Abstract
Primary prevention through lifestyle interventions is a cost-effective alternative for preventing a large burden of chronic and degenerative diseases, including cancer, which is one of the leading causes of morbidity and mortality worldwide. In the past decade, epidemiologic and preclinical evidence suggested that polyphenolic phytochemicals present in many plant foods possess chemopreventive properties against several cancer forms. Thus, there has been increasing interest in the potential cancer chemopreventive agents obtained from natural sources, such as polyphenols, that may represent a new, affordable approach to curb the increasing burden of cancer throughout the world. Several epidemiologic studies showed a relation between a soy-rich diet and cancer prevention, which was attributed to the presence of a phenolic compound, genistein, present in soy-based foods. Genistein acts as a chemotherapeutic agent against different types of cancer, mainly by altering apoptosis, the cell cycle, and angiogenesis and inhibiting metastasis. Targeting caspases, B cell lymphoma 2 (Bcl-2)–associated X protein (Bax), Bcl-2, kinesin-like protein 20A (KIF20A), extracellular signal-regulated kinase 1/2 (ERK1/2), nuclear transcription factor κB (NF-κB), mitogen-activated protein kinase (MAPK), inhibitor of NF-κB (IκB), Wingless and integration 1 β-catenin (Wnt/β-catenin), and phosphoinositide 3 kinase/Akt (PI3K/Akt) signaling pathways may act as the molecular mechanisms of the anticancer, therapeutic effects of genistein. Genistein also shows synergistic behavior with well-known anticancer drugs, such as adriamycin, docetaxel, and tamoxifen, suggesting a potential role in combination therapy. This review critically analyzes the available literature on the therapeutic role of genistein on different types of cancer, focusing on its chemical features, plant food sources, bioavailability, and safety.
Keywords: genistein, cancer, source, bioavailability, safety
Introduction
The International Agency for Research on Cancer, which is part of the WHO, reported that of the estimated 14.1 million adults worldwide who were diagnosed with cancer in 2012, 8.2 million deaths were recorded (1). Moreover, on the basis of recent trends in the incidence of major cancers and projected population growth, >23 million new cancer cases annually are expected by 2030. This means 68% more cases of cancer than in 2012 (2). The most commonly diagnosed cancer types worldwide are lung, breast, and colorectal, whereas those with a higher index of mortality are lung, liver, and stomach (3).
It is therefore necessary to have new, affordable approaches to curb the increasing burden of cancer throughout the world (4). It is widely known that dietary habits have a strong impact on the incidence of chronic diseases such as cancers (5–8).
High intakes of animal fat, energy, and alcohol increase the cancer risk (9–12), whereas foods of plant origin exert their protective effects due to the presence of phytochemicals via different mechanisms of action (i.e., antioxidant capacity, hormonal activity, stimulation of enzymes, interference with DNA replication) (13–18). These biological and functional activities are ascribed both to the micronutrient content of plant foods, such as vitamins and minerals, as well as plant secondary metabolites, such as polyphenols, sulfur-containing compounds, terpenes, and alkaloids (19–25). Among them, the most studied compounds are polyphenols, which have been investigated for their potential protective effects on human health over the past 2 decades (26–29).
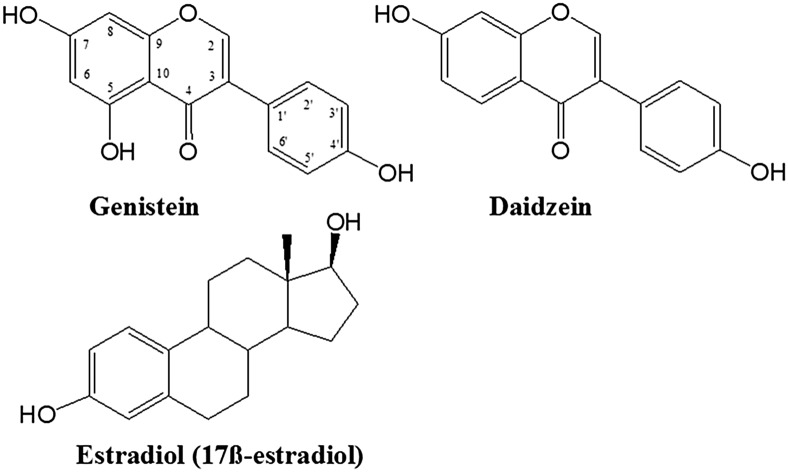
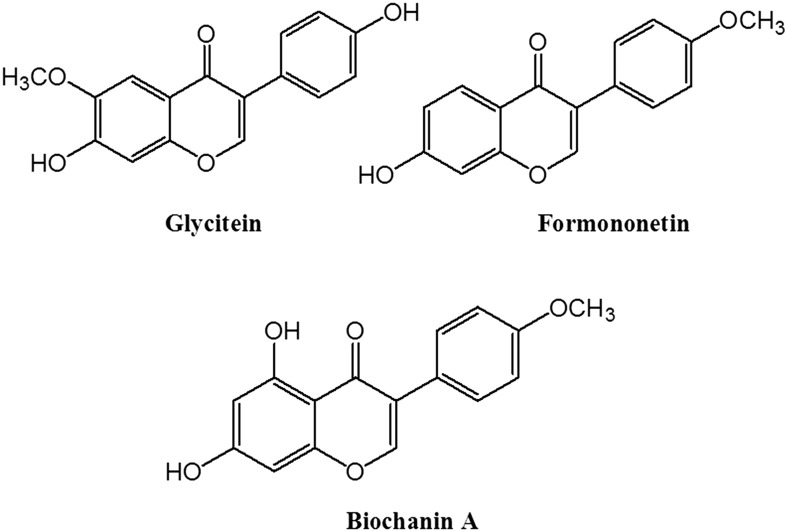
Polyphenols are generally subdivided into 2 large groups: flavonoids and nonflavonoids (30). Isoflavones are flavonoids in which the B ring is linked to the heterocyclic ring at the C3 instead of the C2 position (31, 32). The most important isoflavones are genistein and daidzein (Figure 1), followed by glycitein, formononetin, and biochanin A (Figure 2), which can occur in foods both in free and esterified forms (glycosylated, acetyl- and malonyl-glycosylated forms) (33).
FIGURE 1.
Chemical structure of genistein and related compounds.
FIGURE 2.
Chemical structure of glycitein, formononetin, and biochanin A.
The intake of soy products has been attributed to the lower incidence of breast and prostate cancer in Asian populations, which is mainly due to the presence of an isoflavone called genistein. When compared with the other isoflavones, genistein was observed to have structural similarity to 17β-estradiol and to possess weak estrogenic activity. It competes with 17β-estradiol for the estrogen receptor (ER),11 with 4% binding affinity for ER-α and 87% for ER-β, thereby contributing a favorable role in the treatment of hormone-related cancers (34). Moreover, many in vitro and in vivo studies also support that genistein can be considered a promising chemopreventive agent for the treatment of different types of cancer. In this article we review the beneficial role of genistein against different types of cancer, which were selected among those that are more common and with a high mortality rate. We also discuss the chemistry, plant food sources, bioavailability, and safety of genistein. Finally, we provide some recommendations that could be useful in directing future studies on this isoflavone.
Chemistry
Genistein [4′,5,7-trihydroxyisoflavone or 5,7-dihydroxy-3-(4-hydroxyphenyl) chromen-4-one] (C15H10O5) belongs to a multifunctional natural isoflavonoid class of flavonoids with a 15-carbon skeleton. Similar to other plant constituents, such as lignans, which possess estrogenic effect, genistein is a typical example of a phytoestrogenic compound. It was isolated for the first time from Genista tinctoria L. in 1899 and named after the genus of this plant. Since then, it has been found to occur as the main secondary metabolite of the Trifolium species and in Glycine max L. (synonym Soja hispida) (35). As shown in Figure 1, the chemical structure of genistein is similar to estradiol, leading to its binding ability to ERs (36–39). It possesses a high solubility in polar solvents including dimethylsulfoxide, acetone, and ethanol, although its solubility is much lower in water.
Although total organic synthesis of genistein was achieved in 1928, it has also been obtained by using various other methods. Chemical synthesis of genistein was performed via cyclization of the corresponding ketones by using a conventional microwave oven (40). Biotechnological synthesis of genistein was earlier reported to be achieved through conversion of (2S)-naringen into genisteinin NAD(P)H- and oxygen-dependent conditions as well as by the addition of cytochrome P-450 (CYP) in elicitor-treated soybean cell suspension cultures (41). Moreover, a metabolic-engineering approach to genistein synthesis was set up by using genetically engineered Saccharomyces cerevisiae yeast cells that contained the isoflavone synthase (IFS) gene isolated from Glycyrrhyza echinata L. (42). Similarly, genistein was produced in Nicotiana tabacum L. leaves transformed with IFS, by acting at the phenylpropanoid pathway, although UV-B treatment also enhanced genistein production in Arabidopsis (43).
On this basis and because of the known beneficial biological effects of genistein, chemists have been so far encouraged to synthesize many derivatives of this compound, with improved pharmacologic profile. For instance, FA-esterified (44), 6-carboxymethyl (45), nitroxy (46), 7-O-heterocycle (47), 7-O-β-d-glucoside (48) and 7-O-β-d-glucuronic acid (49), halogenated (50, 51), deoxybenzoin (50), benzylated (52), hydroxylated (53), esterified (54), benzosulfonate (55), dimethylaminomethyl (56), phenoxyalkylcarboxylic acid (57), glycoconjugate, and alkylbenzylamine (58) derivatives of genistein have been reported to date. All of these data reveal that the major structural features of genistein pave the way for synthesis of new genistein derivatives, which may emerge as novel types of anticancer, estrogenic, and antiosteoporetic agents.
Sources
The best known sources of genistein are soy-based foods, such as soy cheese or soy drinks (i.e., soy milk and soy-based beverages). The content of genistein in mature soybeans has been shown to range from 5.6 to 276 mg/100 g, and an average content of 81 mg/100 g is often described for comparative purposes (59). In addition to genistein, soy foods contain another major isoflavone, daidzein, which differs from genistein by the lack of the hydroxyl group at position 5 (Figure 1). Both isoflavones may exist in their aglycone or glycoside forms. The most common glycoside forms of genistein and daidzein are those of O-β-d-glucoside derivatives at position 7 in both compounds. Because numerous traditional Asian foods are made from soybeans, the average dietary isoflavone intake in Asian countries is in the range of 25–50 mg/d, whereas in Western countries the estimated intake is as low as 2 mg/d (60, 61).
Legumes are considered the second most important source of genistein, at 0.2 to 0.6 mg/100 g, which is present together with the other related isoflavone, daidzein (62). The genus Lupinus (commonly known as lupin) represents a typical example of the legume that is now widely cultivated for its seeds, which possess a nutritional value similar to soybean. Other important legumes are broad beans and chick peas, which are known to contain significant amounts of genistein, although less than soybeans. The content of genistein in fruit, nuts, and vegetables can vary considerably; the estimated range is from 0.03 to 0.2 mg/100 g (63). However, in some native cherry cultivars of Hungarian origin, genistein concentrations up to 4.4 mg/100 g have been recorded. An extended list of foods with their genistein content is available online in several databases (59).
