Abstract
The genetic basis of many muscular disorders, including many of the more common muscular dystrophies, is now known. Clinically, the recent genetic advances have improved diagnostic capabilities, but they have not yet provided clues about treatment or management. Thanks to better management strategies and therapeutic interventions, however, many patients with a muscular dystrophy are more active and are living longer. Physical therapists, therefore, are more likely to see a patient with a muscular dystrophy, so understanding these muscle disorders and their management is essential. Physical therapy offers the most promise in caring for the majority of patients with these conditions, because it is unlikely that advances in gene therapy will significantly alter their clinical treatment in the near future. This perspective covers some of the basic molecular biological advances together with the clinical manifestations of the muscular dystrophies and the latest approaches to their management.
Keywords: Muscular dystrophy, Skeletal muscle
The muscular dystrophies (MDs) are a heterogeneous group of inherited disorders characterized by progressive weakness and degeneration of skeletal muscles (Table). They have traditionally been classified by clinical presentation, mode of inheritance, age of onset, and overall progression. The development of molecular genetic mapping techniques has shown that a number of clinically similar conditions are linked to a variety of distinct single-gene disorders. So far, MDs have been mapped to at least 29 different genetic loci that give rise to at least 34 different clinical disorders,1 and additional information is accumulating rapidly.*
Table.
List of Muscular Dystrophiesa
| Disease | Protein Missing/ Deficient |
Age of Onset | Muscles Affected | Complications |
|---|---|---|---|---|
| Duchenne (DMD) | Dystrophin | Early childhood | Muscles of the hips, legs, shoulders, and spine and the heart |
Severe muscle weakness and wasting, scoliosis, contractures, respiratory failure, pneumonia, and dilated cardiomyopathy. Death early 20s. |
| Becker (BMD) | Dystrophin | Adolescence or adulthood | Similar to DMD | Muscle weakness as in DMD, but slower progress and much less severe. Cardiomyopathy. |
| Limb-girdle (LGMD) | ||||
| LGMD1A | Myotilin | Adolescence to early adulthood |
Proximal shoulder/pelvic girdle musculature |
Walking may not be possible within 20 years of onset |
| LGMD1B | Lamin | |||
| LGMD1C | Caveolin-3 | |||
| LGMD1D | Not identified | |||
| LGMD1E | Not identified | |||
| LGMD1F | Not identified | |||
| LGMD2A | Calpain-3 | Infancy to early adulthood |
Proximal shoulder/pelvic girdle musculature |
Type 2 LGMD is much more severe than type 1 LGMD and some result in a DMD-like phenotype. Cardiac complications, sometimes occurring in later stages. |
| LGMD2B | Dysferlin | |||
| LGMD2C | γ-sarcoglycan | |||
| LGMD2D | α-sarcoglycan | |||
| LGMD2E | β-sarcoglycan | |||
| LGMD2F | δ-sarcoglycan | |||
| LGMD2G | TCAP | |||
| LGMD2H | TRIM32 | |||
| LGMD21 | FKRP | |||
| LGMD2J | Titin | |||
| Distal muscular dystrophies | ||||
| Miyoshi myopathy | Dysferlin | Late adolescence |
Posterior compartment of legs |
Can eventually affect anterior compartment and distal arm muscles. Slow progression and able to maintain independent ambulation throughout life. |
| Tibial muscular dystrophy | Titin | Late adulthood | Anterior compartment of legs |
Slowly progressive. Eventually can affect upper extremities and heart. |
| Welander myopathy | Unknown | Fifth decade | Distal muscle of upper limbs |
Slowly progressive. Eventually affects lower limbs. |
| Nonakel myopathy (also known as distal myopathy with rimmed vacuoles) and inclusion body myopathy |
Acetylglucosamine epimerase |
Late adolescence |
Anterior compartment of legs |
Display red-rimmed autophagocytic vacuoles. Weakness can progress to proximal muscles, but quadriceps femoris muscles are spared. |
| Laing myopathy | Unknown | Ranges from infancy to adulthood |
Anterior compartment of legs and neck flexors |
Weakness can progress to proximal muscles |
| Myofibrillary/desmin-related myopathy |
Desmin | Initially distal lower extremities, but eventually proximally |
Aggregation of desmin and other proteins inclusion bodies. Slow progress, eventual cardiomyopathy. |
|
| Congenital muscular dystrophies (CMD) |
||||
| MDC1A | Laminin α2 (merosin) | At birth | Proximal limb muscles | Joint contractures, cognitive and speech problems, seizures. Most do not learn to walk. White matter changes and structural abnormalities on magnetic resonance image. |
| MDC1B | Not identified | At birth | ||
| MDC1C | FKRP | At birth | ||
| MDC1D | LARGE | At birth | ||
| Fukuyama CMD | Fukutin | At birth | Proximal upper limbs, distal lower limbs, face and neck |
Cortex malformation and brainstem hypoplasia. Primarily in Japanese population. |
| α7 integrin congenital myopathy |
α7 integrin | At birth | Mild myopathy | Generalized weakness |
| Rigid spine CMD | Selenoprotein N1 | At birth | Contractures of spinal extensors |
Spine rigidity, early restrictive lung disease |
| Muscle-eye-brain disease | POMGnT1 glycotransferase |
At birth | Generalized and mild | Myopia, cataracts, optic nerve atrophy, mental retardation, delayed motor milestones |
| Walker-Warburg syndrome | POMT1 | At birth | Generalized | Retinal abnormality, myopia, cataracts, optic nerve atrophy, seizures |
| Bethlem myopathy/Ullrich syndrome |
Type VI collagen | At birth | Proximal limb muscles | Early contractures, flat feet. Rare and mild. |
| Other types | ||||
| Emery-Dreifuss (EDMD) | Emerin, lamin | Childhood to early teens |
Proximal upper extremity and distal lower extremities |
Early contractures, cardiomyopathy |
| Epidermolysis bullosa | Plectin | Childhood | Generalized | Skin blistering with range of severity, joint contractures, dysphagia |
| Oculopharyngeal (OPMD) | Poly-A-binding protein 2 |
Age 40–60 y | Eyelids, throat | Ptosis of eyelids, dysphagia, aspiration pneumonia |
| Facioscapulohumeral (FSHD) | Not identified | Childhood to early adolescence |
Face, shoulders, proximal upper extremities |
Cardiac conduction defects, mild hearing loss, retinal abnormalities |
| Myotonic (DM) | Myotinin protein kinase, ZNF9 |
Infancy (more severe) to adulthood |
Distal extremity muscles first, then proximal as well |
Myotonia, cataracts, hypogonadism, cardiac arrythmias. Adult form is mild compared to early onset. |
TCAP=telethonin, a protein that interacts with, or “caps,” another protein in muscle called titin. TRIM32 = one of 37 TRIM proteins containing a tripartite motif (TRIM). FKRP=fukutin-related protein. LARGE = the LARGE gene was so named because it covers over 660 kb of genomic DNA; the protein it encodes is a putative glycosyltransferase. POMT1=protein-O-mannosyltransferase 1. POMGnT1=protein O-linked mannose β-1,2-N-acetylglucosaminyltransferase. ZNF9=zinc finger protein 9.
Since the cloning of the dystrophin gene in the 1980s,2,3 the identification of its protein product, dystrophin,4 the complex it forms in muscle,5 and the mapping of mutations linking several MDs to dystrophin and its associated proteins, we now know a great deal about the genetic basis of these diseases. In many instances, new diagnostic tests have eliminated the need to perform muscle biopsies and, in some cases, even electromyography. Although the molecular advances have greatly improved diagnostic capabilities, they have not greatly altered clinical practice. Thanks to better management strategies and therapeutic interventions, however, many patients with MDs are more active and are living longer.6 Physical therapists, therefore, are more likely to see patients with MDs, so understanding these muscle disorders and their management is essential. Because there is no cure for any of the MDs, physical therapy, in our opinion, offers the most promise to promote a longer and more active life for the majority of patients with these conditions. In this perspective, we review the more common muscular dystrophies7 and discuss new strategies for treating people with these disorders. Although our focus is on skeletal muscle, the expression of affected genes in other organ systems also may result in cognitive, cardiac, or other clinical disturbances.
One of the goals of MD research is to understand how sarcolemmal damage is initiated, how it is repaired, and how the sarcolemma can be protected (or the damage minimized) by pharmacologic or therapeutic interventions. Researchers studying muscle injuries share these same goals. In skeletal muscle injuries, particularly those resulting from lengthening (“eccentric”) contractions, the membrane is damaged and the cytoskeleton is disrupted.8 The initial injury is followed by pain, inflammation, weakness, and often necrosis, which are then followed by regeneration of muscle fibers. Because these findings parallel those in Duchenne muscular dystrophy (DMD), the study of muscle injury—its mechanisms, management, and repair—likely will provide important insights into the mechanisms underlying many of the MDs.
Physical therapists are ideally trained to help care for patients with MD, especially because of the primary involvement of skeletal muscle and the secondary effects of the disease on the joints.
Disorders Associated With Dystrophin
Duchenne Muscular Dystrophy
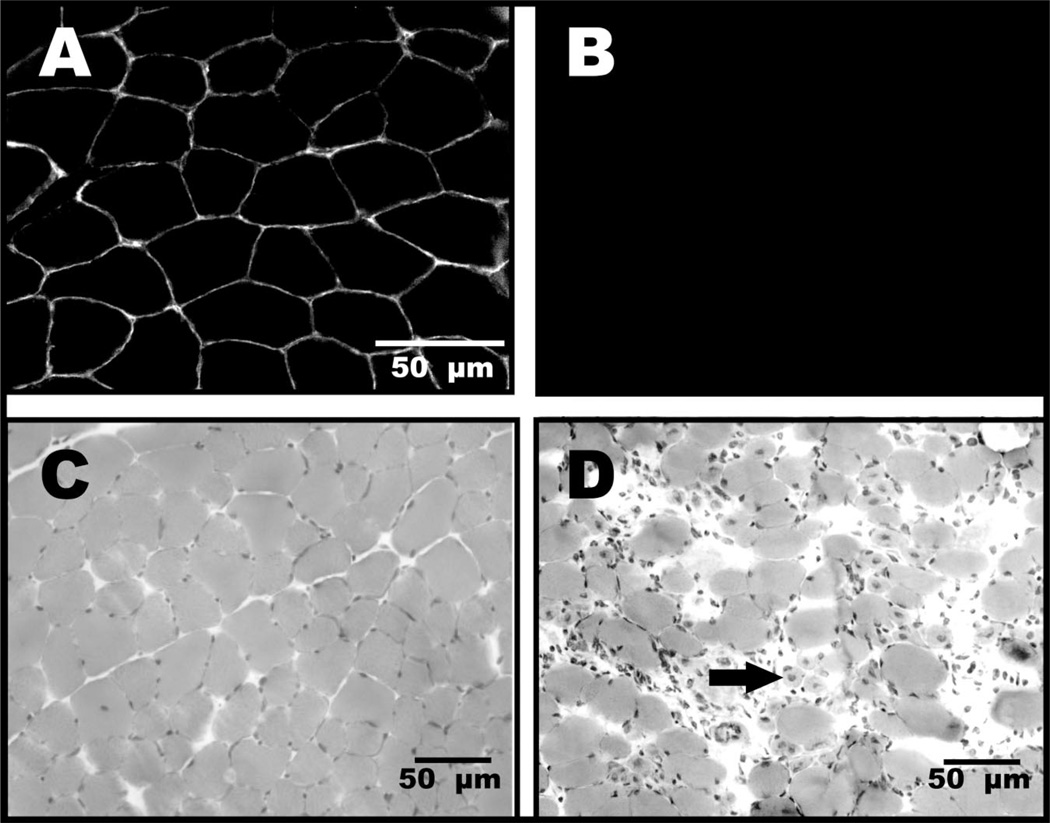
Duchenne muscular dystrophy, the most common form of MD, is an X-linked disorder (ie, associated with a gene on the X chromosome) that was first described over a century ago.9 Duchenne muscular dystrophy is characterized by progressive wasting of skeletal muscles, with the limb-girdle muscles first showing weakness by the age of 5 years, followed by an inability to walk by the ages of 8 to 12 years.10,11 Other findings include elevated creatine kinase levels, pseudohypertrophic calf muscles, decreased levels of activity, and, in some patients, cognitive impairment. At the cellular level, pathological changes include the absence of dystrophin at the membrane of the muscle fibers, increased adipose and connective tissue between muscle fibers, increased variability in muscle fiber size, infiltration of inflammatory cells, and centrally located nuclei, which are indicative of degenerating and regenerating muscle fibers (Fig. 1).
