Abstract
Background
Improved referral algorithms for children with non-severe pneumonia at the community level are desirable. We sought to identify predictors of oral antibiotic failure in children who fulfill the case definition of World Health Organization (WHO) non-severe pneumonia. Predictors of greatest interest were those not currently utilized in referral algorithms and feasible to obtain at the community level.
Methods
We systematically reviewed prospective studies reporting independent predictors of oral antibiotic failure for children 2–59 months of age in resource-limited settings with WHO non-severe pneumonia (either fast breathing for age and/or lower chest wall indrawing without danger signs), with an emphasis on predictors not currently utilized for referral and reasonable for community health workers. We searched PubMed, Cochrane, and Embase and qualitatively analyzed publications from 1997–2014. To supplement the limited published evidence in this subject area we also surveyed respiratory experts.
Results
Nine studies met criteria, seven of which were performed in south Asia. One eligible study occurred exclusively at the community level. Overall, oral antibiotic failure rates ranged between 7.8-22.9 %. Six studies found excess age-adjusted respiratory rate (either WHO-defined very fast breathing for age or 10–15 breaths/min faster than normal WHO age-adjusted thresholds) and four reported young age as predictive for oral antibiotic failure. Of the seven predictors identified by the expert panel, abnormal oxygen saturation and malnutrition were most highly favored per the panel’s rankings and comments.
Conclusions
This review identified several candidate predictors of oral antibiotic failure not currently utilized in childhood pneumonia referral algorithms; excess age-specific respiratory rate, young age, abnormal oxygen saturation, and moderate malnutrition. However, the data was limited and there are clear evidence gaps; research in rural, low-resource settings with community health workers is needed.
Electronic supplementary material
The online version of this article (doi:10.1186/s12887-015-0392-x) contains supplementary material, which is available to authorized users.
Keywords: integrated Community Case Management (iCCM), Community health workers, Childhood pneumonia, Pediatrics, Developing countries, Predictors, Treatment failure, mHealth
Background
Healthcare providers in resource-limited countries use practical, standardized case management guidelines to diagnose pneumonia [1]. Although mortality reductions of 36 % have been attributed to standardized management, pneumonia remains the second leading cause of childhood death globally with nearly one million deaths annually [2]. Three-quarters of all deaths occur in Africa and Asia, and it is estimated that a majority of all childhood pneumonia deaths occur outside of the hospital [3, 4]. Incomplete antibiotic coverage and care-seeking delays negatively influence pneumonia outcomes in low-resource settings and improvements have been inconsistent and difficult to sustain [4].
The World Health Organization (WHO) recommends expansion of basic healthcare beyond traditional facilities to community health workers (CHWs). This strategy, called ‘integrated Community Case Management’ (iCCM), includes childhood pneumonia care [5]. iCCM can address gaps in antibiotic access and care seeking delays by providing antibiotics earlier in a pneumonia illness when the disease is more responsive to treatment [5, 6]. Likewise, iCCM can permit earlier referral if treatment fails - defined as disease persistence or progression despite antibiotics. Evidence from Zambia and Pakistan has demonstrated improved childhood pneumonia antibiotic coverage and reduced treatment failure with iCCM [7–9]. As a result, iCCM expansion is a key strategy against childhood pneumonia [5, 6].
Despite iCCM’s potential there are challenges, such as sustaining quality of care. CHWs receive limited training and are difficult to supervise in remote areas [5, 6]; consistent supervision and retraining is likely important for successful iCCM pneumonia management to ensure standardized recording of subjective clinical danger signs [10]. Strengthening CHW decision-making and identification of higher-risk patients, including those that do not meet current referral criteria, could reduce incorrect pneumonia diagnosis, antibiotic prescription, and referrals. Mobile health, or mHealth, may help to address these weaknesses [11]. mHealth utilizes user-friendly software compatible with mobile phones for healthcare delivery and capitalizes on mobile networks with wide coverage and usage in developing countries [11]. New mHealth applications that provide an array of performance support for CHWs are in development [12, 13].
An iCCM mHealth mobile phone application could assist CHWs to identify two groups of children with pneumonia; (1) those who meet current referral criteria but are mis-classified, and (2) those who do not meet current referral criteria but are still at greater risk for treatment failure based upon their initial clinical assessment. Both groups of children could then be referred for more appropriate treatment or be flagged for closer community-based monitoring. An improved referral mHealth tool could provide the result as a simple audio recommendation translated into the preferred local language, allowing both the CHW and caregiver to hear the recommendation. Facilitating objective decision-making by CHWs with a mHealth tool could complement existing guidelines to expand appropriate, timely antibiotic coverage and further reduce life-threatening treatment delays.
This systematic review and expert opinion survey aimed to identify predictors of WHO non-severe pneumonia treatment failure that are not currently used in referral algorithms and are also realistic for CHWs. The ultimate aim of a prediction algorithm would be to facilitate improved referral pathways originating from the community level, leading both to improved antibiotic stewardship and decreased morbidity and mortality.
Methods
Systematic review
We conducted a systematic review in accordance with PRIMSA (see Additional file 1) [14]. All studies from January 1, 1997 - June 25, 2014 listed in PubMed, Cochrane Central Register of Controlled Trials, and Embase were eligible. The following search terms were used: pneumonia; predictors or risk factors or prognostic; treatment failure or death; and Africa or Asia or developing country. These were combined with the following search filters: clinical trial, humans, and infant or preschool ages. No language restrictions were applied. We conducted the search on June 25, 2014. We also conducted a supplementary, non-systematic search that did not filter for geographical region. Please see Additional file 2 for these findings.
Titles and abstracts were reviewed twice and those which both reviewers agreed were relevant underwent full text review. Reference lists of reviewed manuscripts were screened for additional studies. Eligibility criteria included: 1) Study design: Prospective observational studies and clinical trials. 2) Participants: Aged 2–59 months treated with oral antibiotics for WHO-defined non-severe pneumonia per the 2013 revised WHO guidelines (Fig. 1) [15]. 3) Data collection dates: 1997 or more recent. The current WHO guidelines were formally disseminated in 1997 [1]. 4) Geographic regions: Africa and/or Asia, representing the highest burden of disease. 5) Outcome: A priori defined treatment failure, with statistical analysis for independent baseline clinical predictors of treatment failure.
