Abstract
White adipose tissue plays a critical role in regulating systemic metabolism and can remodel rapidly in response to changes in nutrient availability. Nevertheless, little is known regarding the metabolic changes occurring in adipocytes during obesity. Our laboratory recently addressed this issue in a commonly used, high-fat-diet mouse model of obesity. We found remarkable changes in adipocyte metabolism that occur prior to infiltration of macrophages in expanding adipose tissue. Results of metabolomic analyses, adipose tissue respirometry, electron microscopy, and expression analyses of key genes and proteins revealed dysregulation of several metabolic pathways, loss of mitochondrial biogenetic capacity, and apparent activation of mitochondrial autophagy which were followed in time by downregulation of numerous mitochondrial proteins important for maintaining oxidative capacity. These findings demonstrate the presence of an adipocyte whitening program that may be critical for regulating adipose tissue remodeling under conditions of chronic nutrient excess.
Keywords: adipose tissue, browning, diabetes, insulin resistance, metabolomics, mitochondria, obesity, whitening
The adipocyte is a keystone of systemic metabolism. White adipocytes occupy subcutaneous and internal adipose tissue depots and are the primary site of energy storage and mobilization. Compared with other cell types, they are conspicuously unique: ≥90% of the cell volume of a mature white adipocyte is occupied by a central lipid droplet. This droplet contains triglycerides, prudently placed there via metabolic processes occurring in the cytoplasm, that may be lipolyzed to release the stored energy to maintain or regulate bodily processes during periods of decreased nutrient intake or increased energy expenditure. In addition, white adipocytes secrete numerous factors that regulate feeding behavior, systemic insulin sensitivity, and inflammatory responses.1 These basic functions of the adipocyte, so critical to regulating whole body metabolic homeostasis, depend largely upon adipocyte intermediary metabolism.
Despite the importance of adipocyte metabolism in lipogenesis, lipolysis, and even adipokine secretion, a clear understanding of how it changes with conditions such as nutrient excess have yet to be attained. This is important because such knowledge could prove useful for developing therapies for obesity, which has increased in prevalence remarkably over the past few decades and is a risk factor for developing type 2 diabetes and cardiovascular disease.2,3 To address this, we used a multi-pronged approach to define the metabolic changes that occur in the expanding white adipose organ. Results of metabolomic analyses, adipose tissue respirometry, electron microscopy, and expression analyses of key genes and proteins important for adipocyte metabolism showed evidence of dysregulation of metabolic pathways, loss of mitochondrial biogenetic capacity, mitochondrial remodeling, and activation of mitophagy.4 We propose that these findings demonstrate the presence of a “whitening” program (as opposed to the more commonly considered “browning” program) that occurs in adipocytes under conditions of chronic nutrient excess.
How was the work done?
“We have a habit of writing articles published in scientific journals to make the work as finished as possible, to cover up all the tracks, to not worry about blind alleys or describe how you had the wrong idea first, and so on. So, there isn't any place to publish, in a dignified manner, what you actually did in order to get to do the work.” – Physicist Richard Feynman
This venue offers me the opportunity to tell what was actually done “in order to get to do [and publish] the work.” First things being first, I feel it is important to discuss the considerations we took into account to make the study useful to the scientific community. For solid conclusions to be made and for comparability with the literature, we examined adipose tissue metabolism in a commonly used, reproducible model of obesity—the high fat-fed, C57BL/6J mouse.5-9 Several important methodological issues were considered as well. It was imperative to study metabolism in the intact tissue, as isolation of adipocytes would undoubtedly have confounding effects on their metabolism; however, this approach also had numerous matters with which to deal, primarily, the issue of cell composition.
