Summary
Objective
Inhibitors of the mechanistic target of rapamycin (mTOR) pathway have antiepileptogenic effects in preventing epilepsy and pathological and molecular mechanisms of epileptogenesis in mouse models of tuberous sclerosis complex (TSC). However, long-term treatment with mTOR inhibitors may be required to maintain efficacy and potentially has chronic side effects, such as immunosuppression. Attempts to minimize drug exposure will facilitate translational efforts to develop mTOR inhibitors as antiepileptogenic agents for TSC patients. In this study, we tested intermittent dosing paradigms of mTOR inhibitors for antiepileptogenic properties in a TSC mouse model.
Methods
Western blot analysis of phosphorylation of S6 protein was used to assess the dose- and time-dependence of mTOR inhibition by rapamycin in control mice and conditional knock-out mice with inactivation of the Tsc1 gene in glial fibrillary acidic protein (GFAP)-expressing cells (Tsc1GFAPCKO mice). Based on the western blot studies, different dosing paradigms of rapamycin starting at postnatal day 21 were tested for their ability to prevent epilepsy or pathological abnormalities in Tsc1GFAPCKO mice: 4 days of rapamycin only (4-∞), 4 days on-24 days off (4–24), and 4 days on-10 days off (4–10).
Results
mTOR activity was inhibited by rapamycin in a dose-dependent fashion and recovered to baseline by about 10 days after the last rapamycin dose. 4–10 and 4–24 dosing paradigms almost completely prevented epilepsy and the 4–10 paradigm inhibited glial proliferation and megalencephaly in Tsc1GFAPCKO mice.
Significance
Intermittent dosing of rapamycin, with drug holidays of more than three weeks, maintains significant antiepileptogenic properties in mouse models of TSC. These findings have important translational applications in developing mTOR inhibitors as antiepileptogenic agents in TSC patients by minimizing drug exposure and potential side effects.
Keywords: epilepsy, seizure, rapamycin, mTOR, mice
Introduction
A holy grail of epilepsy research is to develop a disease-modifying or antiepileptogenic treatment for epilepsy. Current medical treatments for epilepsy can suppress seizures symptomatically, but do not have antiepileptogenic properties to prevent the development or progression of epilepsy. The mechanistic/mammalian target of rapamycin (mTOR) signaling pathway has been implicated in mediating epileptogenesis and representing a potential antiepileptogenic target in the genetic epilepsy, tuberous sclerosis complex (TSC).1 In mouse models of TSC, Tsc1 or Tsc2 gene inactivation causes dysregulated mTOR activity and epilepsy. mTOR inhibitors prevent the development of seizures and the associated pathological and molecular abnormalities that promote epileptogenesis in these models, such as glial proliferation and megalencephaly.2–7
Clinical trials are already ongoing testing the effects of an mTOR inhibitor, everolimus, on refractory seizures in TSC patients with established, intractable epilepsy.8 As many TSC patients are identified at a young age prior to the onset of seizures and are at high risk for future epilepsy, TSC may also represent a rational, feasible population to target with an antiepileptogenic approach. However, an antiepileptogenic drug trial of TSC patients has not yet been undertaken due to a number of practical barriers. One significant barrier is that long-term treatment initiated at a very young age (i.e. infancy) is likely required to maintain efficacy in the setting of chronic mTOR hyperactivation due to the underlying TSC gene mutations. Since mTOR inhibitors may have significant side effects, such as immunosuppression, efforts to reduce drug exposure may improve the translational potential and feasibility of mTOR inhibitors as antiepileptogenic drugs in TSC patients. In this study, we tested intermittent dosing paradigms of mTOR inhibitors, with drug holidays of various durations, for antiepileptogenic properties in a TSC mouse model, Tsc1GFAPCKO mice, which develop progressive epilepsy and premature death, as well as pathological brain abnormalities contributing to epileptogenesis.2,5,9
Methods
Animals
Care and use of animals were conducted according to an animal protocol approved by the Washington University Animal Studies Committee. Tsc1flox/flox-GFAP-Cre knock-out (Tsc1GFAPCKO) mice with conditional inactivation of the Tsc1 gene in glial fibrillary acidic protein (GFAP)-containing cells were generated as described previously.2, 9 Tsc1flox/+-GFAP-Cre and Tsc1flox/flox littermates have previously been found to have no abnormal phenotype and were used as control animals in these experiments. Both male and female mice were used, as previous studies have found no significant sex differences in the epilepsy phenotype of Tsc1GFAPCKO mice,10 which was confirmed by subgroup analysis of the current data.
Rapamycin treatment paradigms
Rapamycin (LC Labs, Woburn, MA) was initially dissolved in 100% ethanol, stored at −20°C, and diluted in a vehicle solution containing 5% Tween 80, 5% PEG 400 (Sigma, St. Louis, MO) and 4% ethanol immediately before injection. In initial studies assessing dose-dependence of mTOR inhibition by rapamycin, control and Tsc1GFAPCKO mice were injected with rapamycin for 4 days at doses ranging between 0.1 to 10 mg/kg/d i.p. and harvested 24 hours after the last injection for western blot analysis. In other studies assessing the duration of mTOR inhibition by rapamycin, control and Tsc1GFAPCKO mice were injected with rapamycin for 4 days at 3 mg/kg/d and harvested between 3 hours and 21 days after the last injection for western blot analysis.