Fig. 1.
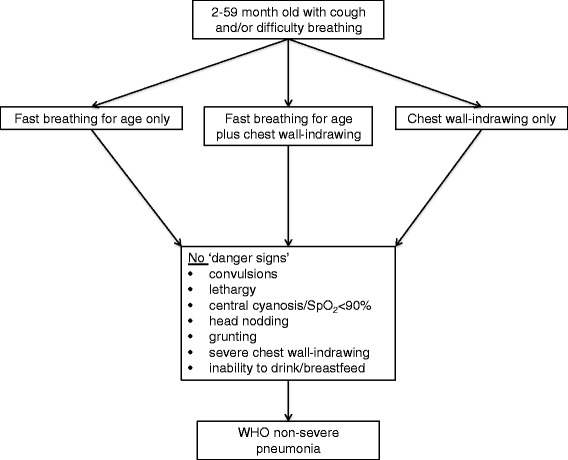
2013 World Health Organization non-severe pneumonia case definition
We used a structured data extraction tool to describe key data from the eligible studies including: region, study design, primary outcome, data collection dates, enrollment criteria, treatment failure definition, sample size, age range of participants, treatment failure rate, and independent predictors of treatment failure. The Cochrane Risk of Bias tool was used to qualitatively assess the risk of bias in individual studies [16].
Expert opinion
We complemented the systematic literature review by surveying childhood pneumonia experts. The experts were all healthcare professionals experienced in childhood pneumonia in the African or Asian region across hospital and community care settings, many with research experience [17]. We used a modified Delphi consensus process, administering two separate structured phone interviews and a final electronic survey to 13 experts, concluding when a general consensus was reached (see Additional file 3 for interview materials). Participants were asked to assess the potential role for a pneumonia treatment prognostic, including where in the health system (the home, a community health clinic, or the hospital) a prognostic would be best utilized.
The literature search provided the initial prognostic indicators to test with interviewees, using WHO definitions [15]. The panel was allowed to adjust these indicators, as well as the definitions of them and we encouraged the panel to use their own knowledge and experience. Fever was modified to an axillary temperature >38.0 °C, high blood lactate levels to >2 mmol/l, and low weight for age as a standardized z score < −2. The definition of ‘human immunodeficiency virus (HIV)-affected’ included HIV-exposed children (i.e. born to an HIV-infected mother but not HIV tested or previously tested HIV-negative with ongoing breastfeeding) or HIV-infected per standard guidelines [18].
The experts qualitatively and quantitatively estimated each predictor’s potential value. Qualitative estimates of each prognostic indicator’s value were allocated using an ordinal scale (0 = unfavorable, 1 = minimally favorable, 2 = favorable, 3 = highly favorable), along with any comments regarding each indicator’s likely performance and feasibility. Panel members provided quantitative estimates by assigning a childhood pneumonia mortality rate to community oral or parenteral hospital antibiotic treatment.
An anonymous summary of panel answers were provided to interviewees after the first interview and each expert was encouraged to revise their answers after considering the panel’s overall results. For the final survey, the expert opinion results were rephrased into binary yes/no questions to determine a final consensus. This research was approved by the Johns Hopkins Institutional Review Board (IRB00047406) and written consent was obtained from survey participants.
Results
Systematic review
Our search yielded 7,649 studies; of these, 59 manuscripts were retrieved for full text review. Nine studies met all eligibility criteria, including quality control, and were qualitatively analyzed (Fig. 2). We found no publications that included children with fast breathing and lower chest indrawing (LCI)-defined pneumonia together, probably because the WHO guidelines were only recently revised and disseminated in 2013.
Fig. 2.
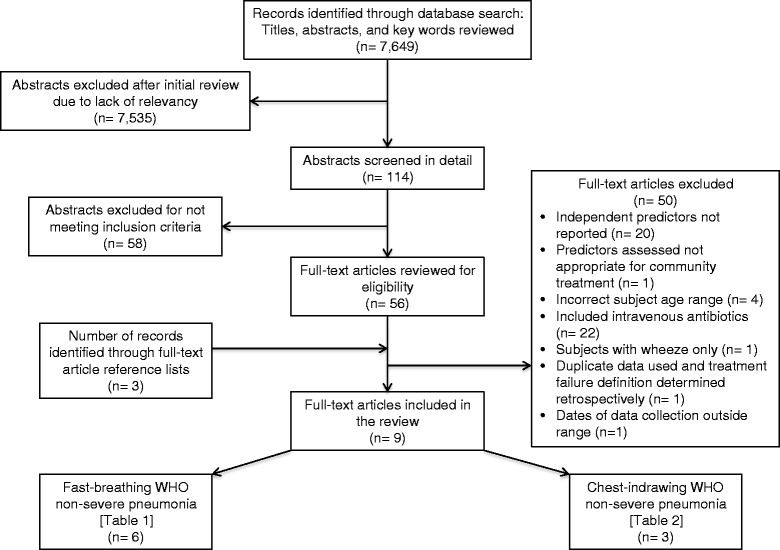
Flow diagram of study identification and selection
Table 1 summarizes the six studies reporting predictors of oral antibiotic failure in fast breathing non-severe pneumonia [19–24]. All studies were from developing countries in south Asia. Five of the six studies included amoxicillin treatment and only one included community clinics. Five studies defined treatment failure as either a persistence of fast breathing after 3–5 days or disease progression (development of LCI or danger signs) between 1–5 days after antibiotic initiation. One study included peripheral oxygen saturation (SpO2) less than 90 % in the definition of treatment failure. The proportion of children that failed oral antibiotic treatment ranged from 7.8 % to 22.9 %. Three studies identified baseline excess age-specific respiratory rate, and two studies found history of difficulty breathing, age, and illness duration of ≥3 days as independent predictors of oral antibiotic failure.
Table 1.