Figure 1.
Immunofluorescent images of control muscle (A) and dystrophic (mdx) muscle (B) from mice illustrate the presence of dystrophin at the sarcolemmal membranes of normal muscle and its loss from the sarcolemmal membranes in dystrophic samples (muscle sections were processed together and labeled for dystrophin, but because mdx muscle lacks dystrophin, the field remains completely dark). Hematoxylin and eosin staining of control (C) and dystrophic muscle (D) from mice show abnormal variance in fiber size, fibrosis, inflammatory cell infiltration, and degenerating and regenerating muscle fibers that are typically of abnormal size and contain centrally located nuclei (arrow). Although they are not as severely affected as humans with Duchenne muscular dystrophy, dystrophic (mdx mice) serve as a model for that disease, which also is due to a lack of dystrophin.
There is currently no explanation for the different rates of disease progression in different muscle groups. Parents typically do not seek medical care early on, because children with DMD look “normal” for the first few years of life. Between the ages of 2 and 5 years, they begin to show signs of clumsiness, falling, and gait changes, as well as difficulty ascending stairs.12 By age 6 years, the child often develops contractures of the calf muscles and an exaggerated lordosis of the spine. By this time, the child has a positive Gowers’ sign, and the loss of strength (the ability of a muscle to produce force) progresses throughout the upper body and lower body.13 The scoliosis often becomes severe, producing secondary pulmonary complications and requiring surgical fusion to stop its progress.7,14 Death usually occurs in the second or third decade of life due to cardiac or respiratory impairment.15
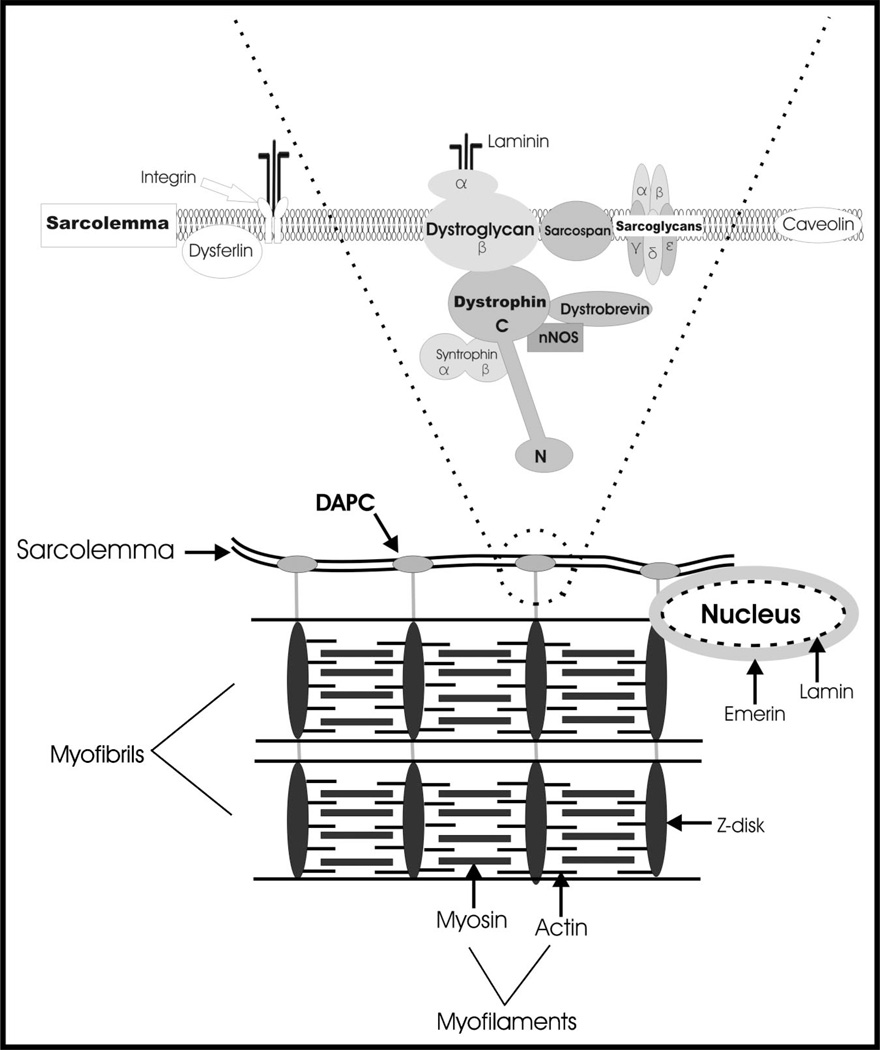
Duchenne muscular dystrophy is caused by the absence of dystrophin, a 427 kDa protein found on the cytoplasmic surface of the plasma membrane of muscle fibers (the sarcolemma) in skeletal and cardiac muscle (Fig. 2). Dystrophin provides mechanical stability to the sarcolemma and is likely involved in force transmission between the intracellular contractile apparatus and the extracellular matrix (ECM), which envelops the fiber and is connected to the tendon.16 Without dystrophin, the sarcolemma becomes fragile and unable to withstand the stress of normal muscle contractions.16 The resulting membrane damage leads to increased intracellular Ca2+, which activates proteases that ultimately result in fiber death or necrosis. The regeneration of myofibers that normally occurs after damage to healthy skeletal muscle, which also occurs in the first few years of life in patients with DMD, does not persist as these patients mature. Instead, regenerative capacity becomes insufficient to replace lost muscle fibers.17–19 Necrotic fibers become replaced by fat and connective tissue, to such an extent that there can be an apparent “pseudohypertrophy,” especially in the calves.
Figure 2.
Schematic model showing the molecular linkages of the sarcolemma via the dystrophin-associated protein complex (DAPC) to the underlying contractile apparatus. Cytoskeletal components that are affected in the more common muscular dystrophies are indicated.
In addition to its mechanical role in stabilizing the sarcolemma, dystrophin probably has a role in signal transduction (eg, sensing mechanical perturbations such as sarcolemmal stress and converting this signal into a biochemical response such as alterations in phosphorylation and changes in the levels of expression of certain proteins). Although dystrophin itself is not a signaling molecule, it anchors signaling proteins, such as neuronal nitric oxide synthase (nNOS), Grb2, and others, to the sarcolemma.20,21 Neuronal nitric oxide synthase is normally localized at the sarcolemma via one of the dystrophin-associated proteins, but it is absent from the sarcolemma in animals lacking dystrophin and in patients with DMD.22
The majority of patients with DMD have unimpaired intelligence, but some have mild intellectual impairments. In 30% of patients with DMD, the mean intelligence quotient is 18 points below normal,23 and these patients may have trouble with attention, verbal learning, and memory.13 The pathological basis of these mild cognitive impairments is likely changes in isoforms of dystrophin expressed in the brain.24 The impairments are likely due to the biological effect of missing dystrophin rather than loss of mobility and a disadvantaged lifestyle, as shown by a comparison with age-matched patients with spinal muscular atrophy.25
Approximately 1 in 3,500 newborn males worldwide are affected with DMD.7,13,14 The gene for dystrophin is one of the largest known in humans (approximately 1.2 million base pairs) and is composed of 79 exons.4 The sheer size and complexity of the dystrophin gene may account for the high frequency of mutations, with approximately one third of DMD cases arising from new, spontaneous mutations.7,26 The remaining cases are inherited in an X-linked recessive fashion. Females who inherit the mutation do not develop DMD, because they also inherit a second, “healthy” X chromosome from their fathers. Consequently, female carriers are usually unaffected, unless there is an abnormality of X chromosome inactivation or a chromosomal anomaly, both of which are extremely rare.6 Thus, essentially only boys acquire DMD.
Becker Muscular Dystrophy
Becker muscular dystrophy (BMD) is an allelic variant of DMD. Whereas DMD is caused by the essential absence of dystrophin, BMD is caused by abnormalities in the quality or quantity of dystrophin. In general, the greater the amount of dystrophin, the less severe the myopathy.27 The onset of BMD is usually between the ages of 5 and 15 years, but can occur as late as the fourth decade of life. The phenotypic presentation of BMD is similar to that of DMD, but is clinically milder and with more variability and a much slower progression. Patients with BMD do not have contractures or severe scoliosis, and many live well into adulthood, sometimes to a normal life span.
The Dystrophin Complex and Its Organization
Dystrophin binds directly or indirectly to a group of proteins at the sarcolemma, collectively known as the dystrophin-associated protein complex (DAPC or DPC) (Fig. 2) or the dystrophin-glycoprotein complex (DGC).5,28,29 Dystrophin binds to this complex through the interaction of its WW domain (a protein-binding module composed of 35 to 40 amino acids that include 2 conserved moieties of tryptophan,30 with the W representing the single letter code for that amino acid) and nearby sequences with the C-terminal cytoplasmic sequence of β-dystroglycan,31 a transmembrane protein.32 The N-terminal and extracellular portion of β-dystroglycan associates with α-dystroglycan, which in turn connects the DAPC to the ECM.32 The major ligand of α-dystroglycan in the ECM is likely laminin-2, a major component of the basement membrane surrounding muscle fibers. Neurexin and agrin, however, also may be significant binding partners.33 Additional components of the DAPC include several sarcoglycans (see below), which also span the sarcolemmal membrane, and peripheral membrane proteins, including syntrophins and dystrobrevins, that bind to the C-terminal region of dystrophin. Additional proteins of the DAPC are still being discovered.34,35
Inside the muscle fiber, the N-terminus of dystrophin binds to F-actin,36 connecting the DAPC to the actin cytoskeleton and ultimately to the contractile apparatus. Thus, dystrophin is the central component of a molecular link that connects the contractile apparatus inside the muscle fiber to the ECM outside the muscle fiber. Dystrophin plays an important structural role in stabilizing the sarcolemma during muscle contractions,16 and it is thought to transmit force laterally across the sarcolemma to the ECM.37,38
Although loss of dystrophin results in DMD, no naturally occurring mutations have been described for the dystroglycan gene, which transcribes both the α and β components. Targeted deletion of this gene in animals results in embryonic death39 rather than progressive muscular weakness. Recently, however, mutations that result in altered glycosylation of α-dystroglycan have been reported to be linked to both murine40 and human MDs,41,42 including muscle-eye-brain disease, Walker-Warburg syndrome (WWS), and a type of congenital MD (MDC1D).42 These mutations alter the ability of α-dystroglycan to bind to its extracellular ligands, including laminin-2. Thus, the structural connection of the ECM with the intracellular actin cytoskeleton appears to be dependent on the state of the DAPC.
Sarcospan is a 25 kD protein in the DAPC that is firmly anchored to the sarcolemmal membrane.43 The amount of sarcospan is dramatically reduced in DMD muscle compared with normal muscle,43 but surprisingly, animals genetically engineered to lack sarcospan maintain normal function.44 Sarcospan is tightly linked to the sarcoglycans. The α-, β-, γ-, δ, possibly ε-sarcoglycans are integral membrane glycoproteins that associate with β-dystroglycan. Loss-of-function mutations in the genes encoding for the sarcoglycans result in various types of limb-girdle muscular dystrophy (LGMD). Because the loss of one sarcoglycan can affect the stability of all the other sarcoglycans,45 these proteins, like dystrophin, play an important role in stabilizing the DAPC.