The biotechnological approach used to maximize the isoflavonoid yield by sprouting seeds is the commonest method used to improve the nutritional and medicinal values of certain foods. The metabolic processes of seed germination, which are characterized by degradation of food reserves and anabolic processes devoted to the developing embryo, have been shown to enhance nutritional value primarily by increasing the content of vitamins and plant secondary metabolites, such as isoflavonoids (59, 64–68). Accordingly, the increased content of genistein and other isoflavonoid aglycones has been well documented in germinated soybean seeds and related products (69). During the process of fermentation of soybean products, the content of genistein and related aglycones increases (70). Through genetic manipulation, it is also possible to obtain genistein from nonlegume plant sources, such as rice. Cloning the enzyme IFS from a genistein-rich soybean cultivar resulted in transgenic rice lines with 30-fold more genistein content (71). With the medicinal value of genistein and related isoflavonoids now well recognized, soy-based meat substitutes, soy milk, soy cheese, and soy yogurt have recently gained popularity in Europe and the United States.
Bioavailability
Various experimental models, including in vivo studies, have shown that genistein from soy extracts, its free form, and its glycoside genistin are readily bioavailable. For example, in freely moving unanesthetized rats with a cannula in the portal vein, genistein was readily bioavailable and was detected in portal vein plasma 15 min after administration with AUC values (0–24 h) of 54 and 24 μmol · h/L for genistein and genistin, respectively (72). Several studies, however, indicated that the oral bioavailability of genistin is higher than that of genistein (73). The limitation of genistein bioavailability after oral administration is generally due to its poor water solubility (74). Genistein also has a bitter taste (75), and formulations to overcome both the limitation of bioavailability and acceptable taste are necessary. Extensive metabolism of genistein in the intestine and postabsorption has been documented both in humans and experimental animals. Among the several metabolites identified in the blood and excreta are dihydrogenistein, dihydrodaidzein, 6′-hydroxy-O-desmethylangolensin, 4-ethylphenol, glucuronoide and sulfate conjugates of genistein and its metabolites, and 4-hydroxyphenyl-2-propionic aid. The gut microflora is known to cleave the C-ring of the isoflavonoid skeleton to give 4-hydroxyphenyl-2-propionic acid and dihydrogenistein (76–78). The metabolism in the gut wall and liver is also known to yield glucuronide and sulfated products (79). Generally, the 3 hydroxyl groups (5, 7, and 4′) are available for conjugation, but genistein-7-glucuronide-4′-sulfate and genistein-4′,7-diglucuronide byproduct appears to be the major metabolite in plasma (80). Some reports also suggest that conjugation plays a role in rapid elimination by biliary and urinary excretion (81).
Safety
There is no clear evidence that the consumption of large amounts of isoflavones in the diet is harmful in humans, although the multiple and complex effects of these compounds suggest that the administration of high doses of isoflavones could induce potentially adverse effects (82). However, minimal clinical toxicity in healthy postmenopausal women was observed after a single dose that exceeded normal dietary intakes of purified unconjugated isoflavones (83). The genotoxicity of anticancer agents, such as genistein, may be beneficial because they promote cancer cell death by inducing apoptosis and other cytotoxic processes. However, these agents would also negatively affect normal cells. Genotoxic and potentially adverse effects of genistein (apoptosis, cell growth inhibition, topoisomerase inhibition, DNA damage) were reported in vitro as well as in experimental animals (84–87). However, genistein concentrations used in these studies were much higher than the physiologically relevant doses achievable by dietary or pharmacologic intake of soy foods or supplements. In contrast, in vivo studies generally showed negative genotoxicity results (88). In a study conducted in postmenopausal women aged 46–68 y, the administration of a purified unconjugated isoflavone mixture (genistein, daidzein, and glycitein) showed minimal toxicity at doses as high as 16 mg genistein/kg body weight (83).
The potential effects of genistein on fertility and fetus development have been largely investigated. Some studies showed that therapeutically relevant doses of genistein have significant negative impacts on ovarian differentiation, estrous cyclicity, and fertility in the rodent model (89). However, data from human trials are lacking, and hence studies on the effect of genistein on the human reproductive function and/or fetal development need to be considered in the future. Studies in animal models showed that the exposure to genistein during uterine development caused several adverse effects (89, 90). The conflicting results are probably attributable to differences in the timing of exposure, doses, and experimental endpoints. It is worth noting that human fetuses can be exposed to isoflavones during the developmental period in the uterus and during infancy via breast milk (91). Serum genistein concentrations in soy formula–fed infants are from 10- to >100-fold those of the general population (92). These concentrations can increase blood genistein concentrations to values compatible with substantial biological estrogenic effects in children. Further epidemiologic studies are required to determine the beneficial (and detrimental) effects of genistein exposure, as well as establishing its safe therapeutic doses.
Genistein and Cancer
Several epidemiologic studies showed a relation between a soy-rich diet and cancer prevention. These studies arose from observations that in Asian countries, such as Japan and China, where diets are high in soy products, the incidence of breast and prostate cancers is lower than that in the United States and Europe. In fact, several meta-analyses suggest that the consumption of soy foods is associated with a reduction in prostate cancer risk in men (93–95) and is inversely associated with breast cancer risk among Asian women. This association was not confirmed in Western women (96–98). Moreover, a recent meta-analysis found that soy isoflavone intake can lower the risk of breast cancer in both pre- and postmenopausal women in Asian countries (99). Furthermore, migration studies showed an increase in prostate and breast cancer incidence in Asians after emigration to the United States (100), suggesting that environmental factors and changes in lifestyle, particularly in dietary practices, affect the etiology of these types of cancers.
These epidemiologic studies provide the rationale to investigate at a molecular level how the predominant isoflavone present in soy (i.e., genistein) is able to prevent cancer with the use of appropriate cellular and animal models. Because of its pleiotropic activity, genistein shows promising results as an anticancer agent in preclinical studies, opening the possibility to verify its clinical efficacy in clinical trials.
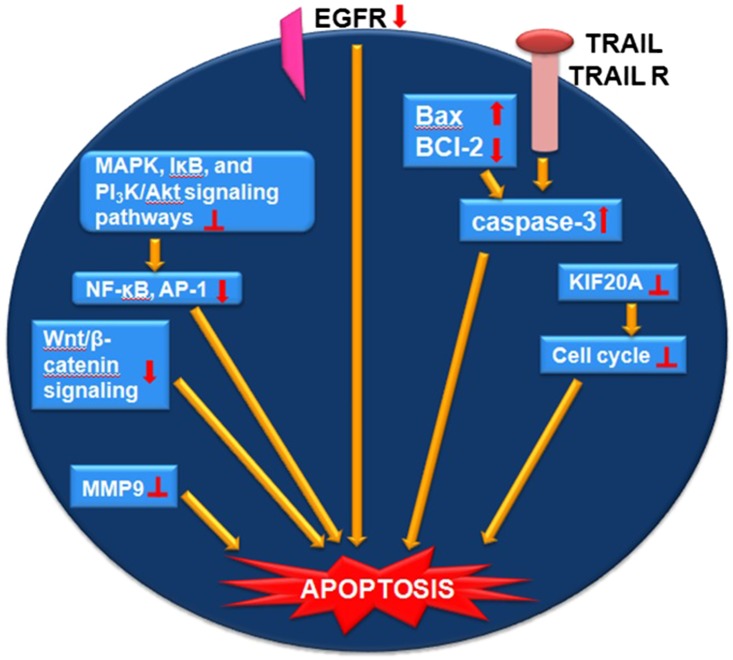
During the biogenesis process, genistein is present essentially in its glycosylated form, mostly with a glucose molecule. Although genistein is ingested as genestein glycoside, after ingestion a deglycosylation process occurs in the small intestine and the free genistein aglycone is absorbed by the body, resulting in different pharmacologic effects including anticancer effects (101). Apart from genistein, the synthetic derivatives, such as genistein glycosides, are also reported to possess anticancer activity when assessed in vitro. The anticancer potency of genistein glycosides varies depending on the sugar groups attached. For example, the addition of acetylated sugar hydroxyls to genistein resulted in more selectivity toward tumor cells (102). It is worthwhile to note that the anticancer potency of genistein and its derivatives differs in different types of cancer, depending on their selectivity toward the target molecules (Figure 3).
FIGURE 3.
Molecular mechanisms mediating the anticancer effect of genistein: downregulation/suppression, inhibition, enhancement. AP-1, activator protein 1; Bax, Bcl-2–associated X protein; Bcl-2, B cell lymphoma 2; EGFR, epidermal growth factor receptor; IκB, inhibitor of NF-κB; KIF20A, kinesin-like protein 20A; PI3K/Akt, phosphoinositide 3 kinase/Akt; TRAIL, tumor necrosis factor–related apoptosis-inducing ligand; TRAIL R, TRAIL death receptors; Wnt/β-catenin, Wingless and integration 1 β-catenin.
Liver cancer
Epidemiologic data.
A recent nested case-control study of a population-based prospective cohort in Japan, which investigated the preventive role of estrogens in primary liver cancer development, verified whether isoflavones were associated with the risk of liver cancer (103). The authors selected patients with either hepatitis B or C virus infection at baseline and measured plasma concentrations of isoflavones (genistein, daidzein, glycitein, and equol). The study indicated no association between isoflavones and the occurrence of primary liver cancer risk in middle-aged Japanese women and men with hepatitis virus infection.
In vitro studies.
In vitro studies support the efficacy of genistein as a chemopreventive and/or chemotherapeutic agent against liver cancer. It induces apoptosis in the following hepatocellular carcinoma cells (HCCs): Bel 7402 (104), HuH-7 (105), Hep3B (106), and HepG2 (107). Genistein may affect HCC progression as a result of its activity on apoptosis and cell cycle regulation (104, 108), acting as a promising inhibitor of the metastatic process in HCCs. In fact, genistein has been shown to inhibit the migration of 3 cell lines (HepG2, SMMC-7721, and Bel-7402 cells) (109). Moreover, it promotes anti-invasive and antimetastatic effects against 12-O-tetradecanoylphorbol-13-acetate-mediated metastasis via downregulation of matrix metalloproteinase 9 (MMP-9) and epidermal growth factor receptor (EGFR) and subsequent suppression of NF-κB and activator protein 1 (AP-1) transcription factors through inhibition of MAPK, inhibitor of NF-κB (IκB), and phosphoinositide 3 kinase/Akt (PI3K/Akt) signaling pathways (110).
