - Avignon), P Perré (Les Oudairies - La Roche sur Yon), C Miodovski (Paris), G Guermonprez, A Dulioust (CMC Bligny - Briis s/Forges), P Boudon, D Malbec (R Ballanger - Aulnay s/bois), O Patey, C Semaille (CH - Villeneuve St Georges), J Deville, G Remy, I Béguinot (CH - Reims).
SEROCO: Hopital Antoine Beclere, Clamart (P Galanaud, F Boue, V Chambrin, C Pignon, GA Estocq, A Levy), Hopital de Bicetre, Le Kremlin Bicetre (JF Delfraissy, C Goujard, M Duracinsky, P Le Bras, MS Ngussan, D Peretti, N Medintzeff, T Lambert, O Segeral, P Lezeau, Y Laurian), Hopital Europeen Georges Pompidou, Paris (L Weiss, M Buisson, C Piketty, M Karmochkine, D Batisse, M Eliaszewitch, D Jayle, D Tisne- Dessus, M Kazatchkine), Hopital Bichat Claude Bernard, Paris (C Leport, U Colasante, C Jadand, C Jestin, X Duval, W Nouaouia, S Boucherit, JL Vilde), Hopital Saint Antoine, Paris (PM Girard, D Bollens, D Binet, B Diallo, MC Meyohas, L Fonquernie, JL Lagneau), Hopital Cochin, Paris (D Salmon, LGuillevin, T Tahi, O Launay, MP Pietrie, D Sicard, N Stieltjes, J Michot), Hopital Henri Mondor, Creteil (A Sobel, Y Levy, F Bourdillon, AS Lascaux, JD Lelievre, C Dumont), Hopital Necker, Paris (B Dupont, G Obenga, JP Viard, A Maignan), Hopital Paul Brousse, Villjuif (D Vittecoq, L Escaut, C Bolliot), Hopital Pitie Salpetriere, Paris (F Bricaire, C Katlama, L Schneider, S Herson, A Simon, M Iguertsira), Hopital de la Conception, Marseille (A Stein, C Tomei, I Ravaux, C Dhiver, H Tissot Dupont, A Vallon, J Gallais, H Gallais), Hopital Sainte Marguerite, Marseille (JA Gastaut, MP Drogoul, G Fabre), Hopital de L’Archet, Nice (P Dellamonica, J Durant, V Mondain, I Perbost, JP Cassuto, JM Karsenti, H Venti, JG Fuzibet, E Rosenthal, C Ceppi, M Quaranta), Hopital Avicenne, Bobigny (JA Krivitsky, M Bentata, O Bouchaud, P Honore), Hopital Saint Louis, Paris (D Sereni, C Lascoux, J Delgado), ACCTES / Hopital Necker, Paris (C Rouzioux, M Burgard, L Boufassa), Hopital Mignot, Le Chesnay (J Peynet).
GEMES: Principal Investigator: R Muga/S Pérez-Hoyos. Data analysis center:S Pérez-Hoyos,A Schiaffino Centro Nacional de Epidemiología: J del Amo,D Alvarez S Monge. Participating centres: Cohorte del Hospital Germans Trias I Pujol, Badalona (R Muga, A Sanvisens, B Clotet, J Tor, F Bolao, I Rivas, G Vallecillo), Cohorte de Madrid-Sandoval (J del Romero, P Raposo, C Rodríguez, M Vera), Cohorte de los CIPS de la Comunidad Valenciana (I Hurtado, J Belda, E Fernandez I Alastrue, C Santos T Tasa, A Juan, J Trullen), Cohortes de los CAS, de las Prisiones de Cataluña y de hemofílicos del Hospital Vall d´Hebron, Barcelona (P Garcia de Olalla, J Cayla, E Masdeu, H Knobel, JM Mirò MA Sambeat, R Guerrero, E Rivera), (R Guerrero, A Marco), Cohorte de hemofílicos del Hospital La Paz, Madrid (M Quintana, C Gonzalez), , Cohorte de Navarra (J Castilla, M Guevara). Laboratory: C de Mendoza, N Zahonero, M Ortíz.