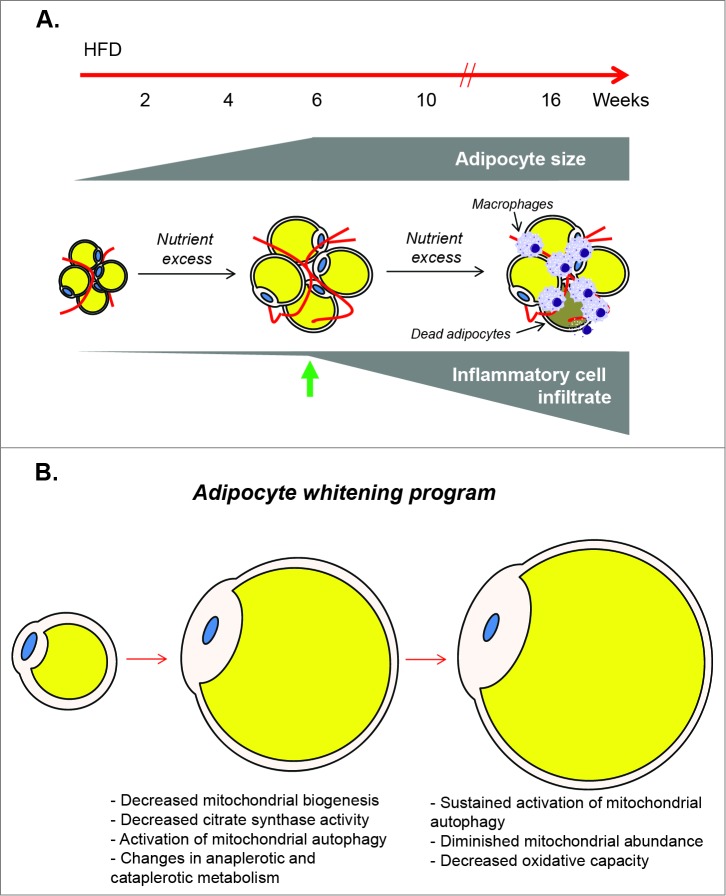
First is the problem of cell infiltrate. Feeding a high fat diet to male C57BL/6J mice has been shown to promote large increases in macrophages and other inflammatory cells in white adipose tissue.10-12 While increases in T cells and neutrophils occur relatively early in the course of high-fat-diet-induced obesity,13 infiltration of the metabolically dynamic macrophage population occurs by 10 weeks of feeding,14,15 with 16 weeks of high fat feeding culminating in massive adipocyte death, termed a “necrotic wave,” that is associated with a profound increase in macrophages.15 Subsequently, the adipose organ is repopulated by new adipocytes and corresponding vasculature.15 Hence, the dynamic nature of the adipose organ, with multiple cell types en voyage into and out of the tissue during its expansion, makes studying adipocyte metabolism in obesity unusually difficult. However, upon six weeks of high fat feeding, we found little evidence of infiltrating macrophages in adipose tissue, despite near maximal increases in adipocyte size and the development of systemic glucose intolerance, insulin intolerance, and hyperinsulinemia.4 Thus, we chose six weeks of high fat feeding as the model to study adipocyte metabolism because it provided a model of near maximal adipocyte hypertrophy occurring in the context of obesity in the relative absence of infiltrating inflammatory cells (Fig. 1A).
Figure 1.
Metabolic changes in white adipocytes during obesity. (A) Considerations for assessing metabolic changes in adipocytes under conditions of nutrient excess: White adipose tissue from mice fed a high fat diet (HFD) was used to identify and measure the metabolic changes that occur in adipocytes during obesity. Near maximal increases in adipocyte size were identified with six weeks of HFD (green arrow), which preceded the increases in macrophage infiltration known to occur with longer durations of nutrient excess. Thus, this time point of high fat feeding provided a model to study adipocyte metabolism in the context of obesity, in the relative absence of infiltrating macrophages which possess high metabolic activity. (B) Putative model of an adipocyte whitening program: Conditions of chronic nutrient excess lead initially to robust adipocyte hypertrophy. When the adipocyte is near its maximal size, Pgc1a is diminished leading to decreased mitochondrial biogenetic potential. Coordinately, citrate synthase activity is decreased, carbon is re-allocated via changes in anaplerotic and cataplerotic metabolism, and mitochondrial autophagy is activated. This leads to overt decreases in mitochondrial abundance and decreased oxidative capacity, i.e. adipocyte whitening.