Independent predictors of oral antibiotic failure in fast breathing WHO non-severe childhood pneumoniaa
| Study overview | Awasthi S et al. | CATCH UP | MASCOT | Agarwal G et al. | Noorani QA et al. | Hazir T et al. | |
|---|---|---|---|---|---|---|---|
| Region (country) | Asia (India) | Asia (Pakistan) | Asia (Pakistan) | Asia (India) | Asia (Pakistan) | Asia (Pakistan) | |
| Data collection dates | 2004–2006 | 1998–1999 | 1999–2001 | 2000–2002 | 2000–2001 | 2005–2008 | |
| Enrollment criteria | 2–59 months old | 2–59 months old | 2–59 months of age | 2–59 months of age | 2–59 months of age | 2–59 months of age | |
| Study design | Cluster randomized study | Randomized multicenter equivalency study | Randomized multicenter equivalency study | Randomized multicenter equivalency study | Multicenter observational study | Randomized multicenter equivalency study | |
| Intervention arm | Oral amoxicillin 31–51 mg/kg/day for 3 days | Oral amoxicillin 50 mg/kg/day for 5 days | Oral amoxicillin 45 mg/kg/day for 3 days | Oral amoxicillin 31–54 mg/kg/day for 3 days | - | Placebo for 3 days | |
| Control arm (or standard of care) | Oral trimethoprim (7–11 mg/kg/day)-sulfamethoxazole for 5 days | Oral trimethoprim (8 mg/kg/day)-sulfamethoxazole for 5 days | Oral amoxicillin 45 mg/kg/day for 5 days | Oral amoxicillin 31–54 mg/kg/day for 5 days | Oral trimethoprim (8 mg/kg/day)-sulfamethoxazole for 5 days | Oral amoxicillin 45 mg/kg/day for 3 days | |
| Blinding | No | Yes | Yes | Yes | - | Yes | |
| Study site types | Health centers | Tertiary care and secondary-level hospitals, and health center | Tertiary care and secondary-level hospitals | Tertiary care hospitals | Health centers | Tertiary care hospitals | |
| Number of sites | 14 | 8 | 7 | 7 | 14 | 4 | |
| Primary outcome | Treatment failure | Equivalency | Equivalency | Equivalency | Treatment failure | Equivalency | |
| Treatment failure definition | |||||||
| Day of assignment | Day 3 (intervention); Day 5 (control) | Days 3 to 5 | Day 5 | Days 3 to 5 | Day 5 | Day 3 | |
| Respiratory ratea | Yes, elevated for age (days 3–5) | Yes, +/−5 breaths/min or higher versus enrollment (days 3–5) | Yes, elevated for age (days 3–5) | Yes, elevated for age (days 3–5) | Yes, +/−5 breaths/min or higher versus enrollment (days 3–5) | No | |
| Fever >38 °C and LCI | No | No | No | No | No | No | |
| Fever >38 °C | Yes (days 3–5) | No | No | No | No | No | |
| LCI | Yes (days 1–5) | Yes (days 3–5) | Yes (days 1–5) | Yes (days 1–5) | Yes (days 1–5) | Yes (days 1–3) | |
| Convulsions | Yes (days 1–5) | Yes (days 3–5) | Not specified | Yes (days 1–5) | Not specified | Yes (days 1–3) | |
| Abnormally sleepy | Yes (days 1–5) | Yes (days 3–5) | Not specified | Yes (days 1–5) | Not specified | Yes (days 1–3) | |
| Inability to drink | Yes (days 1–5) | Yes (days 3–5) | Not specified | Yes (days 1–5) | Not specified | Yes (days 1–3) | |
| Stridor in calm child | Yes (days 1–5) | Yes (days 3–5) | No | No | No | Yes (days 1–3) | |
| Malnutrition | No | Yes (days 3–5) | No | No | No | Yes (days 1–3) | |
| Cyanosis | Yes (days 1–5) | No | Yes | No | Yes | No | |
| SpO2 | No | No | No | Yes, <90 % (on day 3 only) | No | No | |
| Antibiotic change | No | Yes (days 3–5) | Yes (days 1–5) | No | Yes (days 1–5) | No | |
| Hospitalization | No | Yes (days 1–5) | No | No | No | No | |
| Lost to follow-up | Yes (days 3–5) | Yes (days 3–5) | No | Yes (days 1–5) | Yes (days 5–11) | No | |
| Study withdrawal | Yes (days 1–5) | No | No | Yes (days 1–5) | No | No | |
| Death | Yes (days 1–14) | Yes (days 1–5) | Yes (days 1–5) | Yes (days 1–5) | Yes (days 1–5) | Yes (days 1–5) | |
| Study participants and description | |||||||
| Sample sizeb | 2009 | 1459 | 1953 | 2188 | 944 | 873 | |
| Age 2–11 months | 594/2009 (29.6 %) | 732/1459 (50.2 %) | 1051/1953 (53.8 %) | 954/2188 (43.6 %) | 334/944 (35.4 %) | 573/873 (65.6 %) | |
| Age 12–59 months | 1415/2009 (70.4 %) | 727/1459 (49.8 %) | 902/1953 (46.2 %) | 1234/2188 (56.4 %) | 610/944 (64.6 %) | 300/873 (34.4 %) | |
| Treatment failure rateb | 234/2009 (11.6 %) | 256/1459 (17.5 %) | 364/1953 (22.9 %) | 225/2188 (10.3 %) | 110/944 (11.6 %) | 68/873 (7.8 %) | |
| Independent predictors of treatment failure OR (95 % CI)c,d | Diarrhea, 1.65 (1.24–2.19) | History of difficulty breathing, 1.61 (1.13–2.15) | Excess respiratory rate >10 breaths/min for agea, 1.4 (1.1–1.9) | Excess respiratory rate >10 breaths/min for agea, 2.89 (1.83–4.55) | Excess respiratory rate ≥15 breaths/min for agea, 2.0 (1.2–3.4) | History of difficulty breathing, 2.86 (1.29–7.23) | |
| Age 12–59 months, 1.5 (1.12–1.91) | Antibiotic non-adherence, 4.5 (95 % CI 2.9–7.0) | Antibiotic non-adherence, 11.57 (7.4–18.0 | Wheeze on examination, 1.7 (1.1–2.6) | Temperature >37.5 °C at enrollment, 1.99 (1.37–2.90) | |||
| Illness >3 days, 1.4 (1.03–1.8) | Illness ≥3 days, 1.7 (1.3–2.1) | RSV, 1.95 (1.0–3.8) | |||||
| Age 2–11 months, 1.7 (1.1–2.1) | |||||||
| Persistent vomiting, 1.4 (1.0–2.0) | |||||||
Serious adverse event or drug reaction, and new comorbid condition were not considered in the treatment failure definitions for any of the studies. WHO: World Health Organization; LCI: lower chest wall indrawing; SpO2: peripheral oxygen saturation; OR: odds ratio; CI: confidence interval; RSV: human respiratory syncytial virus
aRespiratory rate norms: <50 breaths/min for ages 2–11 months; <40 breaths/min for ages 12–59 months
bIf primary outcome of trial was equivalency or no difference was found then intervention and control arm data was combined
cMultivariate logistic regression modeling was performed to determine independent predictors of treatment failure in all included studies. CATCHUP trial modeled age, history of difficulty breathing, duration of illness, and respiratory rate. The models were not reported for the MASCOT trial, Agarwal G et al., Noorani QA et al., Awasthi S et al., and Hazir T et al
dFor Hazir T et al. multivariate logistic regression modeling was performed on cumulative treatment failure data from day 5 although primary outcome data was analyzed from day 3 treatment failure data
Table 2 summarizes the three studies reporting independent predictors of oral antibiotic failure in LCI non-severe pneumonia [25–27]. Two studies were multi-country with sites in Africa. In two of the three studies, treatment failure was assigned on day six after antibiotic initiation. Treatment failure rates for the three studies ranged from 8.1 % to 19.3 %. All studies found young age and very fast breathing at baseline to be independent predictors of treatment failure. One study collected SpO2 measurements and found low baseline SpO2 to predictive; another study found malnutrition to be predictive of treatment failure.
