Abstract
This study investigates the regulation of hepcidin, the key iron-regulatory molecule, by alcohol and hydrogen peroxide (H2O2) in glutathione peroxidase-1 (gpx-1−/−) and catalase (catalase−/−) knockout mice. For alcohol studies, 10% ethanol was administered in the drinking water for 7 days. Gpx-1−/− displayed significantly higher hepatic H2O2 levels than catalase−/− compared to wild-type mice, as measured by 2'-7'-dichlorodihydrofluorescein diacetate (DCFH-DA). The basal level of liver hepcidin expression was attenuated in gpx-1−/− mice. Alcohol increased H2O2 production in catalase−/− and wild-type, but not gpx-1−/−, mice. Hepcidin expression was inhibited in alcohol-fed catalase−/− and wild-type mice. In contrast, alcohol elevated hepcidin expression in gpx-1−/− mice. Gpx-1−/− mice also displayed higher level of basal liver CHOP protein expression than catalase−/− mice. Alcohol induced CHOP and to a lesser extent GRP78/BiP expression, but not XBP1 splicing or binding of CREBH to hepcidin gene promoter, in gpx-1−/− mice. The up-regulation of hepatic ATF4 mRNA levels, which was observed in gpx-1−/− mice, was attenuated by alcohol. In conclusion, our findings strongly suggest that H2O2 inhibits hepcidin expression in vivo. Synergistic induction of CHOP by alcohol and H2O2, in the absence of gpx-1, stimulates liver hepcidin gene expression by ER stress independent of CREBH.
Keywords: alcoholic liver disease, CHOP, endoplasmic reticulum stress, Hamp, hepatocyte, iron, oxidative stress
1. Introduction
Patients with alcoholic liver disease (ALD) frequently display increased levels of iron and even moderate alcohol intake affects iron homeostasis [1,2]. Hepcidin, a circulatory peptide synthesized in the liver, is the key iron-regulatory hormone, which controls iron absorption through the duodenum and the release of iron from macrophages [3]. A role for hepcidin has been suggested in ALD [4,5,6,7]. We have reported that oxidative stress induced by short-term alcohol-intake is sufficient to suppress liver hepcidin expression by inhibiting the activity of transcription factor C/EBP alpha (C/EBPα) [8]. The suppression of hepatic hepcidin expression by alcohol results in elevated expression of intestinal iron transporters, which is abolished by vitamin E treatment of mice [8].
Ethanol metabolism in the liver produces reactive oxygen species (ROS) including superoxide (O2•−) [9]. O2•− is short-lived and rapidly converted to H2O2 by superoxide dismutase enzymes, SOD1 and SOD2. H2O2 can react with iron to form the highly reactive and damaging hydroxyl radicals [10]. The removal of excess H2O2 and the tight regulation of iron metabolism are therefore important to prevent tissue injury. The antioxidant enzymes, glutathione peroxidase (gpx), catalase and peroxiredoxins catabolize H2O2 [11,12]. Gpx-1 is the most abundant and ubiquitously expressed member of gpx family [13]. Catalase is localized to peroxisomes whereas gpx-1 is present in both cytosol and mitochondria [13]. The protein and activity levels of gpx-1 and catalase have been reported to be significantly reduced in rats livers following intragastric administration of alcohol and fish oil [14]. A decrease in both cytosolic and mitochondrial gpx-1 activity in the liver has also been shown in rats fed with liquid alcohol diets [15].
The activation of unfolding protein response (UPR) signaling is an adaptive mechanism in response to oxidative stress [16]. UPR and endoplasmic reticulum (ER) stress are known to play a role in the pathogenesis of ALD [17,18]. H2O2 synergizes with low doses of alcohol to induce UPR and ER stress in a zebra fish model of ALD [19]. UPR-responsive transcription factor, ATF4 (activating transcription factor-4) plays a role in redox regulation [18,20]. ATF4 is also the dominant transactivator of transcription factor, C/EBP homologous protein (CHOP, a.k.a GADD153) [21]. CHOP can inhibit the function of C/EBP family of transcription factors including C/EBPα by forming heterodimers [22]. CHOP is also involved in the regulation of cellular redox state and apoptosis [23]. Two independent studies have shown a role for ER stress in hepcidin induction either via the CHOP/CEBPα axis or by the direct binding of the transcription factor, cyclic AMP response element binding protein-H (CREBH) to hepcidin gene promoter [24,25]. Studies using tissue culture cells suggested both induction and inhibition of hepcidin by H2O2 in vitro [26,27].
The roles of H2O2 and/or ER stress in hepcidin regulation by alcohol metabolism in vivo are unknown. Understanding these mechanisms is important because iron and alcohol act synergistically to induce liver injury. This study addresses these questions by employing mice with or without impaired H2O2 catabolism and subjecting them to alcohol exposure in an experimental model.
2. Results and Discussion
2.1. The Effect of Alcohol on H2O2 Production and Hepcidin Expression in Catalase−/− and Gpx-1−/− Mice
In order to study the synergistic action of alcohol and hydrogen peroxide (H2O2), transgenic mice, lacking the expression of antioxidant enzymes, glutathione peroxidase-1 (gpx-1−/−) or catalase (catalase−/−) on both alleles, were fed with plain water (control) or ethanol for 1 week. This feeding protocol did not alter liver histology or serum ALT levels, and the body weights were similar between experimental groups at the beginning and end of the 7 day period, as reported previously [7,8]. Both wild-type and transgenic mice consumed around 4 mL of 10% ethanol or 5 mL of plain water per day. The blood alcohol levels in wild-type, catalase−/− and gpx-1−/− mice were similar and matched the values we have published previously with 129/Sv strain mice [8] (Table 1). 7 day-long alcohol feeding induced a weak but significant increase in CYP2E1 activity, which was similar in wild-type and both knockout mice (Table 1). These findings suggest that alcohol exposure and/or the deficiency of catalase or gpx-1 does not induce liver injury in our experimental model.
Table 1.