Daily, chronic injections of rapamycin starting during the first few weeks of life have been shown to prevent epilepsy and associated pathological abnormalities causing epileptogenesis in Tsc1GFAPCKO mice.2 To determine whether intermittent dosing of rapamycin can inhibit pathological abnormalities, we primarily compared two different rapamycin dosing paradigms (3 mg/kg/d), starting at postnatal day 21: 4 days on-24 days off (4–24), and 4 days on-10 days off (4–10). Vehicle-treated and rapamycin-treated control mice and vehicle-treated knock-out mice served as control groups. Control mice treated with the 4–10 and 4–24 paradigms were not significantly different, so these groups were combined. To examine effects on epilepsy, a third rapamycin treatment group was also included, with rapamycin administered for 4 consecutive days starting at postnatal day 21 only (4-∞). Mice were harvested at specific time points depending on the study. Serum levels of rapamycin were measured by the St. Louis Children’s Hospital clinical laboratory.
Western blotting
Western blotting was performed using standard methods as described previously.2 Briefly, neocortex was dissected and homogenized. Equal amounts of total protein extract were separated by gel electrophoresis and transferred to nitrocellulose membranes. Primary antibodies to P-S6 (Ser240/244) and S6, (1:1,000, Cell Signaling Technology, Danvers, MA) were used. The membranes were then reacted with a peroxidase-conjugated secondary antibody. Signals were detected by enzyme chemiluminescence (GE Healthcare, Buckinghamshire, UK) and quantitatively analyzed using ImageJ software. The ratios of P-S6 to total S6 and were used as measures of activation of the mTOR pathway. Normalization to total S6 also served as a control for protein loading.
Histology/Immunohistochemistry
Histological analysis was performed at 7 and 17 weeks of age to assess glial proliferation and megalencephaly previously reported to be associated with epileptogenesis and inhibited by rapamycin in Tsc1GFAPCKO mice, using standard methods as described previously.2 Briefly, mice were transcardially perfused with PBS, pH 7.4, followed by 4% paraformaldehyde in PBS, pH 7.4. The brains were removed and post-fixed in 4% paraformaldehyde in PBS overnight at 4°C. Fixed brains were transferred to 30% sucrose for at least 24 hours and then sectioned coronally with a sliding microtome at a thickness of 45 μm. For cresyl violet staining, sections were mounted and immersed in 0.5% cresyl violet for 3 min. For GFAP staining, sections were incubated with GFAP antibody (anti-rabbit, 1:500, Cell Siginaling Technology, Danvers, MA) overnight at 4°C. After washing with PBS, the sections were incubated with Cy3 conjugated anti-rabbit IgG (1:500, Jackson ImmunoResearch, West Grove, PA) immersed in DAPI solution (Sigma, St. Louis, MO) and then cover-slipped with anti-fade mount solution (Vector Laboratories, Burlingame, CA). Images were acquired with a Hamamatsu NanoZoomer 2.0 pathological microscope. GFAP-immunoreactive cells in neocortex and hippocampus were counted by an investigator blinded to the treatment of the mice. In images from coronal sections at ~ 2 mm posterior to bregma and ~ 1 mm from midline, regions of interest were marked in neocortex by a 200 μm wide box spanning from the neocortical surface to the bottom of layer VI. Both hippocampus and dentate gyrus were quantified using 200 x 200μm2 areas from the CA1 pyramidal cell layer to stratum lacunosum moleculare and the polymorph layer of dentate gyrus. GFAP-immunoreactive cells were quantified bilaterally in the regions of interest from 2 sections per mouse from a total of 6–17 mice per group in a blinded fashion.
Video-EEG Monitoring
Video-EEG monitoring was performed, starting at 3 weeks of age until 17 weeks of age, to assess seizure frequency, as described previously.2 As Tsc1GFAPCKO mice typically start having seizures around 4 weeks of age,2,9 it is important to start video-EEG monitoring before this age. However, due to their small size, EEG tethering significantly limits mobility of Tsc1GFAPCKO mice at 3 weeks of age. Thus, 3 week old Tsc1GFAPCKO mice were monitored for an initial 48 hour recording period, but untethered for the rest of the week. Starting at 4 weeks of age, the mice are larger allowing for continuous (24/7) EEG monitoring for the remainder of the experiment. Briefly, four epidural screw electrodes were surgically implanted in mice under isoflurane anesthesia. Continuous video-EEG data were acquired with Stellate systems or AD Instruments video-EEG systems. Electrographic seizures were identified by their characteristic pattern of discrete periods of rhythmic spike discharges that evolved in frequency and amplitude lasting at least 10 seconds, typically ended with repetitive burst discharges, and followed by severe voltage suppression. On video analysis, the behavioral correlate to these seizures typically involved head bobbing, rearing with forelimb clonus, and occasional generalized convulsive activity. Seizure frequency (# seizures/week) was calculated for each week.
Statistics
SigmaStat (Systat Software, San Jose, CA) was used for statistical analysis. Quantitative differences between groups were analyzed by one-way analysis-of-variance (ANOVA) with Tukey multiple comparisons post-tests. When data did not conform to a normal distribution, a non-parametric (Kruskal-Wallis) ANOVA with Dunn’s multiple comparisons post-tests was used. Survival data was analyzed by a Kaplan-Meier LogRank test or Chi-square analysis. Statistical significance was defined as P<0.05. Data are reported as mean ± SEM.
