Abstract
Streptococcus pneumoniae (the pneumococcus), a leading cause of bacterial disease, is most commonly carried in the human nasopharynx. Colonization induces inflammation that promotes the organism's growth and transmission. This inflammatory response is dependent on intracellular sensing of bacterial components that access the cytosolic compartment via the pneumococcal pore-forming toxin pneumolysin. In vitro, cytosolic access results in cell death that includes release of the proinflammatory cytokine interleukin-1β (IL-1β). IL-1 family cytokines, including IL-1β, are secreted upon activation of inflammasomes, although the role of this activation in the host immune response to pneumococcal carriage is unknown. Using a murine model of pneumococcal nasopharyngeal colonization, we show that mice deficient in the interleukin-1 receptor type 1 (Il1r1−/−) have reduced numbers of neutrophils early after infection, fewer macrophages later in carriage, and prolonged bacterial colonization. Moreover, intranasal administration of Il-1β promoted clearance. Macrophages are the effectors of clearance, and characterization of macrophage chemokines in colonized mice revealed that Il1r1−/− mice have lower expression of the C-C motif chemokine ligand 6 (CCL6), correlating with reduced macrophage recruitment to the nasopharynx. IL-1 family cytokines are known to promote adaptive immunity; however, we observed no difference in the development of humoral or cellular immunity to pneumococcal colonization between wild-type and Il1r1−/− mice. Our findings show that sensing of IL-1 cytokines during colonization promotes inflammation without immunity, which may ultimately benefit the pneumococcus.
INTRODUCTION
Streptococcus pneumoniae (the pneumococcus) is an opportunistic bacterial pathogen that is responsible for over 1 million deaths annually, mostly in children under the age of 5 years (1). The pneumococcus serially colonizes the mucosal surfaces of the human upper respiratory tract, and carriage of the organism provides the reservoir for all pneumococcal disease (2). Colonization induces airway inflammation that is characterized by a suppurative rhinitis and increased mucus secretion. These secretions promote bacterial growth (3), and inflammation is important for bacterial transmission in a viral coinfection model (4). Human studies have demonstrated that higher bacterial burdens are correlated with a more profound rhinitis (5); however, as a result of this inflammatory response, colonization is normally cleared by the host's immune system within several weeks (6).
A well-defined murine model of pneumococcal colonization (7) has elucidated bacterial and host factors that are critical to immune recognition of the pneumococcus, which drives the eventual resolution of the carrier state. Although early colonization triggers the recruitment of neutrophils, these are ineffective at resolving the infection. Clearance of pneumococci from the upper airway over a period of weeks requires a sustained presence of macrophages in the nasopharynx (8). Although S. pneumoniae is an extracellular pathogen, this macrophage influx is the result of intracellular innate immune recognition by the cytosolic Nod-like receptor 2 (Nod2) (9). Nod2 detects peptidoglycan (10, 11) that accesses the macrophage cytosol via the pneumococcal pore-forming toxin pneumolysin following phagocytosis and bacterial degradation (12). Nod2 activation results in nuclear factor κB (NF-κB) activation (13) and drives proinflammatory cytokine production, including the C-C motif chemokine ligand 2 (CCL2), which contributes to monocyte/macrophage-dependent pneumococcal clearance (9). Cytosolic access, however, is a fatal event for the host cell following bacterial uptake (12), although the type of cell death that occurs and its contribution to the host immune response remain unclear.
Our further in vitro studies show that macrophage death results in pneumolysin-dependent release of the proinflammatory cytokine interleukin-1β (IL-1β), indicating activation of the inflammasome. Inflammasomes are multiprotein cytosolic complexes that oligomerize through homotypic domain interactions and are comprised of a sensor that detects bacterial stimuli and adaptor proteins that recruit procaspase-1. This recruitment drives caspase-1 enzymatic activation, which processes pro-IL-1β and pro-interleukin-18 (pro-IL-18) into their mature forms and results in a proinflammatory cell death known as “pyroptosis” (14).
The role of inflammasome activation and the production of IL-1 family cytokines in pneumococcal disease has been characterized (15–18); however, carriage rather than disease is the predominant state for pneumococci in the host. The contribution of proinflammatory cell death to immunity against this extracellular pathogen during colonization has not been determined. Here we show that host sensing of IL-1 family cytokines in vivo is required for the macrophage presence in the nasopharynx and clearance of pneumococcal colonization. IL-1 cytokine production in response to the pneumococcus contributes to inflammation in the upper airway, without driving the eventual development of adaptive immune responses.
MATERIALS AND METHODS
Bacterial strains.
A type 23F strain of Streptococcus pneumoniae, isolated from an experimental human carriage study (6), or an isogenic strain containing a full, in-frame deletion of the pneumolysin gene (pneumolysin deficient), was grown overnight at 37°C on tryptic soy (TS) agar plates containing 5% sheep blood (BD). Cultures were then inoculated into broth TS and grown to mid-log phase (optical density at 620 nm [OD620] of ≈0.5) at 37°C in a nonshaking water bath. Pneumococci were pelleted by centrifugation at 14,000 rpm and resuspended in phosphate-buffered saline (PBS).
Cell culture.
Bone marrow was isolated from the tibiae and femurs of C57BL/6 mice and differentiated into macrophages by being cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 30% L929 enriched supernatant and 20% fetal bovine serum (FBS). Cells were cultured at 37°C with 5% CO2 for 7 to 9 days. One day prior to infection, macrophages were replated in DMEM supplemented with 15% L929 enriched supernatant and 10% FBS.
Macrophage infection and ELISA.
Bone marrow-derived macrophages (BMMs) were seeded in a 48-well plate at a density of 2.5 × 105 cells/well. The next day, BMMs were primed with 400 ng/ml Pam3CSK4 for 4 h and infected with pneumococcal strains at a multiplicity of infection (MOI) of 10 for 2 h. The culture medium was then replaced with DMEM containing 300 μg/ml gentamicin. At 24 h postinfection, supernatants were harvested and assayed for the presence of IL-1β using the Legend Max mouse IL-1β enzyme-linked immunosorbent assay (ELISA) kit (BioLegend) per the manufacturer's instructions.
Murine model of S. pneumoniae nasopharyngeal colonization.
C57BL/6 (wild type [WT]) and Tlr2−/− mice were obtained from The Jackson Laboratory. IL-1 receptor type 1-deficient (Il-1r1−/−) mice (19) were a generous gift from Sunny Shin (University of Pennsylvania). S. pneumoniae cells (107 CFU in 10 μl PBS) were inoculated into the nares of unanesthetized mice. Inocula were serially diluted in PBS and grown overnight on TS agar plates to verify the dose. At the time points indicated in the figures, the mice were sacrificed, and the trachea was cannulated and lavaged with 200 μl PBS through the nares. The resulting lavage fluid was plated in serial dilutions on TS agar plates, cultures were grown overnight at 37°C in 5% CO2, and the following day the CFU were counted. For intranasal administration of cytokine, WT mice colonized with a pneumolysin-deficient S. pneumoniae strain (20) received 100 or 200 ng of recombinant IL-1β (eBioscience) resuspended in 10 μl PBS or 10 μl PBS alone as a vehicle control every other day for 14 days. For secondary challenge experiments, mice of the indicated genotype were colonized with WT S. pneumoniae and allowed 8 weeks to clear colonization. They were then recolonized with an isogenic strain containing a single point mutation that confers resistance to streptomycin and that has no colonization defect (21, 22). All animal work was conducted in accordance with the guidelines provided by National Science Foundation Animal Welfare Requirements and the Public Health Service Policy on the Humane Care and Use of Laboratory Animals. The protocol was approved by the Institutional Animal Care and Use Committee, University of Pennsylvania Animal Welfare Assurance no. A3079-01, protocol no. 803231.
qRT-PCR.