The levels of blood alcohol and liver CYP2E1 enzyme activity in wild-type and knockout mice were measured, as described in the experimental section. (N.D. = not detectable).
| Mouse identity | Blood alcohol (mg/dL) | CYP2E1 activity (nmole 4-nc/h/mg protein) |
|---|---|---|
| Wild-type water-fed | N.D. | 57 ± 3 |
| Wild-type alcohol-fed | 123 ± 16 | 92 ± 3.9 |
| Catalase−/− water-fed | N.D. | 61 ± 6 |
| Catalase−/− alcohol-fed | 125 ± 13 | 100 ± 2 |
| Gpx-1−/− water-fed | N.D. | 62 ± 4 |
| Gpx-1−/− alcohol-fed | 127 ± 14 | 100 ± 3 |
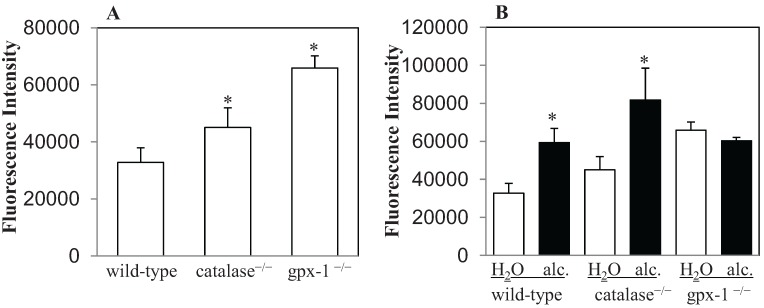
The effect of alcohol exposure on H2O2 production was determined by 2'-7'-dichlorodihydrofluorescein diacetate (DCFH-DA) assays. For these experiments, hepatocytes, freshly isolated by perfusion from the livers of control and ethanol-fed mice, were incubated with DCFH-DA, as described in Experimental Section. After DCFH-DA enters the cell, it is deacetylated to DCFH by intracellular esterases, and then oxidized by peroxides to highly fluorescent 2'-7'-dichlorodihydrofluorescein (DCF) [28]. Significantly higher levels of DCF fluorescence were observed in the hepatocytes of untreated catalase−/− and gpx-1−/− mice than in untreated wild-type mice (Figure 1A). Gpx-1−/− mice exhibited the most prominent hepatic H2O2 accumulation. DFC fluorescence in gpx-1−/− mice was 2-fold higher than wild-type mice (Figure 1A). Similarly, the level of fluorescence in gpx-1−/− was also significantly greater than that in catalase−/− mice (Figure 1A). In contrast, alcohol exposure elevated hepatic H2O2 levels in wild-type and catalase−/−, but not in gpx-1−/−, mice compared to their water-fed counterparts (Figure 1B). Alcohol-induced increase in hepatic H2O2 content was observed most significantly in catalase−/− mice. Untreated or alcohol-fed wild-type and gpx-1−/− mice exhibited significantly less fluorescence intensity than alcohol-fed catalase−/− mice (Figure 1B). Furthermore, the amount of hepatic H2O2 in alcohol-fed wild-type mice was similar to that in gpx-1−/− mice (Figure 1B).
Figure 1.
Intracellular H2O2 levels in hepatocytes freshly isolated by perfusion from the livers of untreated wild-type and catalase−/− or gpx-1−/− transgenic mice (A), and mice fed with 10% ethanol (alc.) or plain water (H2O) for 1 week (B) were measured by 2'-7'-dichlorodihydrofluorescein (DCF) fluorescence. DCF fluorescence detected by spectrophotometer in cells incubated with 2'-7'-dichlorodihydrofluorescein diacetate (DCFH-DA) was normalized to that in cells incubated with 0.1% DMSO (control), and expressed as arbitrary fluorescence per 0.5 × 106 hepatocytes. Asterisks indicate statistical significance (p < 0.05).
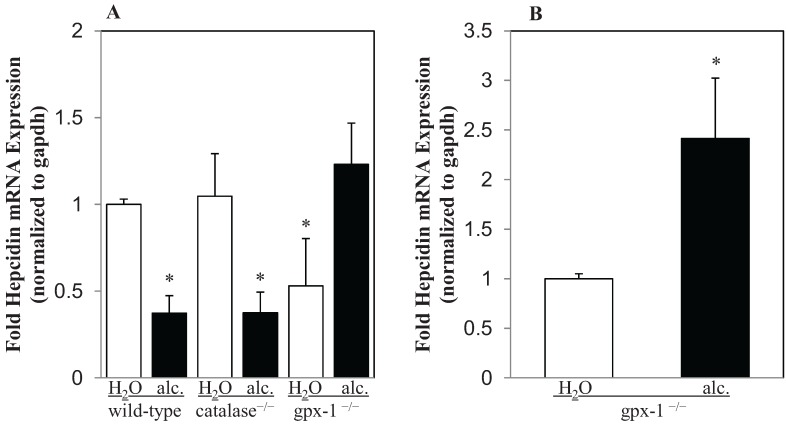
The combined effect of alcohol and H2O2 in the regulation of hepcidin gene expression was determined by real-time PCR, as described in Experimental Section. Alcohol inhibited hepcidin mRNA expression in the livers of wild-type mice (Figure 2A). The deletion of catalase gene in catalase−/− transgenic mice did not significantly alter the basal level of hepcidin expression in the liver (Figure 2A). Similar to wild-type mice, hepcidin expression in the livers of catalase−/− mice was also significantly inhibited by alcohol (Figure 2A). Contrary to catalase−/−, the basal level of hepcidin mRNA expression was decreased by two-fold in gpx-1−/− mice compared to wild-type mice (Figure 2A). Alcohol however up-regulated hepcidin gene expression in gpx-1−/− mice over two-fold compared to that in water-fed gpx-1−/− mice (Figure 2B).
Figure 2.
Hepcidin mRNA expression in the liver. cDNA synthesized from liver RNA of transgenic mice, lacking the expression of either glutathione peroxidase-1 (gpx-1−/−) or catalase (catalase−/−), and wild-type mice, fed with plain water (H2O) or ethanol (alc.), was employed to determine hepcidin mRNA expression by Taqman real-time PCR. (A) Hepcidin gene expression in water or alcohol-fed transgenic mice and alcohol-fed wild-type mice was expressed as-fold hepcidin expression of that in wild-type mice fed with water. (B) Hepcidin gene expression in alcohol-fed gpx-1−/− mice was expressed as-fold hepcidin expression of that in gpx-1−/− mice fed with water. Asterisks indicate statistical significance (p < 0.05).