Several studies also reported the synergistic effect of genistein when administered together with other anticancer drugs. For example, TNF-related apoptosis-inducing ligand (TRAIL) is a member of the TNF superfamily, and it has been shown that many human cancer cell lines are refractory to TRAIL-induced cell death. The treatment with nontoxic concentrations of genistein, sufficient to inhibit MAPK activation, sensitizes human hepatocellular carcinoma Hep3B cells to TRAIL-mediated apoptosis (111, 112). Genistein also potentiates the cytotoxic effect of arsenic trioxide (ATO) against human hepatocellular carcinoma. ATO possesses limited therapeutic benefit in the treatment of solid tumors; genistein, by inhibiting Akt and NF-κB, potentiates the proliferation-inhibiting and apoptosis-inducing effect of ATO on human HCC cell lines in vitro (15–20 μM genistein) and dramatically increases its suppressive effect on both tumor growth and angiogenesis in nude mice (50 mg genistein/kg body weight) (113).
In vivo studies.
Tumor growth in male BALB/C nu/nu mice injected with Bel 7402 cells was significantly retarded when treated with 50 mg genistein/kg body weight in comparison with control mice; genistein also significantly inhibited the invasion of Bel 7402 cells into the renal parenchyma of nude mice with a xenograft transplant by altering cell cycle, apoptosis, and angiogenesis (104). In a different animal model of liver cancer, HCC-bearing rats (male Wistar rats induced with N-nitrosoiethylmine by single intraperitoneal injection and promoted with phenobarbital), it was reported that genistein efficiently inhibited cell proliferation and induced apoptosis. In fact, the administration of a 15-mg genistein/kg body weight suspension in olive oil stimulated caspase-3 activity and remarkably decreased proliferating cell nuclear antigen (PCNA) in these HCC-bearing rats (114).
Gastric cancer
Epidemiologic data.
The beneficial role of soybean products against gastric cancer remains debatable from an interventional point of view. A nested case-control study within the Korean Multicenter Cancer Cohort suggested that high serum concentrations of isoflavones were associated with a decreased risk of gastric cancer (115); on the contrary, a parallel nested case-control study within the Japan Public Health Center–Based Prospective Study indicated a null association between isoflavone intake and gastric cancer risk among Japanese men and women (116).
In vitro studies.
In preclinical models, genistein was able to induce apoptosis in primary gastric cancer cells (20 μM for 24–72 h) by downregulating the expression of the antiapoptotic protein B cell lymphoma 2 (Bcl-2) and upregulating the expression of proapoptotic Bcl-2–associated X protein (Bax) (117). A similar modification of the Bcl-2:Bax ratio was considered responsible for the ability of genistein (0.5, 1, and 1.5 mg/kg) to induce apoptosis in SG7901 cells transplanted into subcutaneous tissue of nude mice (118). In the human gastric cancer cell line BGC-823, genistein treatment inhibited cell proliferation and induced apoptosis in a dose- and time-dependent manner. In this model, the molecule exerted a significant inhibitory effect on activation of the transcription factor NF-κB, causing a reduction in cyclooxygenase 2 (COX-2) protein concentrations (119).
The ability of genistein to induce G2/M cell cycle arrest was tested in SGC-7901 and BGC-823 cells. Here, genistein (20–80 μM) inhibited Akt activation by upregulation of phosphatase and tensin homolog (PTEN). This event resulted in the decreased phosphorylation of Wee1 on Ser642 and increased phospho-activation of cell division cycle protein 2 homolog/cyclin-dependent kinase 1 (Cdc2/Cdk1) on Thr15, leading to G2/M arrest (120).
A stable isotope labeling by/with amino acids in cell culture quantitative proteomics approach was used to identify the genistein-regulated factors and to investigate the anticancer mechanisms of the molecule. In SGC-7901 cells treated with 40 μM genistein for 48 h, the expression of 86 proteins involved in the regulation of G2/M transition, cellular growth, and proliferation resulted modulated by genistein, with 49 being upregulated and 37 being downregulated. In particular, 4 kinesins [kinesin-like protein (KIF) 11, KIF20A, KIF22, and KIF23] and a KIF, centromere protein F (CENPF), were found to be significantly downregulated by genistein, with KIF20A playing an important role in genistein-induced mitotic arrest (121).
Increasing evidence suggests that gastric cancer stem cells (GCSCs), a subpopulation of tumor cells capable of self-renewal and resistant to chemotherapeutic drugs, are responsible for the relapse of the disease. Gastric cancer cells treated with a low dose of genistein (15 μM) inhibited the GCSC-like properties such as self-renewal ability, drug resistance, and tumorigenicity, which were associated with the inhibition of ATP-binding cassette subfamily G member 2 (ABCG2) expression and extracellular signal-regulated kinase (ERK) 1/2 activity (122). In GCSCs, genistein can also inhibit glioma-associated oncogene family zinc finger 1 (Gli1), an activator of Hedgehog signaling, involved not only in oncogenesis but also in cancer stemness and overexpression of CD44, a typical GCSC surface marker. In more detail, it was shown that the levels of Gli1 and CD44 expression are downregulated by genistein in GCSCs sorted from MKN45, a human gastric cancer cell line, according to CD44 expression. In addition, the high cell migration capacity of CD44+ cells was blocked by genistein, suggesting that it can be an effective agent for gastric cancer therapy by targeting cancer stem cell-like features (123).
In vivo studies.
Tatsuta et al. (124) used, as an in vivo model of gastric cancer, Wistar rats induced with N-methyl-N’-nitro-N-nitroso guanidine and treated with sodium chloride to enhance induction of gastric carcinogenesis. They showed that, after 25 wk of the carcinogen treatment, daily injections of genistein (30 mg/kg body weight) decreased the labeling index and vessel counts of the antral mucosa and significantly reduced the incidence of gastric cancers, inducing increased apoptosis and decreased angiogenesis of antral mucosa and gastric cancers.
Moreover, to investigate the development of cancer cachexia and malignant progression of human stomach cancer, MKN45cl85 and highly metastatic 85As2mLuc (2 cachexia-inducing sublines) cells were isolated from the human stomach cancer cell line MKN-45. These 2 cell lines induce cachexia at high frequency in mice. It has been shown that a long duration of treatment with isoflavones induced tumor cytostasis, attenuated cachexia, and prolonged survival in rats (the antitumor effect was graded as AglyMax > daidzein > genistein) (125).
Lung cancer
Epidemiologic data.
Estrogens have been shown to have mitogenic effects in lung cells and interact with growth factor pathways in tumorigenesis; epidemiologic studies have produced conflicting results regarding the association between lung cancer risk and isoflavone intake (126–128). However, prospective studies carried out in Asia indicated an inverse association in never smokers (129). A nested case-control study within a large-scale, population-based prospective study in Japanese women with different isoflavone intakes and a high prevalence of never smokers revealed an inverse association between plasma isoflavone concentration and lung cancer risk (130).
In vitro studies.
Several in vitro and in vivo studies showed a protective effect of genistein on lung carcinogenesis when this compound was either used alone or in association with other compounds (131–134). Genistein showed anticancer effects on the small cell lung cancer (SCLC) cell line H446; the molecule induced cell cycle arrest and apoptosis, deregulating Forkhead box protein M1 (FoxM1) and its target genes [e.g., cell division cycle 25B (Cdc25B), cyclin B1, and survivin] (135). Several articles have also shown a synergistic effect; for example, in A549 lung cancer cells genistein (5–10 μM) enhanced apoptosis induced by trichostatin A (TSA) and increased the expression of the death receptor TNF receptor 1 (TNFR-1), which mediates extrinsic apoptosis pathways (134, 136). Patients with non-SCLC treated with tyrosine kinase inhibitors developed an acquired resistance to this therapy. In a non-SCLC cell line carrying the T790M mutation in EGFR, genistein associated with gefitinib, an EGFR tyrosine kinase inhibitor, showed a synergistic anticancer effect due to apoptosis induction and inhibition of the key regulators of growth signaling pathways, such as Akt (131). The synergistic effect was also confirmed in in vivo experiments.
In vivo studies.
Gu et al. (104, 108) investigated, in vitro and in vivo, the inhibitory effects of genistein on the invasive potential of HCCs (Bel 7402 and MHCC97-H). The authors first proved the ability of genistein (10 μg/mL) to significantly inhibit the growth of HCCs in vitro; subsequently, Bel 7402 or MHCC97-H cells were injected in BALB/C nu/nu mice before the administration of 50 mg genistein/kg body weight. The tumor growth in genistein-treated nude mice was significantly lower than that in control mice. The molecule significantly inhibited the invasion of Bel 7402 cells into the renal parenchyma of nude mice with xenograft transplant. Moreover, in the in situ xenograft transplantation of MHCC97-H cells, the number of pulmonary micrometastatic foci after genistein treatment were significantly lower than in the control group.
Because of the low bioavailability of genistein in vivo, there is a growing interest in its derivative, 7-difluoromethyl-5,4′-dimethoxygenistein (dFMGEN), which possesses a better in vivo bioavailability. An in vitro study showed the efficacy of dFMGEN in reducing the viability of lung carcinoma A549 cells through induction of G1 phase arrest (137). Moreover, dFMGEN suppressed tumor growth in vivo and was well tolerated, confirming its therapeutic potential in the treatment of human lung cancer (137).
Colorectal cancer
Epidemiologic data.
The consumption of soy has been found to reduce colon cancer risk in human and animal studies (138, 139). Epidemiologic evidence indicates that phytoestrogens may protect against the development of colorectal cancer (140, 141).
For example, a case-control study evaluated the association between dietary phytoestrogen intake (isoflavones, lignans, and total phytoestrogens) and colorectal cancer risk among healthy subjects and those belonging to the population-based Ontario Familial Colorectal Cancer Registry. It was reported that dietary lignin and isoflavone intake was associated with a significant reduction in colorectal cancer risk; moreover, it was observed that, with respect to phytoestrogen intake, polymorphic genes encoding enzymes involved in the metabolism of phytoestrogens [CYP, catechol O-methyl transferase, glutathione S-transferases (GSTs), and UDP-glucuronosyltransferase (UGT)] were not subject to modifications (142).
In vitro studies.
Numerous in vitro studies have shown anticancer properties of genistein against colorectal cancer, and the mechanisms whereby it exerts anticancer effects have been widely investigated. Genistein efficiently suppresses colon cancer cell growth by attenuating the activity of the PI3K/Akt pathway (143, 144), which has a critical role in the regulation of colon cancer progression. In colon cancer cells, genistein also affects the expression of ERs and tumor suppressor genes (145, 146). In addition, it can block uncontrolled cell growth in a DLD-1 cell line by inhibiting the Wingless and integration 1 (Wnt) signaling pathway (147). In particular, genistein enhanced gene expression of the Wnt signaling pathway antagonist, Dickkopf-related protein 1 (DKK1), through the induction of histone acetylation at the promoter region in an SW480 human colon cancer cell line (148).
In vivo studies.
An in vivo study that used azoxymethane as a chemical inducer of colon cancer in male Sprague-Dawley rats showed that rats fed 140 mg genistein/kg body weight from gestation to 13 wk of age showed a downregulation of Wingless and integration 1 β-catenin (Wnt/β-catenin) signaling and a reduction in total aberrant crypts, confirming the role of this isoflavone in preventing the development of early colon neoplasia (149).
Clinical trials.