Quantitative reverse transcription-PCR (qRT-PCR) was performed as follows. Following lavage with PBS, sacrificed mice were lavaged with 600 μl RLT RNA lysis buffer (Qiagen) containing 10% 2-mercaptoethanol. Samples were stored at −80°C until RNA was isolated using a QIAshredder kit (Qiagen) followed by an RNeasy minikit (Qiagen) per the manufacturer's protocol. cDNA was reverse transcribed using a high-capacity cDNA kit (Applied Biosystems). Ten nanograms of cDNA was used as a template in each reaction with 0.5 μM forward and reverse primers and Power SYBR green (Applied Biosystems) per the manufacturer's protocol. Reactions were amplified with the StepOnePlus real-time PCR system (Applied Biosystems), and comparisons were calculated using the threshold cycle (ΔΔCT) method. The following primers were used for amplification: GAPDH-F, 5′-AGG TCG GTG TGA ACG GAT TTG-3′; GAPDH-R, 5′-TGT AGA CCA TGT AGT TGA GGT CA-3′; IL-1A-F, 5′-GCA CCT TAC ACC TAC CAG AGT-3′; IL-1A-R, 5′-TGC AGG TCA TTT AAC CAA GTG G-3′; IL-1B-F, 5′-GCA ACT GTT CCT GAA CTC AAC T-3′; IL-1B-R, 5′-ATC TTT TGG GGT CCG TCA ACT-3′; CCL2-F, 5′-AGC TCT CTC TTC CTC CAC CAC-3′; CCL2-R, 5′-CGT TAA CTG CAT CTG GCT GA-3′; CCL6-F, 5′-ATG AGA AAC TCC AAG ACT GCC-3′; CCL6-R, 5′-TTA TTG GAG GGT TAT AGC GAC G-3′; CCL7-F, 5′-GCT GCT TTC AGC ATC CAA GTG-3′; CCL7-R, 5′-CCA GGG ACA CCG ACT ACT G-3′; CCL8-F, 5′-TCT ACG CAG TGC TTC TTT GCC-3′; CCL8-R, 5′-AAG GGG GAT CTT CAG CTT TAG TA-3′; IL-17A-F, 5′-TTT AAC TCC CTT GGC GCA AAA-3′; and IL-17A-R, 5′-CTT TCC CTC CGC ATT GAC AC-3′.
Flow cytometry.
PBS lavage samples from 5 mice were pooled per group, pelleted at 1,500 rpm for 10 min, and resuspended in PBS containing 1% bovine serum albumin (BSA). Samples were first blocked for 10 min in 1% BSA (Sigma-Aldrich) and then blocked again with a rat anti-mouse FcγIII/II receptor antibody (BD). Cells were stained for 30 min at 4°C with a cocktail of the following rat anti-mouse antibodies: CD4-fluorescein isothiocyanate (FITC) (BD), Ly6G-phycoerythrin (PE) (BioLegend), Cd11b-peridinin chlorophyll protein (PerCP) cy5.5 (BioLegend), and F4/80-allophycocyanin (APC) (eBioscience).
Quantification of anti-pneumococcal serum IgG.
The wild-type 23F strain used for colonization was grown to the mid-log phase in TS, pelleted, and resuspended in coating buffer (0.015 M Na2CO3, 0.035 M NaHCO3 [pH 9.6]) to an OD620 of 0.1. Immulon 2HB 96-well plates (Thermo) were coated with pneumococci by incubating 100 μl per well of resuspension overnight at 4°C. Plates were blocked for 1 h at 37°C in 1% BSA in PBS and washed with PBS containing 0.05% Brij-35 (Fisher). Serum samples were serially diluted 2-fold and incubated overnight at 4°C. The plates were washed with 0.05% Brij-35 in PBS, and pneumococcal-specific antibodies were detected by incubation with a goat anti-mouse IgG alkaline phosphatase-conjugated antibody for 1.5 h at room temperature. Plates were developed for 1 h at 37°C using phosphatase substrate (Sigma) and read at 415 nm. Geometric mean titers (GMTs) were calculated based on the sample dilution where absorbance was 0.1.
RESULTS
Pneumococcal infection results in IL-1 family cytokine expression in vitro and in vivo.
We have previously reported that infection of macrophages with a type 23F strain of S. pneumoniae results in host cell death, subsequent to bacterial uptake and pneumolysin-dependent cytosolic access of pneumococcal fragments (12). To determine whether pneumolysin-dependent cytosolic access causes proinflammatory cytokine release upon in vitro infection, we incubated bone marrow-derived macrophages (BMMs) with the wild-type (WT) 23F isolate and 24 h later assayed for the presence of IL-1β in the cell culture supernatants by Western blotting. We observed the release of mature IL-1β by BMMs infected with WT bacteria but not in the supernatants of BMMs infected with an isogenic pneumolysin-deficient strain (Fig. 1A). Furthermore, preincubation with cytochalasin D (CytD), an inhibitor of actin polymerization, reduced the amount of IL-1β secreted, suggesting that proinflammatory cytokine production is dependent on bacterial uptake. Quantification by enzyme-linked immunosorbent assay (ELISA) showed that release of IL-1β by BMMs was significantly reduced during infection with the pneumolysin-deficient strain and when phagocytosis was blocked by CytD treatment (Fig. 1B). Additionally, enzymatic processing of caspase-1 and production of its active p10 fragment correlated with secretion of IL-1β (Fig. 1A). We observed no statistically significant differences in levels of IL-1α as measured by ELISA under any of these infection conditions (data not shown). These results show that following infection with a WT pneumococcal strain, BMMs release the proinflammatory cytokine IL-1β, and this is dependent on bacterial uptake and expression of the pneumococcal pore-forming toxin.
FIG 1.