Results
Dose-dependence and time course of mTOR inhibition by rapamycin
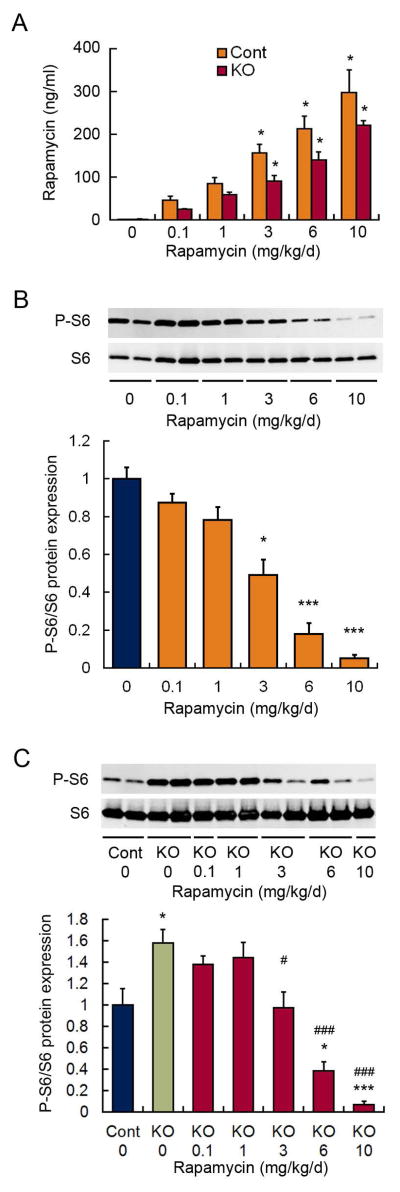
Under the assumption that the degree of mTOR inhibition correlates directly with antiepileptogenic efficacy and side effects, we first systematically assessed the dose-dependence and time course of mTOR inhibition in both control and Tsc1GFAPCKO mice, to help devise rational dosing paradigms that minimize drug exposure but maintain antiepileptogenic efficacy. Rapamycin showed a dose-dependent increase in serum levels in both control and Tsc1GFAPCKO mice (Fig. 1A). There appeared to be a trend toward lower rapamycin levels in Tsc1GFAPCKO mice compared with control mice, but this was not statistically significant. In control mice, rapamycin correspondingly induced a dose-dependent inhibition of mTOR activity, as reflected by P-S6 expression, with 3–6 mg/kg/d causing moderate degrees of mTOR inhibition and 10 mg/kg/d resulting in almost complete suppression of mTOR activity (Fig. 1B). In comparison with control mice, vehicle-treated Tsc1GFAPCKO mice have elevated mTOR activity. In Tsc1GFAPCKO mice, rapamycin also a caused a dose-dependent inhibition of mTOR activity, with 3 mg/kg/d of rapamycin restoring mTOR activity to the level of control mice and 10 mg/kg/d of rapamycin causing almost complete suppression (Fig. 1C). Based on this dose-dependence, it was presumed that 3 mg/kg/d may have antiepileptogenic efficacy but have fewer side effects than higher doses, which inhibit mTOR below control levels.
Figure 1.
Dose-dependence of mTOR inhibition by rapamycin. (A) Rapamycin administered at various doses (i.p.) for four consecutive days causes a dose-dependent increase in serum levels, sampled 24 hours after the last dose, in both control and Tsc1GFAPCKO mice. *p<0.05, compared to vehicle-treated mice by Kruskal-Wallis with Dunn’s; n = 8 per group. (B) Rapamycin similarly causes a dose-dependent inhibition of mTOR activity, as assayed by P-S6 expression in brain homogenates, in control mice. *p<0.05, ***p<0.001, compared to vehicle-treated control mice by ANOVA with Tukey; n = 8 per group. (C) Tsc1GFAPCKO mice have increased mTOR activity compared with control mice, and rapamycin causes a dose-dependent inhibition of mTOR activity in Tsc1GFAPCKO mice. *p<0.05, ***p<0.001, compared to vehicle-treated control mice; #p<0.05, ###p<0.001 compared to vehicle-treated Tsc1GFAPCKO mice by ANOVA with Tukey; n = 6–10 per group.
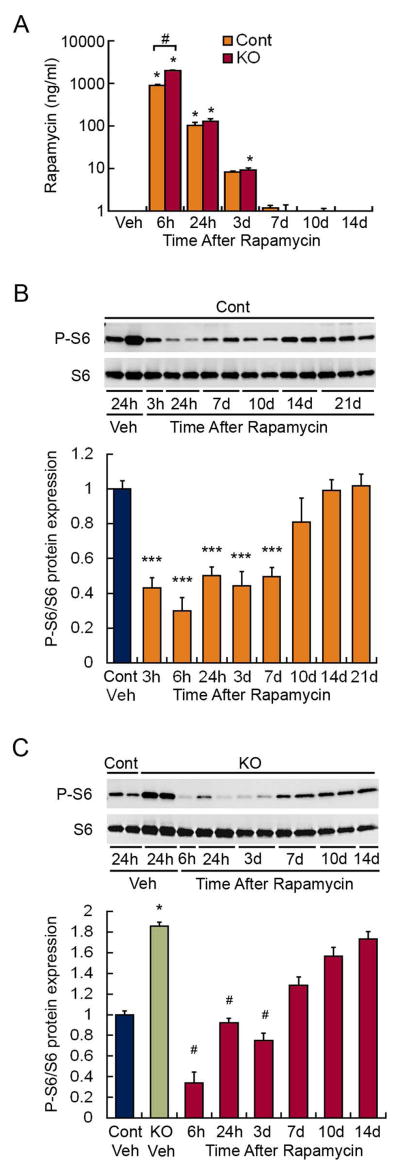
To assess the duration and time course of recovery of mTOR inhibition following rapamycin, P-S6 expression was assessed at various time points after receiving 4 days of rapamycin at 3 mg/kg/d. Serum levels of rapamycin decreased to undetectable by 10 days after the last rapamycin dose in both control and Tsc1GFAPCKO mice (Fig. 2A). In control mice, mTOR inhibition was present 3 hours after the last dose and persisted for at least 7 days, with mTOR activity starting to return toward control levels by 10 days (Fig. 2B). In Tsc1GFAPCKO mice, rapamycin had a similar time course of mTOR inhibition, except recovery started to occur slightly earlier around 7 days (Fig. 2C). Again based on the assumption that inhibition of mTOR activity directly correlates with antiepileptogenic efficacy, these time course studies suggest that intermittent dosing of rapamycin with drug holidays of at least 7 days may be able to maintain antiepileptogenic efficacy.