A phase I/II pilot study of genistein use in the treatment of metastatic colorectal cancer is currently recruiting participants; because of the promising results of the in vitro and in vivo studies it is expected to have interesting findings.
Breast cancer
Epidemiologic data.
Several case-control studies (150, 151) showed an inverse relation between soy intake and breast cancer risk; and a prospective cohort study (152) found that frequent miso soup and isoflavone consumption was associated with a reduced risk of breast cancer in Japan. Clearly, the chemopreventive effects of soybeans and soy-containing foods are related to their isoflavone content.
In vitro studies.
Genistein induced apoptosis in several breast cancer cell lines and promoted synergistic inhibitory effects when combined with anticancer drugs. For example, genistein was shown to induce apoptosis in the low-invasive (ER-positive) MCF-7 and in the high-invasive (ER-negative) MDA-MB-231 breast cancer cell lines (10–100 μM) (153, 154). Synergistic proapoptotic effects were also described when genistein was used in combination with adriamycin and docetaxel in MDA-MB-231 cells (155) and with tamoxifen on BT-474 breast cancer cells (156). The main molecular targets of the molecule in breast cancer cells appear to be NF-κB (157) and Akt pathways (158). Moreover, genistein induces in breast and prostate cancer cells the expression of breast cancer growth suppressor protein (BRCA) 1 and BRCA2 tumor suppressor genes and the overexpression of many genes involved in the BRCA1 and BRCA2 pathways (159). However, it is important to underline the paradoxical effect of genistein, which stimulates proliferation and estrogen-sensitive gene expression of the ER-positive breast cancer cell lines at concentrations of 1–10 μM (160). At these low concentrations, genistein abrogates tamoxifen-associated mammary tumor prevention, but its effect is null on ER-negative and tamoxifen-resistant breast cancer cells (161).
In vivo studies.
The in vitro observations have been confirmed in in vivo studies, suggesting that genistein exposure early in life may reduce the risk of breast cancer (162). On the contrary, in a preclinical mouse model that resulted in 17β-estradiol blood concentrations similar to those found in postmenopausal women, dietary genistein in the presence of low concentrations of circulating E2 acted in an additive manner to stimulate estrogen-dependent tumor growth in vivo (163). Results from this study suggest that the consumption of products containing genistein may not be safe for postmenopausal women with estrogen-dependent breast cancer.
Clinical trials.
These controversial results have been confirmed in human clinical studies, in which, in some cases, purified genistein did not exert any adverse estrogenic effects on breast tissue when consumed at a dose of 54 mg/d (164, 165), whereas others found proestrogenic effects of dietary soy supplementation on breast tissue (166–168). Thus, considering the agonist activity of genistein against ER-α, its use in women with established ER-positive breast cancers must be carefully considered. In this regard, 2 clinical trials based on the use of genistein in breast cancer, a phase II study entitled “Gemcitabine Hydrochloride and Genistein in Treating Women with Stage IV Breast Cancer,” and a phase I study entitled “Genistein in Preventing Breast or Endometrial Cancer in Healthy Postmenopausal Women,” have been completed, although the results are not yet published (169).
Genistein Metabolites and Cancer
Although genistein is reported to be metabolized mainly through oxidation, sulfation, glucuronidation, hydroxylation, or methylation (170), the influence of genistein metabolites on its anticancer property is not understood clearly. Metabolites such as 5,7,3′,4′-tetrahydroxyisoflavone (THIF) and 2 glutathinyl conjugates of THIF were identified in T47D tumorigenic breast epithelial cells that were treated with genistein. Because THIF has been shown to inhibit angiogenesis and endothelial cell proliferation (171), it is worthwhile to note that the formation of THIF during genistein treatment may play a major role in cell cycle arrest, inhibition of cellular proliferation, and activation of signaling pathways such as p38 MAPK, which was observed in T47D cells. Furthermore, oxidation of THIF to o-quinone along with formation of hydrogen peroxides and reactive oxygen species induces DNA strand breakage. This leads to the activation of the ataxia telangiectasia and Rad3-related kinase (ATR) signaling pathway, which activates the kinases involved in DNA damage check-point control (172).
Conclusions and Recommendations
We reviewed the available evidence on the promising role of genistein against cancer. Several experimental and clinical investigations suggest a therapeutic role of genistein on different types of cancer. The emergence of negative phenomena in cancer treatment is well known, such as drug resistance, high risk of relapse, and the unavailability or poor outcome of therapies, such as surgery, chemotherapy, phototherapy, and radiotherapy. Therefore, attention has been paid in recent years to natural remedies possessing the capacity to improve the efficacy of chemotherapeutic treatment and to lower adverse effects. Genistein can be included among these compounds because the molecule shows synergistic behavior when associated with well-known anticancer drugs, such as adriamycin, docetaxel, and tamoxifen, suggesting a potential role in combination therapy. However, genistein, as well as other bioactive phytochemicals, benefits and, at the same time, suffers from 2 apparently opposite features: high pleiotropy and low bioavailability. The former refers to the ability of a given compound to act at several levels in the cells, triggering at the same time several biochemical pathways involved in the occurrence and development of cancer (i.e., cell cycle arrest, apoptosis, cell death). The net result is a synergistic effect that may enhance the efficacy of a specific drug, even if present in the cells at relatively low concentrations. In this regard, in the previous paragraphs, we reviewed several molecular targets of genistein, such as ER, tyrosine kinases, and pro- and antiapoptotic factors. Bioavailability was also discussed above, which brings us to the concept that “what we adsorb” from food is even more important than what we eat: the plasma concentration of genistein present in the diet (similar to other bioactive compounds) is significantly lower than the concentrations needed in experimental models (cell lines and animal studies) to trigger an anticancer response. Therefore, it is reasonable to predict a significant clinical outcome of genistein when applied at pharmacologic doses (hundreds of micromolars) and weak or null effects when the same molecule is administered at chemopreventive doses (<1 μM). The reality is probably more complex than we can expect. In fact, when genistein is adsorbed at low concentrations together with other bioactive compounds present in the diet, we can postulate pleiotropic anticancer effects that result from synergistic mechanisms attributable to the plethora of individual compounds (or their metabolites) deriving from the diet. Alternatively, we can also hypothesize that genistein possesses, at low doses, effects different than those measured at high doses, depending on the cellular background and the molecular target investigated. In this respect, the ability of genistein to inhibit cell growth in both hormone-dependent and -independent cancer cells is dose dependent (173). In fact, when genistein concentration increases from a low nanomolar concentration to hundreds of nanomolars, preferential activation of ER-β is lost and genistein activates both ERs (α and β); therefore, at least in different breast cancers, the ratios of the ER subtypes and the concentrations of genistein strongly influence the final effect on hormone-regulated gene expression and cell fate (174).
Future studies are necessary to clarify the potential therapeutic and chemopreventive use of genistein. In particular, it will be important to investigate the following:
The pharmacodynamics and pharmacokinetics of genistein and related compounds in experimental and clinical studies
Possible strategies to increase the bioavailability of genistein
The ideal therapeutic dose for treatment of specific types of cancer
Other molecular mechanisms explaining the anticancer effects of genistein (e.g., microRNAs)
Possible interactions between genistein and well-known anticancer drugs, by both experimental and clinical studies
Despite the promising results reported in literature, there is still a long way to go.
Acknowledgments
All authors read and approved the final manuscript.
Footnotes
Abbreviations used: ABCG2, ATP-binding cassette subfamily G member 2; AP-1, activator protein 1; ATO, arsenic trioxide; ATR, ataxia telangiectasia and Rad3-related kinase; Bax, Bcl-2–associated X protein; Bcl-2, B cell lymphoma 2; BRCA, breast cancer growth suppressor protein; Cdc2/Cdk1, cell division cycle protein 2 homolog/cyclin-dependent kinase 1; Cdc25B, cell division cycle 25B; CENPF, centromere protein F; COX-2, cyclooxygenase 2; CYP, cytochrome P450; dFMGEN, 7-difluoromethyl-5,4′-dimethoxygenistein; DKK1, Dickkopf-related protein 1; EGFR, epidermal growth factor receptor; ER, estrogen receptor; ERK, extracellular signal-regulated kinase; FoxM1, Forkhead box protein M1; GCSC, gastric cancer stem cell; Gli1, glioma-associated oncogene family zinc finger 1; GST, glutathione S-transferase; HCC, hepatocellular carcinoma cell; IFS, isoflavone synthase; INT-1, integration 1; IκB, inhibitor of NF-κB; KIF, kinesin-like protein; MMP-9, matrix metalloproteinase 9; PCNA, proliferating cell nuclear antigen; PI3K/Akt, phosphoinositide 3 kinase/Akt; PTEN, phosphatase and tensin homolog; SCLC, small cell lung cancer; THIF, 5,7,3′,4′ tetrahydroxyisoflavone; TNFR-1, tumor necrosis factor receptor 1; TRAIL, tumor necrosis factor–related apoptosis-inducing ligand; TSA, trichostatin A; UGT, UDP-glucuronosyltransferase; Wnt/β-catenin, Wingless and integration 1 β-catenin.
References
- 1.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin D, Forman D, Bray F. GLOBOCAN 2012 version 1. 0: cancer incidence and mortality worldwide. IARC CancerBase Vol. 11. Lyon (France): International Agency for Research on Cancer; 2013. [cited 2014 Nov 13]. Available from: http://globocan.iarc.fr. [Google Scholar]
- 2.Bray F, Ren JS, Masuyer E, Ferlay J. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer 2013;132:1133–45. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;61:69–90. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. The World Health Organizationaposs fight against cancer: strategies that prevent, cure and care. Geneva (Switzerland): WHO Press; 2007.