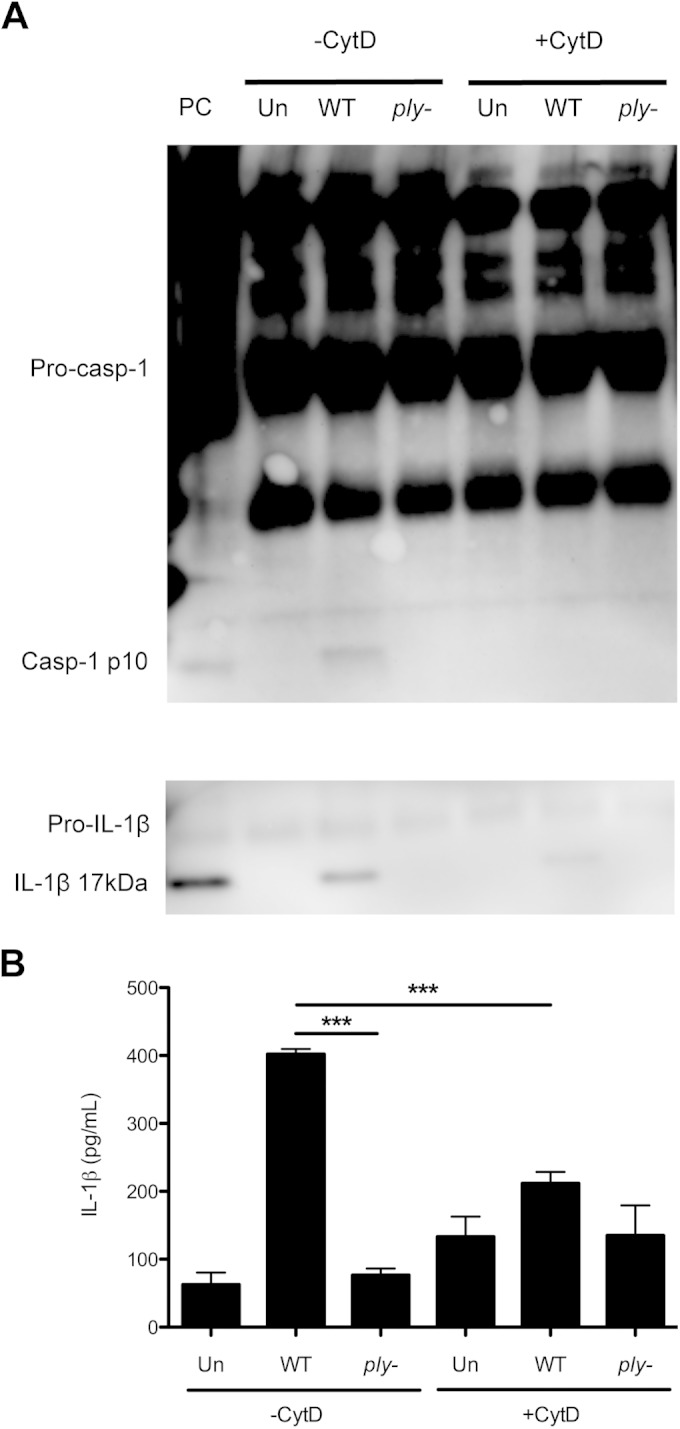
Pneumococcal infection results in IL-1β release in vitro. (A and B) Bone marrow-derived macrophages (BMMs) were infected with wild-type (WT) or pneumolysin-deficient (ply−) S. pneumoniae strains or left uninfected (Un). Where indicated, BMMs were treated with 20 μM cytochalasin D (CytD) to block phagocytosis. Supernatants were collected at 24 h postinfection. (A) Western blot of supernatants for the presence of caspase-1 (Casp-1) and interleukin-1 beta (IL-1β). BMMs primed with 0.5 μg/ml lipopolysaccharide (LPS) and stimulated with ATP were used as a positive control (PC). Representative images are shown. (B) Amounts of IL-1β in supernatants were quantified by ELISA. Results are from 2 independent experiments. Error bars represent ± standard error of the mean (SEM). Significance was determined by one-way analysis of variance (ANOVA) with Newman-Keuls posttest. ***, P < 0.001.
To ascertain whether IL-1 family cytokines are expressed in vivo during pneumococcal colonization, mice were intranasally inoculated with the WT S. pneumoniae strain. Cytokine levels could not be detected because the airway surface fluid is highly diluted in lavages from the upper respiratory tract. Therefore, lysates of the respiratory epithelium were obtained and quantitative RT-PCR (qRT-PCR) was performed to assess Il1a (Fig. 2A) and Il1b (Fig. 2B) gene expression. We observed that upon colonization of WT mice with S. pneumoniae, Il1b (Fig. 2B) was significantly upregulated compared to in mock-colonized mice. A much smaller but significant increase in Il1a (Fig. 2A) expression was observed. Inflammasome components, including pro-IL-1β, are upregulated upon Toll-like receptor (TLR) signaling, and this “priming” provides the first signal required for inflammasome activation (23–25). Previous studies of inflammasome activation and IL-1β release following pneumococcal infection in vitro have characterized TLR2-dependent IL-1β secretion (26). To determine the role of TLR2 in the upregulation of Il1a and Il1b gene transcripts during pneumococcal colonization, we inoculated TLR2-deficient (Tlr2−/−) mice with WT S. pneumoniae, obtained lysates of the epithelial tissue, and performed qRT-PCR to measure Il1a and Il1b transcription. We found that Tlr2−/− mice had no significant upregulation of Il1a (Fig. 2A) or Il1b (Fig. 2B), demonstrating that the increased expression of both genes in vivo is dependent on TLR2.
FIG 2.
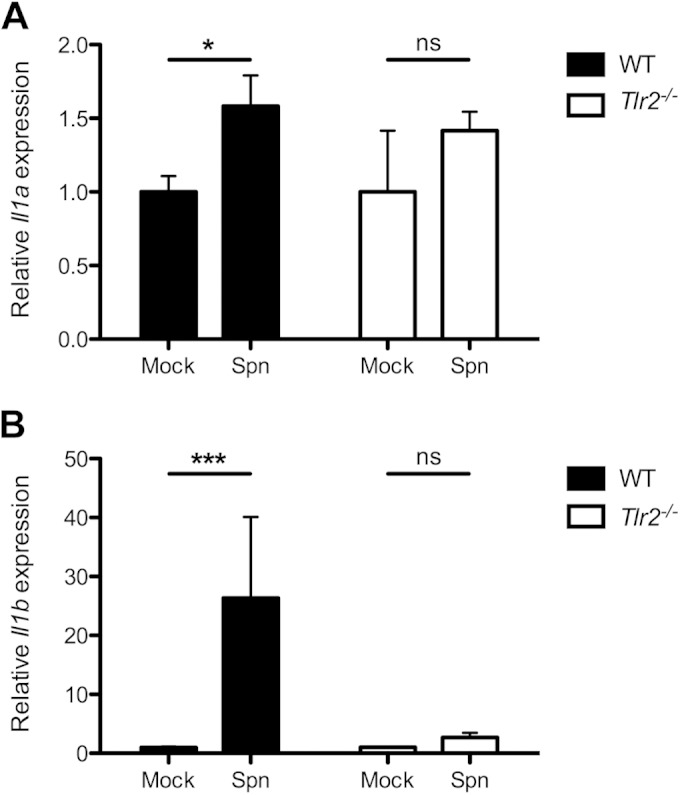
Expression of IL-1 cytokines is upregulated during S. pneumoniae colonization and is dependent on TLR2. (A and B) Wild-type (black bars) or TLR2-deficient (Tlr2−/− [white bars]) mice were intranasally inoculated with 107 CFU of S. pneumoniae (Spn). At 14 days postcolonization, the upper respiratory tracts were lavaged with RLT RNA lysis buffer. Gene expression relative to PBS (mock)-inoculated mice was measured by quantitative RT-PCR for Il1a (A) and Il1b (B). Results are from at least 2 independent experiments (n > 10 mice). Error bars represent ± SEM. Significance was determined by the Mann-Whitney U test. *, P < 0.05; ***, P < 0.001. ns, not significant.
Sensing of IL-1 cytokines is required for inflammation and macrophage-driven bacterial clearance.
The role of IL-1 sensing in early events in pneumococcal colonization was investigated by inoculating wild-type (WT) and IL-1 receptor-deficient (Il1r1−/−) mice with S. pneumoniae and obtaining PBS lavage samples from the upper respiratory tract at 3 days postinfection. We assessed bacterial burden and host immune cell infiltrates by plating for colonizing pneumococci and flow cytometry, respectively. We observed no significant difference in bacterial numbers in the nasopharynx (Fig. 3A); however, there were significantly lower numbers of neutrophils in lavage samples from the Il1r1−/− mice compared to those from WT mice (Fig. 3B; see Fig. S1 in the supplemental material). Macrophage numbers were low, consistent with previous studies (8, 9), and there was no difference between the two groups (Fig. 3C). These results suggest that Il1r1−/− mice have a dampened early inflammatory response to S. pneumoniae colonization.
FIG 3.