Our knockout mice studies indicated a correlation between H2O2 and the inhibition of hepcidin expression in the liver. Namely, H2O2 accumulation in the livers of untreated gpx-1−/− and alcohol-fed wild-type or catalase−/− mice was accompanied by a significant decrease in hepcidin expression. This inhibition might be concentration-dependent because the level of hepatic H2O2 in untreated catalase−/− mice, which was significantly lower than that in untreated gpx-1−/−, was not sufficient to inhibit hepcidin. Miura et al. [26] and Millonig et al. [27] have reported the regulation of hepcidin expression by H2O2 in tissue culture cells. Millonig et al. have shown an induction and inhibition of hepcidin expression by low and high concentrations of H2O2, respectively. They suggested the involvement of Stat3 in the stimulation and cytotoxicity in the inhibition of hepcidin expression by H2O2 in vitro [27]. Miura et al. however have reported a role for elevated histone deacetylase activity in H2O2-mediated inhibition of hepatic hepcidin in Huh7 cells [26]. Although antioxidant defense mechanisms in the liver in vivo are more elaborate than in cultured hepatoma cells, these studies show a concentration-dependent effect of H2O2 on liver hepcidin expression. Given the fact that the intracellular localization of catalase and gpx-1 are not similar, our study also pointed out the spatial dynamics of H2O2 in hepcidin regulation. Namely, the regulation of hepcidin expression was different in catalase−/− and gpx-1−/− mice. Unlike catalase, which is primarily expressed in peroxisomes, gpx-1 is expressed both in cytosol and mitochondria [13]. We have previously reported that mitochondrial superoxide is not involved in inhibition of hepcidin expression by alcohol [29]. Attenuation of hepcidin expression in untreated gpx-1−/− mice, which displayed a higher H2O2 content than untreated catalase−/− mice, suggested that cytosolic and mitochondrial H2O2 might be involved in inhibition of hepcidin expression. However, upon alcohol exposure, gpx-1−/− mice displayed induction of hepcidin expression without changes in H2O2 levels. Nevertheless, our knockout mice studies suggested that both subcellular location and concentration of H2O2 in hepatocytes are equally important in differential regulation of hepcidin expression.
2.2. The Effect of Alcohol and H2O2 on ER Stress in the Liver
Elevated hepcidin expression in alcohol-fed gpx-1−/− mice may involve various mechanisms such as inflammation or ER stress, which are known to induce hepcidin expression [24,25]. Our previous studies have however shown that alcohol can inhibit liver hepcidin expression in the presence of inflammation [30,31]. We have also shown the involvement of alcohol-mediated TLR4 and NF-κB activation in this process [31]. It is therefore not feasible that the induction of hepcidin in alcohol-treated gpx-1−/− mice is mediated by inflammation. We then studied the role of ER stress in this process.
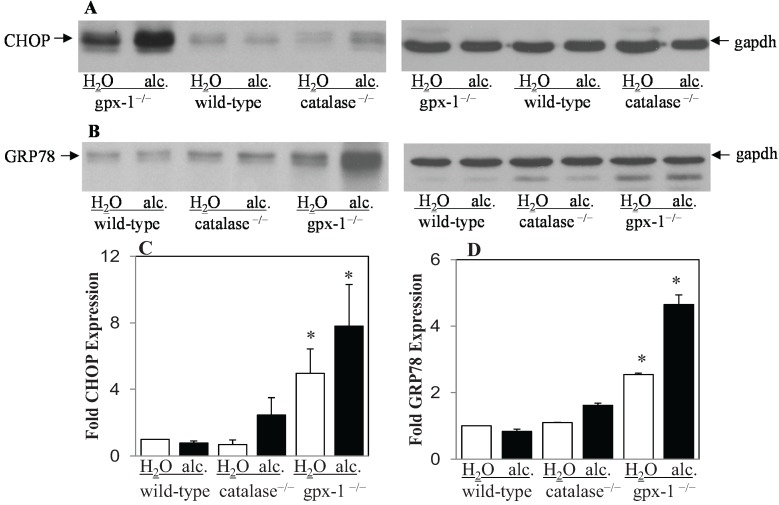
Iron-regulatory genes have been reported to be the downstream targets of the transcription factor, CHOP (GADD153), which is involved in ER stress [24,32]. In untreated cells, CHOP is expressed at very low levels and its expression is induced by stress [21]. We therefore determined the expression level of CHOP in the livers of untreated and alcohol-treated wild-type, gpx-1−/− and catalase−/− mice by western blotting (Figure 3A). CHOP protein expression was significantly elevated in the livers of untreated gpx-1−/−, but not catalase−/− mice, compared to untreated wild-type mice. Alcohol induced liver CHOP expression strongly in gpx-1−/−, and only marginally and not significantly in catalase−/− mice, compared to wild-type mice (Figure 3A,C). To further examine ER stress, the expression of chaperone protein, GRP78 (BiP) in the livers of untreated and alcohol-treated wild-type, catalase−/− and gpx-1−/− mice was detected by western blotting (Figure 3B). GRP78 protein expression was significantly elevated in the livers of untreated gpx-1, but not catalase, knockout mice, compared to untreated wild-type mice. Alcohol treatment further induced liver GRP78 expression significantly in gpx-1−/−, but not catalase−/− or wild-type, mice (Figure 3B,D).
Figure 3.
Liver CHOP (GADD153) and GRP78 (BiP) protein expression. Total cell lysates isolated from the livers of H2O or ethanol (alc.)-fed wild-type and gpx-1−/− or catalase−/− transgenic mice were employed to detect CHOP (A) or GRP78/BiP (B) protein expression by western blotting. An anti-gapdh antibody was used to demonstrate equal protein loading. CHOP (C) and GRP78 (D) expression was quantified by densitometric analysis and normalized to gapdh expression, respectively. Normalized expression in H2O or alcohol-fed knockout and alcohol-fed wild-type mice was expressed as fold expression of that in H2O-fed wild-type mice. Asterisks indicate statistical significance (p < 0.05).