Several proteins bind to dystrophin on the inner surface of sarcolemma, including syntrophin and dystrobrevin. There are 3 related syntrophins (α-, β1-, and β2-syntrophin) and 2 dystrobrevins (α- and β-dystrobrevin). These proteins help to bring together other components of the DAPC in a variety of combinations.46 Syntrophin has been shown to bind nNOS47 and is linked to signaling within the cell,48 although the relevance of this is not clear. Mutations in the genes that encode these proteins have not yet been identified in humans, except for a mutation in α-dystrobrevin that has been described in left ventricular noncompaction, an uncommon genetic disorder of endocardial morphogenesis with a reportedly high mortality rate.49 Mice that have been genetically engineered (so-called “knock out” mice) to lack syntrophin and dystrobrevin, in an attempt to determine their functions,50,51 display only a mild skeletal and cardiac muscle disease.
It is quite clear that dystrophin and its associated proteins that make up the DAPC are important in membrane function, but other membrane proteins also seem to have a role. Integrins are membrane proteins that are important in cell signaling and that bind to laminin in the ECM. Mutation of the most common form of integrins in muscle, α7β1, results in a form of congenital MD in humans and animals,52 implying that they have a role in membrane stability.
Most MDs are associated with missing or truncated structural proteins at the sarcolemma, such as those discussed previously, or mutations in other sarcolemmal components (eg, caveolin-3, dysferlin), extracellular proteins (eg, laminin), and proteins of the nuclear membrane (eg, emerin and lamin) (Fig. 2). However, even disruption of enzymes can give rise to MDs. Calpain is one of the Ca2+-activated enzymes thought to be involved in breakdown of the myofiber after disruption of the DAPC and consequent sarcolemmal damage,53,54 yet the absence of calpain itself results in MD. Dysferlin is another protein considered to be unrelated to sarcolemmal structural stability,55 yet its absence also results in MD. The sarcolemma of dysferlin “knock out” animals (animals that lack dysferlin as a result of homologous recombination) does not have increased susceptibility to mechanical force, such as in DMD.55 Instead, dysferlin seems to be important in the repair of membranes.56 These examples indicate that MD can result from the loss of structural proteins, loss of enzymatic proteins, or loss of another class of proteins involved in membrane repair. Findings such as these illustrate the complex pathways that are involved in maintaining the health of normal muscle and that, when altered, can result in MD.
Other Dystrophies
Limb-Girdle Muscular Dystrophies
The LGMDs are genotypically and phenotypically heterogeneous. As their name implies, these mypopathies are characterized by weakness of the proximal muscles in the upper and lower extremities. Onset can occur in childhood and the clinical presentation can mimic DMD, but onset more often occurs in late adolescence or early adulthood. Some of the most severe forms of LGMD present at birth, falling into the category of congenital muscular dystrophy (CMD). The heart is usually not affected, but patients with LGMD should be screened routinely because some will develop cardiomyopathy.
Limb-girdle muscular dystrophies can either be autosomal dominant (single gene defect on a chromosome from either parent or one copy of a mutant gene and one normal gene, known as type 1 LGMD) or autosomal recessive (a defect or mutation on the gene from the chromosome of each parent is needed, known as type 2 LGMD). The type 2 LGMDs are more severe, with some resembling DMD in severity. Sixteen genetically different LGMDs have been identified, with a correspondingly wide range of phenotypes. The various types of LGMDs are listed in the Table. It should be noted that a small percentage of patients diagnosed with LGMD actually have mutations in the gene for dystrophin, although they do not lack the protein as in DMD and BMD, which also are characterized by proximal weakness. Therefore, careful genetic screening is indicated, and a muscle biopsy may be needed to confirm the diagnosis.
The majority of LGMDs are autosomal recessive. Patients exhibit a variable severity of muscle disease, usually involving scapular winging and weakness of proximal limb and trunk muscles.7 The most common LGMD, LGMD2A, is the result of a mutation in the gene for calpain-3, a muscle-specific enzyme (discussed earlier). Clinical findings include limb-girdle atrophy and weakness that begins in the gluteal and hip adductor muscles,57 with highly variable patterns of progression. Onset also is variable and can occur at any time from 2 years of age to middle age.58 Another type 2 LGMD, LGMD2B, is the result of deficiency or absence of dysferlin, a protein involved with trafficking of vesicles to the sarcolemma for repair after membrane damage.55 Patients with LGMD2B display normal mobility in childhood, but eventually develop slowly progressive muscle weakness without signs of inflammation.59 It is not clear whether the amount of dysferlin loss correlates to the phenotypic severity.59,60
Except for LGMD2A and LGMD2B, the other LGMDs are caused by deficiencies in specific structural proteins. Four of the type 2 LGMDs are due to the loss of sarcoglycans. There are 5 known sarcoglycans (α-, β-, γ-, δ, and possibly ε) (Fig. 1) at the muscle fiber membrane that form part of the DAPC, and genetic defects affecting 4 of these proteins cause specific type 2 LGMDs, also known as “sarcoglycanopathies” (Table). A deficiency in one of the sarcoglycans (ε) has not been associated with any primary muscle disease so far.61,62 In general, proximal muscles of the lower extremities are affected early in sarcoglycanopathies, followed by gradual weakness of the shoulder girdle muscles with consequent scapular winging. There is considerable heterogeneity among the sarcoglycanopathies in the patterns of muscles affected and the rate of progression, compared with dystrophinopathies.63
Facioscapulohumeral Muscular Dystrophy
After DMD and LGMDs, facioscapulohumeral muscular dystrophy (FSHD) is the third most common inherited muscle disease, affecting approximately 1 in 20,000 people in the United States. It is an autosomal dominant disorder with a variable age of onset, but it usually is first detected in early adolescence. As indicated by its name, FSHD is characterized by weakness in muscles of the face and proximal upper extremity, including those muscles that stabilize the scapula. This muscle weakness results in winging and anterior tilting of the scapula. Although extraocular muscles are not affected, weakness in muscles around the eye (ie, obicularis oculi, a facial muscle) may be evident when patients sleep with their eyes slightly open, a symptom that may manifest itself before other symptoms develop. There is also difficulty in whistling or blowing (eg, blowing out candles). Distal extremity muscles also can be affected, and the weakness is often asymmetric. There is a wide range of clinical severity overall, with symptoms that can include cardiac, cognitive, visual, and auditory impairments.64–66 Progression of FSHD usually is slow, however, and these patients have a near-normal life expectancy.
Clinical diagnosis of FSHD is based initially on the pattern of muscle involvement, but genetic tests, which can detect FSHD with a 98% success rate, are now preferred. Although the specific gene responsible for FSHD has not been identified, FSHD is associated with a deletion of 3.3 kb repeats (called D4Z4) mapped to the telomeric end of the long arm of chromosome 4.67,68 This region, termed 4q35, normally has 11 to 150 D4Z4 repeats, but in patients with FSHD, the number of repeats is less than 11. Although it was thought until recently that the severity of clinical presentation increased as the number of D4Z4 repeats under 11 decreased,69 this has recently been challenged.70 The telomeric D4Z4 repeats have most recently been thought to affect anchoring of chromosome 4 to the inner nuclear membrane.71 If true, FSHD would resemble other MDs, such as Emery-Dreifuss muscular dystrophy (EDMD), that are nuclear in origin.72,73
Emery-Dreifuss Muscular Dystrophy
Emery-Dreifuss muscular dystrophy can have 2 different genetic forms: X-linked or autosomal dominant. Although both genetic forms have the same phenotype, the X-linked form is more common. Emery-Dreifuss muscular dystrophy affects proteins in the inner nuclear membrane of muscle fibers (and other tissues), specifically lamin, a nuclear form of intermediate filament protein (70 kD) that lines the nucleoplasmic surface of the inner membrane, and emerin, an integral protein (34 kD) of the inner nuclear membrane that is thought to anchor lamins74 (Fig. 2). It is not clear how defects in these nuclear membrane proteins result in the phenotype. It has been suggested that these mutated proteins increase the susceptibility of the nucleus to mechanical stress or alter gene expression.73
Emery-Dreifuss muscular dystrophy presents clinically with the triad of early contractures, muscle weakness, and cardiac conduction defects.72 Weakness occurs in the shoulder girdle and distal lower extremities (“humeroperoneal” weakness) and usually starts in childhood, although symptoms can begin at any time between the neonatal period and the third decade. Contractures are usually out of proportion to the weakness and affect many joints, especially the elbows, followed by the ankles and cervical spine. Although patients with EDMD are not wheelchair-bound, contractures are a major cause of morbidity, creating more functional impairment than the weakness. The major cause of mortality is cardiac disease, which often results in premature and sudden death.75
Oculopharyngeal Muscular Dystrophy
Oculopharyngeal muscular dystrophy (OPMD) is an autosomal dominant disorder that is characterized by progressive eyelid ptosis and progressive dysphagia, followed by involvement of other muscles of the head and neck, and eventually proximal limb weakness.15 The extraocular muscles are usually spared, although not always.76 Onset occurs in late adulthood, and a clinical test that is sometimes used is timed swallowing, which is 2 times slower than normal in people with OPMD.77
Oculopharyngeal muscular dystrophy is caused by an abnormal number of GCG trinucleotide repeats in the PABPN1 gene that encodes the polyadenylate binding protein, nuclear 1 protein. This trinucleotide expansion in the PABPN1 gene interferes with a nuclear protein involved in gene transciption. The PABPN1 gene was previously known as the PAB2 or PABP2 gene (polyadenylation binding protein 2), the OPMD gene, or the nuclear 1 gene, and these names are still encountered in the literature.78 Interestingly, OPMD can be inherited in an autosomal dominant or autosomal recessive fashion.77 Testing for the disorder is now commercially available.79
Myotonic Dystrophy
Myotonic dystrophies are the most common form of MD in adults. Myotonic dystrophies are now recognized as genetically heterogeneous diseases, caused by 2 distinct mutations. Myotonic dystrophy type 1 (DM1) is caused by an expansion of a CTG trinucleotide repeat in a gene for an enzyme (the DMPK gene that encodes the enzyme dystrophia myotonica protein kinase, a serine/threonine kinase).80,81 The CTG expansion is in an untranslated region of the gene, so that the exact pathogenesis is still uncertain (the most promising theory posits that the mutation leads to abnormal processing and splicing of certain species of RNA). The number of repeats tends to increase from one generation to the next, which results in an earlier age of onset for subsequent generations (called “anticipation”) as well as an increase in severity. The features of classic myotonic dystrophy include myotonia (skeletal muscle hyperexcitability and slowed relaxation time), slowly progressive muscle weakness, cardiac conduction defects, pain, peripheral neuropathy, frontal balding, temporal wasting, cataracts, and endocrine disturbances such as diabetes. Myotonic dystrophy type 1 may present in infancy (CMD) or childhood, but the disorder typically presents in young adults, with a prevalence estimated to be as high as 1/8,000.7,82
Myotonic dystrophy type 2 (DM2) is caused by an expansion of a CCTG repeat in intron 1 of the gene ZNF9, which encodes zinc finger protein 9 on chromosome 3q.83–85 Both DM1 and DM2 are inherited in an autosomal dominant fashion, and both affect multiple organ systems. Although the 2 types can be clinically indistinguishable in some cases, DM2 is more likely to be associated with more proximal weakness (proximal myotonic myopathy [PROMM]) as opposed to the predominantly distal weakness seen in classic myotonic dystrophy (DM1). Before the genetics were clarified, DM2 and PROMM were thought to be distinct disorders. Overall the DM2/PROMM phenotype appears to be less severe than the DM1 phenotype, with less cognitive impairment.86 The pathophysiology of DM2 is unclear; however, it may be similar to DM1, in that noncoding nucleotide expansions (in transcribed, but untranslated, portions of the gene) seem to interfere with proper RNA splicing.87
Congenital Muscular Dystrophy
Congenital muscular dystrophies are a class of relatively rare conditions that present in infancy. Because of the vagaries of the naming system, many forms of CMD are classified with the limb-girdle muscular dystrophies (eg, severe congenital autosomal recessive muscular dystrophy [SCARMD]). The typical CMD cases are often those associated with disturbances in the central nervous system. Newborns and infants with CMD have significant weakness and up to a 10-fold increase in the blood level of the enzyme creatine kinase,88 a general indicator of muscle damage. Clinical manifestations include muscle weakness, hypotonia, delayed motor development, and severe contractures with consequent joint deformities.