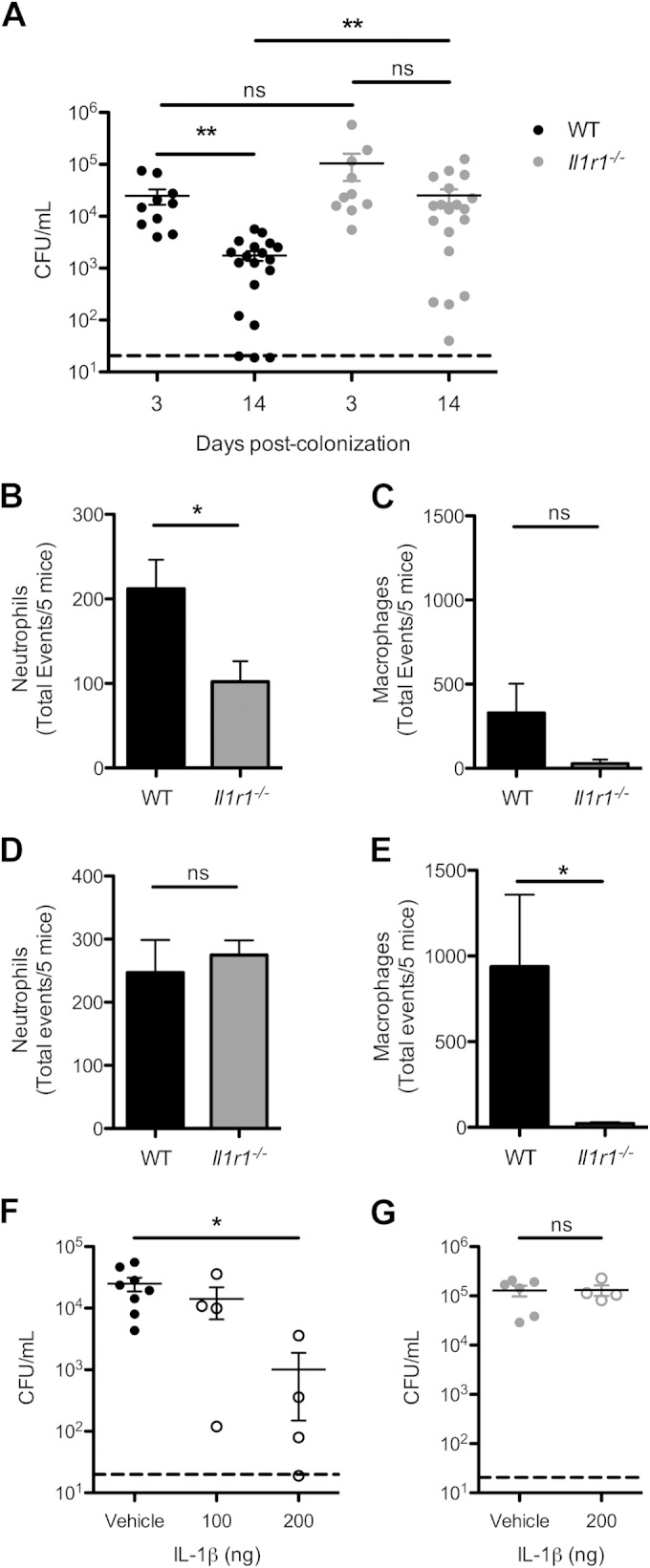
Sensing of IL-1 family cytokines is important for bacterial clearance and macrophage recruitment. (A to E) Wild-type (WT [black]) or IL-1 receptor-deficient (Il1r1−/− [gray]) mice were intranasally inoculated with 107 CFU of S. pneumoniae, and at the time points indicated, PBS lavages of the upper respiratory tract were obtained. (A) Bacterial numbers of colonizing pneumococci were quantified by plating of nasal lavages. The dashed line indicates the limit of detection. Results are from 2 to 4 independent experiments (n ≥ 10 mice per group). Error bars represent ± SEM. Significance was determined by Kruskal-Wallis test with Dunn's posttest. **, P < 0.01; ns, not significant. (B to E) Cellular infiltrates in the nasal lavage samples from 5 mice were measured by flow cytometry. Quantification of neutrophils (Ly6G+, CD11b+) and monocytes/macrophages (F4/80+, CD11b−) after 3 (B and C) and 14 (D and E) days of colonization is shown. The total events available for capture were used due to the low cell counts that are commonly seen in nasal lavage samples. Results are from 4 independent experiments. Error bars represent ± SEM. Significance was determined by Student's t test or Mann-Whitney U test as appropriate. *, P < 0.05; ns, not significant. (F and G) WT (F) or Il1r1−/− (G) mice were inoculated with 107 CFU of a pneumolysin-deficient S. pneumoniae strain and intranasally administered recombinant IL-1β (open circles) or vehicle control (PBS [closed circles]) every other day for 14 days. Nasal lavage samples were obtained, and numbers of pneumococci were measured by plating serial dilutions. The dashed line indicates the limit of detection. Results are from 2 experiments (n ≥ 4 mice per group). Error bars represent ± SEM. Significance was determined by Kruskal-Wallis test with Dunn's posttest or Student's t test as appropriate. *, P < 0.05; ns, not significant.
To address the role of IL-1 sensing in subsequent clearance of colonization, WT and Il1r1−/− mice were inoculated with S. pneumoniae, and bacterial counts and cellular infiltrates were quantified at 14 days postinfection. We observed that WT mice had a significantly lower bacterial load than at day 3 postinfection (Fig. 3A), indicating a partial clearance of pneumococcal colonization. In contrast, Il1r1−/− mice had no difference in bacterial numbers between days 3 and 14 postcolonization (Fig. 3A). Additionally, 2 weeks after inoculation there was a significantly higher bacterial burden in the Il1r1−/− mice than in WT mice (Fig. 3A). There were similar quantities of neutrophils present in the nasopharynx (Fig. 3D) of WT and Il1r1−/− mice; however, the numbers of macrophages, the effectors of pneumococcal clearance (8), were significantly decreased in the Il1r1−/− group (Fig. 3E; see Fig. S1 in the supplemental material). This attenuated clearance and delayed macrophage recruitment resemble previous findings in Tlr2−/− mice and WT mice colonized with a pneumolysin-deficient mutant (8, 27), both factors implicated in inflammasome activation. From these findings, we conclude that sensing by the IL-1 receptor is required for increased macrophage presence in the nasopharynx and clearance of pneumococci.
The contribution of IL-1β to clearance of pneumococci from the nasopharynx was assessed by administration of recombinant cytokine after colonization with a pneumolysin-deficient S. pneumoniae strain, which was used to minimize endogenous production of cytokine. Following the establishment of colonization, the mice were intranasally dosed with a small (100 ng) or large (200 ng) amount of IL-1β every other day. At 14 days postinfection, PBS lavage samples from the respiratory tract were obtained, and bacterial quantity was measured. We observed a significant dose-dependent decrease in bacterial numbers when mice received intranasal IL-1β (Fig. 3F). Il1r1−/− mice that were similarly colonized and received the high dose of IL-1β did not have any significant decrease in bacterial burden (Fig. 3G). This suggests that IL-1β is sufficient to promote clearance of pneumococcal colonization.
Il1r1−/− mice have altered macrophage chemokine expression.