Figure 2.
Time-dependence of mTOR inhibition by rapamycin. (A) Rapamycin administered at 3 mg/kg/d (i.p.) for four consecutive days causes an increase in serum levels within 6 hours, which decreases to undetectable levels by about 10 days after the last dose. *p<0.05, compared to vehicle-treated mice; #p<0.05 comparing control to Tsc1GFAPCKO mice at 6h by Kruskal-Wallis with Dunn’s; n = 6–9 per group. (B) Rapamycin administered at 3 mg/kg/d (i.p.) for four consecutive days causes an inhibition of mTOR activity in control mice, as assayed by P-S6 expression in brain homogenates, which persists for at least 7 days after the last dose. ***p<0.001, compared to vehicle-treated control mice by ANOVA with Tukey; n = 5–12 per group. (C) Rapamycin administered at 3 mg/kg/d (i.p.) for four consecutive days causes an inhibition of mTOR activity in Tsc1GFAPCKO mice, as assayed by P-S6 expression in brain homogenates, which persists for about 7 days after the last dose. *p<0.05 compared to vehicle-treated control mice #p<0.05, compared to vehicle-treated Tsc1GFAPCKO mice by Kruskal-Wallis with Dunn’s; n = 5–12 per group.
Intermittent dosing of rapamycin prevents epilepsy in Tsc1GFAPCKO mice
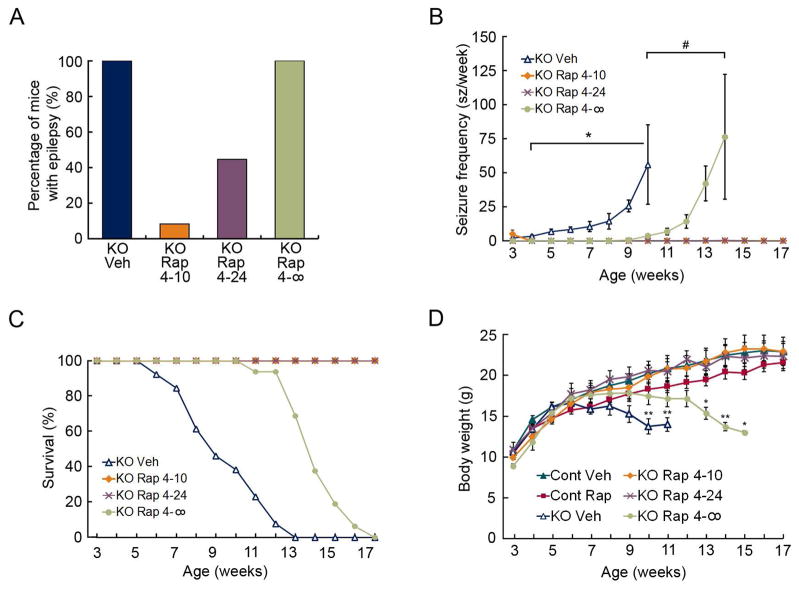
Daily, chronic injections of rapamycin prevent epilepsy in Tsc1GFAPCKO mice.2 To determine whether intermittent dosing of rapamycin can also inhibit epilepsy, we compared two different rapamycin dosing paradigms (3 mg/kg/d), starting at postnatal day 21: 4 days on-24 days off (4–24) and 4 days on-10 days off (4–10), and followed by continuous video-EEG monitoring up to 17 weeks of age. Seizures develop in 100% of vehicle-treated Tsc1GFAPCKO mice around 3–4 weeks of age and become progressively more frequent over the following 6–8 weeks until they die prematurely, with all mice dying by 13 weeks of age (Fig. 3A–C). Although a few mice had seizures in the immediate post-surgical period (3 weeks), the 4–10 treatment paradigm completely prevented the subsequent development of seizures in 11 of 12 Tsc1GFAPCKO mice, with only one mouse having a single seizure at 10 weeks of age. The 4–24 treatment paradigm was also very effective in preventing epilepsy development in 5 of 9 Tsc1GFAPCKO mice, with three mice having just two seizures each. All 4–10 and 4–24 treatment mice survived through the 17 week monitoring period. To assess the longest duration of effectiveness of rapamycin, an additional group of mice were given 4 consecutive daily doses of rapamycin at 3 weeks of age and monitored until death (4-∞ group). All these mice started having seizures between 9–12 weeks of age, which increased in frequency over the next few weeks, and all died by 17 weeks of age. While vehicle-treated and 4-∞ treated Tsc1GFAPCKO mice developed progressive weight loss correlating with the progression of seizures, the 4–10 and 4–24 groups maintained body weight at the same rate as control mice (Fig. 3D). Thus, overall the 4–10 and 4–24 treatment groups were very effective in preventing the progression of epilepsy in Tsc1GFAPCKO mice and had no significant side effects on weight gain.
Figure 3.