AMACS: Steering Committee: Antoniadou A., Daikos G., Gargalianos-Kakolyris P., Katsarou O., Kordossis T., Lazanas M., Panos G., Paparizos V., Paraskevis D., Sambatakou H., Skoutelis A., Touloumi G. (Chair). Coordinating Center: Department of Hygiene, Epidemiology and Medical Statistics, Athens University Medical School, Greece (Touloumi G., Pantazis N., Bakoyannis G., Gioukari V.) Participating Centers: 4th Dept of Internal Medicine, Athens Medical School, Attikon University Hospital (Antoniadou A., Papadopoulos A., Petrikkos G); 1st Dept of Propedeutic Medicine, Athens University, Medical School “Laikon” General Hopsital (Daikos G, Psichogiou M); 1st Dept of Medicine, Infectious Diseases Unit, "G. Gennimatas" Athens General Hospital (Gargalianos-Kakolyris P., Xylomenos G.); Haemophilia Centre, 2nd Blood Transfusion Centre, “Laikon” Athens General Hospital (Katsarou O., Kouramba A., Ioannidou P.); AIDS Unit, Dept of Pathophysiology, “Laikon” Athens General Hospital and Athens University, Medical School (Kordossis T., Kontos A); Infectious Diseases Unit, Red Cross General Hospital of Athens (Lazanas M., Chini M., Tsogas N); HIV Unit, 2nd Internal Medicine Clinic, 1st IKA (Panos G.); AIDS Unit, Clinic of Venereologic & Dermatologic Diseases, Athens University, Medical School, Syngros Hospital (Paparizos V., Leuow K., Kourkounti S.); HIV Unit, 2nd Dpt. of Internal Medicine, Athens University, Medical School, Hippokration General Hospital (Sambatakou H., Mariolis I); Infectious Diseases & HIV Division, Dept of Internal Medicine, Evaggelismos Athens General Hospital (Skoutelis A., Papastamopoulos V., Baraboutis I)
Footnotes
All authors have contributed to the interpretation of the data and have read and approved the final version of the manuscript
Principal contributions made by the authors.
Data collection: Julia del Amo, Santiago Moreno, Heiner C. Bucher, Hansjakob Furrer, Santiago Pérez-Hoyos, Inma Jarrín, Andrew Phillips, Ashley Olson, Ard van Sighem, Peter Reiss, Caroline Sabin, Sophie Jose, Amy Justice, Joseph Goulet, José M. Miró, Elena Ferrer, Laurence Meyer, Rémonie Seng, Georgia Vourli, Anastasia Antoniadou, Francois Dabis, Mari-Anne Vandenhede, Dominique Costagliola, Sophie Abgrall; Study design: Sara Lodi, Julia del Amo, Santiago Moreno, Miguel Hernan; Statistical analyses: Sara Lodi, Roger Logan; Interpretation of results: All authors; Read and approved the manuscript: All authors; Drafted the manuscript: Sara Lodi, Miguel Hernan. Sara Lodi is the guarantor.
Ethics statement
All cohorts in the HIV-CAUSAL collaboration received approval from their individual ethics review boards. Approval was also given by all ethics review boards to pool anonymised data for analyses and dissemination. Signed informed consent was obtained from all patients.