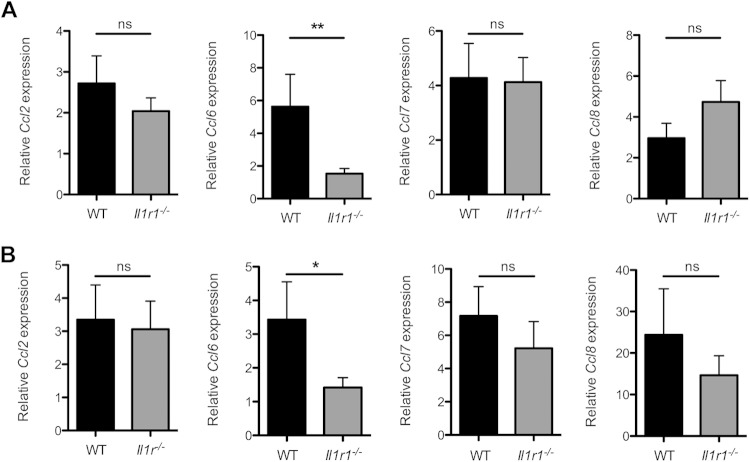
Previous studies investigating clearance of pneumococcal colonization have characterized an essential role for the host C-C chemokine receptor type 2 (CCR2) in macrophage recruitment and retention in the nasopharynx (9). CCR2 binds multiple ligands in the C-C motif chemokine family, which act as macrophage attractants (28). To assess the role of IL-1 sensing in macrophage recruitment to the nasopharynx, we investigated the expression of several CCR2 ligands during pneumococcal colonization. WT and Il1r1−/− mice were inoculated with S. pneumoniae, and at days 3 and 14 postcolonization, we obtained lysates of the upper respiratory tract and determined chemokine expression by qRT-PCR. We observed a significant difference between WT and Il1r1−/− mice in expression of Ccl6 at day 3 postinoculation (Fig. 4A). At day 14 postinfection, when clearance had initiated, Il1r1−/− mice still had significantly lower expression of Ccl6 than WT mice (Fig. 4B). These findings demonstrate that Il1r1−/− mice have an altered macrophage chemokine profile characterized by a reduction in Ccl6 expression, which correlates with lower macrophage presence and delayed clearance.
FIG 4.
Il1r1−/− mice have altered C-C chemokine profiles during S. pneumoniae colonization. (A and B) Wild-type (WT [black bars]) or IL-1 receptor-deficient (Il1r1−/− [gray bars]) mice were inoculated with 107 CFU of S. pneumoniae. At 3 (A) and 14 (B) days postcolonization, RLT RNA lysis buffer lavage samples from the upper respiratory tract were obtained, and gene expression of Ccl2, Ccl6, Ccl7, and Ccl8 was measured by quantitative RT-PCR. Values are relative to PBS (mock)-inoculated mice. Results are from 2 to 4 independent experiments (n ≥ 9 mice). Error bars represent ± SEM. Significance was determined by Mann-Whitney U test. *, P < 0.05; **, P < 0.01. ns, not significant.
Il1r1−/− mice do not have altered adaptive immunity to the pneumococcus.
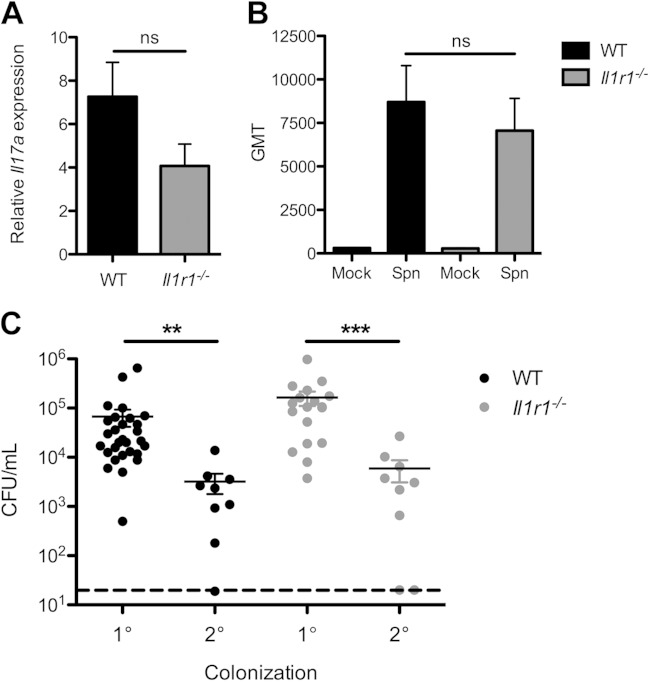
Although there is a contribution of type-specific antibody to protection induced by either vaccination or prior exposure (29, 30), effective clearance of primary colonization requires Th17-dependent cellular immunity rather than humoral responses (8, 31). Il-1β is a known driver of Th17 responses (32), which contribute to macrophage recruitment during colonization (8). We investigated this potential role of IL-1 sensing in cell-mediated immunity by comparing expression of Il17a in colonized WT and Il1r1−/− mice by qRT-PCR. Expression of Il17a was increased in colonized mice; however, we observed no significant difference between WT and Il1r1−/− mice (Fig. 5A). To address a possible effect on humoral immunity, we measured the geometric mean titer (GMT) of whole anti-pneumococcal serum IgG from colonized WT and Il1r1−/− mice. Both groups had detectable antibody titers, although there was no significant difference in GMTs (Fig. 5B). The development of adaptive immunity to the pneumococcus was interrogated using a secondary challenge model. WT and Il1r1−/− mice were colonized with S. pneumoniae and allowed to clear for a period of 8 weeks. The mice were then rechallenged with a marked isogenic strain that could be distinguished from the primary challenge strain, and at day 4 postcolonization, nasal lavage samples were obtained and bacterial quantity measured. Compared to the bacterial burden observed at day 3 during primary colonization, WT mice had significantly lower CFU in the nasopharynx upon secondary challenge (Fig. 5C). Similarly, the Il1r1−/− mice also exhibited a significant drop in bacterial levels upon secondary colonization of an immune host (Fig. 5C). Together these data show that signaling through the IL-1 receptor contributes to inflammation without impacting adaptive immune responses to pneumococcal colonization.
FIG 5.
Sensing of IL-1 does not alter adaptive immunity to the pneumococcus. (A and B) Wild-type (WT [black bars]) or IL-1 receptor-deficient (Il1r1−/− [gray bars]) mice were intranasally colonized with S. pneumoniae (Spn) for 14 days. (A) RLT RNA lysis buffer lavage samples from the respiratory tract were obtained, and expression of Il17a was measured by quantitative RT-PCR. Values are reported as fold change relative to PBS (mock)-inoculated mice. Significance was determined by Student's t test. ns, not significant. (B) Total anti-pneumococcal serum IgG levels were measured by ELISA. Values are expressed as geometric mean titer (GMT). Significance was determined by Kruskal-Wallis test with Dunn's posttest. ns, not significant. (C) WT (black circles) or Il1r1−/− (gray circles) mice were inoculated with 107 CFU of S. pneumoniae. For primary (1°) colonization, mice were sacrificed at day 3 postcolonization, PBS nasal lavage samples were obtained, and bacterial density was measured by plating. For secondary (2°) colonization, mice were allowed 8 weeks to clear the pneumococci and then rechallenged with an isogenic S. pneumoniae strain distinguishable by antibiotic resistance. Nasal lavage samples were obtained at day 4 post-secondary colonization, and bacterial density was quantified by plating. The dashed line indicates the limit of detection. Results are from 2 to 5 independent experiments (n ≥ 10 mice). Error bars represent ± SEM. Significance was determined by the Kruskal-Wallis test with Dunn's posttest. **, P < 0.01; ***, P < 0.001.
DISCUSSION
Host defense against S. pneumoniae requires the activity of professional phagocytes, and previous studies have defined a critical role for macrophages in the clearance of colonization (8). This macrophage presence in the nasopharynx is driven by sensing of pneumococcal components that access the host cell cytosol through the action of the pore-forming toxin pneumolysin (9, 12). Cytosolic access results in death of the phagocytes that clear the organism; however, this appears to result in proinflammatory cytokine production, mediated by activation of the inflammasome, which further contributes to sustaining the inflammatory response against the colonizing pneumococci.
Pneumococcal strains have been reported to vary in their ability to activate inflammasome signaling (26); however, our observation that macrophages activate caspase-1 and secrete IL-1β following pneumococcal infection aligns with previous studies that have reported similar findings in other cell types with different pneumococcal strains (15–17). These investigations have demonstrated a pneumolysin-dependent activation of both the NLRP3 (16, 17) and AIM2 (15) inflammasomes, which sense membrane perturbations and cytosolic DNA, respectively. While recombinant pneumolysin alone, presumably acting at the plasma membrane, can activate the NLRP3 inflammasome (16), caspase-1 maturation and IL-1β secretion following infection with whole bacteria require phagocytosis (15). This suggests that the virulence activity of pneumolysin, which triggers inflammasome formation, occurs at the phagosome membrane.