Intermittent rapamycin dosing prevents epilepsy progression, prolongs survival, and improves body weight of Tsc1GFAPCKO mice. (A) All vehicle-treated Tsc1GFAPCKO mice and Tsc1GFAPCKO mice treated with rapamycin for 4 consecutive days at 3 weeks of age only (4-∞) develop epilepsy. Tsc1GFAPCKO mice, with intermittent rapamycin treatment of 4 days on-10 days off (4–10) or 4 days on-24 days off (4–24) only developed epilepsy in 8% and 44% of mice, respectively. p<0.001 by Chi-Square test; n=9–12 per group. (B) Vehicle-treated Tsc1GFAPCKO mice exhibit a progressive increase in seizure frequency between 3–9 weeks of age. Treatment with rapamycin for 4 consecutive days at 3 weeks of age (4-∞) significantly delayed the progression of seizure frequency. 4–10 and 4–24 almost completely inhibited seizures in Tsc1GFAPCKO mice, with even the mice that developed epilepsy having a very low seizure frequency. *p<0.05 compared to rapamycin-treated Tsc1GFAPCKO mice by Kruskal-Wallis with Dunn’s; #p<0.05 compared to (4–10) and (4–24) rapamycin-treated Tsc1GFAPCKO mice by Kruskal-Wallis with Dunn’s; n = 9–12 per group. (C) Vehicle-treated Tsc1GFAPCKO mice have reduced survival, with all mice dying by 13 weeks of age. The 4-∞ rapamycin treatment paradigm significantly prolonged survival by about 4–5 weeks, whereas all mice treated with the 4–10 and 4–24 paradigms survived through the duration of the study. p<0.001 by Chi-Square test; n=15–18 per group. (D) Vehicle-treated and 4-∞ rapamycin-treated Tsc1GFAPCKO mice develop progressive weight loss. The 4–10 and 4–24 rapamyin paradigms had no significant effect on body weight compared with control mice. *p<0.05, **p<0.01, compared to vehicle-treated control mice by ANOVA with Tukey; n = 8–10 per group.
Intermittent dosing of rapamycin prevents pathological abnormalities in Tsc1GFAPCKO mice
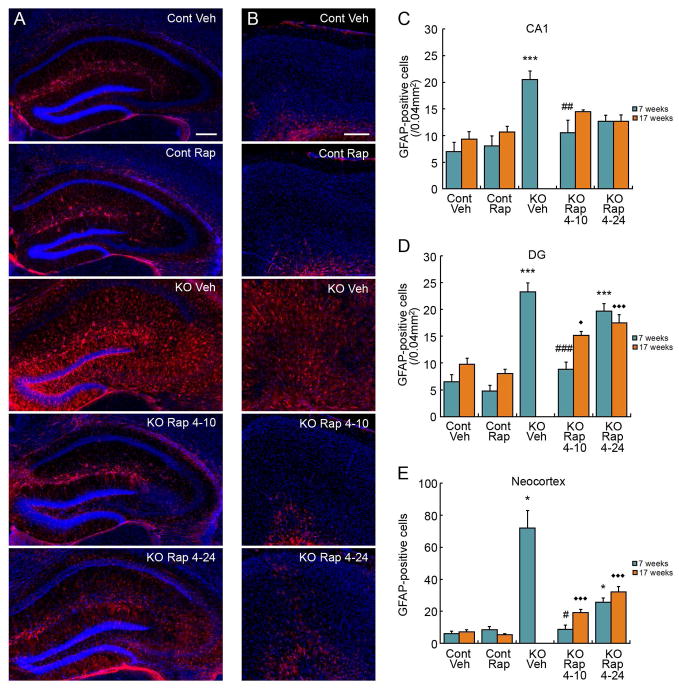
A number of pathological abnormalities in astrocytes and neurons correlate with development of epilepsy in Tsc1GFAPCKO mice. Daily, chronic injections of rapamycin prevent these pathological abnormalities in Tsc1GFAPCKO mice.2 As previously reported, 7 week old vehicle-treated Tsc1GFAPCKO mice show a dramatic increase in GFAP-positive astrocytes in hippocampus and neocortex compared with control mice (Fig. 4). The 4–10 rapamycin treatment paradigm completely prevented the increase in GFAP-positive astrocytes in all regions in Tsc1GFAPCKO mice at 7 weeks of age, maintaining astrocyte numbers at control levels. With the 4–24 rapamycin treatment paradigm, there also appeared to be a decrease in the elevated astrocytes, especially in CA1, where astrocyte number was not significantly different than control mice, although the decrease compared with vehicle-treated Tsc1GFAPCKO mice did not reach statistical significance. At 17 weeks of age, no vehicle-treated Tsc1GFAPCKO mice survived to allow direct pathological comparisons of treatment effectiveness. However, the astrocyte number in CA1 in both the 4–10 and 4–24 treatment groups were again not significantly different than control mice at 17 weeks, suggesting that these regimens maintained effectiveness at 17 weeks. In dentate gyrus and neocortex, astrocyte number was increased in 4–10 and 4–24 paradigms at 17 weeks compared with control mice.
Figure 4.
Intermittent rapamycin dosing partially inhibits astrocyte proliferation in Tsc1GFAPCKO mice. Representative images of GFAP-labeled astrocytes (red) and DAPI counterstaining (blue) in control and Tsc1GFAPCKO mice, treated with vehicle or intermittent 3 mg/kg rapamycin dosing (4 days on-24 days off [4–24], and 4 days on-10 days off [4–10]) in hippocampus (A) and neocortex (B). Quantitative analysis of GFAP-positive cells at 7 and 17 weeks of age in CA1 (C), dentate gyrus (D), and neocortex (E). Note that no vehicle-treated Tsc1GFAPCKO mice survived to 17 weeks for comparison. *p<0.05,***p<0.001, compared to vehicle-treated control mice; #p<0.05, ##p<0.01, ###p<0.001 compared to vehicle-treated Tsc1GFAPCKO mice at 7 weeks by ANOVA with Tukey or Kruskal-Wallis with Dunn’s depending on normality; n = 6–13 per group. ◆p<0.05, ◆◆◆p<0.001 compared to vehicle-treated control mice at 17 weeks by ANOVA with Tukey; n=6–17 per group.