A common form of CMD is associated with a protein in the DAPC. Laminin is a component of the ECM that binds to dystroglycan in the DAPC (Fig. 1). The α2-chain of laminin, also known as “merosin,” is absent or depleted in this “merosin-deficient CMD.” Children with CMD have marked weakness and never acquire the ability to walk independently. The muscle weakness usually is not progressive, and life expectancy is nearly normal. There is a slight increase in incidence of seizures and mental retardation in children lacking merosin,89 but the majority of children have no structural brain abnormalities or academic deficits.6 Abnormalities of myelin maturation are extremely common, however, and help in making the diagnosis.
A more severe form of CMD associated with brain abnormalities is WWS. Walker-Warburg syndrome is due to mutations in the gene encoding protein O-mannosyltransferase. The disorder is associated with abnormal glycosylation of α-dystroglycan (Fig. 1), disrupting the linkage of the cell membrane to the ECM in brain and muscle.33 A condition that bears some resemblance to WWS, known as muscle-eye-brain disease, is caused by mutations in the gene for the protein O-mannose β-1,2-N-acetylglucosaminyltransferase (POMGNT1 gene). Abnormal glycosylation of α-dystroglycan probably plays a role in this condition as well. Lastly, Fukuyama CMD, a very common form of CMD in Japan, is associated with less profound brain abnormalities than WWS and muscle-eye-brain disease, but nonetheless is characterized by profound mental retardation, weakness, and developmental delay. Fukuyama disease, which is caused by mutations in the Fukutin gene,41 also appears to be associated with abnormal glycosylation of α-dystroglycan.
Animal Models of Muscular Dystrophy
One obvious challenge in studying MDs is the heterogeneous nature of these diseases. This has led to the development of several animal models that are used experimentally to study some of the MDs, but more are clearly needed. The most universally used laboratory animal model of DMD is the mdx mouse. The X-linked recessive mutation in the dystrophin gene of the mdx mouse resembles that seen in boys with DMD. As a result, the mouse lacks dystrophin.90 Although mdx mice do have a muscle pathology consistent with MD, the phenotype is much less severe than that seen with DMD in humans, and the validity of the mdx mouse as a model of DMD, therefore, is disputable.29 Mdx mice have pseudohypertrophy of certain muscles, large variability in muscle fiber size, fibrosis, and fatty infiltrates as well as an increased susceptibility to injury. However, they show only minimal weakness, and mechanical function is much less compromised than in DMD, so much so that the lifespan of the mdx mouse is normal. Although the mdx mouse has been used as a model for DMD for years, other mutations in the dystrophin gene have been found in mutant mice that result in a phenotype much more similar to DMD (mdx2-5cv mice).
Because of availability and knowledge of the mouse genome, mouse models of many of the MDs have been identified or genetically engineered. For example, disruption of the genes encoding the sarcoglycans has provided models for all the known sarcoglycanopathies (subtypes of type 2 LGMD), and there are at least 5 mouse models for deficiency of the laminin α2 chain,91–94 providing models for one type of CMD. The myd mouse provides a model for CMD with brain malformation,95 associated with altered glycosylation of α-dystroglycan. There are also models of muscular dystrophy in other species, such as dogs that lack dystrophin (CXMD dog)96 and the cardiomyopathic hamster,97 which lacks δ-sarcoglycan.
Animal studies have used methods such as running,98 swimming,99 and electrical stimulation to determine the effects of training on animals with muscular dystrophy. Heavy resistance training has deleterious effects on dystrophic skeletal muscle,100,101 particularly if it involves eccentric contractions.16,102 In addition to the risk of further muscle damage, there is no evidence of beneficial adaptation to heavy resistance training in dystrophic animals or in humans with MD.103,104 Several well-controlled studies, however, do indicate that light to moderate exercise can have beneficial effects in patients with MD, such as increased strength. Most of these studies, regardless of species and differences in methodology, indicate a beneficial adaptation to exercise in dystrophic animals that is similar to that in control animals.105–109 Unfortunately, there are relatively few controlled studies available that are easily translated to the human population. Detailed studies to determine the forms of exercise that are most beneficial to patients with different types of MDs are greatly needed.
Potential New Treatments
One area of research has focused on various pharmaceuticals, such as protease inhibitors and antioxidants, to minimize the inflammation that results from muscle damage.110–112 Although these and other drugs may slow the progression of the disease,113,114 they do not substantially affect the long-term outcome. Therefore, new strategies are being developed.
Because dystrophin is the central component of a large complex of proteins at the cell membrane that is missing in DMD, an ideal treatment would be simply to replace the missing protein. Much of the focus in DMD is on gene therapy to do just that, but delivery of the dystrophin gene to all muscles of the body has presented some serious challenges. First, the dystrophin gene is enormous (2.4 Mb and is not readily inserted into the “vectors” that are best able to deliver it. Even the 14 kb complementary DNA (cDNA) sequence is too large for most viral vectors. There are a few viruses used as vectors that can carry the full-length dystrophin cDNA plus a promoter, but their ability to persist in muscle is transient and their safety is unclear.101 Some adeno-associated viral vectors efficiently infect muscle, where they can persist for years, but they have a limited cloning capacity (~6 kb) and, therefore, are unable to carry cargo as large as the dystrophin cDNA. One method used to circumvent this is based on the fact that dystrophin can retain a large part of its function even when missing much of its middle region, as long as the “mini-dystrophin” contains the N-terminal and C-terminal sequences responsible for actin and dystroglycan binding, respectively.31,101,115,116 As a consequence, smaller, truncated dystrophins with functional capacities near that of the full-length protein have been used successfully in studies with mdx mice, in which they rescued the dystrophic muscle to a nearly normal phenotype.101 Alternative therapies being explored substitute nonviral carriers (eg, polymers) to deliver the dystrophin cDNA to muscle.117
Although these and other gene therapies suggest potentially exciting new ways to manage MDs, significant hurdles to use in humans still exist.117 Gene therapy for MD requires efficient delivery to all striated muscles of the body, usually including the heart. This means that the delivery has to overcome the physical barriers (ie, the blood vessels, basement membranes, and the fascia) surrounding each muscle. Furthermore, expressing a protein in a patient who lacks a functional copy of the gene can stimulate an immune response, as can the vector used to deliver the transgene.116,118,119
One possible solution is to use utrophin. Utrophin, short for “ubiquitous dystrophin,” is highly homologous to dystrophin.120 During fetal muscle development, utrophin is found along the entire muscle sarcolemma. Once dystrophin is expressed, however, utrophin disappears from most of the sarcolemmal membrane so that, in normal adult muscle, it is located only at the neuromuscular and myotendinous junctions.121 One reason the mdx mice do not display pathology equivalent to that seen in DMD may be that utrophin is up-regulated to levels sufficient to compensate, in part, for the lack of dystrophin. This idea has been supported by the observation that mice lacking dystrophin and utrophin have a much more severe myopathy.121–123 This suggests that utrophin could replace dystrophin if it could be delivered at sufficient quantities to the muscles of patients with DMD or, even better, if its local production in each myofiber could be increased.121–122 One large advantage of this strategy over that of delivering dystrophin is that patients with DMD already make utrophin, so they are unlikely to initiate an immune response to the protein. Attempts to induce utrophin expression in adult muscle so far have focused on characterization of its promoter region, which, if activated, could up-regulate utrophin in patients with DMD.124
Still other potential treatments involve stem cell therapy125,126 and myoblast transfer.127,128 Stem cell studies in the mdx mouse show that bone marrow–derived stem cells injected intravenously can migrate into muscle, differentiate into muscle fibers, and result in partial restoration of dystrophin.126 Myoblasts, muscle precursor cells that can proliferate and produce thousands of daughter cells, can be obtained from biopsies and grown in vitro. Transplantation of these cells from donors (or genetically corrected myoblasts from the host) can result in some myofibers that express dystrophin.127,129 Despite the early success in animal studies, clinical trials in boys with DMD have failed to yield significant benefit. The levels of dystrophin restoration have been low, and it is questionable whether myoblast transfer results in a functional change.129–131 Other concerns with myoblast transfer include the need for hundreds of intramuscular injections, overcoming immunological rejection, and high costs. At present, the most promising technologies involve the use of microdystrophins expressed by adeno-associated viruses,132 but the problems of low delivery efficiency and immune reactions still need to be addressed.
Management of Duchenne Muscular Dystrophy
Pharmacological Treatment
Most of the treatments for MD have focused on DMD because of its frequency and severity. The pharmacological treatment of choice for DMD has historically been the corticosteroid prednisone, a potent anti-inflammatory that improves strength in mdx mice as well as in boys with DMD.133–138 Unfortunately, long-term administration of prednisone has side effects, such as significant weight gain, growth retardation, and osteoporosis. Deflazacort, an alternative prednisone derivative, appears to be more beneficial than prednisone, causing less immunosuppression, smaller weight gain, reduced osteoporosis, longer time before surgery for scoliosis, and a prolonged period of ambulation before a wheelchair is needed.14,113,114,139,140 Deflazacort, however, does not affect death rate or life expectancy in people with DMD. Recently, the antibiotic gentamicin has been tested in boys with DMD, about 10% of whom have inherited a dystrophin gene with a premature stop codon that halts the synthesis of the protein N-terminal to the dystroglycan binding region. Gentamicin suppresses chain termination, allowing some full-length dystrophin to be produced. This results in improved muscle function in animal studies,141 and trials are now under way in humans.142–144 Regardless of the medical interventions attempted to date, however, DMD is a progressive disease, the symptoms and outcome of which are currently unavoidable.
Physical Therapy
Physical therapists are ideally trained to help care for patients with MD, especially because of the primary involvement of skeletal muscle and the secondary effects of the disease on the joints. Aside from developing a safe exercise program, patients need to be monitored for progressive scoliosis, homes need to be evaluated for safety, assistive devices are usually needed, and family members need to be taught to perform passive-range-of-motion exercises, transfers, and repositioning. These skills and many more are already practiced by most physical therapists, yet knowledge of the specific MD being treated is essential in developing an appropriate treatment strategy.
Although DMD is incurable, it is not untreatable. The principles for treating people with DMD and other MDs are similar, but vary in degree. The major goals are to prevent the progression of joint contractures and spinal deformity, to prolong ambulation for as long as possible, and to maintain the best level of health and function possible.27,145 Physical therapy and pain management should be started as early as possible. Physical therapy, particularly in combination with night splinting, can help delay the onset of contractures,146 which are more of a limitation to ambulation than muscle weak-ness.145,147 Several researchers148–150 have suggested that light to moderate resistive exercise also can retard the progression of muscle weakness without negative effects. Although many factors can contribute to fatigue,151–153 exercise has been used in many neuro-muscular diseases to combat the deconditioning that occurs with immobility.149,150,152,154
The role of strength training in MDs is controversial, particularly with DMD. Although there are very few randomized or controlled studies in patients with DMD, studies involving patients with DMD indicate little to no physical decline with exercise.145,155 On the contrary, exercise appears to be beneficial to patients with DMD148 or patients with other MDs.156 Because of the increased susceptibility to muscle damage, patients with MD should be advised to avoid exhaustive or maximal effort during exercise and to use exercise regimens that minimize exercise-induced muscle damage.102 Thus, resistive eccentric exercises, which are associated with muscle damage, should be avoided.157–162 Hydrotherapy is likely to result in minimal muscle damage, because it minimizes the need for eccentric contractions. Hydrotherapy appears especially useful in the later stages of DMD in order to help maintain mobility in the absence of gravity,27 although there is very little objective evidence published to support this commonly held hypothesis.
In addition to hydrotherapy, land-based exercises also are useful; however, home exercise programs must be reasonable and easy to follow in order to prevent nonadherence.163 Muscle weakness typically progresses at different rates in different muscles. Although the reasons for this are unclear, the larger limb-girdle muscles are affected first, whereas smaller muscles are spared until later.27 For example, in DMD, weakness of the quadriceps femoris muscles occurs early and plays a significant role in deterioration of gait.164 As in any research on exercise, the intensity, frequency, duration, and mode of exercise can vary between studies, making comparisons difficult. This is especially true with studies involving neuromuscular diseases such as MDs, because the patient populations often are not homogeneous and the timing of the intervention may play a role in the outcome.103 Establishing clearly defined exercise protocols from the available evidence, therefore, is difficult.145 Future randomized and controlled research is needed to fully ascertain the effects of exercise in MDs.