References
- 1.CASCADE collaboration. Changes over calendar time in the risk of specific first AIDS-defining events following HIV seroconversion, adjusting for competing risks. Int J Epidemiol. 2002;31:951–958. doi: 10.1093/ije/31.5.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.HIV-CAUSAL collaboration. The effect of combined antiretroviral therapy on the overall mortality of HIV-infected individuals. AIDS. 2010;24:123–137. doi: 10.1097/QAD.0b013e3283324283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ledergerber B, Egger M, Erard V, Weber R, Hirschel B, Furrer H, et al. AIDS-related opportunistic illnesses occurring after initiation of potent antiretroviral therapy: the Swiss HIV Cohort Study. JAMA. 1999;282:2220–2226. doi: 10.1001/jama.282.23.2220. [DOI] [PubMed] [Google Scholar]
- 4.Mocroft A, Ledergerber B, Katlama C, Kirk O, Reiss P, d’Arminio Monforte A, et al. Decline in the AIDS and death rates in the EuroSIDA study: an observational study. Lancet. 2003;362:22–29. doi: 10.1016/s0140-6736(03)13802-0. [DOI] [PubMed] [Google Scholar]
- 5.DeSimone JA, Pomerantz RJ, Babinchak TJ. Inflammatory reactions in HIV-1-infected persons after initiation of highly active antiretroviral therapy. Ann Intern Med. 2000;133:447–454. doi: 10.7326/0003-4819-133-6-200009190-00013. [DOI] [PubMed] [Google Scholar]
- 6.French MA, Price P, Stone SF. Immune restoration disease after antiretroviral therapy. AIDS. 2004;18:1615–1627. doi: 10.1097/01.aids.0000131375.21070.06. [DOI] [PubMed] [Google Scholar]
- 7.Shelburne SA, Montes M, Hamill RJ. Immune reconstitution inflammatory syndrome: more answers, more questions. J Antimicrob Chemother. 2006;57:167–170. doi: 10.1093/jac/dki444. [DOI] [PubMed] [Google Scholar]
- 8.Achenbach CJ, Harrington RD, Dhanireddy S, Crane HM, Casper C, Kitahata MM. Paradoxical immune reconstitution inflammatory syndrome in HIV-infected patients treated with combination antiretroviral therapy after AIDS-defining opportunistic infection. Clin Infect Dis. 2012;54:424–433. doi: 10.1093/cid/cir802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bower M, Nelson M, Young AM, Thirlwell C, Newsom-Davis T, Mandalia S, et al. Immune reconstitution inflammatory syndrome associated with Kaposi’s sarcoma. J Clin Oncol. 2005;23:5224–5228. doi: 10.1200/JCO.2005.14.597. [DOI] [PubMed] [Google Scholar]
- 10.Knysz B, Kuliszkiewicz-Janus M, Jelen M, Podlasin R, Gladysz A. Non-Hodgkin’s lymphoma as a rare manifestation of immune reconstitution disease in HIV-1 positive patients. Postepy Hig Med Dosw (Online) 2006;60:547–551. [PubMed] [Google Scholar]
- 11.Muller M, Wandel S, Colebunders R, Attia S, Furrer H, Egger M. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:251–261. doi: 10.1016/S1473-3099(10)70026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Novak RM, Richardson JT, Buchacz K, Chmiel JS, Durham MD, Palella FJ, et al. Immune reconstitution inflammatory syndrome: incidence and implications for mortality. AIDS. 2012;26:721–730. doi: 10.1097/QAD.0b013e3283511e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Post MJ, Thurnher MM, Clifford DB, Nath A, Gonzalez RG, Gupta RK, et al. CNS-Immune Reconstitution Inflammatory Syndrome in the Setting of HIV Infection, Part 1: Overview and Discussion of Progressive Multifocal Leukoencephalopathy-Immune Reconstitution Inflammatory Syndrome and Cryptococcal-Immune Reconstitution Inflammatory Syndrome. AJNR Am J Neuroradiol. 2013;34:1297–1307. doi: 10.3174/ajnr.A3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shelburne SA, 3rd, Darcourt J, White AC, Jr, Greenberg SB, Hamill RJ, Atmar RL, et al. The role of immune reconstitution inflammatory syndrome in AIDS-related Cryptococcus neoformans disease in the era of highly active antiretroviral therapy. Clin Infect Dis. 2005;40:1049–1052. doi: 10.1086/428618. [DOI] [PubMed] [Google Scholar]
- 15.Haddow LJ, Colebunders R, Meintjes G, Lawn SD, Elliott JH, Manabe YC, et al. Cryptococcal immune reconstitution inflammatory syndrome in HIV-1-infected individuals: proposed clinical case definitions. Lancet Infect Dis. 2010;10:791–802. doi: 10.1016/S1473-3099(10)70170-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cinque P, Pierotti C, Vigano MG, Bestetti A, Fausti C, Bertelli D, et al. The good and evil of HAART in HIV-related progressive multifocal leukoencephalopathy. J Neurovirol. 2001;7:358–363. doi: 10.1080/13550280152537247. [DOI] [PubMed] [Google Scholar]