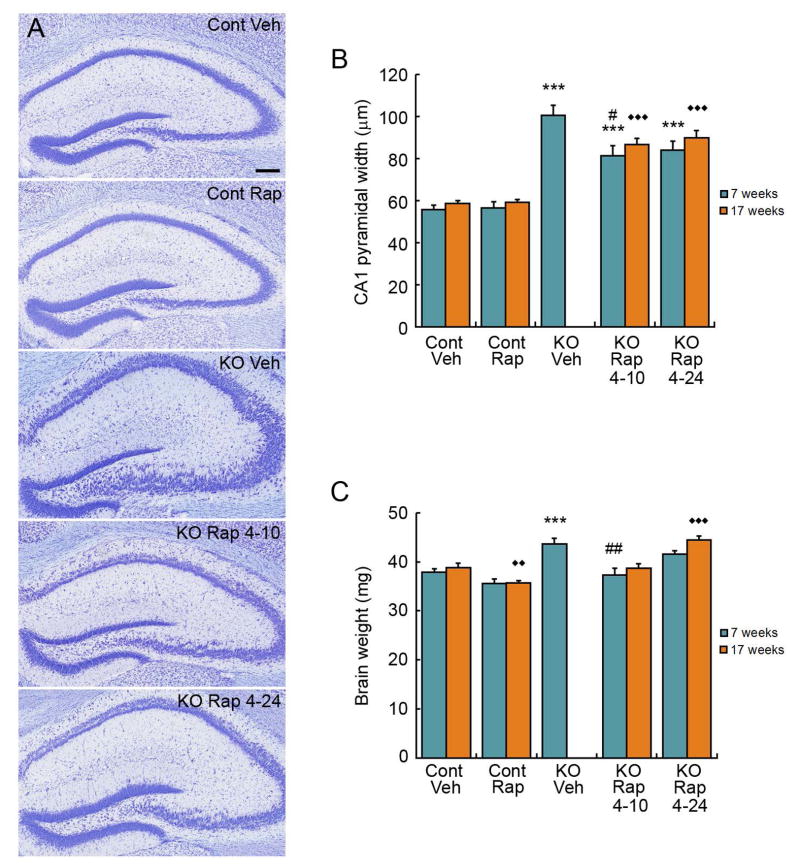
Dispersion of the pyramidal cell layer in hippocampus is another pathological abnormality that may contribute to epileptogenesis in Tsc1GFAPCKO mice. Seven week-old vehicle-treated knock-out mice have a significantly increased width of the CA1 region compared to control mice (Fig. 5A,B). The 4–10, but not the 4–24, rapamycin treatment paradigm partially reversed this abnormality in Tsc1GFAPCKO mice.
Figure 5.
Intermittent rapamycin dosing partially inhibits pyramidal neuron dispersion and megalencephaly in hippocampus in Tsc1GFAPCKO mice. (A) Representative images of hippocampal pyramidal layers in control and Tsc1GFAPCKO mice, treated with vehicle or intermittent 3 mg/kg rapamycin dosing (4 days on-24 days off [4–24], and 4 days on-10 days off [4–10]). (B) Quantitative analysis of CA1 pyramidal cell layer width. Note that no vehicle-treated Tsc1GFAPCKO mice survived to 17 weeks for comparison. ***p<0.001 compared to vehicle-treated control mice; #p<0.05 compared to vehicle-treated Tsc1GFAPCKO mice at 7 weeks by ANOVA with Tukey; n = 6–13 per group. ◆◆ ◆p<0.001 compared to vehicle-treated control at 17 weeks by ANOVA with Tukey; n=6–17 per group. (C) Tsc1GFAPCKO mice have increased brain weight compared with control mice. The 4–10 intermittent rapamycin paradigm, but not the 4–24 paradigm, inhibits this increased brain weight in Tsc1GFAPCKO mice. ***p<0.001 compared to vehicle-treated control mice; ##p<0.01 compared to vehicle-treated Tsc1GFAPCKO mice at 7 weeks by ANOVA with Tukey; n = 6–13 per group. ◆◆p<0.01, ◆◆◆p<0.001 compared to vehicle-treated control mice at 17 weeks by ANOVA with Tukey; n=6–17 per group.
The cellular abnormalities in astrocytes and neurons are associated with overall increased brain size or megalencephaly in Tsc1GFAPCKO mice. The brain weight of Tsc1GFAPCKO mice is significantly increased compared with control mice (Fig. 5C). The 4–10 rapamycin treatment paradigm, but not the 4–24 paradigm, prevented this megalencephaly in Tsc1GFAPCKO mice. Overall, the 4–10 treatment paradigm was effective in completely or partially reversing pathological abnormalities in Tsc1GFAPCKO mice, whereas the 4–24 paradigm was less effective.
Discussion
In this study, we tested the effect of intermittent dosing of rapamycin in preventing pathological abnormalities and epilepsy in a mouse model of TSC. We find that intermittent rapamycin dosing, with drug holidays as long as 24 days, is still effective in preventing the development or progression of seizures in Tsc1GFAPCKO mice, which normally have severe, progressive epilepsy. Intermittent rapamycin also inhibited the pathological abnormalities associated with epileptogenesis, although the pathological effects were not necessarily complete. These findings have important translational applications for designing rational antiepileptogenic treatment protocols for TSC patients that minimize drug exposure and side effects.
Current medications for epilepsy directly decrease neuronal excitability via modulation of ion channels or neurotransmitters, but do not have proven disease-modifying properties to prevent the development or progression of epilepsy in patients.11 In contrast, mTOR inhibitors have limited or no direct effects on neuronal excitability and acute seizures12–17, but modulate other molecular and pathological abnormalities associated with epileptogenesis and decrease or prevent epilepsy development in animal models of TSC.2–7 An important point is that these effects in TSC models are only maintained with continued rapamycin treatment, as the underlying genetic defect driving mTOR hyperactivation persists after rapamycin is stopped. However, unlike conventional antiseizure drugs, which do not correct the underlying mechanistic abnormalities of epileptogenesis, rapamycin modifies most of the underlying pathological and molecular defects driving epileptogenesis, such as astrocyte proliferation, pyramidal cell dispersion, and glutamate transporter expression in Tsc1GFAPCKO mice,2 indicating that mTOR inhibition has true antiepileptogenic effects, at least during the duration of treatment.12
For clinical reasons TSC patients may also represent a model population for developing an antiepileptogenic therapy. To prevent epilepsy, high risk patients must be identified before the onset of seizures. A subset of TSC patients are diagnosed during infancy due to non-neurological findings, such as cardiac rhabdomyomas.18 As TSC patients are at high risk for developing epilepsy, with a prevalence of up to 90%,19 initiating a potential antiepileptogenic treatment at a young age to attempt to prevent epilepsy is both feasible and justifiable.