Contractures
Joint contractures are a major problem in many MDs. They clearly limit joint function, but also may contribute to muscle weakness, because the force generated by a muscle is related to its length. In some instances, muscle weakness can be compensated for during gait, such as by maintaining exaggerated anterior pelvic tilt and decreased hip extension in the stance phase (moving the line of gravity anterior to the knee), by using an equine gait, or by changing the base of support.147,164 All of these compensations are directed at altering the moment of inertia at the knee in an attempt to compensate for quadriceps femoris muscle insufficiency. Contractures, however, often are permanent, and it is difficult to compensate for them. Although contractures may be the result of postural compensations for muscle weakness, they are more likely the result of a maintained position over time. For example, flexion contractures of the elbows are rare in patients with DMD who are ambulatory, but usually develop gradually after wheelchair use.165 Common treatments include frequent changes in position, passive or active stretching, and night splints, specifically ankle-foot orthoses (AFOs). A combination of these is likely to yield a better outcome and prolong independent ambulation.146,166 Daytime AFOs may be prescribed to help retard the progress of equine deformity; however, the use of an AFO also can interfere with independent gait, because the hyperlordotic and equine gait of many patients with DMD is a compensation for weakness of the hip extensor and quadriceps femoris muscles.147,164 The use of aggressive physical therapy or surgical release to delay contractures, combined with the proper use of orthoses, may help to prolong ambulation by 1 or 2 years.167
Summary
The phenotypic similarity of muscular dystrophies has made it challenging to diagnose which form of a MD a patient has; the genetic basis of many muscular disorders is now known, however. Clinically, the recent genetic advances have improved diagnostic capabilities, but they have not yet provided rational approaches to treatment or management. Because of the progressive nature of most MDs, genuine rehabilitation (ie, a return to normal function) is not likely. Nonetheless, the application of physical therapy interventions is mandated by the chronicity and disabling effects of MD. Despite the exciting progress being made in the laboratory to understand the molecular mechanisms underlying MDs, the best therapies to use in their treatment are still primarily supportive, with the use of anti-inflammatory drugs and physical therapy to maximize function.
Much of the research regarding treatment has focused on DMD, because of its severity and frequency. Current evidence suggests that exercise can improve muscle function in patients with DMD, yet the appropriate frequency, intensity, and duration are still unclear. Different amounts and types of exercise are indicated for other forms of MD,145 suggesting that what we learn about possible treatments for one of these diseases will not be fully applicable to others. Furthermore, it is still not clear whether the benefits of increased muscle strength, which appropriate exercise regimens might facilitate, have a significant effect on functional outcome. Contractures are common in MDs, especially in the more severe diseases and when the patient is no longer ambulatory. Early treatment, with stretching, active and passive range of motion, and appropriate orthoses, is essential. There are obvious ethical dilemmas in withdrawing current standards of treatment in order to perform controlled studies, but animal models will likely continue to provide some guidelines toward prescribing exercises. The benefits of steroids appear to outweigh the side effects, yet up to now no drug substantially affects the overall natural history of MDs. More effective drugs and therapies will likely be developed when specific pathologic mechanisms are more clearly understood. There is great hope that gene therapy will someday be possible, but even when such therapies are available, physical therapy will remain essential for treating patients with MDs, and it will behoove physical therapists to have an understanding of the benefits, limitations, and expected results of various gene delivery systems.
Footnotes
Dr Lovering and Dr Bloch provided concept/idea/project design and writing. Dr Porter and Dr Bloch provided consultation (including review of the manuscript before submission).
For more information on muscular dystrophy, contact: Muscular Dystrophy Association (MDA), National Headquarters, 3300 E Sunrise Dr, Tucson, AZ 85718, or visit the MDA Web site (http://www.mdausa.org/).
Contributor Information
Richard M Lovering, Email: rlovering@som.umaryland.edu, Department of Physiology, University of Maryland, School of Medicine, 685 W Baltimore St, Baltimore, MD 21201 (USA).
Neil C Porter, Department of Neurology, University of Maryland, School of Medicine.
Robert J Bloch, Department of Physiology, University of Maryland, School of Medicine.
References
- 1.Dalkilic I, Kunkel LM. Muscular dystrophies: genes to pathogenesis. Curr Opin Genet Dev. 2003;13:231–238. doi: 10.1016/s0959-437x(03)00048-0. [DOI] [PubMed] [Google Scholar]
- 2.Kunkel LM, Monaco AP, Middlesworth W, et al. Specific cloning of DNA fragments absent from the DNA of a male patient with an X chromosome deletion. Proc Natl Acad Sci U S A. 1985;82:4778–4782. doi: 10.1073/pnas.82.14.4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray JM, Davies KE, Harper PS, et al. Linkage relationship of a cloned DNA sequence on the short arm of the X chromosome to Duchenne muscular dystrophy. Nature. 1982;300:69–71. doi: 10.1038/300069a0. [DOI] [PubMed] [Google Scholar]
- 4.Hoffman EP, Brown RH, Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 5.Ehmsen J, Poon E, Davies K. The dystrophin-associated protein complex. J Cell Sci. 2002;115:2801–2803. doi: 10.1242/jcs.115.14.2801. [DOI] [PubMed] [Google Scholar]
- 6.Mathews KD. Muscular dystrophy overview: genetics and diagnosis. Neurol Clin. 2003;21:795–816. doi: 10.1016/s0733-8619(03)00065-3. [DOI] [PubMed] [Google Scholar]
- 7.Wagner KR. Genetic diseases of muscle. Neurol Clin. 2002;20:645–678. doi: 10.1016/s0733-8619(02)00002-6. [DOI] [PubMed] [Google Scholar]
- 8.Lovering RM, De Deyne PG. Contractile function, sarcolemma integrity, and the loss of dystrophin after skeletal muscle eccentric contraction-induced injury. Am J Physiol Cell Physiol. 2004;286:C230–C238. doi: 10.1152/ajpcell.00199.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duchenne G. L’Electrisation Localisee at de son Application a la Pathologie at a la Therapeutique. Paris, France: Bailliere et Fils; 1861. [Google Scholar]
- 10.McDonald CM, Abresch RT, Carter GT, et al. Profiles of neuromuscular diseases: Duchenne muscular dystrophy. Am J Phys Med Rehabil. 1995;74:S70–S92. doi: 10.1097/00002060-199509001-00003. [DOI] [PubMed] [Google Scholar]
- 11.Brooke MH, Fenichel GM, Griggs RC, et al. Duchenne muscular dystrophy: patterns of clinical progression and effects of supportive therapy. Neurology. 1989;39:475–481. doi: 10.1212/wnl.39.4.475. [DOI] [PubMed] [Google Scholar]
- 12.Roland EH. Muscular dystrophy. Pediatr Rev. 2000;21:233–237. doi: 10.1542/pir.21-7-233. [DOI] [PubMed] [Google Scholar]
- 13.Metules T. Duchenne muscular dystrophy. RN. 2002;65:39–44. 47. [PubMed] [Google Scholar]
- 14.Biggar WD, Gingras M, Fehlings DL, et al. Deflazacort treatment of Duchenne muscular dystrophy. J Pediatr. 2001;138:45–50. doi: 10.1067/mpd.2001.109601. [DOI] [PubMed] [Google Scholar]
- 15.Emery AE. The muscular dystrophies. BMJ. 1998;317:991–995. doi: 10.1136/bmj.317.7164.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petrof BJ, Shrager JB, Stedman HH, et al. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci U S A. 1993;90:3710–3714. doi: 10.1073/pnas.90.8.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blau HM, Webster C, Pavlath GK. Defective myoblasts identified in Duchenne muscular dystrophy. Proc Natl Acad Sci U S A. 1983;80:4856–4860. doi: 10.1073/pnas.80.15.4856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Webster C, Blau HM. Accelerated age-related decline in replicative life-span of Duchenne muscular dystrophy myoblasts: implications for cell and gene therapy. Somat Cell Mol Genet. 1990;16:557–565. doi: 10.1007/BF01233096. [DOI] [PubMed] [Google Scholar]
- 19.Bockhold KJ, Rosenblatt JD, Partridge TA. Aging normal and dystrophic mouse muscle: analysis of myogenicity in cultures of living single fibers. Muscle Nerve. 1998;21:173–183. doi: 10.1002/(sici)1097-4598(199802)21:2<173::aid-mus4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 20.Grozdanovic Z, Gosztonyi G, Gossrau R. Nitric oxide synthase I (NOS-I) is deficient in the sarcolemma of striated muscle fibers in patients with Duchenne muscular dystrophy, suggesting an association with dystrophin. Acta Histochem. 1996;98:61–69. doi: 10.1016/S0065-1281(96)80051-1. [DOI] [PubMed] [Google Scholar]
- 21.Wehling M, Spencer MJ, Tidball JG. A nitric oxide synthase transgene ameliorates muscular dystrophy in mdx mice. J Cell Biol. 2001;155:123–131. doi: 10.1083/jcb.200105110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brenman JE, Chao DS, Xia H, et al. Nitric oxide synthase complexed with dystrophin and absent from skeletal muscle sarcolemma in Duchenne muscular dystrophy. Cell. 1995;82:743–752. doi: 10.1016/0092-8674(95)90471-9. [DOI] [PubMed] [Google Scholar]
- 23.Bresolin N, Castelli E, Comi GP, et al. Cognitive impairment in Duchenne muscular dystrophy. Neuromuscul Disord. 1994;4:359–369. doi: 10.1016/0960-8966(94)90072-8. [DOI] [PubMed] [Google Scholar]
- 24.Culligan K, Ohlendieck K. Diversity of the brain dystrophin-glycoprotein complex. J Biomed Biotechnol. 2002;2:31–36. doi: 10.1155/S1110724302000347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Billard C, Gillet P, Barthez M, et al. Reading ability and processing in Duchenne muscular dystrophy and spinal muscular atrophy. Dev Med Child Neurol. 1998;40:12–20. doi: 10.1111/j.1469-8749.1998.tb15351.x. [DOI] [PubMed] [Google Scholar]
- 26.Barbujani G, Russo A, Danieli GA, et al. Segregation analysis of 1885 DMD families: significant departure from the expected proportion of sporadic cases. Hum Genet. 1990;84:522–526. doi: 10.1007/BF00210802. [DOI] [PubMed] [Google Scholar]