The second issue relates to the composition of the adipocytes themselves. When normalized to explant mass, our early interpretations from studies on adipose tissue from obese mice suggested a decrease in many metabolites; however, as pointed out in the early stages of manuscript review by the astute and supportive Reviewers and Associate Editor of the American Journal of Physiology Endocrinology and Metabolism, many of our initial conclusions were wrong! Expansion of adipocytes results in less adipocytes and more triglyceride content per explant, which when normalized to tissue mass, led to a specious decrease in many of our metabolic endpoints. How to deal with such an issue is extremely important, not only for our study, but for that of others studying metabolism in tissue explants. And it is of particular importance with respect to adipocytes, where the metabolic action occurs in an area comprising <3% of the total cell volume; in that thin, peripheral rim that contains cytoplasm and forms the boundaries of the cell. This unique structure dictates that a normalizing factor representative of cytoplasmic cell contents would be an appropriate way of accounting for changes in adipocyte number in explants from obese mice. Protein content was the most logical choice and was found to be diminished by ∼40% (per mg tissue) in adipose tissue from high fat-fed mice. This information allowed us to correct our data and helped to elucidate veritable metabolic changes that occur in white adipose tissue of obese mice. Of note, it would not have been appropriate to normalize values to mitochondrial content, as this was unchanged at the six week time point of high fat diet studied. It is our hope that this rather simple, yet critical point helps others to correct or normalize data to interpret results from adipose explant studies more accurately.
What insights does the study bestow?
“Data does not equal information; information does not equal knowledge; and, most importantly of all, knowledge does not equal wisdom. We have oceans of data, rivers of information, small puddles of knowledge, and the odd drop of wisdom.” – Ecologist Henry Nix.
The results of the study gave us large amounts of data to consider and integrate into a conceptual framework. In our metabolomics analysis, we found changes consistent with adipocyte inflammation, osmotic stress, and re-allocation of carbon, likely via anaplerotic and cataplerotic processes. Although mitochondrial abundance as detected by mitochondrial DNA abundance (relative to nuclear DNA) as well as mitochondrial protein abundance was not changed with 6 weeks of nutrient excess, citrate synthase activity was remarkably diminished, and Pgc1a was downregulated, indicating decreased mitochondrial biogenetic capacity. At this time point in the obesity protocol, indicators of mitophagy were elevated and there was ultrastructural and biochemical evidence of activation of autophagy. By 12 weeks of high fat diet, there were extensive decreases in the abundance of multiple mitochondrial matrix and electron transport chain proteins.4
Collectively, this information enables construction of a new working model of how adipocyte metabolism remodels under conditions of nutrient excess. We posit that once an adipocyte reaches near maximal size, a coordinated adipocyte “whitening” program is triggered. Sensing that it is nearly overladen with triglycerides, the adipocyte downregulates citrate synthase, allocates carbon to metabolic intermediates important for fat storage, diminishes mitochondrial biogenetic potential, and activates mitophagy to decrease mitochondrial mass (Fig. 1B). The term whitening seems appropriate by analogy. Because adipocyte “browning” occurs when mitochondrial abundance (and typically uncoupling protein 1 as well) is increased in adipose tissue,17 a loss of mitochondrial cytochromes, which give mitochondria their characteristic brown pigmentation, promotes a loss of color—a whitening.
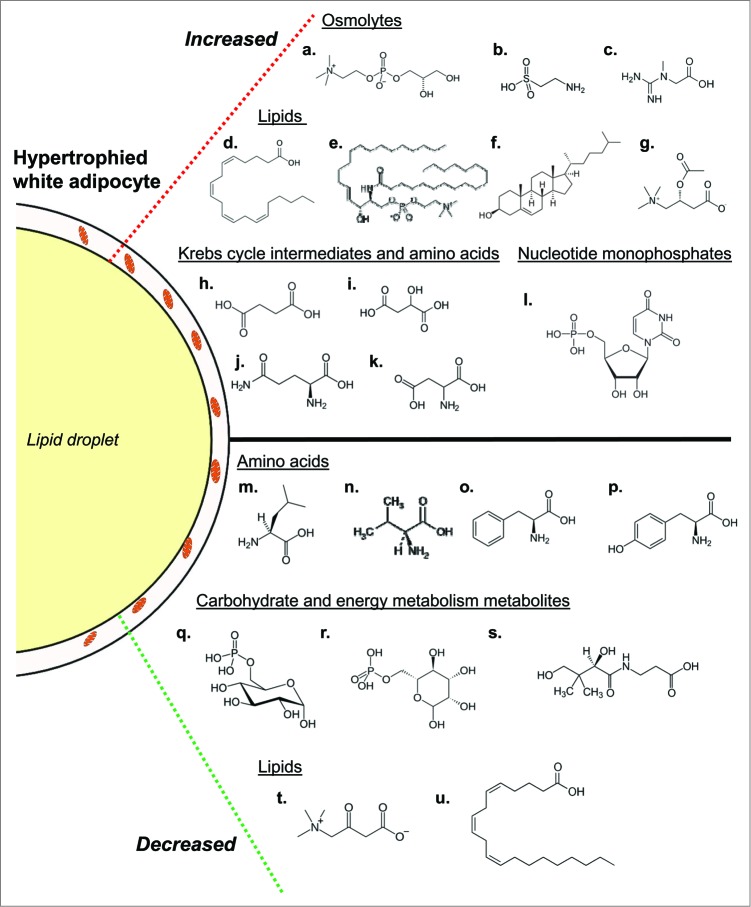
Figure 2.