Table 2.
Independent predictors of oral antibiotic failure in chest indrawing WHO non-severe childhood pneumonia
| Study overview | Addo-Yobo E, Chisaka N et al. | Hazir T, Fox LM et al. | Addo-Yobo E, Anh DD et al. | |
|---|---|---|---|---|
| Region (countries) | Africa (Ghana, South Africa, Zambia) Asia (India, Pakistan, Vietnam) South America (Columbia, Mexico) | Pakistan (Asia) | Africa (Egypt, Ghana) Asia (Bangladesh, Vietnam) | |
| Data collection dates | 1999–2002 | 2005–2006 | 2005–2008 | |
| Enrollment criteria | 3–59 months | 3–59 months | 3–59 months | |
| Study design | Randomized multicenter equivalency study | Randomized multicenter equivalency study | Multicenter observational study | |
| Intervention arm | Oral amoxicillin 45 mg/kg/day for 5 days | Oral amoxicillin 80–90 mg/kg/day for 5 days | - | |
| Control arm (or standard of care) | Intravenous 200,000 IU penicillin G for 2 days then oral amoxicillin 45 mg/kg/day for 3 days (total 5 days) | Intravenous ampicillin 100 mg/kg/day for 2 days then oral amoxicillin for 3 days (total 5 days) | Amoxicillin 80–90 mg/kg/day for 5 days | |
| Blinding | No | No | - | |
| Study site type(s) | Pediatric department of tertiary care hospitals | Pediatric department of tertiary care hospitals | Pediatric department of tertiary care and second-level hospitals, health centers | |
| Number of study sites | 9 | 7 | 5 | |
| Primary outcome | Equivalence | Equivalence | Treatment failure | |
| Treatment failure definition | ||||
| Day of assignment | Day 3 | Day 6 | Day 6 | |
| Respiratory ratea | No | No | No | |
| Fever >38 °C and LCI | No | Yes (days 3–6) | Yes (day 3) | |
| Fever >38 °C | No | Yes (day 6) | Yes (day 6) | |
| LCI | Yes (day 3) | Yes (day 6) | Yes (day 6) | |
| Convulsions | Yes (days 1–3) | Yes (days 1–6) | Yes (days 1–6) | |
| Abnormally sleepy | Yes (days 1–3) | Yes (days 1–6) | Yes (days 1–6) | |
| Inability to drink | Yes (days 1–3) | Yes (days 1–6) | Yes (days 1–6) | |
| Stridor in calm child | No | No | No | |
| Malnutrition | No | No | No | |
| Cyanosis | Yes (days 1–3) | Yes (days 1–6) | Yes (days 1–6) | |
| SpO2 | <80 % at sea-level or <75 % below sea-level (days 1–3) | No | No | |
| Antibiotic change | Yes (days 1–3) | No | Yes (days 1–6) | |
| Hospitalization | No | Yes, if related to pneumonia (days 1–6) | No | |
| Serious drug reaction | Yes (days 1–3) | No | Yes (days 1–6) | |
| Serious adverse event | No | Yes (days 1–6) | No | |
| New comorbid condition | Yes (days 1–3) | Yes, if required antibiotic (days 1–6) | Yes (days 1–6) | |
| Lost to follow-up | Yes (days 1–3) | Yes (days 1–6) | Yes (day 6) | |
| Study withdrawal | Yes (days 1–3) | Yes (days 1–6) | No | |
| Death | Yes (days 1–3) | Yes (days 1–14) | Yes (days 1–6) | |
| Study participants and description | ||||
| Sample sizeb | 1702 | 2037 | 823 | |
| Age: | 3–11 months | 1045/1669 (62.6 %) | 1311/2037 (64.4 %) | 562/873 (64.4 %) |
| 12–59 months | 624/1669 (37.4 %) | 726/2037 (35.6 %) | 310/873 (35.5 %) | |
| Treatment failure ratec | 328/1702 (19.3 %) | 164/2037 (8.1 %) | 76/823 (9.2 %) | |
| Independent predictors of treatment failurec OR/RR (95 % CI) | Age 3–11 months, 2.72 OR (95 % CI 1.95–3.79) | Age 3–5 months, 3.22 OR, (95 % CI 1.87–5.52) | Age 3–5 months, 1.96 RR (95 % CI 1.09–3.51) | |
| Very fast breathing, 1.94 OR (95 % CI 1.42–2.65)d | Very fast breathing, 1.65 OR (95 % CI 1.07–2.57)d | Very fast breathing, 12.5 RR (95 % CI 1.74–89.1)d | ||
| SpO2 < 90 %, 2.11 OR (95 % CI 1.6–2.78) | Weight for age z score <−2, 1.79 OR (95 % CI 1.23–2.60) | |||
WHO: World Health Organization; IU: International Units; LCI: lower chest wall indrawing; SpO 2: peripheral oxygen saturation; OR: odds ratio; RR: relative risk; CI, confidence interval
aRespiratory rate norms: <50 breaths/min for ages 2–11 months; <40 breaths/min for ages 12–59 months
bIf primary outcome of trial was equivalency then intervention and control arm data was combined
cMultivariate logistic regression modeling was used by Addo-Yobo E, Chisaka N et al. and Hazir T while log-linear regression modeling was used by Fox LM et al. Addo-Yobo E, Chisaka N et al. modeled age, very fast breathing, hypoxemia, and amoxicillin. Hazir T, Fox LM et al. modeled sex, age, breastfeeding, weight-for-age z score <−2, very fast breathing, and treatment group (home vs hospital). Addo-Yobo E, Anh DD et al. modeled sex, age, and respiratory rate
dVery fast breathing defined as ≥70 breaths/min for ages 3–11 months and ≥60 breaths/min for ages 12–59 months
Expert opinion
Of the 13 experts interviewed, eight reported experience in Africa, four in Asia, and one in both. All interviewees agreed that the development of a pneumonia treatment prognostic could significantly advance childhood pneumonia care and that the community-level was the most attractive setting for implementation.