The NLRP3 receptor and ASC signaling adaptor protein have both been implicated in defense against pneumococcal pneumonia (15, 16) and corneal infection (17). However, nasopharyngeal colonization is the most common pneumococcal-host interaction, and until now the role of inflammasome-derived innate immune signaling during carriage has not been addressed. While we have defined a critical role for sensing by the IL-1 receptor during S. pneumoniae colonization, the specific IL-1 receptor agonists that contribute to bacterial clearance remain to be defined. Nevertheless, IL-1β is highly expressed during colonization and is sufficient to reduce S. pneumoniae density. Prolonged pneumococcal carriage has been associated with increased expression of immunosuppressive chemokines, such as transforming growth factor beta (33), which complements our findings that a proinflammatory cytokine promotes bacterial clearance.
Our observation that sensing of IL-1 family cytokines contributes to inflammation in the nasopharynx following pneumococcal colonization is consistent with previous reports that IL-1β and IL-1 receptor signaling is important for host defense against disease states caused by other mucosal pathogens, including Staphylococcus aureus (34) and group B Streptococcus (GBS) (35). Both of these organisms trigger IL-1β secretion through toxin-dependent activation of the inflammasome (36, 37), which drives neutrophil activation and proinflammatory cytokine production that controls infection (38, 39). The contribution of inflammasome signaling during natural carriage of these organisms remains unexplored. Unlike S. aureus or GBS, neutrophil responses are insufficient to clear S. pneumoniae during infection (8), and IL-1β appears to contribute to the macrophage recruitment that promotes reduction in bacterial burden. The requirement for IL-1 sensing in macrophage recruitment during bacterial infection is less well understood; however, IL-1 sensing in the lung during fungal infection is known to enhance expression of both neutrophil and macrophage chemokines (40). Macrophage presence during pneumococcal colonization is dependent on host expression of CCR2, which binds multiple ligands. We observe that Il1r1−/− mice have diminished macrophage numbers in the nasopharynx 2 weeks after pneumococcal colonization, which correlates with significantly lower expression of Ccl6.
CCL6 is a C-C motif chemokine originally identified in murine bone marrow (41). It acts as a macrophage chemoattractant (42) and is highly expressed in, peripheral eosinophils and elicited macrophages (42, 43), as well as lung tissue (44, 45) and epithelial cells of the intestinal mucosa (46). The mRNA present in RLT lysis lavage samples from the upper respiratory tract is predominantly derived from epithelial cells of the mucosal barrier, suggesting that these cells are a likely source of CCL6 during pneumococcal colonization. In addition to its chemokine functions, CCL6 may have intrinsic antibacterial properties (46), and overexpression of CCL6 in transgenic mice confers protection against bacterial sepsis (47). Multiple studies have observed that in contrast to CCL2, which is expressed early during inflammation, CCL6 induction occurs later and is sustained for several days to over a week (43, 48). These results, from a model of peritonitis, suggest that CCL2 acts in initial macrophage recruitment and that CCL6 sustains macrophage presence. This raises the possibility that IL-1 cytokines, generated as a result of proinflammatory macrophage death during pneumococcal colonization, contribute to the chemokine production that sustains macrophage presence throughout the weeks required for bacterial clearance.
Previous studies have defined an important role for the intracellular receptor Nod2 in innate immune defense against pneumococci (9). However, in these studies, a minimal clearance defect was observed in Nod2-deficient mice, although mice lacking both Nod2 and TLR2 had a significantly higher bacterial burden at a time point when WT mice had no bacterial carriage. Combined with our observation that upregulation of IL-1 cytokines is dependent on TLR2, this suggests that cytosolic access of pneumococcal components triggers multiple innate immune sensing pathways, both of which contribute to the orchestrated immune response that clears colonization. Activation of multiple sensing pathways may also be critical for controlling pneumococcal disease. Patients deficient in myeloid differentiation primary response gene 88 (MyD88) or interleukin-1 receptor-associated kinase 4 (IRAK-4), adaptor proteins for both TLRs and the IL-1 receptor, are acutely susceptible to recurrent pneumococcal sepsis (49–53). However, polymorphisms in TLRs alone do not correlate with significant increases in S. pneumoniae disease (54), suggesting that sensing by both TLRs and the IL-1 receptor may be critical for host defense.
Our findings that WT and Il1r1−/− mice do not differ in either antibody titers or expression of Il17a during primary colonization suggest that IL-1 sensing does not significantly contribute to the development of adaptive immunity to S. pneumoniae; however, it is also possible that these phenotypes are affected by the more prolonged antigenic burden during primary colonization. Nevertheless we also observe that WT and Il1r1−/− mice have similar responses to secondary colonization, demonstrating that the absence of IL-1 sensing does impact the dynamics of subsequent colonization events.
Although pneumolysin-mediated cytosolic access triggers innate immune responses that eventually clear S. pneumoniae, this may still be to the benefit of the bacterium. Cytosolic access results in the death of the phagocytes that clear the organism and generates IL-1 family cytokines that drive inflammation, an important factor in both bacterial growth (3) and transmission (4). Sensing of IL-1 cytokines, however, does not appear to contribute to the development of an adaptive immune response, suggesting that toxin expression and cytosolic access drive IL-1 cytokine-mediated inflammation that benefits the organism without inducing further immunity that is detrimental to its persistence.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the United States Public Health Service (R01AI038446 and T32AI060516).
We thank Cierra Casson for technical expertise.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00224-15.