- 5.Kris-Etherton PM, Hecker KD, Bonanome A, Coval SM, Binkoski AE, Hilpert KF, Griel AE, Etherton TD. Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. Am J Med 2002;113:71S–88S. [DOI] [PubMed] [Google Scholar]
- 6.Giacosa A, Barale R, Bavaresco L, Gatenby P, Gerbi V, Janssens J, Johnston B, Kas K, La Vecchia C, Mainguet P, et al. Cancer prevention in Europe: the Mediterranean diet as a protective choice. Eur J Cancer Prev 2013;22:90–5. [DOI] [PubMed] [Google Scholar]
- 7.Sung B, Prasad S, Yadav VR, Lavasanifar A, Aggarwal BB. Cancer and diet: how are they related? Free Radic Res 2011;45:864–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martínez ME, Marshall JR, Giovannucci E. Diet and cancer prevention: the roles of observation and experimentation. Nat Rev Cancer 2008;8:694–703. [DOI] [PubMed] [Google Scholar]
- 9.Le Marchand L, Kolonel LN, Wilkens LR, Myers BC, Hirohata T. Animal fat consumption and prostate cancer: a prospective study in Hawaii. Epidemiology 1994;5:276–82. [DOI] [PubMed] [Google Scholar]
- 10.Giacosa A, Franceschi S, La Vecchia C, Favero A, Andreatta R. Energy intake, overweight, physical exercise and colorectal cancer risk. Eur J Cancer Prev 1999;8:S53–60. [PubMed] [Google Scholar]
- 11.Chen WY, Rosner B, Hankinson SE, Colditz GA, Willett WC. Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. JAMA 2011;306:1884–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pelucchi C, Tramacere I, Boffetta P, Negri E, Vecchia CL. Alcohol consumption and cancer risk. Nutr Cancer 2011;63:983–90. [DOI] [PubMed] [Google Scholar]
- 13.Soobrattee MA, Bahorun T, Aruoma OI. Chemopreventive actions of polyphenolic compounds in cancer. Biofactors 2006;27:19–35. [DOI] [PubMed] [Google Scholar]
- 14.Liu RH. Potential synergy of phytochemicals in cancer prevention: mechanism of action. J Nutr 2004;134(Suppl):3479S–85S. [DOI] [PubMed] [Google Scholar]
- 15.Messina M, Barnes S, Setchell KD. Phyto-oestrogens and breast cancer. Lancet 1997;350:971–2. [DOI] [PubMed] [Google Scholar]
- 16.Surh Y-J. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer 2003;3:768–80. [DOI] [PubMed] [Google Scholar]
- 17.Rajendran P, Ho E, Williams DE, Dashwood RH. Dietary phytochemicals, HDAC inhibition, and DNA damage/repair defects in cancer cells. Clin Epigenetics 2011;3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nabavi SF, Nabavi SM, Setzer W, Nabavi SA, Nabavi SA, Ebrahimzadeh MA. Antioxidant and antihemolytic activity of lipid-soluble bioactive substances in avocado fruits. Fruits 2013;68:185–93. [Google Scholar]
- 19.Nabavi SF, Nabavi SM, Habtemariam S, Moghaddam AH, Sureda A, Jafari M, Latifi AM. Hepatoprotective effect of gallic acid isolated from Peltiphyllum peltatum against sodium fluoride-induced oxidative stress. Ind Crops Prod 2013;44:50–5. [Google Scholar]
- 20.Stoner GD, Mukhtar H. Polyphenols as cancer chemopreventive agents. J Cell Biochem Suppl 1995;22:169–80. [DOI] [PubMed] [Google Scholar]
- 21.Amtmann E, Zöller M, Wesch H, Schilling G. Antitumoral activity of a sulphur-containing platinum complex with an acidic pH optimum. Cancer Chemother Pharmacol 2001;47:461–6. [DOI] [PubMed] [Google Scholar]
- 22.Gould MN. Cancer chemoprevention and therapy by monoterpenes. Environ Health Perspect 1997;105:977–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang MZ, Wang Y, Ai N, Hou Z, Sun Y, Lu H, Welsh W, Yang CS. Tea polyphenol (−)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res 2003;63:7563–70. [PubMed] [Google Scholar]
- 24.Piyanuch R, Sukhthankar M, Wandee G, Baek SJ. Berberine, a natural isoquinoline alkaloid, induces NAG-1 and ATF3 expression in human colorectal cancer cells. Cancer Lett 2007;258:230–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howes MJR, Simmonds MS. The role of phytochemicals as micronutrients in health and disease. Curr Opin Clin Nutr Metab Care 2014;17:558–66. [DOI] [PubMed] [Google Scholar]
- 26.Nabavi SM, Nabavi SF, Eslami S, Moghaddam AH. In vivo protective effects of quercetin against sodium fluoride-induced oxidative stress in the hepatic tissue. Food Chem 2012;132:931–5. [Google Scholar]
- 27.Arts IC, Hollman PC. Polyphenols and disease risk in epidemiologic studies. Am J Clin Nutr 2005;81(Suppl):317S–25S. [DOI] [PubMed] [Google Scholar]
- 28.Khan N, Mukhtar H. Multitargeted therapy of cancer by green tea polyphenols. Cancer Lett 2008;269:269–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daglia M. Polyphenols as antimicrobial agents. Curr Opin Biotechnol 2012;23:174–81. [DOI] [PubMed] [Google Scholar]
- 30.Daglia M, Di Lorenzo A, Nabavi SF, Talas ZS, Nabavi SM. Polyphenols: well beyond the antioxidant capacity: gallic acid and related compounds as neuroprotective agents: you are what you eat! Curr Pharm Biotechnol 2014;15:362–72. [DOI] [PubMed] [Google Scholar]
- 31.Jacob V, Hagai T, Soliman K. Structure-activity relationships of flavonoids. Curr Org Chem 2011;15:2641–57. [Google Scholar]
- 32.Jaganath IB, Crozier A. Dietary flavonoids and phenolic compounds. In: Fraga CG, editor. Plant phenolics and human health: biochemistry, nutrition, and pharmacology. Hoboken (NJ): Wiley; 2010. p. 1–49. [Google Scholar]
- 33.Preedy VR. Isoflavones: chemistry, analysis, function and effects. Royal Society of Chemistry. Cambridge (United Kingdom); 2012. [Google Scholar]
- 34.Banerjee S, Li Y, Wang Z, Sarkar FH. Multi-targeted therapy of cancer by genistein. Cancer Lett 2008;269:226–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Polkowski K, Mazurek AP. Biological properties of genistein: a review of in vitro and in vivo data. Acta Pol Pharm 2000;57:135–55. [PubMed] [Google Scholar]
- 36.Klinge CM. Estrogen receptor interaction with co-activators and co-repressors. Steroids 2000;65:227–51. [DOI] [PubMed] [Google Scholar]
- 37.Kurzer MS. Hormonal effects of soy in premenopausal women and men. J Nutr 2002;132(Suppl):570S–3S. [DOI] [PubMed] [Google Scholar]
- 38.Yoon K, Kwack SJ, Kim HS, Lee BM. Estrogenic endocrine-disrupting chemicals: molecular mechanisms of actions on putative human diseases. J Toxicol Environ Health B Crit Rev 2014;17:127–74. [DOI] [PubMed] [Google Scholar]
- 39.Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology 1998;139:4252–63. [DOI] [PubMed] [Google Scholar]
- 40.Chang YC, Nair MG, Santell RC, Helferich WG. Microwave-mediated synthesis of anticarcinogenic isoflavones from soybeans. J Agric Food Chem 1994;42:1869–71. [Google Scholar]
- 41.Kochs G, Grisebach H. Enzymic synthesis of isoflavones. Eur J Biochem 1986;155:311–8. [DOI] [PubMed] [Google Scholar]
- 42.Katsuyama Y, Miyahisa I, Funa N, Horinouchi S. One-pot synthesis of genistein from tyrosine by coincubation of genetically engineered Escherichia coli and Saccharomyces cerevisiae cells. Appl Microbiol Biotechnol 2007;73:1143–9. [DOI] [PubMed] [Google Scholar]
- 43.Yu O, Jung W, Shi J, Croes RA, Fader GM, McGonigle B, Odell JT. Production of the isoflavones genistein and daidzein in non-legume dicot and monocot tissues. Plant Physiol 2000;124:781–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meng Q-H, Wähälä K, Adlercreutz H, Tikkanen MJ. Antiproliferative efficacy of lipophilic soy isoflavone phytoestrogens delivered by low density lipoprotein particles into cultured U937 cells. Life Sci 1999;65:1695–705. [DOI] [PubMed] [Google Scholar]
- 45.Somjen D, Amir-Zaltsman Y, Gayer B, Kulik T, Knoll E, Stern N, Lu LJ, Toldo L, Kohen F. 6-Carboxymethyl genistein: a novel selective oestrogen receptor modulator (SERM) with unique, differential effects on the vasculature, bone and uterus. J Endocrinol 2002;173:415–27. [DOI] [PubMed] [Google Scholar]
- 46.Matsumoto T, Kobayashi T, Kikuchi T, Honda T, Kamata K. Effects of dual-action genistein derivatives on relaxation in rat aorta. J Smooth Muscle Res 2005;41:23–33. [DOI] [PubMed] [Google Scholar]
- 47.Zhang LN, Xiao ZP, Ding H, Ge HM, Xu C, Zhu HL, Tan RX. Synthesis and cytotoxic evaluation of novel 7‐o‐modified genistein derivatives. Chem Biodivers 2007;4:248–55. [DOI] [PubMed] [Google Scholar]
- 48.Shimoda K, Kobayashi T, Akagi M, Hamada H, Hamada H. Synthesis of oligosaccharides of genistein and quercetin as potential anti-inflammatory agents. Chem Lett 2008;37:876–7. [Google Scholar]
- 49.Kgomotso T, Chiu F, Ng K. Genistein‐and daidzein 7‐O‐β‐D‐glucuronic acid retain the ability to inhibit copper‐mediated lipid oxidation of low density lipoprotein. Mol Nutr Food Res 2008;52:1457–66. [DOI] [PubMed] [Google Scholar]
- 50.Fu XH, Wang L, Zhao H, Xiang H-L, Cao JG. Synthesis of genistein derivatives and determination of their protective effects against vascular endothelial cell damages caused by hydrogen peroxide. Bioorg Med Chem Lett 2008;18:513–7. [DOI] [PubMed] [Google Scholar]
- 51.Zheng X, Yao X, Liu Y, Zheng Z, Cao J, Liao D. Synthesis and cytotoxic activity of genistein derivatives. Med Chem Res 2010;19:1296–306. [Google Scholar]
- 52.Maniewska J, Grynkiewicz G, Szeja W, Hendrich A. Interaction of genistein benzyl derivatives with lipid bilayers-fluorescence spectroscopic and calorimetric study. Bioorg Med Chem 2009;17:2592–7. [DOI] [PubMed] [Google Scholar]
- 53.Choi JN, Kim D, Choi HK, Yoo KM, Kim J, Lee CH. 2′-Hydroxylation of genistein enhanced antioxidant and antiproliferative activities in Mcf-7 human breast cancer cells. J Microbiol Biotechnol 2009;19:1348–54. [DOI] [PubMed] [Google Scholar]