The panel qualitatively evaluated seven prognostic indicators for performance and feasibility at the community level (Table 3). Abnormal SpO2 and malnutrition were ranked highest while fever and high blood lactate levels were lowest. Abnormal SpO2 was judged to be objective, although the panel identified the need for a robust, precise, low-cost pulse oximeter for young children. Malnutrition was also valued by the experts as it is likely to have a high positive predictive value and the feasibility of reliable malnutrition screening was attractive, with tools such as tapes and scales being simple, accurate, and relatively inexpensive.
Table 3.
Expert panel qualitative assessments of potential prognostic indicators
| Prognostic indicator | Overall favorability | Selected performance comments | Selected feasibility comments |
|---|---|---|---|
| Fever | Unfavorable | Low specificity for identifying children who will not fail treatment | High feasibility since already implemented and measurement tools simple |
| Rapid breathing | Minimally favorable | Normal range highly variable | Measurement tools inaccurate |
| Low sensitivity for hypoxemic children | Low feasibility with respect to sequential monitoring of respiratory rates over time | ||
| Performance profile improves with sequential monitoring of respiratory rates over time | |||
| Lower chest wall indrawing | Favorable | Low specificity for identifying children who will not fail treatment | Subjective sign with variable inter-provider agreement levels |
| Abnormal oxygen saturation | Highly favorable | High specificity for identifying children who will not fail treatment | Objective measurement but inter-provider agreement levels unknown |
| Later indicator decreases sensitivity for identifying children who will fail treatment at the community-level | Robust, precise, low-cost point-of-care instrument needed | ||
| Likely costly to implement and sustain | |||
| High blood lactate levels | Unfavorable | Low specificity for identifying children who will not fail treatment | Point-of-care tool appropriate for community use not available, likely costly |
| Very late indicator decreases sensitivity for identifying children who will fail treatment at the community-level | |||
| Moderate malnutrition | Highly favorable | Mid-range sensitivity and specificity which likely improves greatly in combination with other indicators | High feasibility since measurement tools simple and accurate |
| High positive predictive value | |||
| HIV-affected | Minimally favorable | Geographically limited in relevancy (e.g. primarily in eastern and southern Africa) | Low feasibility since difficult to obtain HIV status at community-level (e.g. stigma) |
| High positive predictive value |
HIV: Human Immunodeficiency Virus
The panel’s quantitative performance estimates for each prognostic indicator are presented in Table 4. Regardless of setting, children that are malnourished, HIV-affected or have an abnormal SpO2, were estimated to be at the highest mortality risk by the panel. Estimated median mortality rates of community treatment with amoxicillin for children with abnormal SpO2, malnutrition, or an HIV-affected status ranged from 15.5–20 %.
Table 4.
Expert panel quantitative assessments of potential prognostic indicators
| Identified prognostic indicatora | Mortality rate according to location of care received if prognostic indictor positive | |
|---|---|---|
| Community treatment with oral amoxicillin | Hospital treatment with parenteral antibiotics | |
| Fever | 0.5 % (0.5–1.0) | 0.1 % (0.1–0.2) |
| Rapid breathing | 1.0 % (1.0–2.0) | 0.2 % (0.1–0.5) |
| Lower chest indrawing | 5.8 % (5.1–7.4) | 1.0 % (0.6–1.0) |
| Abnormal SpO2 | 15.5 % (11.3–23.0) | 6.3 % (5.0–7.5) |
| Moderate malnutrition | 20.0 % (18.5–25.0) | 10.5 % (5.0–12.8) |
| HIV-infected or HIV-exposed status | 16.5 % (15.0–20.0) | 7.5 % (6.1–8.6) |
IQR: interquartile range; SpO 2: peripheral oxygen saturation
aLactate not assessed quantitatively
Discussion
WHO guidelines standardize pneumonia management in low-resource settings and stratify pneumonia severity according to the presence of severe clinical features [1, 15]. Currently, these guidelines do not further categorize non-severe pneumonia patients according to their treatment failure risk even though some children with non-severe disease have failure rates as high as 23 % [20]. Given the increased decentralization of pneumonia care to CHWs, improving baseline risk stratification with objective clinical measures may be an important next step for reducing pneumonia morbidity. To assist in algorithm development, we performed a systematic review and surveyed experts to identify predictors of oral antibiotic failure in children that fit the WHO case definition for non-severe pneumonia (2013 guidelines) [15]. We placed the greatest emphasis on predictors not currently incorporated into referral algorithms and those that were also measureable by CHWs. From the literature and expert review, we found some agreement for excess age-specific respiratory rate, abnormal baseline SpO2 and moderate malnutrition as important predictors of treatment failure. However, the literature was limited and highlighted several key evidence gaps, and there were notable differences between those predictors supported by the literature and those favored by the experts.