- 27.Kakulas BA. Problems and solutions in the rehabilitation of patients with progressive muscular dystrophy. Scand J Rehabil Med Suppl. 1999;39:23–37. doi: 10.1080/003655098443869. [DOI] [PubMed] [Google Scholar]
- 28.Straub V, Rafael JA, Chamberlain JS, Campbell KP. Animal models for muscular dystrophy show different patterns of sarcolemmal disruption. J Cell Biol. 1997;139:375–385. doi: 10.1083/jcb.139.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allamand V, Campbell KP. Animal models for muscular dystrophy: valuable tools for the development of therapies. Hum Mol Genet. 2000;9:2459–2467. doi: 10.1093/hmg/9.16.2459. [DOI] [PubMed] [Google Scholar]
- 30.Bork P, Sudol M. The WW domain: a signalling site in dystrophin? Trends Biochem Sci. 1994;19:531–533. doi: 10.1016/0968-0004(94)90053-1. [DOI] [PubMed] [Google Scholar]
- 31.Jung D, Yang B, Meyer J, et al. Identification and characterization of the dystrophin anchoring site on beta-dystroglycan. J Biol Chem. 1995;270:27305–27310. doi: 10.1074/jbc.270.45.27305. [DOI] [PubMed] [Google Scholar]
- 32.Petrucci TC, Macchia G, Macioce P, et al. Functional flexibility of dystroglycan, a transmembrane linker between the extracellular matrix and the cytoskeleton. Cell Mol Biol Lett. 2001;6:226. [PubMed] [Google Scholar]
- 33.Michele DE, Barresi R, Kanagawa M, et al. Post-translational disruption of dystroglycan-ligand interactions in congenital muscular dystrophies. Nature. 2002;418:417–422. doi: 10.1038/nature00837. [DOI] [PubMed] [Google Scholar]
- 34.Newey SE, Howman EV, Ponting CP, et al. Syncoilin, a novel member of the intermediate filament superfamily that interacts with alpha-dystrobrevin in skeletal muscle. J Biol Chem. 2001;276:6645–6655. doi: 10.1074/jbc.M008305200. [DOI] [PubMed] [Google Scholar]
- 35.Poon E, Howman EV, Newey SE, Davies KE. Association of syncoilin and desmin: linking intermediate filament proteins to the dystrophin-associated protein complex. J Biol Chem. 2002;277:3433–3439. doi: 10.1074/jbc.M105273200. [DOI] [PubMed] [Google Scholar]
- 36.Norwood FL, Sutherland-Smith AJ, Keep NH, Kendrick-Jones J. The structure of the N-terminal actin-binding domain of human dystrophin and how mutations in this domain may cause Duchenne or Becker muscular dystrophy. Structure Fold Des. 2000;8:481–491. doi: 10.1016/s0969-2126(00)00132-5. [DOI] [PubMed] [Google Scholar]
- 37.Patel TJ, Lieber RL. Force transmission in skeletal muscle: from actomyosin to external tendons. Exerc Sport Sci Rev. 1997;25:321–363. [PubMed] [Google Scholar]
- 38.Tidball JG. Force transmission across muscle cell membranes. J Biomech. 1991;24(suppl 1):43–52. doi: 10.1016/0021-9290(91)90376-x. [DOI] [PubMed] [Google Scholar]
- 39.Williamson RA, Henry MD, Daniels KJ, et al. Dystroglycan is essential for early embryonic development: disruption of Reichert’s membrane in Dag1-null mice. Hum Mol Genet. 1997;6:831–841. doi: 10.1093/hmg/6.6.831. [DOI] [PubMed] [Google Scholar]
- 40.Grewal PK, Holzfeind PJ, Bittner RE, Hewitt JE. Mutant glycosyl-transferase and altered glycosylation of alpha-dystroglycan in the myodystrophy mouse. Nat Genet. 2001;28:151–154. doi: 10.1038/88865. [DOI] [PubMed] [Google Scholar]
- 41.Brockington M, Blake DJ, Prandini P, et al. Mutations in the fukutin-related protein gene (FKRP) cause a form of congenital muscular dystrophy with secondary laminin alpha2 deficiency and abnormal glycosylation of alpha-dystroglycan. Am J Hum Genet. 2001;69:1198–1209. doi: 10.1086/324412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Longman C, Brockington M, Torelli S, et al. Mutations in the human LARGE gene cause MDC1D, a novel form of congenital muscular dystrophy with severe mental retardation and abnormal glycosylation of alpha-dystroglycan. Hum Mol Genet. 2003;12:2853–2861. doi: 10.1093/hmg/ddg307. [DOI] [PubMed] [Google Scholar]
- 43.Crosbie RH, Heighway J, Venzke DP, et al. Sarcospan, the 25-kDa transmembrane component of the dystrophin-glycoprotein complex. J Biol Chem. 1997;272:31221–31224. doi: 10.1074/jbc.272.50.31221. [DOI] [PubMed] [Google Scholar]
- 44.Lebakken CS, Venzke DP, Hrstka RF, et al. Sarcospan-deficient mice maintain normal muscle function. Mol Cell Biol. 2000;20:1669–1677. doi: 10.1128/mcb.20.5.1669-1677.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vainzof M, Passos-Bueno MR, Canovas M, et al. The sarcoglycan complex in the six autosomal recessive limb-girdle muscular dystrophies. Hum Mol Genet. 1996;5:1963–1969. doi: 10.1093/hmg/5.12.1963. [DOI] [PubMed] [Google Scholar]
- 46.O’Brien KF, Kunkel LM. Dystrophin and muscular dystrophy: past, present, and future. Mol Genet Metab. 2001;74:75–88. doi: 10.1006/mgme.2001.3220. [DOI] [PubMed] [Google Scholar]
- 47.Hillier BJ, Christopherson KS, Prehoda KE, et al. Unexpected modes of PDZ domain scaffolding revealed by structure of nNOS-syntrophin complex. Science. 1999;284:812–815. [PubMed] [Google Scholar]
- 48.Oak SA, Russo K, Petrucci TC, Jarrett HW. Mouse alpha1-syntrophin binding to Grb2: further evidence of a role for syntrophin in cell signaling. Biochemistry. 2001;40:11270–11278. doi: 10.1021/bi010490n. [DOI] [PubMed] [Google Scholar]
- 49.Hamamichi Y, Ichida F, Hashimoto I, et al. Isolated noncompaction of the ventricular myocardium: ultrafast computed tomography and magnetic resonance imaging. Int J Cardiovasc Imaging. 2001;17:305–314. doi: 10.1023/a:1011658926555. [DOI] [PubMed] [Google Scholar]
- 50.Grady RM, Grange RW, Lau KS, et al. Role for alpha-dystrobrevin in the pathogenesis of dystrophin-dependent muscular dystrophies. Nat Cell Biol. 1999;1:215–220. doi: 10.1038/12034. [DOI] [PubMed] [Google Scholar]
- 51.Adams ME, Kramarcy N, Krall SP, et al. Absence of alpha-syntrophin leads to structurally aberrant neuromuscular synapses deficient in utrophin. J Cell Biol. 2000;150:1385–1398. doi: 10.1083/jcb.150.6.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mayer U, Saher G, Fassler R, et al. Absence of integrin alpha 7 causes a novel form of muscular dystrophy. Nat Genet. 1997;17:318–323. doi: 10.1038/ng1197-318. [DOI] [PubMed] [Google Scholar]
- 53.Dayton WR, Goll DE, Zeece MG, et al. A Ca2+-activated protease possibly involved in myofibrillar protein turnover: purification from porcine muscle. Biochemistry. 1976;15:2150–2158. doi: 10.1021/bi00655a019. [DOI] [PubMed] [Google Scholar]
- 54.Goll DE, Thompson VF, Li H, et al. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- 55.Bansal D, Miyake K, Vogel SS, et al. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature. 2003;423:168–172. doi: 10.1038/nature01573. [DOI] [PubMed] [Google Scholar]
- 56.Lennon NJ, Kho A, Bacskai BJ, et al. Dysferlin interacts with annexins A1 and A2 and mediates sarcolemmal wound-healing. J Biol Chem. 2003;278:50466–50473. doi: 10.1074/jbc.M307247200. [DOI] [PubMed] [Google Scholar]
- 57.Fardeau M, Eymard B, Mignard C, et al. Chromosome 15-linked limb-girdle muscular dystrophy: clinical phenotypes in Reunion Island and French metropolitan communities. Neuromuscul Disord. 1996;6:447–453. doi: 10.1016/s0960-8966(96)00387-2. [DOI] [PubMed] [Google Scholar]
- 58.Richard I, Roudaut C, Saenz A, et al. Calpainopathy: a survey of mutations and polymorphisms. Am J Hum Genet. 1999;64:1524–1540. doi: 10.1086/302426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prelle A, Sciacco M, Tancredi L, et al. Clinical, morphological and immunological evaluation of six patients with dysferlin deficiency. Acta Neuropathol (Berl) 2003;105:537–542. doi: 10.1007/s00401-002-0654-1. [DOI] [PubMed] [Google Scholar]
- 60.Weiler T, Bashir R, Anderson LV, et al. Identical mutation in patients with limb girdle muscular dystrophy type 2B or Miyoshi myopathy suggests a role for modifier gene(s) Hum Mol Genet. 1999;8:871–877. doi: 10.1093/hmg/8.5.871. [DOI] [PubMed] [Google Scholar]
- 61.McNally EM, Ly CT, Kunkel LM. Human epsilon-sarcoglycan is highly related to alpha-sarcoglycan (adhalin), the limb girdle muscular dystrophy 2D gene. FEBS Lett. 1998;422:27–32. doi: 10.1016/s0014-5793(97)01593-7. [DOI] [PubMed] [Google Scholar]
- 62.Zimprich A, Grabowski M, Asmus F, et al. Mutations in the gene encoding epsilon-sarcoglycan cause myoclonus-dystonia syndrome. Nat Genet. 2001;29:66–69. doi: 10.1038/ng709. [DOI] [PubMed] [Google Scholar]
- 63.Angelini C, Fanin M, Freda MP, et al. The clinical spectrum of sarcoglycanopathies. Neurology. 1999;52:176–179. doi: 10.1212/wnl.52.1.176. [DOI] [PubMed] [Google Scholar]
- 64.Brouwer OF, Padberg GW, Ruys CJ, et al. Hearing loss in facioscapulohumeral muscular dystrophy. Neurology. 1991;41:1878–1881. doi: 10.1212/wnl.41.12.1878. [DOI] [PubMed] [Google Scholar]
- 65.Padberg GW, Brouwer OF, de Keizer RJ, et al. On the significance of retinal vascular disease and hearing loss in facioscapulohumeral muscular dystrophy. Muscle Nerve. 1995;2:S73–S80. [PubMed] [Google Scholar]
- 66.Funakoshi M, Goto K, Arahata K. Epilepsy and mental retardation in a subset of early onset 4q35-facioscapulohumeral muscular dystrophy. Neurology. 1998;50:1791–1794. doi: 10.1212/wnl.50.6.1791. [DOI] [PubMed] [Google Scholar]
- 67.van Deutekom JC, Wijmenga C, van Tienhoven EA, et al. FSHD associated DNA rearrangements are due to deletions of integral copies of a 3.2 kb tandemly repeated unit. Hum Mol Genet. 1993;2:2037–2042. doi: 10.1093/hmg/2.12.2037. [DOI] [PubMed] [Google Scholar]
- 68.Deidda GC, Cacurri S, La Cesa I, et al. 4q35 molecular probes for the diagnosis and genetic counseling of facioscapulohumeral muscular dystrophy. Ann Neurol. 1994;36:117–118. doi: 10.1002/ana.410360128. [DOI] [PubMed] [Google Scholar]
- 69.Tawil R, Forrester J, Griggs RC, et al. The FSH-DY Group. Evidence for anticipation and association of deletion size with severity in facioscapulohumeral muscular dystrophy. Ann Neurol. 1996;39:744–748. doi: 10.1002/ana.410390610. [DOI] [PubMed] [Google Scholar]
- 70.Jiang G, Yang F, van Overveld PG, et al. Testing the position-effect variegation hypothesis for facioscapulohumeral muscular dystrophy by analysis of histone modification and gene expression in subtelomeric 4q. Hum Mol Genet. 2003;12:2909–2921. doi: 10.1093/hmg/ddg323. [DOI] [PubMed] [Google Scholar]
- 71.Masny PS, Bengtsson U, Chung SA, et al. Localization of 4q35.2 to the nuclear periphery: is FSHD a nuclear envelope disease? Hum Mol Genet. 2004;13:1857–1871. doi: 10.1093/hmg/ddh205. [DOI] [PubMed] [Google Scholar]