- 17.Murdoch DM, Venter WD, Van Rie A, Feldman C. Immune reconstitution inflammatory syndrome (IRIS): review of common infectious manifestations and treatment options. AIDS Res Ther. 2007;4:9. doi: 10.1186/1742-6405-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen F, Day SL, Metcalfe RA, Sethi G, Kapembwa MS, Brook MG, et al. Characteristics of autoimmune thyroid disease occurring as a late complication of immune reconstitution in patients with advanced human immunodeficiency virus (HIV) disease. Medicine. 2005;84:98–106. doi: 10.1097/01.md.0000159082.45703.90. [DOI] [PubMed] [Google Scholar]
- 19.Calabrese LH, Kirchner E, Shrestha R. Rheumatic complications of human immunodeficiency virus infection in the era of highly active antiretroviral therapy: emergence of a new syndrome of immune reconstitution and changing patterns of disease. Seminars in arthritis and rheumatism. 2005;35:166–174. doi: 10.1016/j.semarthrit.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Cheng VC, Ho PL, Lee RA, Chan KS, Chan KK, Woo PC, et al. Clinical spectrum of paradoxical deterioration during antituberculosis therapy in non-HIV-infected patients. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology. 2002;21:803–809. doi: 10.1007/s10096-002-0821-2. [DOI] [PubMed] [Google Scholar]
- 21.French MA, Mallal SA, Dawkins RL. Zidovudine-induced restoration of cell-mediated immunity to mycobacteria in immunodeficient HIV-infected patients. AIDS. 1992;6:1293–1297. doi: 10.1097/00002030-199211000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Narita M, Ashkin D, Hollender ES, Pitchenik AE. Paradoxical worsening of tuberculosis following antiretroviral therapy in patients with AIDS. Am J Respir Crit Care Med. 1998;158:157–161. doi: 10.1164/ajrccm.158.1.9712001. [DOI] [PubMed] [Google Scholar]
- 23.Shelburne SA, 3rd, Hamill RJ, Rodriguez-Barradas MC, Greenberg SB, Atmar RL, Musher DW, et al. Immune reconstitution inflammatory syndrome: emergence of a unique syndrome during highly active antiretroviral therapy. Medicine (Baltimore) 2002;81:213–227. doi: 10.1097/00005792-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Shelburne SA, Visnegarwala F, Darcourt J, Graviss EA, Giordano TP, White AC, Jr, et al. Incidence and risk factors for immune reconstitution inflammatory syndrome during highly active antiretroviral therapy. AIDS. 2005;19:399–406. doi: 10.1097/01.aids.0000161769.06158.8a. [DOI] [PubMed] [Google Scholar]
- 25.HIV CAUSAL collaboration. Impact of antiretroviral therapy on tuberculosis incidence among HIV-positive patients in high-income countries. Clin Infect Dis. 2012;54:1364–1372. doi: 10.1093/cid/cis203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gopal S, Patel MR, Achenbach CJ, Yanik EL, Cole SR, Napravnik S, et al. Lymphoma immune reconstitution inflammatory syndrome in the center for AIDS research network of integrated clinical systems cohort. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014;59:279–286. doi: 10.1093/cid/ciu270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ancelle-Park R. Expanded European AIDS case definition. Lancet. 1993;341:441. doi: 10.1016/0140-6736(93)93040-8. [DOI] [PubMed] [Google Scholar]
- 28.Kalbfleisch J, Prentice R. The statistical analysis of failure time data. New York: John Wiley and Sons; 1980. [Google Scholar]
- 29.Hernan MA, Hernandez-Diaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15:615–625. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 30.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 31.Jaffe HW, De Stavola BL, Carpenter LM, Porter K, Cox DR. Immune reconstitution and risk of Kaposi sarcoma and non-Hodgkin lymphoma in HIV-infected adults. AIDS. 2011;25:1395–1403. doi: 10.1097/QAD.0b013e3283489c8b. [DOI] [PubMed] [Google Scholar]
- 32.Lodi S, Guiguet M, Costagliola D, Fisher M, de Luca A, Porter K. Kaposi sarcoma incidence and survival among HIV-infected homosexual men after HIV seroconversion. J Natl Cancer Inst. 2010;102:784–792. doi: 10.1093/jnci/djq134. [DOI] [PMC free article] [PubMed] [Google Scholar]