Our systematic review and expert survey yielded several interesting comparisons. The panel strongly favored abnormal SpO2 as a predictor, however, SpO2 was evaluated in only two studies [21, 25], none of which examined SpO2 at community clinics or assessed less severe, but still abnormal SpO2 ranges (90–94 %). Similarly, malnutrition was highlighted by the panel but was only found to be predictive for oral antibiotic failure in one study conducted at tertiary hospital clinics [26]. Milder degrees of malnutrition were not evaluated, and the experts defined malnutrition in the moderate range. Although young age was found to be predictive in multiple studies [20, 25–27], the experts did not recommend young age as a candidate predictor. Haemophilus influenzae type B (HiB) vaccine or pneumococcal conjugate vaccine (PCV) status were not examined as predictors in our systematic review since most studies were performed before introduction of these vaccines, but it was also not recommended by the panel. These differences between the published literature and expert panel may in part reflect the panel’s emphasis on personal experience to guide their answers and the overall lack of publications meeting eligibility criteria. In addition, a majority of the panel worked in Africa while in contrast the published literature was heavily represented by Asia.
The systematic review suggested that fast breathing and LCI pneumonia may be distinct groups with different drivers and predictors of failure. Our review of the literature consistently found excess age-specific respiratory rate as an important predictor in both LCI- and fast breathing non-severe pneumonia [20–22, 25–27], but abnormal baseline SpO2 and young age were clearer predictors of treatment failure in children solely with LCI [25–27]. In those studies looking at fast-breathing defined pneumonia, the independent predictors were less consistent and included persistent vomiting, diarrhea, wheeze, human respiratory syncytial virus infection and history of difficult breathing; these may suggest a primarily viral cause of illness. Other studies support the notion that a low proportion of fast breathing pneumonia cases may be due to bacterial pneumonia. Hazir and colleagues reported that only 1.7 % of Pakistani children with fast breathing pneumonia had a radiographic consolidation consistent with a bacterial etiology [28], and the same group also published findings of equivalency when fast breathing cases were treated with either placebo or amoxicillin [23]. At this time listening for wheeze with a stethoscope or testing for respiratory syncytial virus are not feasible for routine CHW care. The results from this systematic review should be interpreted within this context; LCI and fast breathing pneumonia may differ etiologically and the published literature on this subject has primarily focused on fast breathing pneumonia (only three studies meeting eligibility criteria for this work included LCI pneumonia).
Our systematic review found an overall paucity of community-level data, potentially limited by publication bias. However, we also found other important evidence gaps. Firstly, it is likely that predictors will differ between African and Asian populations, in part because HIV is endemic in southern Africa and a key driver of treatment unresponsive pneumonia [29]. However, our systematic review demonstrated that African data is limited, with only two studies including data from African countries. These studies did not explore region as a predictor of treatment failure, and did not report adjusting for region in their multivariate models. The experts also did not address this evidence gap since they did not explicitly factor regional differences into their responses, although the majority of experts worked in Africa and this is likely to have influenced their answers. The development of a sound prognostic algorithm for the southern African region that accounts for HIV status may be challenging since HIV stigma persists, particularly in more rural, intimate community settings where anonymous testing is difficult to achieve [30]. Second, it is likely that pneumonia epidemiology, and thus treatment failure predictors, will shift after HiB vaccine and PCV introduction. However, data from developing countries after full vaccine introduction is only more recently becoming available [31]. Due to these limitations in the literature, the findings from the literature review need to be interpreted cautiously.
Although validated prognostic models that identify higher risk pneumonia patients exist, only two studies (both secondary analyses from the Amoxicillin Penicillin Pneumonia International Study (APPIS) [25]) present predictive models for children who fulfill the LCI non-severe pneumonia case definition [32, 33]. Mamtani et al. validated a clinical tool for predicting oral amoxicillin failure in children with LCI pneumonia and no danger signs, using the child’s age and excess age-specific respiratory rate after 24 h of treatment to stratify patients’ risk [32]. Fu et al. predicted the risk of oral amoxicillin treatment failure after 48 h, with the best performing model including a second clinical evaluation after 12 h of treatment and SpO2 [33]. However both studies likely reflect different pneumonia epidemiology due to increasing HiB and PCV availability and current treatment failure definitions have been simplified [26, 27]. The feasibility of using models that rely on a single patient observation for an extended period or multiple clinical observations will need careful assessment at the CHW level.
At this time more evidence is needed to demonstrate effective use of pulse oximetry by CHWs, whether milder degrees of altitude-adjusted hypoxemia (90–94 %) are predictive of treatment failure, and if pulse oximetry can be cost-effective at the community level in low-resource countries where equipment maintenance can be challenging. Several low-cost respiratory rate counters, pulse oximeters and combination devices have recently been developed or are in development for low-resource settings [34–36]; there is an on-going evaluation of the clinical performance, usability, and acceptability of these devices currently underway by Malaria Consortium (D. Hay Burgess, personal communication).
Conclusion
In conclusion, we found that young age, excess age-specific respiratory rate, abnormal baseline SpO2 and moderate malnutrition should be carefully considered for an automated mHealth iCCM tool for predicting oral antibiotic failure in children with pneumonia. However, the evidence is currently limited. Additional formative research to identify antibiotic failure predictors is needed at the community-level, in HIV and non-HIV endemic regions, with and without malaria, and that have introduced the HiB vaccine and PCV. Modeling patient and health system-level consequences of a prognostic, along with a cost analysis of device scale-up, are important next steps.