- 54.Zengin G. Synthesis, antimicrobial activity, and structure–activity relationships of eugenol, menthol, and genistein esters. Chem Nat Compd 2011;47:550–5. [Google Scholar]
- 55.Fan YE, Li J, Liu R, Ye ZG, Hu JN, Deng ZY, Peng Y. Oral bioavailability of novel genistein sulfonates and their pre-clinical pharmacokinetics. Lat Am J Pharm 2011;30:1582–9. [Google Scholar]
- 56.Hyz K, Kawęcki R, Misior A, Bocian W, Bednarek EB, Sitkowski J, Kozerski L. Genistein binding mode to doubly nicked dumbbell DNA: dynamic and diffusion ordered NMR study. J Med Chem 2011;54:8386–93. [DOI] [PubMed] [Google Scholar]
- 57.Li W, Jia HY, He XH, Shi WG, Zhong BH. Novel phenoxyalkylcarboxylic acid derivatives as hypolipidaemic agents. J Enzyme Inhib Med Chem 2012;27:311–8. [DOI] [PubMed] [Google Scholar]
- 58.Qiang X, Sang Z, Yuan W, Li Y, Liu Q, Bai P, Shi Y, Ang W, Tan Z, Deng Y. Design, synthesis and evaluation of genistein-O-alkylbenzylamines as potential multifunctional agents for the treatment of Alzheimer's disease. Eur J Med Chem 2014;76:314–31. [DOI] [PubMed] [Google Scholar]
- 59.Bhagwat S, Haytowitz DB, Holden JM. USDA database for the isoflavone content of selected foods. Release 2.0. Bethesda (MD): USDA; 2008. [Google Scholar]
- 60.Messina M, Nagata C, Wu AH. Estimated Asian adult soy protein and isoflavone intakes. Nutr Cancer 2006;55:1–12. [DOI] [PubMed] [Google Scholar]
- 61.van Erp-Baart M-AJ, Brants HA, Kiely M, Mulligan A, Turrini A, Sermoneta C, Kilkkinen A, Valsta LM. Isoflavone intake in four different European countries: the VENUS approach. Br J Nutr 2003;89:S25–30. [DOI] [PubMed] [Google Scholar]
- 62.Liggins J, Bluck L, Runswick S, Atkinson C, Coward W, Bingham S. Daidzein and genistein contents of vegetables. Br J Nutr 2000;84:717–25. [PubMed] [Google Scholar]
- 63.Liggins J, Bluck LJ, Runswick S, Atkinson C, Coward WA, Bingham SA. Daidzein and genistein content of fruits and nuts. J Nutr Biochem 2000;11:326–31. [DOI] [PubMed] [Google Scholar]
- 64.Ahmad S, Pathak D. Nutritional changes in soybean during germination. J Food Sci Technol 2000;37:665–6. [Google Scholar]
- 65.Kim WJ, Lee HY, Won MH, Yoo SH. Germination effect of soybean on its contents of isoflavones and oligosaccharides. Food Sci Biotechnol 2005;14:498–502. [Google Scholar]
- 66.Paucar-Menacho LM, Berhow MA, Mandarino JMG, Chang YK, Mejia EG. Effect of time and temperature on bioactive compounds in germinated Brazilian soybean cultivar BRS 258. Food Res Int 2010;43:1856–65. [Google Scholar]
- 67.Shi H, Nam PK, Ma Y. Comprehensive profiling of isoflavones, phytosterols, tocopherols, minerals, crude protein, lipid, and sugar during soybean (Glycine max) germination. J Agric Food Chem 2010;58:4970–6. [DOI] [PubMed] [Google Scholar]
- 68.Yuan JP, Liu YB, Peng J, Wang JH, Liu X. Changes of isoflavone profile in the hypocotyls and cotyledons of soybeans during dry heating and germination. J Agric Food Chem 2009;57:9002–10. [DOI] [PubMed] [Google Scholar]
- 69.Quinhone A Jr.,Ida E. Profile of the contents of different forms of soybean isoflavones and the effect of germination time on these compounds and the physical parameters in soybean sprouts. Food Chem 2015;166:173–8. [DOI] [PubMed] [Google Scholar]
- 70.Lee SY, Lee S, Lee S, Oh JY, Jeon EJ, Ryu HS, Lee CH. Primary and secondary metabolite profiling of doenjang, a fermented soybean paste during industrial processing. Food Chem 2014;165:157–66. [DOI] [PubMed] [Google Scholar]
- 71.Sohn SI, Kim YH, Kim SL, Lee JY, Oh YJ, Chung JH, Lee KR. Genistein production in rice seed via transformation with soybean IFS genes. Plant Sci 2014;217–218:27–35. [DOI] [PubMed] [Google Scholar]
- 72.Steensma A, Faassen-Peters MA, Noteborn HP, Rietjens IM. Bioavailability of genistein and its glycoside genistin as measured in the portal vein of freely moving unanesthetized rats. J Agric Food Chem 2006;54:8006–12. [DOI] [PubMed] [Google Scholar]
- 73.Kwon SH, Kang MJ, Huh JS, Ha KW, Lee JR, Lee SK, Lee BS, Han IH, Lee MS, Lee MW, et al. Comparison of oral bioavailability of genistein and genistin in rats. Int J Pharm 2007;337:148–54. [DOI] [PubMed] [Google Scholar]
- 74.Motlekar N, Khan MA, Youan BBC. Preparation and characterization of genistein containing poly (ethylene glycol) microparticles. J Appl Polym Sci 2006;101:2070–8. [Google Scholar]
- 75.Huang AS, Hsieh OAL, Chang SS. Characterization of the nonvolatile minor constituents responsible for the objectionable taste of defatted soybean flour. J Food Sci 1982;47:19–23. [Google Scholar]
- 76.Kobayashi S, Shinohara M, Nagai T, Konishi Y. Transport mechanisms for soy isoflavones and microbial metabolites dihydrogenistein and dihydrodaidzein across monolayers and membranes. Biosci Biotechnol Biochem 2013;77:2210–7. [DOI] [PubMed] [Google Scholar]
- 77.Schoefer L, Mohan R, Braune A, Birringer M, Blaut M. Anaerobic C‐ring cleavage of genistein and daidzein by Eubacterium ramulus. FEMS Microbiol Lett 2002;208:197–202. [DOI] [PubMed] [Google Scholar]
- 78.Tamura M, Ohnishi-Kameyama M, Nakagawa H, Tsushida T. Dihydrogenistein-producing bacterium TM-40 isolated from human feces. Food Sci Technol Res 2007;13:129–32. [Google Scholar]
- 79.Shelnutt SR, Cimino CO, Wiggins PA, Ronis MJ, Badger TM. Pharmacokinetics of the glucuronide and sulfate conjugates of genistein and daidzein in men and women after consumption of a soy beverage. Am J Clin Nutr 2002;76:588–94. [DOI] [PubMed] [Google Scholar]
- 80.Hosoda K, Furuta T, Yokokawa A, Ishii K. Identification and quantification of daidzein-7-glucuronide-4′-sulfate, genistein-7-glucuronide-4′-sulfate and genistein-4′,7-diglucuronide as major metabolites in human plasma after administration of kinako. Anal Bioanal Chem 2010;397:1563–72. [DOI] [PubMed] [Google Scholar]
- 81.Sfakianos J, Coward L, Kirk M, Barnes S. Intestinal uptake and biliary excretion of the isoflavone genistein in rats. J Nutr 1997;127:1260–8. [DOI] [PubMed] [Google Scholar]
- 82.Sirtori CR. Risks and benefits of soy phytoestrogens in cardiovascular diseases, cancer, climacteric symptoms and osteoporosis. Drug Saf 2001;24:665–82. [DOI] [PubMed] [Google Scholar]
- 83.Bloedon LT, Jeffcoat AR, Lopaczynski W, Schell MJ, Black TM, Dix KJ, Thomas BF, Albright C, Busby MG, Crowell JA, et al. Safety and pharmacokinetics of purified soy isoflavones: single-dose administration to postmenopausal women. Am J Clin Nutr 2002;76:1126–37. [DOI] [PubMed] [Google Scholar]
- 84.Klein CB, King AA. Genistein genotoxicity: critical considerations of in vitro exposure dose. Toxicol Appl Pharmacol 2007;224:1–11. [DOI] [PubMed] [Google Scholar]
- 85.Touny LHE, Banerjee PP. Identification of both Myt‐1 and Wee‐1 as necessary mediators of the p21‐independent inactivation of the Cdc‐2/cyclin B1 complex and growth inhibition of TRAMP cancer cells by genistein. Prostate 2006;66:1542–55. [DOI] [PubMed] [Google Scholar]
- 86.Snyder RD, Gillies PJ. Reduction of genistein clastogenicity in Chinese hamster V79 cells by daidzein and other flavonoids. Food Chem Toxicol 2003;41:1291–8. [DOI] [PubMed] [Google Scholar]
- 87.Ramos S. Effects of dietary flavonoids on apoptotic pathways related to cancer chemoprevention. J Nutr Biochem 2007;18:427–42. [DOI] [PubMed] [Google Scholar]
- 88.Michael McClain R, Wolz E, Davidovich A, Bausch J. Genetic toxicity studies with genistein. Food Chem Toxicol 2006;44:42–55. [DOI] [PubMed] [Google Scholar]
- 89.Jefferson WN, Williams CJ. Circulating levels of genistein in the neonate, apart from dose and route, predict future adverse female reproductive outcomes. Reprod Toxicol 2011;31:272–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rozman KK, Bhatia J, Calafat AM, Chambers C, Culty M, Etzel RA, Flaws JA, Hansen DK, Hoyer PB, Jeffery EH, et al. NTP-CERHR expert panel report on the reproductive and developmental toxicity of genistein. Birth Defects Res B Dev Reprod Toxicol 2006;77:485–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Franke AA, Custer LJ, Wang W, Shi CY. HPLC analysis of isoflavonoids and other phenolic agents from foods and from human fluids. Proc Soc Exp Biol Med 1998;217:263–73. [DOI] [PubMed] [Google Scholar]
- 92.Cao Y, Calafat AM, Doerge DR, Umbach DM, Bernbaum JC, Twaddle NC, Ye X, Rogan WJ. Isoflavones in urine, saliva, and blood of infants: data from a pilot study on the estrogenic activity of soy formula. J Expo Sci Environ Epidemiol 2009;19:223–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yan L, Spitznagel EL. Soy consumption and prostate cancer risk in men: a revisit of a meta-analysis. Am J Clin Nutr 2009;89:1155–63. [DOI] [PubMed] [Google Scholar]
- 94.Yan L, Spitznagel EL. Meta-analysis of soy food and risk of prostate cancer in men. Int J Cancer 2005;117:667–9. [DOI] [PubMed] [Google Scholar]
- 95.Hwang YW, Kim SY, Jee SH, Kim YN, Nam CM. Soy food consumption and risk of prostate cancer: a meta-analysis of observational studies. Nutr Cancer 2009;61:598–606. [DOI] [PubMed] [Google Scholar]
- 96.Wu AH, Yu MC, Tseng CC, Pike MC. Epidemiology of soy exposures and breast cancer risk. Br J Cancer 2008;98:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhong X, Zhang C. Soy food intake and breast cancer risk: a meta-analysis. Wei Sheng Yan Jiu 2012;41:670–6. [PubMed] [Google Scholar]
- 98.Dong JY, Qin LQ. Soy isoflavones consumption and risk of breast cancer incidence or recurrence: a meta-analysis of prospective studies. Breast Cancer Res Treat 2011;125:315–23. [DOI] [PubMed] [Google Scholar]