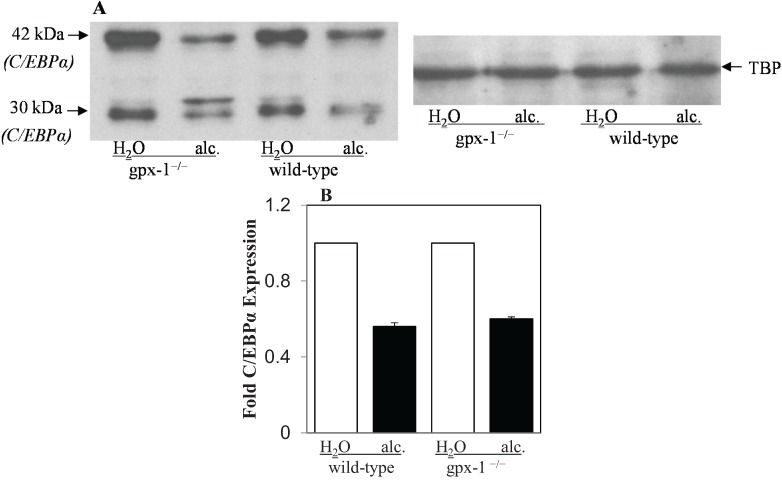
CHOP acts as a negative regulator of the C/EBP family of transcription factors. We have previously shown that alcohol inhibits C/EBPα in wild-type mice livers [8]. Since CHOP was induced by alcohol in gpx-1 mice, we determined nuclear C/EBPα expression in gpx-1−/− mice livers by western blotting. The absence of gpx-1 did not significantly alter the basal expression level of liver C/EBPα protein in gpx-1−/− mice compared to wild-type mice (Figure 4A,B). Alcohol inhibited C/EBPα expression to the same extent in gpx-1−/− and wild-type mice (Figure 4A,B).
Figure 4.
Liver C/EBPα protein expression. (A) Nuclear cell lysates isolated from the livers of H2O or ethanol (alc.)-fed wild-type and gpx-1−/− transgenic mice were employed to detect C/EBPα protein expression by western blotting. An anti-TATA-binding protein (TBP) antibody was used to demonstrate equal nuclear protein loading. (B) C/EBPα protein (30 kDa and 42 kDa) expression was quantified by densitometric analysis and normalized to TBP expression. Normalized expression in H2O or alcohol-fed gpx-1−/− and alcohol-fed wild-type mice was expressed as fold expression of that in H2O-fed wild-type mice.
The up-regulation of CHOP and Grp78 protein expression in the livers of gpx-1, but not catalase, knockout mice, suggest that accumulation of H2O2 in cytosol and mitochondria of hepatocytes together with alcohol metabolism can cause ER stress, thereby leading to the induction of hepcidin gene expression. C/EBPα, which is known to be inhibited by CHOP, is involved in the regulation of hepcidin gene expression [22,33]. We have previously shown that alcohol suppresses liver hepcidin expression via the inhibition of C/EBPα [7,8]. Oliveira et al. have also suggested that ER-stress-mediated biphasic regulation of hepcidin in vitro involves CHOP and C/EBPα [24]. However, our western blot analysis did not establish a correlation between CHOP and C/EBPα protein expression patterns in the livers of alcohol-fed mice. Namely, independent of differences in CHOP expression levels, both wild-type and gpx-1−/− mice displayed inhibition of nuclear C/EBPα expression upon alcohol exposure. Based on these data and previous studies by us and others, we believe that CHOP does not associate with C/EBPα in alcohol-mediated induction of hepcidin expression in gpx-1 knockout mice (see Scheme 1 below). Similarly, Miura et al. have also shown that inhibition of C/EBPα by hepatitis C viral proteins does not involve CHOP [26]. Our findings nonetheless suggest that ER stress signaling may be involved in induction of liver hepcidin expression by alcohol in gpx-1−/− mice.
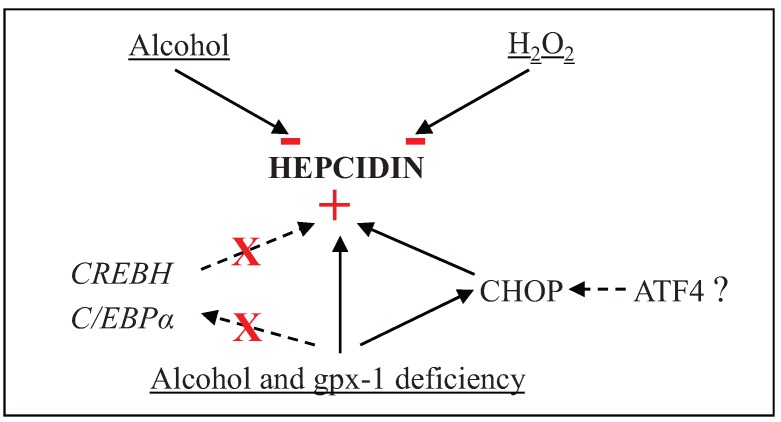
Scheme 1.
H2O2 and/or alcohol-mediated regulation of hepatic hepcidin gene expression. The signs, − and + indicate inhibition and induction of gene expression, respectively, and X represents the lack of CREBH or C/EBPα involvement in the induction of hepatic hepcidin gene expression.
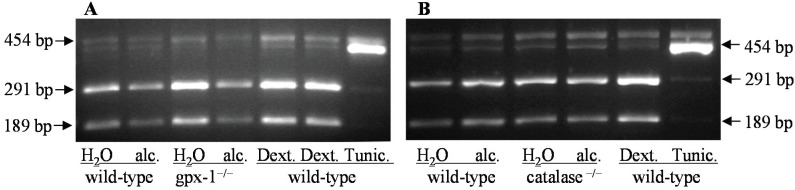
The effect of H2O2 on ER stress was further analyzed by determining the splicing of transcription factor, X-box binding protein 1 (XBP1), as described in Experimental Section (Figure 5). XBP1 is a specific substrate of the endoribonuclease, inositol-requiring enzyme 1 (IRE1). XBP1 mRNA splicing is therefore used as a marker for IRE1 activation. Splicing of XBP1 alters Pst1 recognition sequence present within the IRE1 excision site, and spliced XBP1 becomes resistant to Pst1 digestion. XBP1 was not spliced in the livers of untreated or alcohol-fed catalase−/−, gpx-1−/− and wild-type mice, as confirmed by the presence of Pst1 digested 291 and 189 bp XBP1 DNA fragments (Figure 5A,B). In contrast, XBP1 was spliced in the livers of wild-type mice injected with an ER-inducer, tunicamycin, but not with dextrose (as control), as shown by the presence of a Pst1-resistant 454 bp XBP1 amplicon, and thereby validating our XBP1 splicing assay (Figure 5A,B). These findings show that unlike tunicamycin, alcohol and/or H2O2 did not activate the unfolded protein response transducer, IRE1 in the liver.