Acknowledgements
We would like to thank Saul S. Morris for his contributions to this research. EDM was supported by the Fogarty International Center of the National Institutes of Health under Award Number K01TW009988 for the research reported in this publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- WHO
World Health Organization
- iCCM
integrated Community Case Management
- CHW
Community health worker
- mHealth
Mobile health
- HIV
Human immunodeficiency virus
- LCI
Lower chest indrawing
- SpO2
Peripheral oxygen saturation
- HiB
Haemophilus influenzae type B
- PCV
Pneumococcal conjugate vaccine
Additional files
ᅟ
ᅟ
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
Conceived and designed the experiments: EDM, CK, RH, JZ, DM, DCHB. Performed the literature review and expert review: EDM, RH, JZ, DCHB. Analyzed the data: EDM, CK, RH, JZ, TC, BN. Wrote first draft of paper: EDM. Edited paper for content: EDM, CK, RH, JZ, TC, BN, DM, DCHB. All authors read and approved the final manuscript. EDM is the guarantor of the paper.
Contributor Information
Eric D. McCollum, Phone: 410-955-2035, Email: Eric.D.McCollum@gmail.com
Carina King, Email: c.king@ucl.ac.uk.
Robert Hollowell, Email: Hollowell.Robert@bcg.com.
Janet Zhou, Email: Janet.Zhou@gatesfoundation.org.
Tim Colbourn, Email: t.colbourn@ucl.ac.uk.
Bejoy Nambiar, Email: b.nambiar@ucl.ac.uk.
David Mukanga, Email: David.Mukanga@gatesfoundation.org.
Deborah C. Hay Burgess, Email: Debbie.Burgess@gatesfoundation.org.
References
- 1.Handbook IMCI . Integrated management of childhood illness. World Health Organization. 2005. [Google Scholar]
- 2.Sazawal S, Black RE. Effect of pneumonia case management on mortality in neonates, infants, and preschool children: a meta-analysis of community-based trials. Lancet Infect Dis. 2003;3(9):547–56. doi: 10.1016/S1473-3099(03)00737-0. [DOI] [PubMed] [Google Scholar]
- 3.Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2014;385(9966):430–40. doi: 10.1016/S0140-6736(14)61698-6. [DOI] [PubMed] [Google Scholar]
- 4.Pneumonia: the forgotten killer of children. United Nations Children’s Fund. 2006. http://www.unicef.org/publications/files/Pneumonia_The_Forgotten_Killer_of_Children.pdf. Accessed 28 Aug 2014.
- 5.Management of pneumonia in community settings. United Nations Children’s Fund/World Health Organization. 2006. http://whqlibdoc.who.int/hq/2004/WHO_FCH_CAH_04.06.pdf?ua=1. Accessed 28 Aug 2014.
- 6.Integrated Community Case Management (iCCM). World Health Organization/United Nations Children’s Fund. 2012. http://www.unicef.org/health/files/iCCM_Joint_Statement_2012.pdf. Accessed 28 Aug 2014.
- 7.Yeboah-Antwi K, Pilingana P, Macleod WB, Semrau K, Siazeele K, Kalesha P, et al. Community case management of fever Due to malaria and pneumonia in children under five in Zambia: a cluster randomized controlled trial. PLoS Med. 2010;7(9):e1000340. doi: 10.1371/journal.pmed.1000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bari A, Sadruddin S, Khan A, Khan I, Khan A, Lehri IA, et al. Community case management of severe pneumonia with oral amoxicillin in children aged 2–59 months in Haripur district, Pakistan: a cluster randomised trial. Lancet. 2011;378(9805):1796–803. doi: 10.1016/S0140-6736(11)61140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soofi S, Ahmed S, Fox MP, MacLeod WB, Thea DM, Qazi SA, et al. Effectiveness of community case management of severe pneumonia with oral amoxicillin in children aged 2–59 months in Matiari district, rural Pakistan: a cluster-randomised controlled trial. Lancet. 2012;379(9817):729–37. doi: 10.1016/S0140-6736(11)61714-5. [DOI] [PubMed] [Google Scholar]
- 10.Kahigwa E, Schellenberg D, Schellenberg JA, Aponte JJ, Alonso PL, Menendez C. Inter-observer variation in the assessment of clinical signs in sick Tanzanian children. Trans R Soc Trop Med Hyg. 2002;96(2):162–6. doi: 10.1016/S0035-9203(02)90290-7. [DOI] [PubMed] [Google Scholar]
- 11.Mobile technologies and empowerment: enhancing human development through participation and innovation. United Nations Development Programme. 2012. http://www.undp.org/content/dam/undp/library/Democratic%20Governance/Access%20to%20Information%20and%20E-governance/Mobile%20Technologies%20and%20Empowerment_EN.pdf. Accessed 28 Aug 2014.
- 12.Mechael PN. The case for mHealth in developing countries. Innovations: Technology, Governance, Globalization. 2009;4:103–18. doi: 10.1162/itgg.2009.4.1.103. [DOI] [Google Scholar]
- 13.Kallander K, Tibenderana JK, Akpogheneta OJ, Strachan DL, Hill Z, ten Asbroek AH, et al. Mobile health (mHealth) approaches and lessons for increased performance and retention of community health workers in low- and middle-income countries: a review. J Med Internet Res. 2013;15(1):e17. doi: 10.2196/jmir.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pocket book of hospital care for children: guidelines for the management of common illnesses with limited resources. World Health Organization. 2013. http://apps.who.int/iris/bitstream/10665/81170/1/9789241548373_eng.pdf?ua=1. Accessed 28 Aug 2014. [PubMed]
- 16.Cochrane handbook for systematic reviews of interventions. The Cochrane Collaboration. 2011. http://handbook.cochrane.org. Accessed 3 June 2014.