Despite the strong rationale, there are significant limitations and concerns about testing mTOR inhibitors as antiepileptogenic therapy in TSC, particularly related to potential side effects. The mTOR pathway has been implicated in regulating a number of important physiological processes related to normal development and learning, such as cell growth, neurogenesis, and synaptic plasticity. mTOR inhibitors are known to have significant side effects, such as hyperlipidemia, liver toxicity, hematological abnormalities and chronic immunosuppression with the associated risk of serious, opportunistic infections.20 These theoretical and known adverse effects are especially relevant in TSC, as antiepileptogenic treatment would likely need to be initiated at a very young age, probably infancy, and maintained for a long time, possibly indefinitely.
Given concerns about chronic side effects, dosing regimens to minimize drug exposure may reduce side effects but maintain efficacy. One rational principle particularly relevant to TSC is that the goal of mTOR inhibitor treatment is to restore “normal” control levels of mTOR activity, but not to completely shut down mTOR activity, which presumably would increase risk of side effects. Although it’s difficult to directly extrapolate the absolute dosing and serum levels from mice to humans, in Tsc1GFAPCKO mice, moderate doses of rapamycin (~3 mg/kg/d) returned the elevated P-S6 to the levels of control mice, whereas high doses (10 mg/kg/d) completely inhibited P-S6 expression. Thus, we used mid-range doses of rapamycin to test antiepileptogenic effects in Tsc1GFAPCKO mice.
In addition to minimizing dose, another approach to reduce drug exposure and side effects is intermittent dosing or drug holidays. For example, compared with daily dosing, weekend dosing of prednisone had similar efficacy in maintaining strength in boys with Duchenne muscular dystrophy, but had less severe side effects on linear growth and bone density.21 In TSC, intermittent dosing of mTOR inhibitors is effective in suppressing tumor growth in rodent models of TSC-related tumors.22, 23 While intermittent rapamycin has not previously been tested against epilepsy in TSC models, in a related Pten knock-out mouse, an intermittent treatment pattern of 2 weeks rapamycin alternating with 4 weeks of no treatment was able to suppress epileptiform activity.24 In our present study with Tsc1GFAPCKO mice, intermittent rapamycin treatment with drug holidays of at least 24 days was able to almost completely prevent development of epilepsy. Interestingly, the antiepileptogenic effects of intermittent rapamycin dosing outlasted the duration of mTOR inhibition as reflected by P-S6 expression, indicating that the pharmacodynamic actions of mTOR inhibition persist beyond pharmacokinetic properties. Furthermore, the effects on the pathologic abnormalities were not as complete as the effects on seizures, especially for the 4–24 paradigm, suggesting that there is a threshold effect of pathological abnormalities in causing seizures or there are additional non-pathological molecular abnormalities that are more critical for epileptogenesis.
While intermittent dosing maintains efficacy, an important corollary is that ideally it will reduce side effects. A limitation of the current study is the lack of documented side effects, as there are no overt behavioral or pathological side effects of rapamycin in Tsc1GFAPCKO mice and, in fact, rapamycin appears to improve the general health of Tsc1GFAPCKO mice as seen by dramatically increased survival. However, we and others have previously shown that continuous rapamycin treatment decreases body growth in normal mice.2, 3, 7 In the current study, there was a slight “trend” towards decreased body weight between 7–17 weeks in the intermittent rapamycin-treated control mice versus vehicle-treated control mice (Fig. 5D), but this was not statistically significant, indicating that intermittent treatment does not significantly affect body weight in control mice. Furthermore, the growth curves of 4–10 and 4–24 rapamycin-treated Tsc1GFAPCKO mice were virtually identical to vehicle-treated control mice, again suggesting that intermittent rapamycin treatment does not cause significant impairment of growth.
Overall, the present study provides “proof-of-principle” that intermittent rapamycin treatment paradigms can prevent the development and progression of epilepsy in a TSC mouse model, as well as have at least partial effects on pathological abnormalities associated with epileptogenesis. Given concerns about possible side effects of long-term treatment with mTOR inhibitors, these findings have translational applications for designing antiepileptogenic drug trials for TSC patients.