- 99.Chen M, Rao Y, Zheng Y, Wei S, Li Y, Guo T, Yin P. Association between soy isoflavone intake and breast cancer risk for pre- and post-menopausal women: a meta-analysis of epidemiological studies. PLoS ONE 2014;9:e89288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shimizu H, Ross RK, Bernstein L, Yatani R, Henderson BE, Mack TM. Cancers of the prostate and breast among Japanese and white immigrants in Los Angeles County. Br J Cancer 1991;63:963–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Polkowski K, Popiołkiewicz J, Krzeczyński P, Ramza J, Pucko W, Zegrocka-Stendel O, Boryski J, Skierski JS, Mazurek AP, Grynkiewicz G , et al. Cytostatic and cytotoxic activity of synthetic genistein glycosides against human cancer cell lines. Cancer Lett 2004;203:59–69. [DOI] [PubMed] [Google Scholar]
- 102.Popiołkiewicz J, Polkowski K, Skierski JS, Mazurek AP. In vitro toxicity evaluation in the development of new anticancer drugs-genistein glycosides. Cancer Lett 2005;229:67–75. [DOI] [PubMed] [Google Scholar]
- 103.Michikawa T, Inoue M, Sawada N, Tanaka Y, Yamaji T, Iwasaki M, Shimazu T, Sasazuki S, Mizokami M, Tsugane S. Plasma isoflavones and risk of primary liver cancer in Japanese women and men with hepatitis virus infection: a nested case-control study. Cancer Epidemiol Biomarkers Prev 2015;24:532–7. [DOI] [PubMed] [Google Scholar]
- 104.Gu Y, Zhu CF, Iwamoto H, Chen JS. Genistein inhibits invasive potential of human hepatocellular carcinoma by altering cell cycle, apoptosis, and angiogenesis. World J Gastroenterol 2005;11:6512–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mansoor TA, Ramalho RM, Luo X, Ramalhete C, Rodrigues CM, Ferreira MJ. Isoflavones as apoptosis inducers in human hepatoma HuH-7 cells. Phytother Res 2011;25:1819–24. [DOI] [PubMed] [Google Scholar]
- 106.Yeh TC, Chiang PC, Li TK, Hsu JL, Lin CJ, Wang SW, Peng CY, Guh JH. Genistein induces apoptosis in human hepatocellular carcinomas via interaction of endoplasmic reticulum stress and mitochondrial insult. Biochem Pharmacol 2007;73:782–92. [DOI] [PubMed] [Google Scholar]
- 107.Chodon D, Ramamurty N, Sakthisekaran D. Preliminary studies on induction of apoptosis by genistein on HepG2 cell line. Toxicol In Vitro 2007;21:887–91. [DOI] [PubMed] [Google Scholar]
- 108.Gu Y, Zhu CF, Dai YL, Zhong Q, Sun B. Inhibitory effects of genistein on metastasis of human hepatocellular carcinoma. World J Gastroenterol 2009;15:4952–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dai W, Wang F, He L, Lin C, Wu S, Chen P, Zhang Y, Shen M, Wu D, Wang C. Genistein inhibits hepatocellular carcinoma cell migration by reversing the epithelial-mesenchymal transition: partial mediation by the transcription factor NFAT. Mol Carcinog 2015;54:301–11. [DOI] [PubMed] [Google Scholar]
- 110.Wang SD, Chen BC, Kao ST, Liu CJ, Yeh CC. Genistein inhibits tumor invasion by suppressing multiple signal transduction pathways in human hepatocellular carcinoma cells. BMC Complement Altern Med 2014;14:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jin CY, Park C, Kim GY, Lee SJ, Kim WJ, Choi YH. Genistein enhances TRAIL-induced apoptosis through inhibition of p38 MAPK signaling in human hepatocellular carcinoma Hep3B cells. Chem Biol Interact 2009;180:143–50. [DOI] [PubMed] [Google Scholar]
- 112.Jin CY, Park C, Moon SK, Kim GY, Kwon TK, Lee SJ, Kim WJ, Choi YH. Genistein sensitizes human hepatocellular carcinoma cells to TRAIL-mediated apoptosis by enhancing Bid cleavage. Anticancer Drugs 2009;20:713–22. [DOI] [PubMed] [Google Scholar]
- 113.Ma Y, Wang J, Liu L, Zhu H, Chen X, Pan S, Sun X, Jiang H. Genistein potentiates the effect of arsenic trioxide against human hepatocellular carcinoma: role of Akt and nuclear factor-kappa B. Cancer Lett 2011;301:75–84. [DOI] [PubMed] [Google Scholar]
- 114.Chodon D, Banu SM, Padmavathi R, Sakthisekaran D. Inhibition of cell proliferation and induction of apoptosis by genistein in experimental hepatocellular carcinoma. Mol Cell Biochem 2007;297:73–80. [DOI] [PubMed] [Google Scholar]
- 115.Ko KP, Park SK, Park B, Yang JJ, Cho LY, Kang C, Kim CS, Gwack J, Shin A, Kim Y. Isoflavones from phytoestrogens and gastric cancer risk: a nested case-control study within the Korean Multicenter Cancer Cohort. Cancer Epidemiol Biomarkers Prev 2010;19:1292–300. [DOI] [PubMed] [Google Scholar]
- 116.Hara A, Sasazuki S, Inoue M, Miura T, Iwasaki M, Sawada N, Shimazu T, Yamaji T, Tsugane S. Plasma isoflavone concentrations are not associated with gastric cancer risk among Japanese men and women. J Nutr 2013;143:1293–8. [DOI] [PubMed] [Google Scholar]
- 117.Zhou HB, Chen JJ, Wang WX, Cai JT, Du Q. Apoptosis of human primary gastric carcinoma cells induced by genistein. World J Gastroenterol 2004;10:1822–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhou HB, Chen JM, Cai JT, Du Q, Wu CN. Anticancer activity of genistein on implanted tumor of human SG7901 cells in nude mice. World J Gastroenterol 2008;14:627–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li YS, Wu LP, Li KH, Liu YP, Xiang R, Zhang SB, Zhu LY, Zhang LY. Involvement of nuclear factor kappa B (NF-kappa B) in the downregulation of cyclooxygenase-2 (COX-2) by genistein in gastric cancer cells. J Int Med Res 2011;39:2141–50. [DOI] [PubMed] [Google Scholar]
- 120.Liu YL, Zhang GQ, Yang Y, Zhang CY, Fu RX, Yang YM. Genistein induces G2/M arrest in gastric cancer cells by increasing the tumor suppressor PTEN expression. Nutr Cancer 2013;65:1034–41. [DOI] [PubMed] [Google Scholar]
- 121.Yan GR, Zou FY, Dang BL, Zhang Y, Yu G, Liu X, He QY. Genistein-induced mitotic arrest of gastric cancer cells by downregulating KIF20A: a proteomics study. Proteomics 2012;12:2391–9. [DOI] [PubMed] [Google Scholar]
- 122.Huang W, Wan C, Luo Q, Huang Z. Genistein-inhibited cancer stem cell-like properties and reduced chemoresistance of gastric cancer. Int J Mol Sci 2014;15:3432–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yu D, Shin HS, Lee YS, Lee D, Kim S, Lee YC. Genistein attenuates cancer stem cell characteristics in gastric cancer through the downregulation of Gli1. Oncol Rep 2014;31:673–8. [DOI] [PubMed] [Google Scholar]
- 124.Tatsuta M, Iishi H, Baba M, Yano H, Uehara H, Nakaizumi A. Attenuation by genistein of sodium‐chloride‐enhanced gastric carcinogenesis induced by N‐methyl‐N′‐nitro‐N‐nitrosoguanidine in Wistar rats. Int J Cancer 1999;80:396–9. [DOI] [PubMed] [Google Scholar]
- 125.Yanagihara K, Takigahira M, Mihara K, Kubo T, Morimoto C, Morita Y, Terawaki K, Uezono Y, Seyama T. Inhibitory effects of isoflavones on tumor growth and cachexia in newly established cachectic mouse models carrying human stomach cancers. Nutr Cancer 2013;65:578–89. [DOI] [PubMed] [Google Scholar]
- 126.Schabath MB, Hernandez LM, Wu X, Pillow PC, Spitz MR. Dietary phytoestrogens and lung cancer risk. JAMA 2005;294:1493–504. [DOI] [PubMed] [Google Scholar]
- 127.Seow A, Koh WP, Wang R, Lee HP, Yu MC. Reproductive variables, soy intake, and lung cancer risk among nonsmoking women in the Singapore Chinese Health Study. Cancer Epidemiol Biomarkers Prev 2009;18:821–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cutler GJ, Nettleton JA, Ross JA, Harnack LJ, Jacobs DR Jr, Scrafford CG, Barraj LM, Mink PJ, Robien K. Dietary flavonoid intake and risk of cancer in postmenopausal women: the Iowa Women's Health Study. Int J Cancer 2008;123:664–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shimazu T, Inoue M, Sasazuki S, Iwasaki M, Sawada N, Yamaji T, Tsugane S. Isoflavone intake and risk of lung cancer: a prospective cohort study in Japan. Am J Clin Nutr 2010;91:722–8. [DOI] [PubMed] [Google Scholar]
- 130.Shimazu T, Inoue M, Sasazuki S, Iwasaki M, Sawada N, Yamaji T, Tsugane S. Plasma isoflavones and the risk of lung cancer in women: a nested case-control study in Japan. Cancer Epidemiol Biomarkers Prev 2011;20:419–27. [DOI] [PubMed] [Google Scholar]
- 131.Zhu H, Cheng H, Ren Y, Liu ZG, Zhang YF, De Luo B. Synergistic inhibitory effects by the combination of gefitinib and genistein on NSCLC with acquired drug-resistance in vitro and in vivo. Mol Biol Rep 2012;39:4971–9. [DOI] [PubMed] [Google Scholar]
- 132.Mahmood J, Jelveh S, Calveley V, Zaidi A, Doctrow SR, Hill RP. Mitigation of radiation-induced lung injury by genistein and EUK-207. Int J Radiat Biol 2011;87:889–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gadgeel SM, Ali S, Philip PA, Wozniak A, Sarkar FH. Genistein enhances the effect of epidermal growth factor receptor tyrosine kinase inhibitors and inhibits nuclear factor kappa B in nonsmall cell lung cancer cell lines. Cancer 2009;115:2165–76. [DOI] [PubMed] [Google Scholar]
- 134.Wu TC, Yang YC, Huang PR, Wen YD, Yeh SL. Genistein enhances the effect of trichostatin A on inhibition of A549 cell growth by increasing expression of TNF receptor-1. Toxicol Appl Pharmacol 2012;262:247–54. [DOI] [PubMed] [Google Scholar]
- 135.Tian T, Li J, Li B, Wang Y, Li M, Ma D, Wang X. Genistein exhibits anti-cancer effects via down-regulating FoxM1 in H446 small-cell lung cancer cells. Tumour Biol 2014;35:4137–45. [DOI] [PubMed] [Google Scholar]
- 136.Shiau RJ, Chen KY, Wen YD, Chuang CH, Yeh SL. Genistein and β-carotene enhance the growth-inhibitory effect of trichostatin A in A549 cells. Eur J Nutr 2010;49:19–25. [DOI] [PubMed] [Google Scholar]