REFERENCES
- 1.World Health Organization. 2007. Pneumococcal conjugate vaccine for childhood immunization—WHO position paper. Wkly Epidemiol Rec 82:93–104. [PubMed] [Google Scholar]
- 2.Bogaert D, de Groot R, Hermans PWM. 2004. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis 4:144–154. doi: 10.1016/S1473-3099(04)00938-7. [DOI] [PubMed] [Google Scholar]
- 3.Siegel SJ, Roche AM, Weiser JN. 2014. Influenza promotes pneumococcal growth during coinfection by providing host sialylated substrates as a nutrient source. Cell Host Microbe 16:55–67. doi: 10.1016/j.chom.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Short KR, Reading PC, Wang N, Diavatopoulos DA, Wijburg OL. 2012. Increased nasopharyngeal bacterial titers and local inflammation facilitate transmission of Streptococcus pneumoniae. mBio 3(5):e00255-12. doi: 10.1128/mBio.00255-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodrigues F, Foster D, Nicoli E, Trotter C, Vipond B, Muir P, Gonçalves G, Januário L, Finn A. 2013. Relationships between rhinitis symptoms, respiratory viral infections and nasopharyngeal colonization with Streptococcus pneumoniae, Haemophilus influenzae and Staphylococcus aureus in children attending daycare. Pediatr Infect Dis J 32:227–232. doi: 10.1097/INF.0b013e31827687fc. [DOI] [PubMed] [Google Scholar]
- 6.McCool T, Cate T, Moy G, Weiser J. 2002. The immune response to pneumococcal proteins during experimental human carriage. J Exp Med 195:359–365. doi: 10.1084/jem.20011576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCool TL, Weiser JN. 2004. Limited role of antibody in clearance of Streptococcus pneumoniae in a murine model of colonization. Infect Immun 72:5807–5813. doi: 10.1128/IAI.72.10.5807-5813.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Z, Clarke TB, Weiser JN. 2009. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. J Clin Invest 119:1899–1909. doi: 10.1172/JCI36731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis KM, Nakamura S, Weiser JN. 2011. Nod2 sensing of lysozyme-digested peptidoglycan promotes macrophage recruitment and clearance of S. pneumoniae colonization in mice. J Clin Invest 121:3666–3676. doi: 10.1172/JCI57761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanabe T, Chamaillard M, Ogura Y, Zhu L, Qiu S, Masumoto J, Ghosh P, Moran A, Predergast MM, Tromp G, Williams CJ, Inohara N, Núñez G. 2004. Regulatory regions and critical residues of NOD2 involved in muramyl dipeptide recognition. EMBO J 23:1587–1597. doi: 10.1038/sj.emboj.7600175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ. 2003. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem 278:8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 12.Lemon JK, Weiser JN. 2015. Degradation products of the extracellular pathogen Streptococcus pneumoniae access the cytosol via its pore-forming toxin. mBio 6(1):e02110-14. doi: 10.1128/mBio.02110-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogura Y, Inohara N, Benito A, Chen FF, Yamaoka S, Nunez G. 2001. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J Biol Chem 276:4812–4818. doi: 10.1074/jbc.M008072200. [DOI] [PubMed] [Google Scholar]
- 14.Cookson BT, Brennan MA. 2001. Pro-inflammatory programmed cell death. Trends Microbiol 9:113–114. doi: 10.1016/S0966-842X(00)01936-3. [DOI] [PubMed] [Google Scholar]
- 15.Fang R, Tsuchiya K, Kawamura I, Shen Y, Hara H, Sakai S, Yamamoto T, Fernandes-Alnemri T, Yang R, Hernandez-Cuellar E, Dewamitta SR, Xu Y, Qu H, Alnemri ES, Mitsuyama M. 2011. Critical roles of ASC inflammasomes in caspase-1 activation and host innate resistance to Streptococcus pneumoniae infection. J Immunol 187:4890–4899. doi: 10.4049/jimmunol.1100381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McNeela EA, Burke Á, Neill DR, Baxter C, Fernandes VE, Ferreira D, Smeaton S, El-Rachkidy R, McLoughlin RM, Mori A, Moran B, Fitzgerald KA, Tschopp J, Pétrilli V, Andrew PW, Kadioglu A, Lavelle EC. 2010. Pneumolysin activates the NLRP3 inflammasome and promotes proinflammatory cytokines independently of TLR4. PLoS Pathog 6:e1001191. doi: 10.1371/journal.ppat.1001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karmakar M, Katsnelson M, Malak HA, Greene NG, Howell SJ, Hise AG, Camilli A, Kadioglu A, Dubyak GR, Pearlman E. 2015. Neutrophil IL-1β processing induced by pneumolysin is mediated by the NLRP3/ASC inflammasome and caspase-1 activation and is dependent on K+ efflux. J Immunol 194:1763–1775. doi: 10.4049/jimmunol.1401624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kafka D, Ling E, Feldman G, Benharroch D, Voronov E, Givon-Lavi N, Iwakura Y, Dagan R, Apte RN, Mizrachi-Nebenzahl Y. 2008. Contribution of IL-1 to resistance to Streptococcus pneumoniae infection. Int Immunol 20:1139–1146. doi: 10.1093/intimm/dxn071. [DOI] [PubMed] [Google Scholar]
- 19.Glaccum MB, Stocking KL, Charrier K, Smith JL, Willis CR, Maliszewski C, Livingston DJ, Peschon JJ, Morrissey PJ. 1997. Phenotypic and functional characterization of mice that lack the type I receptor for IL-1. J Immunol 159:3364–3371. [PubMed] [Google Scholar]
- 20.Ratner AJ, Aguilar JL, Shchepetov M, Lysenko ES, Weiser JN. 2007. Nod1 mediates cytoplasmic sensing of combinations of extracellular bacteria. Cell Microbiol 9:1343–1351. doi: 10.1111/j.1462-5822.2006.00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sung C, Li H, Claverys J, Morrison D. 2001. An rpsL cassette, Janus, for gene replacement through negative selection in Streptococcus pneumoniae. Appl Environ Microbiol 67:5190–5196. doi: 10.1128/AEM.67.11.5190-5196.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeBardeleben HK, Lysenko ES, Dalia AB, Weiser JN. 2014. Tolerance of a phage element by Streptococcus pneumoniae leads to a fitness defect during colonization. J Bacteriol 196:2670–2680. doi: 10.1128/JB.01556-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bauernfeind F, Bartok E, Rieger A, Franchi L, Núñez G, Hornung V. 2011. Cutting edge: reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. J Immunol 187:613–617. doi: 10.4049/jimmunol.1100613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bulua AC, Simon A, Maddipati R, Pelletier M, Park H, Kim K-Y, Sack MN, Kastner DL, Siegel RM. 2011. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS). J Exp Med 208:519–533. doi: 10.1084/jem.20102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kahlenberg JM, Lundberg KC, Kertesy SB, Qu Y, Dubyak GR. 2005. Potentiation of caspase-1 activation by the P2X7 receptor is dependent on TLR signals and requires NF-kappaB-driven protein synthesis. J Immunol 175:7611–7622. doi: 10.4049/jimmunol.175.11.7611. [DOI] [PubMed] [Google Scholar]
- 26.Witzenrath M, Pache F, Lorenz D, Koppe U, Gutbier B, Tabeling C, Reppe K, Meixenberger K, Dorhoi A, Ma J, Holmes A, Trendelenburg G, Heimesaat MM, Bereswill S, van der Linden M, Tschopp J, Mitchell TJ, Suttorp N, Opitz B. 2011. The NLRP3 inflammasome is differentially activated by pneumolysin variants and contributes to host defense in pneumococcal pneumonia. J Immunol 187:434–440. doi: 10.4049/jimmunol.1003143. [DOI] [PubMed] [Google Scholar]
- 27.van Rossum AMC, Lysenko ES, Weiser JN. 2005. Host and bacterial factors contributing to the clearance of colonization by Streptococcus pneumoniae in a murine model. Infect Immun 73:7718–7726. doi: 10.1128/IAI.73.11.7718-7726.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsukawa A, Lukacs NW, Hogaboam CM, Chensue SW, Kunkel SL. 2001. Chemokines and other mediators. 8. Chemokines and their receptors in cell-mediated immune responses in the lung. Microsc Res Tech 53:298–306. [DOI] [PubMed] [Google Scholar]
- 29.Malley R, Stack AM, Ferretti ML, Thompson CM, Saladino RA. 1998. Anticapsular polysaccharide antibodies and nasopharyngeal colonization with Streptococcus pneumoniae in infant rats. J Infect Dis 178:878–882. doi: 10.1086/597600. [DOI] [PubMed] [Google Scholar]