- 17.Powell C. The Delphi technique: myths and realities. J Adv Nurs. 2003;41(4):376–82. doi: 10.1046/j.1365-2648.2003.02537.x. [DOI] [PubMed] [Google Scholar]
- 18.WHO recommendations on the diagnosis of HIV infection in infants and children. World Health Organization. 2010. http://whqlibdoc.who.int/publications/2010/9789241599085_eng.pdf?ua=1. Accessed 28 Aug 2014. [PubMed]
- 19.CATCH UP study group Clinical efficacy of co-trimoxazole versus amoxicillin twice daily for treatment of pneumonia: a randomised controlled clinical trial in Pakistan. Arch Dis Child. 2002;86(2):113–8. doi: 10.1136/adc.86.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MASCOT pneumonia study group Clinical efficacy of 3 days versus 5 days of oral amoxicillin for treatment of childhood pneumonia: a multicentre double-blind trial. Lancet. 2002;360(9336):835–41. doi: 10.1016/S0140-6736(02)09994-4. [DOI] [PubMed] [Google Scholar]
- 21.Agarwal G, Awasthi S, Kabra SK, Kaul A, Singhi S, Walter SD. Three day versus five day treatment with amoxicillin for non-severe pneumonia in young children: a multicentre randomised controlled trial. BMJ (Clin Res Ed) 2004;328(7443):791. doi: 10.1136/bmj.38049.490255.DE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noorani QA, Qazi SA, Rasmussen ZA, Rehman GN, Khan SS, Muhammadullah I, et al. Response to cotrimoxazole in the management of childhood pneumonia in first-level health care facilities. Int J Tuberc Lung Dis Off J Int Union Tuberc Lung Dis. 2006;10(8):932–8. [PubMed] [Google Scholar]
- 23.Hazir T, Nisar YB, Abbasi S, Ashraf YP, Khurshid J, Tariq P, et al. Comparison of oral amoxicillin with placebo for the treatment of world health organization-defined nonsevere pneumonia in children aged 2–59 months: a multicenter, double-blind, randomized, placebo-controlled trial in pakistan. Clin Infect Dis Off Publ Infect Dis Soc Am. 2011;52(3):293–300. doi: 10.1093/cid/ciq142. [DOI] [PubMed] [Google Scholar]
- 24.Awasthi S, Agarwal G, Singh JV, Kabra SK, Pillai RM, Singhi S, et al. Effectiveness of 3-day amoxycillin vs. 5-day co-trimoxazole in the treatment of non-severe pneumonia in children aged 2–59 months of age: a multi-centric open labeled trial. J Trop Pediatr. 2008;54(6):382–9. doi: 10.1093/tropej/fmn050. [DOI] [PubMed] [Google Scholar]
- 25.Addo-Yobo E, Chisaka N, Hassan M, Hibberd P, Lozano JM, Jeena P, et al. Oral amoxicillin versus injectable penicillin for severe pneumonia in children aged 3 to 59 months: a randomised multicentre equivalency study. Lancet. 2004;364(9440):1141–8. doi: 10.1016/S0140-6736(04)17100-6. [DOI] [PubMed] [Google Scholar]
- 26.Hazir T, Fox LM, Nisar YB, Fox MP, Ashraf YP, MacLeod WB, et al. Ambulatory short-course high-dose oral amoxicillin for treatment of severe pneumonia in children: a randomised equivalency trial. Lancet. 2008;371(9606):49–56. doi: 10.1016/S0140-6736(08)60071-9. [DOI] [PubMed] [Google Scholar]
- 27.Addo-Yobo E, Anh DD, El-Sayed HF, Fox LM, Fox MP, MacLeod W, et al. Outpatient treatment of children with severe pneumonia with oral amoxicillin in four countries: the MASS study. Trop Med Int Health. 2011;16(8):995–1006. doi: 10.1111/j.1365-3156.2011.02787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hazir T, Nisar YB, Qazi SA, Khan SF, Raza M, Zameer S, et al. Chest radiography in children aged 2–59 months diagnosed with non-severe pneumonia as defined by World Health Organization: descriptive multicentre study in Pakistan. BMJ (Clin Res Ed) 2006;333(7569):629. doi: 10.1136/bmj.38915.673322.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McNally LM, Jeena PM, Gajee K, Thula SA, Sturm AW, Cassol S, et al. Effect of age, polymicrobial disease, and maternal HIV status on treatment response and cause of severe pneumonia in South African children: a prospective descriptive study. Lancet. 2007;369(9571):1440–51. doi: 10.1016/S0140-6736(07)60670-9. [DOI] [PubMed] [Google Scholar]
- 30.Goodson JL, Finkbeiner T, Davis NL, Lyimo D, Rwebembera A, Swartzendruber AL, et al. Evaluation of using routine infant immunization visits to identify and follow-up HIV-exposed infants and their mothers in Tanzania. J Acquir Immune Defic Syndr (1999) 2013;63(1):e9–15. doi: 10.1097/QAI.0b013e31828a3e3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levine OS, O’Brien KL, Deloria-Knoll M, Murdoch DR, Feikin DR, DeLuca AN, et al. The pneumonia etiology research for child health project: a 21st century childhood pneumonia etiology study. Clin Infect Dis Off Publ Infect Dis Soc Am. 2012;54(Suppl 2):S93–101. doi: 10.1093/cid/cir1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mamtani M, Patel A, Hibberd PL, Tuan TA, Jeena P, Chisaka N, et al. A clinical tool to predict failed response to therapy in children with severe pneumonia. Pediatr Pulmonol. 2009;44(4):379–86. doi: 10.1002/ppul.21014. [DOI] [PubMed] [Google Scholar]
- 33.Fu LY, Ruthazer R, Wilson I, Patel A, Fox LM, Tuan TA, et al. Brief hospitalization and pulse oximetry for predicting amoxicillin treatment failure in children with severe pneumonia. Pediatrics. 2006;118(6):e1822–30. doi: 10.1542/peds.2005-2673. [DOI] [PubMed] [Google Scholar]
- 34.Hudson J, Nguku SM, Sleiman J, Karlen W, Dumont GA, Petersen CL, et al. Usability testing of a prototype phone oximeter with healthcare providers in high- and low-medical resource environments. Anaesthesia. 2012;67(9):957–67. doi: 10.1111/j.1365-2044.2012.07196.x. [DOI] [PubMed] [Google Scholar]
- 35.Karlen W, Gan H, Chiu M, Dunsmuir D, Zhou G, Dumont GA, et al. Improving the accuracy and efficiency of respiratory rate measurements in children using mobile devices. PLoS One. 2014;9(6):e99266. doi: 10.1371/journal.pone.0099266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petersen CL, Chen TP, Ansermino JM, Dumont GA. Design and evaluation of a low-cost smartphone pulse oximeter. Sensors (Basel, Switzerland) 2013;13(12):16882–93. doi: 10.3390/s131216882. [DOI] [PMC free article] [PubMed] [Google Scholar]