- 72.Emery AE. Emery-Dreifuss muscular dystrophy: a 40 year retrospective. Neuromuscul Disord. 2000;10:228–232. doi: 10.1016/s0960-8966(00)00105-x. [DOI] [PubMed] [Google Scholar]
- 73.Ostlund C, Worman HJ. Nuclear envelope proteins and neuromuscular diseases. Muscle Nerve. 2003;27:393–406. doi: 10.1002/mus.10302. [DOI] [PubMed] [Google Scholar]
- 74.Tews DS. Emerin. Int J Biochem Cell Biol. 1999;31:891–894. doi: 10.1016/s1357-2725(99)00040-0. [DOI] [PubMed] [Google Scholar]
- 75.Emery AE. Emery-Dreifuss syndrome. J Med Genet. 1989;26:637–641. doi: 10.1136/jmg.26.10.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hill ME, Creed GA, McMullan TF, et al. Oculopharyngeal muscular dystrophy: phenotypic and genotypic studies in a UK population. Brain. 2001;124:522–526. doi: 10.1093/brain/124.3.522. [DOI] [PubMed] [Google Scholar]
- 77.Bouchard JP, Brais B, Brunet D, et al. Recent studies on oculopharyngeal muscular dystrophy in Quebec. Neuromuscul Disord. 1997;7(suppl 1):S22–S29. doi: 10.1016/s0960-8966(97)00077-1. [DOI] [PubMed] [Google Scholar]
- 78.Brais B, Bouchard JP, Xie YG, et al. Short GCG expansions in the PABP2 gene cause oculopharyngeal muscular dystrophy. Nat Genet. 1998;18:164–167. doi: 10.1038/ng0298-164. [DOI] [PubMed] [Google Scholar]
- 79.Robinson DO, Hammans SR, Read SP, Sillibourne J. Oculopharyngeal muscular dystrophy (OPMD): analysis of the PABPN1 gene expansion sequence in 86 patients reveals 13 different expansion types and further evidence for unequal recombination as the mutational mechanism. Hum Genet. 2005;116:267–271. doi: 10.1007/s00439-004-1235-2. [DOI] [PubMed] [Google Scholar]
- 80.Mahadevan M, Tsilfidis C, Sabourin L, et al. Myotonic dystrophy mutation: an unstable CTG repeat in the 3′ untranslated region of the gene. Science. 1992;255:1253–1255. doi: 10.1126/science.1546325. [DOI] [PubMed] [Google Scholar]
- 81.Fu YH, Pizzuti A, Fenwick RG, Jr, et al. An unstable triplet repeat in a gene related to myotonic muscular dystrophy. Science. 1992;255:1256–1258. doi: 10.1126/science.1546326. [DOI] [PubMed] [Google Scholar]
- 82.Roig M, Balliu PR, Navarro C, et al. Presentation, clinical course, and outcome of the congenital form of myotonic dystrophy. Pediatr Neurol. 1994;11:208–213. doi: 10.1016/0887-8994(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 83.Schoser BG, Kress W, Walter MC, et al. Homozygosity for CCTG mutation in myotonic dystrophy type 2. Brain. 2004;127:1868–1877. doi: 10.1093/brain/awh210. [DOI] [PubMed] [Google Scholar]
- 84.Bachinski LL, Udd B, Meola G, et al. Confirmation of the type 2 myotonic dystrophy (CCTG)n expansion mutation in patients with proximal myotonic myopathy/proximal myotonic dystrophy of different European origins: a single shared haplotype indicates an ancestral founder effect. Am J Hum Genet. 2003;73:835–848. doi: 10.1086/378566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liquori CL, Ricker K, Moseley ML, et al. Myotonic dystrophy type 2 caused by a CCTG expansion in intron 1 of ZNF9. Science. 2001;293:864–867. doi: 10.1126/science.1062125. [DOI] [PubMed] [Google Scholar]
- 86.Day JW, Ricker K, Jacobsen JF, et al. Myotonic dystrophy type 2: molecular, diagnostic and clinical spectrum. Neurology. 2003;60:657–664. doi: 10.1212/01.wnl.0000054481.84978.f9. [DOI] [PubMed] [Google Scholar]
- 87.Ranum LP, Day JW. Myotonic dystrophy: RNA pathogenesis comes into focus. Am J Hum Genet. 2004;74:793–804. doi: 10.1086/383590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gilboa N, Swanson JR. Serum creatine phosphokinase in normal newborns. Arch Dis Child. 1976;51:283–285. doi: 10.1136/adc.51.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jones KJ, Morgan G, Johnston H, et al. The expanding phenotype of laminin alpha2 chain (merosin) abnormalities: case series and review. J Med Genet. 2001;38:649–657. doi: 10.1136/jmg.38.10.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sicinski P, Geng Y, Ryder-Cook AS, et al. The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science. 1989;244:1578–1580. doi: 10.1126/science.2662404. [DOI] [PubMed] [Google Scholar]
- 91.Kuang W, Xu H, Vachon PH, et al. Merosin-deficient congenital muscular dystrophy: partial genetic correction in two mouse models. J Clin Invest. 1998;102:844–852. doi: 10.1172/JCI3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xu H, Christmas P, Wu XR, et al. Defective muscle basement membrane and lack of M-laminin in the dystrophic dy/dy mouse. Proc Natl Acad Sci U S A. 1994;91:5572–5576. doi: 10.1073/pnas.91.12.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xu H, Wu XR, Wewer UM, Engvall E. Murine muscular dystrophy caused by a mutation in the laminin alpha 2 (Lama2) gene. Nat Genet. 1994;8:297–302. doi: 10.1038/ng1194-297. [DOI] [PubMed] [Google Scholar]
- 94.Miyagoe Y, Hanaoka K, Nonaka I, et al. Laminin alpha2 chain-null mutant mice by targeted disruption of the Lama2 gene: a new model of merosin (laminin 2)-deficient congenital muscular dystrophy. FEBS Lett. 1997;415:33–39. doi: 10.1016/s0014-5793(97)01007-7. [DOI] [PubMed] [Google Scholar]
- 95.Mathews KD, Rapisarda D, Bailey HL, et al. Phenotypic and pathologic evaluation of the myd mouse: a candidate model for facioscapulohumeral dystrophy. J Neuropathol Exp Neurol. 1995;54:601–606. doi: 10.1093/whq/54.4.601. [DOI] [PubMed] [Google Scholar]
- 96.Cooper BJ, Winand NJ, Stedman H, et al. The homologue of the Duchenne locus is defective in X-linked muscular dystrophy of dogs. Nature. 1988;334:154–156. doi: 10.1038/334154a0. [DOI] [PubMed] [Google Scholar]
- 97.Nigro V, Okazaki Y, Belsito A, et al. Identification of the Syrian hamster cardiomyopathy gene. Hum Mol Genet. 1997;6:601–607. doi: 10.1093/hmg/6.4.601. [DOI] [PubMed] [Google Scholar]
- 98.Hayes A, Williams DA. Beneficial effects of voluntary wheel running on the properties of dystrophic mouse muscle. J Appl Physiol. 1996;80:670–679. doi: 10.1152/jappl.1996.80.2.670. [DOI] [PubMed] [Google Scholar]
- 99.Soltan HC. Swimming stress and adaptation by dystrophic and normal mice. Am J Physiol. 1962;203:91–94. doi: 10.1152/ajplegacy.1962.203.1.91. [DOI] [PubMed] [Google Scholar]
- 100.Dellorusso C, Crawford RW, Chamberlain JS, Brooks SV. Tibialis anterior muscles in mdx mice are highly susceptible to contraction-induced injury. J Muscle Res Cell Motil. 2001;22:467–475. doi: 10.1023/a:1014587918367. [DOI] [PubMed] [Google Scholar]
- 101.Harper SQ, Hauser MA, Dellorusso C, et al. Modular flexibility of dystrophin: implications for gene therapy of Duchenne muscular dystrophy. Nat Med. 2002;8:253–261. doi: 10.1038/nm0302-253. [DOI] [PubMed] [Google Scholar]
- 102.Petrof BJ. The molecular basis of activity-induced muscle injury in Duchenne muscular dystrophy. Mol Cell Biochem. 1998;179:111–123. doi: 10.1023/a:1006812004945. [DOI] [PubMed] [Google Scholar]
- 103.Milner-Brown HS, Miller RG. Muscle strengthening through high-resistance weight training in patients with neuromuscular disorders. Arch Phys Med Rehabil. 1988;69:14–19. [PubMed] [Google Scholar]
- 104.Milner-Brown HS, Miller RG. Muscle strengthening through electric stimulation combined with low-resistance weights in patients with neuromuscular disorders. Arch Phys Med Rehabil. 1988;69:20–24. [PubMed] [Google Scholar]
- 105.Taylor RG, Fowler WM, Jr, Doerr L. Exercise effect on contractile properties of skeletal muscle in mouse muscular dystrophy. Arch Phys Med Rehabil. 1976;57:174–180. [PubMed] [Google Scholar]
- 106.Dangain J, Vrbova G. Long term effect of low frequency chronic electrical stimulation on the fast hind limb muscles of dystrophic mice. J Neurol Neurosurg Psychiatry. 1989;52:1382–1389. doi: 10.1136/jnnp.52.12.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Luthert P, Vrbova G, Ward KM. Effects of slow frequency electrical stimulation on muscles of dystrophic mice. J Neurol Neurosurg Psychiatry. 1980;43:803–809. doi: 10.1136/jnnp.43.9.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Luthert P, Vrbova G, Ward KM. Functional improvement of skeletal muscles of dystrophic mice following electrical stimulation [proceedings] J Physiol. 1979;291:31P. [PubMed] [Google Scholar]
- 109.Carter GT, Abresch RT, Fowler WM., Jr Adaptations to exercise training and contraction-induced muscle injury in animal models of muscular dystrophy. Am J Phys Med Rehabil. 2002;81(11 suppl):S151–S161. doi: 10.1097/00002060-200211001-00016. [DOI] [PubMed] [Google Scholar]
- 110.Spencer MJ, Croall DE, Tidball JG. Calpains are activated in necrotic fibers from mdx dystrophic mice. J Biol Chem. 1995;270:10909–10914. doi: 10.1074/jbc.270.18.10909. [DOI] [PubMed] [Google Scholar]
- 111.Tidball JG, Spencer MJ. Calpains and muscular dystrophies. Int J Biochem Cell Biol. 2000;32:1–5. doi: 10.1016/s1357-2725(99)00095-3. [DOI] [PubMed] [Google Scholar]
- 112.Hayes A, Williams DA. Examining potential drug therapies for muscular dystrophy utilising the dy/dy mouse, I: clenbuterol. J Neurol Sci. 1998;157:122–128. doi: 10.1016/s0022-510x(98)00084-7. [DOI] [PubMed] [Google Scholar]
- 113.Mesa LE, Dubrovsky AL, Corderi J, et al. Steroids in Duchenne muscular dystrophy: deflazacort trial. Neuromuscul Disord. 1991;1:261–266. doi: 10.1016/0960-8966(91)90099-e. [DOI] [PubMed] [Google Scholar]
- 114.Campbell C, Jacob P. Deflazacort for the treatment of Duchenne dystrophy: a systematic review. BMC Neurol. 2003;3:7. doi: 10.1186/1471-2377-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Crawford GE, Faulkner JA, Crosbie RH, et al. Assembly of the dystrophin-associated protein complex does not require the dystrophin COOH-terminal domain. J Cell Biol. 2000;150:1399–1410. doi: 10.1083/jcb.150.6.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.DelloRusso C, Scott JM, Hartigan-O’Connor D, et al. Functional correction of adult mdx mouse muscle using gutted adenoviral vectors expressing full-length dystrophin. Proc Natl Acad Sci U S A. 2002;99:12979–12984. doi: 10.1073/pnas.202300099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.van Deutekom JC, van Ommen GJ. Advances in Duchenne muscular dystrophy gene therapy. Nat Rev Genet. 2003;4:774–783. doi: 10.1038/nrg1180. [DOI] [PubMed] [Google Scholar]
- 118.Burton EA, Davies KE. Muscular dystrophy: reason for optimism? Cell. 2002;108:5–8. doi: 10.1016/s0092-8674(01)00626-2. [DOI] [PubMed] [Google Scholar]