- 137.Peng B, Cao J, Yi S, Wang C, Zheng G, He Z. Inhibition of proliferation and induction of G1-phase cell-cycle arrest by dFMGEN, a novel genistein derivative, in lung carcinoma A549 cells. Drug Chem Toxicol 2013;36:196–204. [DOI] [PubMed] [Google Scholar]
- 138.Messina M, Bennink M. Soyfoods, isoflavones and risk of colonic cancer: a review of the in vitro and in vivo data. Baillieres Clin Endocrinol Metab 1998;12:707–28. [DOI] [PubMed] [Google Scholar]
- 139.Thiagarajan DG, Bennink MR, Bourquin LD, Kavas FA. Prevention of precancerous colonic lesions in rats by soy flakes, soy flour, genistein, and calcium. Am J Clin Nutr 1998;68:1394S–9S. [DOI] [PubMed] [Google Scholar]
- 140.Spector D, Anthony M, Alexander D, Arab L. Soy consumption and colorectal cancer. Nutr Cancer 2003;47:1–12. [DOI] [PubMed] [Google Scholar]
- 141.Rossi M, Negri E, Talamini R, Bosetti C, Parpinel M, Gnagnarella P, Franceschi S, Dal Maso L, Montella M, Giacosa A, et al. Flavonoids and colorectal cancer in Italy. Cancer Epidemiol Biomarkers Prev 2006;15:1555–8. [DOI] [PubMed] [Google Scholar]
- 142.Cotterchio M, Boucher BA, Manno M, Gallinger S, Okey A, Harper P. Dietary phytoestrogen intake is associated with reduced colorectal cancer risk. J Nutr 2006;136:3046–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kim EJ, Shin HK, Park JH. Genistein inhibits insulin-like growth factor-I receptor signaling in HT-29 human colon cancer cells: a possible mechanism of the growth inhibitory effect of Genistein. J Med Food 2005;8:431–8. [DOI] [PubMed] [Google Scholar]
- 144.Su SJ, Chow NH, Kung ML, Hung TC, Chang KL. Effects of soy isoflavones on apoptosis induction and G2-M arrest in human hepatoma cells involvement of caspase-3 activation, Bcl-2 and Bcl-XL downregulation, and Cdc2 kinase activity. Nutr Cancer 2003;45:113–23. [DOI] [PubMed] [Google Scholar]
- 145.Bielecki A, Roberts J, Mehta R, Raju J. Estrogen receptor-beta mediates the inhibition of DLD-1 human colon adenocarcinoma cells by soy isoflavones. Nutr Cancer 2011;63:139–50. [DOI] [PubMed] [Google Scholar]
- 146.Qi W, Weber CR, Wasland K, Savkovic SD. Genistein inhibits proliferation of colon cancer cells by attenuating a negative effect of epidermal growth factor on tumor suppressor FOXO3 activity. BMC Cancer 2011;11:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhang Y, Chen H. Genistein attenuates WNT signaling by up-regulating sFRP2 in a human colon cancer cell line. Exp Biol Med (Maywood) 2011;236:714–22. [DOI] [PubMed] [Google Scholar]
- 148.Wang H, Li Q, Chen H. Genistein affects histone modifications on Dickkopf-related protein 1 (DKK1) gene in SW480 human colon cancer cell line. PLoS ONE 2012;7:e40955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang Y, Li Q, Zhou D, Chen H. Genistein, a soya isoflavone, prevents azoxymethane-induced up-regulation of WNT/beta-catenin signalling and reduces colon pre-neoplasia in rats. Br J Nutr 2013;109:33–42. [DOI] [PubMed] [Google Scholar]
- 150.Dai Q, Shu XO, Jin F, Potter JD, Kushi LH, Teas J, Gao YT, Zheng W, et al. Population-based case-control study of soyfood intake and breast cancer risk in Shanghai. Br J Cancer 2001;85:372–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wu AH, Yu MC, Tseng CC, Twaddle NC, Doerge DR. Plasma isoflavone levels versus self-reported soy isoflavone levels in Asian-American women in Los Angeles County. Carcinogenesis 2004;25:77–81. [DOI] [PubMed] [Google Scholar]
- 152.Yamamoto S, Sobue T, Kobayashi M, Sasaki S, Tsugane S. Soy, isoflavones, and breast cancer risk in Japan. J Natl Cancer Inst 2003;95:906–13. [DOI] [PubMed] [Google Scholar]
- 153.Liu Y, Zhang YM, Song DF, Cui HB. Effect of apoptosis in human breast cancer cells and its probable mechanisms by genistein. Wei Sheng Yan Jiu 2005;34:67–9. [PubMed] [Google Scholar]
- 154.Hsieh CY, Santell RC, Haslam SZ, Helferich WG. Estrogenic effects of genistein on the growth of estrogen receptor-positive human breast cancer (MCF-7) cells in vitro and in vivo. Cancer Res 1998;58:3833–8. [PubMed] [Google Scholar]
- 155.Satoh H, Nishikawa K, Suzuki K, Asano R, Virgona N, Ichikawa T, Hagiwara K, Yano T. Genistein, a soy isoflavone, enhances necrotic-like cell death in a breast cancer cell treated with a chemotherapeutic agent. Res Commun Mol Pathol Pharmacol 2003;113–114:149–58. [PubMed] [Google Scholar]
- 156.Mai Z, Blackburn GL, Zhou JR. Genistein sensitizes inhibitory effect of tamoxifen on the growth of estrogen receptor-positive and HER2-overexpressing human breast cancer cells. Mol Carcinog 2007;46:534–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Li Y, Ahmed F, Ali S, Philip PA, Kucuk O, Sarkar FH. Inactivation of nuclear factor kappaB by soy isoflavone genistein contributes to increased apoptosis induced by chemotherapeutic agents in human cancer cells. Cancer Res 2005;65:6934–42. [DOI] [PubMed] [Google Scholar]
- 158.Gong L, Li Y, Nedeljkovic-Kurepa A, Sarkar FH. Inactivation of NF-kappaB by genistein is mediated via Akt signaling pathway in breast cancer cells. Oncogene 2003;22:4702–9. [DOI] [PubMed] [Google Scholar]
- 159.Ca ëtano B, Le Corre L, Chalabi N, Delort L, Bignon YJ, Bernard-Gallon DJ. Soya phytonutrients act on a panel of genes implicated with BRCA1 and BRCA2 oncosuppressors in human breast cell lines. Br J Nutr 2006;95:406–13. [DOI] [PubMed] [Google Scholar]
- 160.Seo HS, DeNardo DG, Jacquot Y, Laios I, Vidal DS, Zambrana CR, Leclercq G, Brown PH. Stimulatory effect of genistein and apigenin on the growth of breast cancer cells correlates with their ability to activate ER alpha. Breast Cancer Res Treat 2006;99:121–34. [DOI] [PubMed] [Google Scholar]
- 161.Liu B, Edgerton S, Yang X, Kim A, Ordonez-Ercan D, Mason T, Alvarez K, McKimmey C, Liu N, Thor A. Low-dose dietary phytoestrogen abrogates tamoxifen-associated mammary tumor prevention. Cancer Res 2005;65:879–86. [PubMed] [Google Scholar]
- 162.Lamartiniere CA, Zhang JX, Cotroneo MS. Genistein studies in rats: potential for breast cancer prevention and reproductive and developmental toxicity. Am J Clin Nutr 1998;68:1400S–5S. [DOI] [PubMed] [Google Scholar]
- 163.Ju YH, Allred KF, Allred CD, Helferich WG. Genistein stimulates growth of human breast cancer cells in a novel, postmenopausal animal model, with low plasma estradiol concentrations. Carcinogenesis 2006;27:1292–9. [DOI] [PubMed] [Google Scholar]
- 164.Marini H, Bitto A, Altavilla D, Burnett BP, Polito F, Di Stefano V, Minutoli L, Atteritano M, Levy RM, D'Anna R, et al. Breast safety and efficacy of genistein aglycone for postmenopausal bone loss: a follow-up study. J Clin Endocrinol Metab 2008;93:4787–96. [DOI] [PubMed] [Google Scholar]
- 165.Atteritano M, Pernice F, Mazzaferro S, Mantuano S, Frisina A, D'Anna R, Cannata ML, Bitto A, Squadrito F, Frisina N, et al. Effects of phytoestrogen genistein on cytogenetic biomarkers in postmenopausal women: 1 year randomized, placebo-controlled study. Eur J Pharmacol 2008;589:22–6. [DOI] [PubMed] [Google Scholar]
- 166.McMichael-Phillips DF, Harding C, Morton M, Roberts SA, Howell A, Potten CS, Bundred NJ. Effects of soy-protein supplementation on epithelial proliferation in the histologically normal human breast. Am J Clin Nutr 1998;68(Suppl):1431S–5S. [DOI] [PubMed] [Google Scholar]
- 167.Hargreaves DF, Potten CS, Harding C, Shaw LE, Morton MS, Roberts SA, Howell A, Bundred NJ. Two-week dietary soy supplementation has an estrogenic effect on normal premenopausal breast. J Clin Endocrinol Metab 1999;84:4017–24. [DOI] [PubMed] [Google Scholar]
- 168.Khan SA, Chatterton RT, Michel N, Bryk M, Lee O, Ivancic D, Heinz R, Zalles CM, Helenowski IB, Jovanovic BD, et al. Soy isoflavone supplementation for breast cancer risk reduction: a randomized phase II trial. Cancer Prev Res (Phila) 2012;5:309–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.[cited 2014 Nov 13]. Available from: www.clinicaltrials.gov.
- 170.Nguyen DT, Hernandez-Montes E, Vauzour D, Schönthal AH, Rice-Evans C, Cadenas E, Spencer JP. The intracellular genistein metabolite 5,7,3′,4′-tetrahydroxyisoflavone mediates G2-M cell cycle arrest in cancer cells via modulation of the p38 signaling pathway. Free Radic Biol Med 2006;41:1225–39. [DOI] [PubMed] [Google Scholar]
- 171.Kiriakidis S, Högemeier O, Starcke S, Dombrowski F, Hahne JC, Pepper M, Jha HC, Wernert N. Novel tempeh (fermented soyabean) isoflavones inhibit in vivo angiogenesis in the chicken chorioallantoic membrane assay. Br J Nutr 2005;93:317–23. [DOI] [PubMed] [Google Scholar]
- 172.Vauzour D, Vafeiadou K, Rice-Evans C, Cadenas E, Spencer JP. Inhibition of cellular proliferation by the genistein metabolite 5,7,3′,4′-tetrahydroxyisoflavone is mediated by DNA damage and activation of the ATR signalling pathway. Arch Biochem Biophys 2007;468:159–66. [DOI] [PubMed] [Google Scholar]
- 173.Russo M, Spagnuolo C, Tedesco I, Russo GL. Phytochemicals in cancer prevention and therapy: truth or dare? Toxins 2010;2:517–51. [cited 2014 Nov 13]. Available from: http://www.clinicaltrials.gov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chang EC, Charn TH, Park SH, Helferich WG, Komm B, Katzenellenbogen JA, Katzenellenbogen BS. Estrogen receptors alpha and beta as determinants of gene expression: influence of ligand, dose, and chromatin binding. Mol Endocrinol 2008;22:1032–43. [DOI] [PMC free article] [PubMed] [Google Scholar]