Acknowledgments
This work was supported by NIH grants R01 NS056872, P20NS080199, and S10 RR027552. The authors thank the TSC Clinical Research Consortium as collaborators on the P20 grant, for providing the clinical background and context for these preclinical studies. The authors have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Wong M. Mammalian target of rapamycin (mTOR) inhibition as a potential antiepileptogenic therapy: From tuberous sclerosis to common acquired epilepsies. Epilepsia. 2010;51:27–36. doi: 10.1111/j.1528-1167.2009.02341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeng LH, Xu L, Gutmann DH, et al. Rapamycin prevents epilepsy in a mouse model of tuberous sclerosis complex. Ann Neurol. 2008;63:444–453. doi: 10.1002/ana.21331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meikle L, Pollizzi K, Egnor A, et al. Response of a neuronal model of tuberous sclerosis to mammalian target of rapamycin (mTOR) inhibitors: effects on mTORC1 and Akt signaling lead to improved survival and function. J Neurosci. 2008;28:5422–5432. doi: 10.1523/JNEUROSCI.0955-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goto J, Talos DM, Klein P, et al. Regulable neural progenitor-specific Tsc1 loss yields giant cells with organellar dysfunction in a model of tuberous sclerosis complex. Proc Natl Acad Sci USA. 2011;108:E1070–1079. doi: 10.1073/pnas.1106454108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeng LH, Rensing NR, Zhang B, et al. Tsc2 gene inactivation causes a more severe epilepsy phenotype than Tsc1 inactivation in a mouse model of tuberous sclerosis complex. Hum Mol Genet. 2011;20:445–454. doi: 10.1093/hmg/ddq491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderl S, Freeland M, Kwiatkowski DJ, et al. Therapeutic value of prenatal rapamycin treatment in a mouse brain model of tuberous sclerosis complex. Hum Mol Gen. 2011;20:4597–4604. doi: 10.1093/hmg/ddr393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carson RP, Van Nielen DL, Winzenburger PA, et al. Neuronal and glia abnormalities in Tsc1-deficient forebrain and partial rescue by rapamycin. Neurobiol Dis. 2012;45:369–380. doi: 10.1016/j.nbd.2011.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krueger DA, Wilfong AA, Holland-Bouley K, et al. Everolimus treatment of refractory epilepsy in tuberous sclerosis complex. Ann Neurol. 2013;74:679–687. doi: 10.1002/ana.23960. [DOI] [PubMed] [Google Scholar]
- 9.Uhlmann EJ, Wong M, Baldwin RL, et al. Astrocyte-specific TSC1 conditional knockout mice exhibit abnormal neuronal organization and seizures. Ann Neurol. 2002;52:285–296. doi: 10.1002/ana.10283. [DOI] [PubMed] [Google Scholar]
- 10.Zeng LH, Bero AW, Zhang B, Holtzman DH, Wong M. Modulation of astrocyte glutamate transporters decreases seizures in a mouse model of Tuberous Sclerosis Complex. Neurobiol Dis. 2010;37:764–771. doi: 10.1016/j.nbd.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Temkin NR. Preventing and treating posttraumatic seizures: the human experience. Epilepsia. 2009;50(Suppl 2):10–13. doi: 10.1111/j.1528-1167.2008.02005.x. [DOI] [PubMed] [Google Scholar]
- 12.Wong M. A critical review of mTOR inhibitors and epilepsy: from basic science to clinical trials. Expert Rev Neurotherap. 2013;13:657–669. doi: 10.1586/ern.13.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daoud D, Scheld HH, Speckmann EJ, et al. Rapamycin: brain excitability studied in vitro. Epilepsia. 2007;48:834–836. doi: 10.1111/j.1528-1167.2006.00976.x. [DOI] [PubMed] [Google Scholar]
- 14.Ruegg S, Baybis M, Juul H, et al. Effects of rapamycin on gene expression, morphology, and electrophysiological properties of rat hippocampal neurons. Epilepsy Res. 2007;77:85–92. doi: 10.1016/j.eplepsyres.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartman AL, Santos P, Dolce A, et al. The mTOR inhibitor rapamycin has limited acute anticonvulsant effects in mice. PloS One. 2012;7:e45156. doi: 10.1371/journal.pone.0045156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chachua T, Poon KL, Yum MS, et al. Rapamycin has age-, treatment paradigm-, and model-specific anticonvulsant effects and modulates neuropeptide Y expression in rats. Epilepsia. 2012;53:2015–2025. doi: 10.1111/j.1528-1167.2012.03674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang X, McMahon J, Yang J, et al. Rapamycin down-regulates KCC2 expression and increases seizure susceptibility to convulsants in immature rats. Neurosci. 2012;219:33–47. doi: 10.1016/j.neuroscience.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Datta AN, Hahn CD, Sahin M. Clinical presentation and diagnosis of tuberous sclerosis complex in infancy. J Child Neurol. 2008;23:268–273. doi: 10.1177/0883073807309250. [DOI] [PubMed] [Google Scholar]
- 19.Chu-Shore CJ, Major P, Camposano S, et al. The natural history of epilepsy in tuberous sclerosis complex. Epilepsia. 2010;51:1236–1241. doi: 10.1111/j.1528-1167.2009.02474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pallet N, Legendre C. Adverse events associated with mTOR inhibitors. Expert Opinion in Drug Safety. 2013;12:177–186. doi: 10.1517/14740338.2013.752814. [DOI] [PubMed] [Google Scholar]
- 21.Escolar DM, Hache LP, Clemens PR, et al. Randomized, blinded trial of weekend vs daily prednisone in Duchenne muscular dystrophy. Neurology. 2011;77:444–452. doi: 10.1212/WNL.0b013e318227b164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boulay A, Zumstein-Mecker S, Stephan C, et al. Antitumor efficacy of intermittent treatment schedules with the rapamycin derivative RAD001 correlates with prolonged inactivation of ribosomal protein S6 kinase 1 in peripheral blood mononuclear cells. Cancer Res. 2004;64:252–261. doi: 10.1158/0008-5472.can-3554-2. [DOI] [PubMed] [Google Scholar]
- 23.Woodrum C, Nobil A, Dabora SL. Comparison of three rapamycin dosing schedules in A/J Tsc2+/− mice and improved survival with angiogenesis inhibitor or asparaginase treatment in mice with subcutaneous tuberous sclerosis related tumors. J Translational Med. 2010;8:14. doi: 10.1186/1479-5876-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sunnen CN, Brewster AL, Lugo JN, et al. Inhibition of the mammalian target of rapamycin blocks epilepsy progression in NS-Pten conditional knockout mice. Epilepsia. 2011;52:2065–2075. doi: 10.1111/j.1528-1167.2011.03280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]