Figure 5.
XBP1 mRNA splicing in the livers of H2O or ethanol (alc.)-fed wild-type and catalase−/− or gpx-1−/− transgenic mice was determined by RT-PCR and Pst1 restriction enzyme digestion. Wild-type mice injected with dextrose as control (Dext.) or tunicamycin (Tunic.) were used as controls. 454 bp, and 291bp and 189 bp amplicons refer to spliced (Pst1-resistant) and unspliced XBP1, respectively.
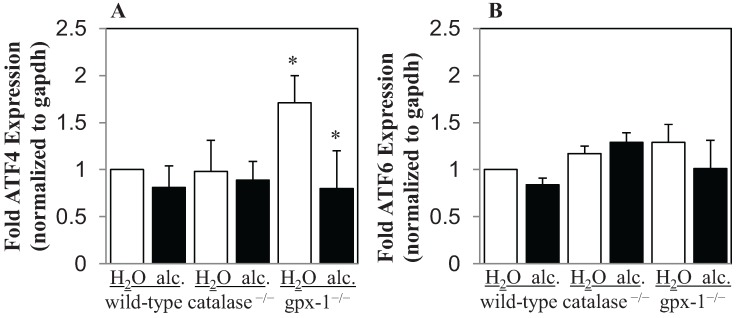
The transcription factors, ATF4 and ATF6, which are activated by ER stress, have been shown to regulate CHOP expression [21]. The level of ATF4 and ATF6 mRNA expression in the livers of untreated and alcohol-treated transgenic and wild-type mice was determined by real-time PCR (Figure 6). The expression of ATF4 in the liver was significantly increased in gpx-1−/− and was unchanged in catalase−/− mice, compared to wild-type mice (Figure 6A). Alcohol however significantly inhibited ATF4 mRNA expression in gpx-1−/−, but not catalase−/− or wild-type, mice (Figure 6A). No significant changes in ATF6 mRNA expression were observed in transgenic mice compared to wild-type mice (Figure 6B). Similarly, ATF6 mRNA expression was not altered by alcohol treatment in wild-type or transgenic mice (Figure 6B).
Figure 6.
Liver ATF4 and ATF6 mRNA expression. cDNA synthesized from liver RNA of wild-type and catalase−/− or gpx-1−/− transgenic mice, fed with plain water (H2O) or ethanol (alc.), was employed to determine ATF4 (A) and ATF6 (B) mRNA expression by real-time PCR. Gene expression levels in alcohol fed wild-type and untreated or alcohol-fed transgenic mice were expressed as-fold ATF expression of that in wild-type mice fed with water. Asterisks indicate statistical significance (p < 0.05).
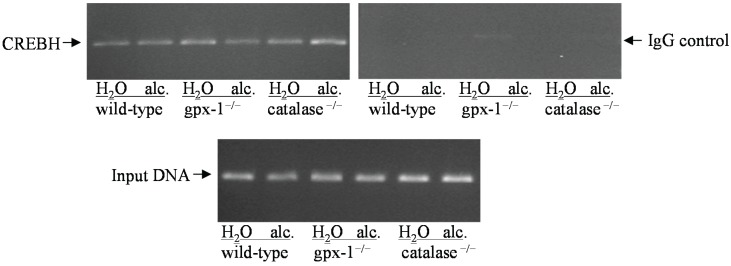
The transcription factor, cyclic AMP-responsive element binding protein H (CREBH) has been shown to be involved in ER stress-mediated regulation of hepcidin transcription. To study the effect of alcohol and/or H2O2 on hepcidin gene promoter, chromatin immunoprecipitation (CHIP) assays were performed, as described in Experimental Section. The occupancy of mouse hepcidin gene promoter by CREBH in the livers of untreated and alcohol-fed gpx-1−/−, catalase−/− and wild-type mice was determined (Figure 7). Alcohol and/or H2O2 did not stimulate the binding of CREBH to hepcidin gene promoter in the livers of transgenic or wild-type mice (Figure 7). The amplification of total input DNA was similar in all the samples confirming that different liver chromatins contained equal amounts of DNA (Figure 7).
Figure 7.
CREBH binding to hepcidin gene promoter. Chromatin isolated from the livers of water (H2O) or ethanol (alc.)-fed wild-type mice and gpx-1−/− or catalase−/− transgenic mice were immunoprecipitated by an anti-CREBH antibody or normal rabbit IgG, as control. The co-immunoprecipitated and total input (control) DNA were used as templates in PCR to amplify a 321 bp mouse hepcidin gene promoter region, which harbors CREBH DNA-binding site, as described in Experimental Section.
The elevation of ER stress is characterized by the activation of three distinct signaling pathways mediated by IRE1, ATF6 and PERK/eIF2α [34]. However, the lack of XBP1 splicing in the livers of knockout and wild-type mice suggests that the IRE1 arm of ER stress signaling is not activated by alcohol in these mice. Transcription factors, ATF4 and ATF6 are involved in the regulation of CHOP expression [21]. The expression of ATF4, but not ATF6, was elevated in gpx-1−/− mice livers. It is therefore feasible that CHOP induction in untreated gpx-1−/− mice is regulated by ATF4. However, alcohol inhibited ATF4 expression but induced CHOP expression in gpx-1−/− mice suggesting the presence of other mechanisms. Different stress conditions have been shown to induce variable ATF4 expression [35]. Furthermore, ATF4 and CHOP are regulated at both the transcriptional and posttranscriptional levels [35,36]. The transcription factor, CREBH has also been reported to play a role in hepcidin up-regulation by tunicamycin-mediated ER stress [25]. The binding of CREBH to hepcidin gene promoter in gpx-1−/−, catalase−/− or wild-type mice was however not stimulated by alcohol exposure, as shown by our CHIP assays. Compared to experimental ER stress models, other transcription factors besides CREBH may play a role in the regulation of hepcidin gene expression by ER stress in liver diseases. Future studies will investigate the mechanisms involved in synergistic regulation of CHOP by alcohol and H2O2.