- 119.Yang Y, Jooss KU, Su Q, et al. Immune responses to viral antigens versus transgene product in the elimination of recombinant adenovirus-infected hepatocytes in vivo. Gene Ther. 1996;3:137–144. [PubMed] [Google Scholar]
- 120.Love DR, Hill DF, Dickson G, et al. An autosomal transcript in skeletal muscle with homology to dystrophin. Nature. 1989;339:55–58. doi: 10.1038/339055a0. [DOI] [PubMed] [Google Scholar]
- 121.Tinsley J, Deconinck N, Fisher R, et al. Expression of full-length utrophin prevents muscular dystrophy in mdx mice. Nat Med. 1998;4:1441–1444. doi: 10.1038/4033. [DOI] [PubMed] [Google Scholar]
- 122.Squire S, Raymackers JM, Vandebrouck C, et al. Prevention of pathology in mdx mice by expression of utrophin: analysis using an inducible transgenic expression system. Hum Mol Genet. 2002;11:3333–3344. doi: 10.1093/hmg/11.26.3333. [DOI] [PubMed] [Google Scholar]
- 123.Dowling P, Culligan K, Ohlendieck K. Distal mdx muscle groups exhibiting up-regulation of utrophin and rescue of dystrophin-associated glycoproteins exemplify a protected phenotype in muscular dystrophy. Naturwissenschaften. 2002;89:75–78. doi: 10.1007/s00114-001-0289-4. [DOI] [PubMed] [Google Scholar]
- 124.Krag TO, Bogdanovich S, Jensen CJ, et al. Heregulin ameliorates the dystrophic phenotype in mdx mice. Proc Natl Acad Sci U S A. 2004;101:13856–13860. doi: 10.1073/pnas.0405972101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ferrari G, Cusella-De Angelis G, Coletta M, et al. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- 126.Gussoni E, Soneoka Y, Strickland CD, et al. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 1999;401:390–394. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- 127.Gussoni E, Blau HM, Kunkel LM. The fate of individual myoblasts after transplantation into muscles of DMD patients. Nat Med. 1997;3:970–977. doi: 10.1038/nm0997-970. [DOI] [PubMed] [Google Scholar]
- 128.Partridge TA, Morgan JE, Coulton GR, et al. Conversion of mdx myofibres from dystrophin-negative to -positive by injection of normal myoblasts. Nature. 1989;337:176–179. doi: 10.1038/337176a0. [DOI] [PubMed] [Google Scholar]
- 129.Mendell JR, Kissel JT, Amato AA, et al. Myoblast transfer in the treatment of Duchenne’s muscular dystrophy. N Engl J Med. 1995;333:832–838. doi: 10.1056/NEJM199509283331303. [DOI] [PubMed] [Google Scholar]
- 130.Law PK, Goodwin TG, Fang Q, et al. Feasibility, safety, and efficacy of myoblast transfer therapy on Duchenne muscular dystrophy boys. Cell Transplant. 1992;1:235–244. doi: 10.1177/0963689792001002-305. [DOI] [PubMed] [Google Scholar]
- 131.Karpati G, Ajdukovic D, Arnold D, et al. Myoblast transfer in Duchenne muscular dystrophy. Ann Neurol. 1993;34:8–17. doi: 10.1002/ana.410340105. [DOI] [PubMed] [Google Scholar]
- 132.Blankinship MJ, Gregorevic P, Allen JM, et al. Efficient transduction of skeletal muscle using vectors based on adeno-associated virus serotype 6. Mol Ther. 2004;10:671–678. doi: 10.1016/j.ymthe.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 133.Brooke MH, Fenichel GM, Griggs RC, et al. Clinical investigation of Duchenne muscular dystrophy: interesting results in a trial of prednisone. Arch Neurol. 1987;44:812–817. doi: 10.1001/archneur.1987.00520200016010. [DOI] [PubMed] [Google Scholar]
- 134.Mendell JR, Moxley RT, Griggs RC, et al. Randomized, double-blind six-month trial of prednisone in Duchenne’s muscular dystrophy. N Engl J Med. 1989;320:1592–1597. doi: 10.1056/NEJM198906153202405. [DOI] [PubMed] [Google Scholar]
- 135.DeSilva S, Drachman DB, Mellits D, Kuncl RW. Prednisone treatment in Duchenne muscular dystrophy: long-term benefit. Arch Neurol. 1987;44:818–822. doi: 10.1001/archneur.1987.00520200022012. [DOI] [PubMed] [Google Scholar]
- 136.Fenichel GM, Florence JM, Pestronk A, et al. Long-term benefit from prednisone therapy in Duchenne muscular dystrophy. Neurology. 1991;41:1874–1877. doi: 10.1212/wnl.41.12.1874. [DOI] [PubMed] [Google Scholar]
- 137.Griggs RC, Moxley RT, III, Mendell JR, et al. Duchenne dystrophy: randomized, controlled trial of prednisone (18 months) and azathio-prine (12 months) Neurology. 1993;43:520–527. doi: 10.1212/wnl.43.3_part_1.520. [DOI] [PubMed] [Google Scholar]
- 138.Oexle K. Prednisone therapy for Duchenne’s muscular dystrophy. N Engl J Med. 1989;321:1481–1482. doi: 10.1056/NEJM198911233212117. [DOI] [PubMed] [Google Scholar]
- 139.Reitter B. Deflazacort vs prednisone in Duchenne muscular dystrophy: trends of an ongoing study. Brain Dev. 1995;17(suppl):39–43. [PubMed] [Google Scholar]
- 140.Angelini C, Pegoraro E, Turella E, et al. Deflazacort in Duchenne dystrophy: study of long-term effect. Muscle Nerve. 1994;17:386–391. doi: 10.1002/mus.880170405. [DOI] [PubMed] [Google Scholar]
- 141.Barton-Davis ER, Cordier L, Shoturma DI, et al. Aminoglycoside antibiotics restore dystrophin function to skeletal muscles of mdx mice. J Clin Invest. 1999;104:375–381. doi: 10.1172/JCI7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Matsuo M, Takeshima Y, Yagi M, et al. Treatment of Duchenne muscular dystrophy with gentamicin [in Japanese] No To Hattatsu. 2004;36:125–129. [PubMed] [Google Scholar]
- 143.Wagner KR, Hamed S, Hadley DW, et al. Gentamicin treatment of Duchenne and Becker muscular dystrophy due to nonsense mutations. Ann Neurol. 2001;49:706–711. [PubMed] [Google Scholar]
- 144.Senior K. Duchenne muscular dystrophy improved by gentamicin. Mol Med Today. 1999;5:461. doi: 10.1016/s1357-4310(99)01588-9. [DOI] [PubMed] [Google Scholar]
- 145.Eagle M. Report on the muscular dystrophy campaign workshop: exercise in neuromuscular diseases Newcastle, January 2002. Neuromuscul Disord. 2002;12:975–983. doi: 10.1016/s0960-8966(02)00136-0. [DOI] [PubMed] [Google Scholar]
- 146.Hyde SA, Fløytrup I, Glent S, et al. A randomized comparative study of two methods for controlling Tendo Achilles contracture in Duchenne muscular dystrophy. Neuromuscul Disord. 2000;10:257–263. doi: 10.1016/s0960-8966(99)00135-2. [DOI] [PubMed] [Google Scholar]
- 147.Khodadadeh S, McClelland MR, Patrick JH. Variations of gait parameters in Duchenne muscular dystrophy. Proc Inst Mech Eng [H] 1990;204:241–243. doi: 10.1243/PIME_PROC_1990_204_262_02. [DOI] [PubMed] [Google Scholar]
- 148.de Lateur BJ, Giaconi RM. Effect on maximal strength of submaximal exercise in Duchenne muscular dystrophy. Am J Phys Med. 1979;58:26–36. [PubMed] [Google Scholar]
- 149.Lindeman E, Spaans F, Reulen J, et al. Progressive resistance training in neuromuscular patients: effects on force and surface EMG. J Electromyogr Kinesiol. 1999;9:379–384. doi: 10.1016/s1050-6411(99)00003-6. [DOI] [PubMed] [Google Scholar]
- 150.Lindeman E, Leffers P, Spaans F, et al. Strength training in patients with myotonic dystrophy and hereditary motor and sensory neuropathy: a randomized clinical trial. Arch Phys Med Rehabil. 1995;76:612–620. doi: 10.1016/s0003-9993(95)80629-6. [DOI] [PubMed] [Google Scholar]
- 151.Sockolov R, Irwin B, Dressendorfer RH, Bernauer EM. Exercise performance in 6-to-11-year-old boys with Duchenne muscular dystrophy. Arch Phys Med Rehabil. 1977;58:195–201. [PubMed] [Google Scholar]
- 152.Sharma KR, Mynhier MA, Miller RG. Muscular fatigue in Duchenne muscular dystrophy. Neurology. 1995;45:306–310. doi: 10.1212/wnl.45.2.306. [DOI] [PubMed] [Google Scholar]
- 153.Blank A, Gonen B, Magora A. The electrophysiologic pattern of development of muscular fatigue in muscular dystrophy. Electromyogr Clin Neurophysiol. 1980;20:3–18. [PubMed] [Google Scholar]
- 154.Sharma KR, Kent-Braun JA, Majumdar S, et al. Physiology of fatigue in amyotrophic lateral sclerosis. Neurology. 1995;45:733–740. doi: 10.1212/wnl.45.4.733. [DOI] [PubMed] [Google Scholar]
- 155.Scott OM, Hyde SA, Goddard C, et al. Effect of exercise in Duchenne muscular dystrophy. Physiotherapy. 1981;67:174–176. [PubMed] [Google Scholar]
- 156.Vignos PJ, Jr, Watkins MP. The effect of exercise in muscular dystrophy. JAMA. 1966;197:843–848. [PubMed] [Google Scholar]
- 157.Brooks SV, Zerba E, Faulkner JA. Injury to muscle fibres after single stretches of passive and maximally stimulated muscles in mice. J Physiol. 1995;488:459–469. doi: 10.1113/jphysiol.1995.sp020980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Faulkner JA, Brooks SV, Opiteck JA. Injury to skeletal muscle fibers during contractions: conditions of occurrence and prevention. Phys Ther. 1993;73:911–921. doi: 10.1093/ptj/73.12.911. [DOI] [PubMed] [Google Scholar]
- 159.Armstrong RB, Ogilvie RW, Schwane JA. Eccentric exercise-induced injury to rat skeletal muscle. J Appl Physiol. 1983;54:80–93. doi: 10.1152/jappl.1983.54.1.80. [DOI] [PubMed] [Google Scholar]
- 160.Friden J, Lieber RL. Eccentric exercise-induced injuries to contractile and cytoskeletal muscle fibre components. Acta Physiol Scand. 2001;171:321–326. doi: 10.1046/j.1365-201x.2001.00834.x. [DOI] [PubMed] [Google Scholar]
- 161.Lieber RL, Friden J. Mechanisms of muscle injury after eccentric contraction. J Sci Med Sport. 1999;2:253–265. doi: 10.1016/s1440-2440(99)80177-7. [DOI] [PubMed] [Google Scholar]
- 162.MacIntyre DL, Reid WD, Lyster DM, et al. Presence of WBC, decreased strength, and delayed soreness in muscle after eccentric exercise. J Appl Physiol. 1996;80:1006–1013. doi: 10.1152/jappl.1996.80.3.1006. [DOI] [PubMed] [Google Scholar]
- 163.Chappell F, Williams B. Rates and reasons for non-adherence to home physiotherapy in paediatrics. Physiotherapy. 2002;88:138–147. [Google Scholar]
- 164.Sutherland DH, Olshen R, Cooper L, et al. The pathomechanics of gait in Duchenne muscular dystrophy. Dev Med Child Neurol. 1981;23:3–22. doi: 10.1111/j.1469-8749.1981.tb08442.x. [DOI] [PubMed] [Google Scholar]
- 165.McDonald CM. Limb contractures in progressive neuromuscular disease and the role of stretching, orthotics, and surgery. Phys Med Rehabil Clin N Am. 1998;9:187–211. [PubMed] [Google Scholar]
- 166.Scott OM, Hyde SA, Goddard C, Dubowitz V. Prevention of deformity in Duchenne muscular dystrophy: a prospective study of passive stretching and splintage. Physiotherapy. 1981;67:177–180. [PubMed] [Google Scholar]
- 167.Bakker JP, de Groot IJ, Beckerman H, et al. The effects of knee-ankle-foot orthoses in the treatment of Duchenne muscular dystrophy: review of the literature. Clin Rehabil. 2000;14:343–359. doi: 10.1191/0269215500cr319oa. [DOI] [PubMed] [Google Scholar]