- 30.Dagan R, Melamed R, Muallem M, Piglansky L, Greenberg D, Abramson O, Mendelman PM, Bohidar N, Yagupsky P. 1996. Reduction of nasopharyngeal carriage of pneumococci during the second year of life by a heptavalent conjugate pneumococcal vaccine. J Infect Dis 174:1271–1278. doi: 10.1093/infdis/174.6.1271. [DOI] [PubMed] [Google Scholar]
- 31.Lu Y-J, Gross J, Bogaert D, Finn A, Bagrade L, Zhang Q, Kolls JK, Srivastava A, Lundgren A, Forte S, Thompson CM, Harney KF, Anderson PW, Lipsitch M, Malley R. 2008. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathog 4:e1000159. doi: 10.1371/journal.ppat.1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, Dong C. 2009. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity 30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neill DR, Coward WR, Gritzfeld JF, Richards L, Garcia-Garcia FJ, Dotor J, Gordon SB, Kadioglu A. 2014. Density and duration of pneumococcal carriage is maintained by transforming growth factor β1 and T regulatory cells. Am J Respir Crit Care Med 189:1250–1259. doi: 10.1164/rccm.201401-0128OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hultgren OH, Svensson L, Tarkowski A. 2002. Critical role of signaling through IL-1 receptor for development of arthritis and sepsis during Staphylococcus aureus infection. J Immunol 168:5207–5212. doi: 10.4049/jimmunol.168.10.5207. [DOI] [PubMed] [Google Scholar]
- 35.Biondo C, Mancuso G, Midiri A, Signorino G, Domina M, Lanza Cariccio V, Venza M, Venza I, Teti G, Beninati C. 2014. Essential role of interleukin-1 signaling in host defenses against group B Streptococcus. mBio 5(5):e01428-14. doi: 10.1128/mBio.01428-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta R, Ghosh S, Monks B, Deoliveira R, Tzeng T, Kalantari P, Nandy A, Bhattacharjee B, Chan J, Ferreira F, Rathinam V, Sharma S, Lien E, Silverman N, Fitzgerald K, Firon A, Trieu-Cuot P, Henneke P, Golenbock D. 2014. RNA and β-hemolysin of group B Streptococcus induce IL-1β by activating NLRP3 inflammasomes in mouse macrophages. J Biol Chem 289:13701–13705. doi: 10.1074/jbc.C114.548982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. 2006. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 38.Biondo C, Mancuso G, Midiri A, Signorino G, Domina M, Lanza Cariccio V, Mohammadi N, Venza M, Venza I, Teti G, Beninati C. 2014. The interleukin-1β/CXCL1/2/neutrophil axis mediates host protection against group B streptococcal infection. Infect Immun 82:4508–4517. doi: 10.1128/IAI.02104-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mölne L, Verdrengh M, Tarkowski A. 2000. Role of neutrophil leukocytes in cutaneous infection caused by Staphylococcus aureus. Infect Immun 68:6162–6167. doi: 10.1128/IAI.68.11.6162-6167.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caffrey AK, Lehmann MM, Zickovich JM, Espinosa V, Shepardson KM, Watschke CP, Hilmer KM, Thammahong A, Barker BM, Rivera A, Cramer RA, Obar JJ. 2015. IL-1α signaling is critical for leukocyte recruitment after pulmonary Aspergillus fumigatus challenge. PLoS Pathog 11:e1004625. doi: 10.1371/journal.ppat.1004625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orlofsky A, Berger MS, Prystowsky MB. 1991. Novel expression pattern of a new member of the MIP-1 family of cytokine-like genes. Cell Regul 2:403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.LaFleur AM, Lukacs NW, Kunkel SL, Matsukawa A. 2004. Role of CC chemokine CCL6/C10 as a monocyte chemoattractant in a murine acute peritonitis. Mediators Inflamm 13:349–355. doi: 10.1080/09629350400014172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu Y, Prystowsky MB, Orlofsky A. 1999. Sustained high-level production of murine chemokine C10 during chronic inflammation. Cytokine 11:523–530. doi: 10.1006/cyto.1998.0436. [DOI] [PubMed] [Google Scholar]
- 44.Kanno M, Suzuki S, Fujiwara T, Yokoyama A, Sakamoto A, Takahashi H, Imai Y, Tanaka J. 2005. Functional expression of CCL6 by rat microglia: a possible role of CCL6 in cell-cell communication. J Neuroimmunol 167:72–80. doi: 10.1016/j.jneuroim.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 45.Hogaboam CM, Chensue SW, Steinhauser ML, Huffnagle GB, Lukacs NW, Strieter RM, Kunkel SL. 1997. Alteration of the cytokine phenotype in an experimental lung granuloma model by inhibiting nitric oxide. J Immunol 159:5585–5593. [PubMed] [Google Scholar]
- 46.Kotarsky K, Sitnik KM, Stenstad H, Kotarsky H, Schmidtchen A, Koslowski M, Wehkamp J, Agace WW. 2010. A novel role for constitutively expressed epithelial-derived chemokines as antibacterial peptides in the intestinal mucosa. Mucosal Immunol 3:40–48. doi: 10.1038/mi.2009.115. [DOI] [PubMed] [Google Scholar]
- 47.Coelho AL, Schaller MA, Benjamim CF, Orlofsky AZ, Hogaboam CM, Kunkel SL. 2007. The chemokine CCL6 promotes innate immunity via immune cell activation and recruitment. J Immunol 179:5474–5482. doi: 10.4049/jimmunol.179.8.5474. [DOI] [PubMed] [Google Scholar]
- 48.Orlofsky A, Lin EY, Prystowsky MB. 1994. Selective induction of the beta chemokine C10 by IL-4 in mouse macrophages. J Immunol 152:5084–5091. [PubMed] [Google Scholar]
- 49.Conway DH, Dara J, Bagashev A, Sullivan KE. 2010. Myeloid differentiation primary response gene 88 (MyD88) deficiency in a large kindred. J Allergy Clin Immunol 126:172–175. doi: 10.1016/j.jaci.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 50.Picard C, Casanova J-L, Puel A. 2011. Infectious diseases in patients with IRAK-4, MyD88, NEMO, or I kappa B alpha deficiency. Clin Microbiol Rev 24:490–497. doi: 10.1128/CMR.00001-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.von Bernuth H, Picard C, Jin Z, Pankla R, Xiao H, Ku C-L, Chrabieh M, Mustapha IB, Ghandil P, Camcioglu Y, Vasconcelos J, Sirvent N, Guedes M, Vitor AB, Herrero-Mata MJ, Aróstegui JI, Rodrigo C, Alsina L, Ruiz-Ortiz E, Juan M, Fortuny C, Yagüe J, Antón J, Pascal M, Chang H-H, Janniere L, Rose Y, Garty B-Z, Chapel H, Issekutz A, Maródi L, Rodriguez-Gallego C, Banchereau J, Abel L, Li X, Chaussabel D, Puel A, Casanova J-L. 2008. Pyogenic bacterial infections in humans with MyD88 deficiency. Science 321:691–696. doi: 10.1126/science.1158298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ku C-L, von Bernuth H, Picard C, Zhang S-Y, Chang H-H, Yang K, Chrabieh M, Issekutz AC, Cunningham CK, Gallin J, Holland SM, Roifman C, Ehl S, Smart J, Tang M, Barrat FJ, Levy O, McDonald D, Day-Good NK, Miller R, Takada H, Hara T, Al-Hajjar S, Al-Ghonaium A, Speert D, Sanlaville D, Li X, Geissmann F, Vivier E, Maródi L, Garty B-Z, Chapel H, Rodriguez-Gallego C, Bossuyt X, Abel L, Puel A, Casanova J-L. 2007. Selective predisposition to bacterial infections in IRAK-4-deficient children: IRAK-4-dependent TLRs are otherwise redundant in protective immunity. J Exp Med 204:2407–2422. doi: 10.1084/jem.20070628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Picard C, Puel A, Bonnet M, Ku CL, Bustamante J, Yang K, Soudais C, Dupuis S, Feinberg J, Fieschi C, Elbim C, Hitchcock R, Lammas D, Davies G, Al-Ghonaium A, Al-Rayes H, Al-Jumaah S, Al-Hajjar S, Al-Mohsen IZ, Frayha HH, Rucker R, Hawn TR, Aderem A, Tufenkeji H, Haraguchi S, Day NK, Good RA, Gougerot-Pocidalo MA, Ozinsky A, Casanova JL. 2003. Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science 299:2076–2079. doi: 10.1126/science.1081902. [DOI] [PubMed] [Google Scholar]
- 54.Moens L, Verhaegen J, Pierik M, Vermeire S, De Boeck K, Peetermans WE, Bossuyt X. 2007. Toll-like receptor 2 and Toll-like receptor 4 polymorphisms in invasive pneumococcal disease. Microbes Infect 9:15–20. doi: 10.1016/j.micinf.2006.10.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.