3. Experimental Section
3.1. Animal Experiments
Animal experiments were approved by the animal ethics committee at the University of Nebraska Medical Center. Transgenic mice, homozygous for the null allele of glutathione peroxidase-1 (gpx-1−/−) or catalase (catalase−/−), on C57BL/6 genetic background, were generated, as described previously [37,38]. For alcohol treatment, male transgenic and wild-type C57BL/6 mice housed individually and maintained on rodent chow diet-7012 (Harlan Teklad), were administered 10% ethanol in the drinking water or plain water for 7 days, as described previously [7]. These experiments were performed 6 times with 2 mice in each group. Blood alcohol levels were measured by using a diagnostic alcohol kit (Sigma Aldrich Inc., St. Louis, MO, USA; product no. N7160), as published previously [8], and expressed as mg alcohol per dL blood.
3.2. Cytochrome P4502E1 (CYP2E1) Activity
CYP2E1 activity was measured in crude liver homogenates by the hydroxylation of p-nitrophenol to form 4-nitrocatechol (4-nc), as published previously [39,40].
3.3. Liver Perfusion
To isolate viable hepatocytes, mice livers were perfused, as described [29,41]. Briefly, livers were perfused (7 mL/min) with warm and gassed KRH buffer (25 mM HEPES, 114.9 mM NaCl, 4.5 mM KCl, 1 mM KH2PO4, 0.5 mM EGTA, pH 7.6) followed by KRH buffer containing 2mM Ca2+ and collagenase (0.214 mg/mL, Sigma C5138). Hepatocytes were washed thrice with ice-cold KRH buffer containing 2 mM Ca2+ and 2% BSA. Hepatocyte viability was ≥ 80%, as determined by Trypan Blue staining.
3.4. Measurement of Intracellular H2O2 Levels
Hepatocytes, freshly isolated by liver perfusion, were washed twice in KRH buffer containing 2 mM Ca2+. 0.5 × 106 hepatocytes were incubated with 15 µM of the fluorescent probe, 2',7'-dichlorodihydrofluorescein-diacetate (DCFH-DA, Molecular Probes) or 0.1% DMSO (control) in KRH buffer for 30 min at 37 °C with constant shaking in the dark. Subsequently, hepatocytes were washed once with KRH buffer and 2'-7'-dichlorodihydrofluorescein (DCF) fluorescence was detected by a spectrophotometer plate reader (Molecular Devices, SpectraMax M5, Sunnyvale, CA, USA) at an excitation wavelength of 488 nm and an emission wavelength of 520 nm. DCFH-DA studies were conducted three times for each experimental group with 2 mice per group.
3.5. RNA Isolation, cDNA Synthesis and Real-time Quantitative PCR Analysis
Liver tissues washed with PBS were lysed in Trizol® (Invitrogen Corporation, Grand Island, NY, USA) and total RNA was isolated according to the manufacturer’s instructions. cDNA synthesis and quantitative PCR were performed, as published previously [30]. Primers (sense: 5'-ACTCGGACCCAGGCTGC-3'; antisense: 5'-AGATAGGTGGTGCTGCTCAGG-3') and Taqman fluorescent probe (5' 6-[FAM]-TGTCTCCTGCTTCTCCTCCTTGCCA-3' [TAMRA-Q]) flanking about 70 base pairs of open reading frame sequences of mouse hepcidin genes, Hamp1 and Hamp2 were designed by the Primer Express 1.5 program (Applied Biosystems). ATF4 and ATF6 mRNA expression were determined by SYBR Green real-time PCR by using specific primers for ATF4 (sense: 5'-CCT GAA CAG CGA AGT GTT GG-3'; antisense: 5'-TGG AGA ACC CAT GAG GTT TCA A-3') and ATF6 (sense: 5'-TCG CCT TTT AGT CCG GTT CTT-3'; antisense: 5'-GGC TCC ATA GGT CTG ACT CC-3') mouse genes. Glyceraldehyde-3-phosphate dehydrogenase (gapdh) gene was used as the endogenous control (sense: 5'-GTGGAGATTGTTGCCATCAACGA -3'; antisense: 5'-CCCATTCTCGGCCTTGACTGT-3').
3.6. Analysis of X-Box Binding Protein 1 Splicing
ER stress-induced splicing of X-box binding protein 1 (XBP1) was determined by RT-PCR and restriction enzyme digestion. cDNA synthesized from mice liver RNA were employed to amplify XBP1 region containing inositol-requiring enzyme 1 (IRE1) excision site using specific primers (sense: 5'-AAACAGAGTAGCAGCGCAGACTGC-3'; antisense: 5'-TCCTTCTGGGTAGACCTCTGGGAA-3'). Amplified DNA was subsequently digested with Pst1 enzyme at 37 °C for 2 h and resolved on a 2% agarose gel, which was stained with ethidium bromide to detect XBP1 DNA fragments. The splicing experiments were repeated three times.
3.7. Chromatin Immunoprecipitation (CHIP)
CHIP assays were performed, as described previously [42]. Briefly, sheared chromatin were immunoprecipitated by using commercial antibodies (Cell Signaling, Santa Cruz, CA, USA). An aliquot of pre-cleared chromatin was saved as total input DNA prior to the immunoprecipitation. Eluted DNA were analyzed by PCR using primers specific for mouse Hamp1 promoter (sense: 5'-GCCATACTGAAGGCACTGA-3'; antisense: 5'-GTGTGGTGGCTGTCTAGG-3'). CHIP assays were repeated three times with different sets of sheared chromatin.
3.8. Western Blotting
Preparation of total or nuclear cell lysates and western blots were performed, as described previously [42]. Each blot was repeated three times with different lysate preparations. All antibodies were obtained commercially (Cell Signaling, Santa Cruz).
3.9. Statistical Analysis
SPSS software was used for statistical analysis. The significance of difference between groups was determined by Student’s t-test, and non-parametric Kruskal-Wallis ANOVA and Wilcoxon Mann-Whitney tests. A value of p < 0.05 was accepted as statistically significant.
4. Conclusions
Hepcidin, synthesized primarily by the liver, protects the body from the harmful effects of iron by tightly controlling iron uptake and release. We have previously demonstrated that alcohol-induced oxidative stress suppresses liver hepcidin expression and thereby elevates intestinal iron transporter expression. A role for superoxide free radicals has been excluded in this process [29]. In this study, we investigated the role of H2O2 in hepcidin regulation by alcohol by using two different knockout mouse models with defective H2O2 catabolism. Comparison of catalase and gpx-1 knockout mice suggested that H2O2 inhibits hepcidin expression in the liver in vivo. In synergy with alcohol and in the absence of gpx-1, H2O2 induced ER stress via CHOP, independent of C/EBPα or CREBH, and thereby up-regulated hepcidin expression (Scheme 1). These studies will help us to further understand the role of iron and redox metabolism in the pathogenesis of alcoholic liver disease.
Acknowledgments
These studies were supported by funds from National Institutes of Health (R01 AA017738 to Duygu Dee Harrison-Findik) and University of Nebraska Medical Center Graduate Studies Research Fellowship (to Sizhao Lu). We thank Emily Zmijewski for technical assistance.
Author Contributions
Duygu Dee Harrison-Findik designed, performed experiments and wrote the manuscript. Sizhao Lu performed experiments and helped with the preparation of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Ioannou G.N., Dominitz J.A., Weiss N.S., Heagerty P.J., Kowdley K.V. The effect of alcohol consumption on the prevalence of iron overload, iron deficiency, and iron deficiency anemia. Gastroenterology. 2004;126:1293–1301. doi: 10.1053/j.gastro.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 2.Tavill A.S., Qadri A.M. Alcohol and iron. Semin. Liver Dis. 2004;24:317–325. doi: 10.1055/s-2004-832943. [DOI] [PubMed] [Google Scholar]
- 3.Nemeth E., Ganz T. Regulation of iron metabolism by hepcidin. Annu. Rev. Nutr. 2006;26:323–342. doi: 10.1146/annurev.nutr.26.061505.111303. [DOI] [PubMed] [Google Scholar]
- 4.Harrison-Findik D.D. Is the iron regulatory hormone hepcidin a risk factor for alcoholic liver disease? World J. Gastroenterol. 2009;15:1186–1193. doi: 10.3748/wjg.15.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bridle K., Cheung T.K., Murphy T., Walters M., Anderson G., Crawford D.G., Fletcher L.M. Hepcidin is down-regulated in alcoholic liver injury: Implications for the pathogenesis of alcoholic liver disease. Alcohol Clin. Exp. Res. 2006;30:106–112. doi: 10.1111/j.1530-0277.2006.00002.x. [DOI] [PubMed] [Google Scholar]
- 6.Ohtake T., Saito H., Hosoki Y., Inoue M., Miyoshi S., Suzuki Y., Fujimoto Y., Kohgo Y. Hepcidin is down-regulated in alcohol loading. Alcohol Clin. Exp. Res. 2007;31:S2–S8. doi: 10.1111/j.1530-0277.2006.00279.x. [DOI] [PubMed] [Google Scholar]
- 7.Harrison-Findik D.D., Klein E., Crist C., Evans J., Timchenko N., Gollan J. Iron-mediated regulation of liver hepcidin expression in rats and mice is abolished by alcohol. Hepatology. 2007;46:1979–1985. doi: 10.1002/hep.21895. [DOI] [PubMed] [Google Scholar]
- 8.Harrison-Findik D.D., Schafer D., Klein E., Timchenko N.A., Kulaksiz H., Clemens D., Fein E., Andriopoulos B., Pantopoulos K., Gollan J. Alcohol metabolism-mediated oxidative stress down-regulates hepcidin transcription and leads to increased duodenal iron transporter expression. J. Biol. Chem. 2006;281:22974–22982. doi: 10.1074/jbc.M602098200. [DOI] [PubMed] [Google Scholar]
- 9.Wu D., Cederbaum A.I. Oxidative stress and alcoholic liver disease. Semin. Liver Dis. 2009;29:141–154. doi: 10.1055/s-0029-1214370. [DOI] [PubMed] [Google Scholar]
- 10.Graf E., Mahoney J.R., Bryant R.G., Eaton J.W. Iron-catalyzed hydroxyl radical formation. Stringent requirement for free iron coordination site. J. Biol. Chem. 1984;259:3620–3624. [PubMed] [Google Scholar]
- 11.Sies H. Strategies of antioxidant defense. Eur. J. Biochem. 1993;215:213–219. doi: 10.1111/j.1432-1033.1993.tb18025.x. [DOI] [PubMed] [Google Scholar]
- 12.Rhee S.G., Chang T.S., Bae Y.S., Lee S.R., Kang S.W. Cellular regulation by hydrogen peroxide. J. Am. Soc. Nephrol. 2003;14:S211–S215. doi: 10.1097/01.ASN.0000077404.45564.7E. [DOI] [PubMed] [Google Scholar]
- 13.Lubos E., Loscalzo J., Handy D.E. Glutathione peroxidase-1 in health and disease: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2011;15:1957–1997. doi: 10.1089/ars.2010.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polavarapu R., Spitz D.R., Sim J.E., Follansbee M.H., Oberley L.W., Rahemtulla A., Nanji A.A. Increased lipid peroxidation and impaired antioxidant enzyme function is associated with pathological liver injury in experimental alcoholic liver disease in rats fed diets high in corn oil and fish oil. Hepatology. 1998;27:1317–1323. doi: 10.1002/hep.510270518. [DOI] [PubMed] [Google Scholar]
- 15.Bailey S.M., Patel V.B., Young T.A., Asayama K., Cunningham C.C. Chronic ethanol consumption alters the glutathione/glutathione peroxidase-1 system and protein oxidation status in rat liver. Alcohol Clin. Exp. Res. 2001;25:726–733. doi: 10.1111/j.1530-0277.2001.tb02273.x. [DOI] [PubMed] [Google Scholar]
- 16.Malhotra J.D., Kaufman R.J. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid. Redox Signal. 2007;9:2277–2293. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- 17.Ji C., Kaplowitz N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology. 2003;124:1488–1499. doi: 10.1016/S0016-5085(03)00276-2. [DOI] [PubMed] [Google Scholar]
- 18.Ji C. Mechanisms of alcohol-induced endoplasmic reticulum stress and organ injuries. Biochem. Res. Int. 2012 doi: 10.1155/2012/216450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsedensodnom O., Vacaru A.M., Howhart L.D., Yin C., Sadler K.C. Ethanol metabolism and oxidative stress are required for unfolded protein response activation and steatosis in zebrafish with alcoholic liver disease. Dis. Models Mech. 2013;6:1213–1226. doi: 10.1242/dmm.012195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang C., Li H., Meng Q., Du Y., Xiao F., Zhang Q., Yu J., Li K., Chen S., Huang Z., et al. ATF4 deficiency protects hepatocytes from oxidative stress via inhibiting CYP2E1 expression. J. Cell Mol. Med. 2014;18:80–90. doi: 10.1111/jcmm.12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oyadomari S., Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 22.Ron D., Habener J.F. CHOP, a novel developmentally regulated nuclear protein that dimerizes with transcription factors C/EBP and LAP and functions as a dominant-negative inhibitor of gene transcription. Genes Dev. 1992;6:439–453. doi: 10.1101/gad.6.3.439. [DOI] [PubMed] [Google Scholar]
- 23.McCullough K.D., Martindale J.L., Klotz L.O., Aw T.Y., Holbrook N.J. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol. Cell Biol. 2001;21:1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oliveira S.J., Pinto J.P., Picarote G., Costa V.M., Carvalho F., Rangel M., de Sousa M., de Almeida S.F. ER stress-inducible factor CHOP affects the expression of hepcidin by modulating C/EBPalpha activity. PLOS ONE. 2009;4:e6618. doi: 10.1371/journal.pone.0006618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vecchi C., Montosi G., Zhang K., Lamberti I., Duncan S.A., Kaufman R.J., Pietrangelo A. ER stress controls iron metabolism through induction of hepcidin. Science. 2009;325:877–880. doi: 10.1126/science.1176639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miura K., Taura K., Kodama Y., Schnabl B., Brenner D.A. Hepatitis C virus-induced oxidative stress suppresses hepcidin expression through increased histone deacetylase activity. Hepatology. 2008;48:1420–1429. doi: 10.1002/hep.22486. [DOI] [PubMed] [Google Scholar]
- 27.Millonig G., Ganzleben I., Peccerella T., Casanovas G., Brodziak-Jarosz L., Breitkopf-Heinlein K., Dick T.P., Seitz H.K., Muckenthaler M.U., Mueller S. Sustained submicromolar H2O2 levels induce hepcidin via signal transducer and activator of transcription 3 (STAT3) J. Biol. Chem. 2012;287:37472–37482. doi: 10.1074/jbc.M112.358911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LeBel C.P., Ischiropoulos H., Bondy S.C. Evaluation of the probe 2',7'-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem. Res. Toxicol. 1992;5:227–231. doi: 10.1021/tx00026a012. [DOI] [PubMed] [Google Scholar]
- 29.Harrison-Findik D.D., Lu S., Zmijewski E.M., Jones J., Zimmerman M.C. Effect of alcohol exposure on hepatic superoxide generation and hepcidin expression. World J. Biol. Chem. 2013;4:119–130. doi: 10.4331/wjbc.v4.i4.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harrison-Findik D.D., Klein E., Evans J., Gollan J. Regulation of liver hepcidin expression by alcohol in vivo does not involve Kupffer cell activation or TNF-α signaling. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;296:G112–G118. doi: 10.1152/ajpgi.90550.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zmijewski E., Lu S., Harrison-Findik D.D. TLR4 signaling and the inhibition of liver hepcidin expression by alcohol. World J. Gastroenterol. 2014;20:12161–12170. doi: 10.3748/wjg.v20.i34.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.You K.R., Liu M.J., Han X.J., Lee Z.W., Kim D.G. Transcriptional regulation of the human transferrin gene by GADD153 in hepatoma cells. Hepatology. 2003;38:745–755. doi: 10.1053/jhep.2003.50367. [DOI] [PubMed] [Google Scholar]
- 33.Courselaud B., Pigeon C., Inoue Y., Inoue J., Gonzalez F.J., Leroyer P., Gilot D., Boudjema K., Guguen-Guillouzo C., Brissot P., et al. C/EBPα regulates hepatic transcription of hepcidin, an antimicrobial peptide and regulator of iron metabolism. Cross-talk between C/EBP pathway and iron metabolism. J. Biol. Chem. 2002;277:41163–41170. doi: 10.1074/jbc.M202653200. [DOI] [PubMed] [Google Scholar]
- 34.Malhi H., Kaufman R.J. Endoplasmic reticulum stress in liver disease. J. Hepatol. 2011;54:795–809. doi: 10.1016/j.jhep.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dey S., Baird T.D., Zhou D., Palam L.R., Spandau D.F., Wek R.C. Both transcriptional regulation and translational control of ATF4 are central to the integrated stress response. J. Biol. Chem. 2010;285:33165–33174. doi: 10.1074/jbc.M110.167213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bruhat A., Jousse C., Wang X.Z., Ron D., Ferrara M., Fafournoux P. Amino acid limitation induces expression of CHOP, a CCAAT/enhancer binding protein-related gene, at both transcriptional and post-transcriptional levels. J. Biol. Chem. 1997;272:17588–17593. doi: 10.1074/jbc.272.28.17588. [DOI] [PubMed] [Google Scholar]
- 37.Ho Y.S., Magnenat J.L., Bronson R.T., Cao J., Gargano M., Sugawara M., Funk C.D. Mice deficient in cellular glutathione peroxidase develop normally and show no increased sensitivity to hyperoxia. J. Biol. Chem. 1997;272:16644–16651. doi: 10.1074/jbc.272.26.16644. [DOI] [PubMed] [Google Scholar]
- 38.Ho Y.S., Xiong Y., Ma W., Spector A., Ho D.S. Mice lacking catalase develop normally but show differential sensitivity to oxidant tissue injury. J. Biol. Chem. 2004;279:32804–32812. doi: 10.1074/jbc.M404800200. [DOI] [PubMed] [Google Scholar]
- 39.Donohue T.M., Curry-McCoy T.V., Todero S.L., White R.L., Kharbanda K.K., Nanji A.A., Osna N.A. L-Buthionine (S,R) sulfoximine depletes hepatic glutathione but protects against ethanol-induced liver injury. Alcohol Clin. Exp. Res. 2007;31:1053–1060. doi: 10.1111/j.1530-0277.2007.00393.x. [DOI] [PubMed] [Google Scholar]
- 40.Cederbaum A.I. Methodology to assay CYP2E1 mixed function oxidase catalytic activity and its induction. Redox Biol. 2014;2C:1048–1054. doi: 10.1016/j.redox.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seglen P.O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- 42.Gerjevic L.N., Liu N., Lu S., Harrison-Findik D.D. Alcohol activates TGF-β but inhibits BMP receptor-mediated Smad signaling and Smad4 binding to hepcidin promoter in the liver. Int. J. Hepatol. 2012 doi: 10.1155/2012/459278. [DOI] [PMC free article] [PubMed] [Google Scholar]