Overview of biochemical changes occurring during adipocyte whitening. Conditions of chronic nutrient excess leading to adipocyte hypertrophy are associated with changes in several metabolic pathways. Increased in abundance (top; a-l) are several osmolytes including glycerophosphorylcholine (a), taurine (b) and hypotaurine (not shown), and creatine (c). Also increased are numerous fatty acids. Shown is arachidonate (d) as well as its downstream product 12-HETE (not shown), which may be involved in adipocyte inflammation. Other indicators of adipocyte inflammation that were higher in abundance in adipose tissues from obese mice include stearoyl sphingomyelin (e) and cholesterol (f). Evidence of lipid buffering is suggested by elevated levels of the carnitine lipid derivatives acetylcarnitine (g) and propionylcarnitine (not shown). Several Krebs cycle intermediates and amino acids were increased in abundance as well; these include succinate (h), malate (i), glutamine (j), glutamate (not shown), and aspartate (k). Additionally, the nucleotide monosphosphates uridine monophosphate (l), cytidine monophosphate (not shown) and inosine monophosphate (not shown) were increased. Also increased in obese mice were the 6-carbon sugars glucose and maltose (not shown). In tandem with the higher levels of these metabolites were decreases in numerous other biochemicals. Decreases in several amino acids involved in anaplerosis, including leucine (m), valine (n), phenylalanine (o) and tyrosine (p), were observed in adipose tissues of high fat-fed mice. Levels of phosphorylated glucose (q) and mannose (r) as well as pantothenate (s), which is important in coenzyme A synthesis, were diminished as well. Finally, the carnitine degradation product 3-dehydrocarnitine (t) and mead acid (u) were metabolites in the lipid family that were lower in abundance in adipose tissues from high fat-fed mice.
Many biochemical features are suggestive of a metabolic remodeling that would be consistent, or complementary, to a whitening program. It is possible that the decrease in citrate synthase is a line of defense triggered by adipocytes to help prevent ectopic lipid deposition. By decreasing synthesis of citrate, fatty acid synthesis from glucose would be limited, thereby diminishing de novo lipogenesis and possibly empowering traditional lipogenesis pathways to esterify and store free fatty acids derived from the circulation. Metabolomics analysis also identified elevated levels of the Krebs cycle intermediates malate and succinate, which may be particularly important for fat storage: malate is known to be used for the cataplerotic production of precursors of glycerol-3-phosphate, which is used for fat esterification18; and the higher levels of succinate could function as an autocrine, biochemical mitokine that inhibits lipolysis, likely via activation of Gpr91.18 The coordinate changes in these metabolic pathways could act in concert with the mitochondrial biogenetic circuitry and autophagic processes to promote or maintain maximal lipid storage and limit ectopic deposition of fat.
As summarized in the illustration in Figure 2, the wealth of metabolomic data also helped identify other pathways markedly affected in adipocytes under conditions of nutrient excess.4 Key changes in lipid pathways were apparent, with increases in the inflammatory mediator 12-HETE as well as most metabolites in the arachidonate synthesis pathway occurring in adipose tissues from high fat-fed mice. Other biochemical evidence for adipocyte inflammation included an increase in cholesterol and osmolytes, both of which could activate the inflammasome.20-23 Recent evidence suggests that such increases in adipocyte inflammation could be an adaptive response to promote adipose tissue remodeling and expansion under conditions of nutrient excess.24
The heightened levels of carnitine derivatives such as acetylcarnitine and propionylcarnitine, which could act as a buffer or repository of excess carbon, suggest that adipocyte mitochondria in near maximally enlarged adipocytes might be “flooded” with substrate under conditions of nutrient excess. That respiratory responses to a mitochondrial uncoupler were higher in hypertrophied adipocytes provides further support to this notion. We speculate that the adipocyte responds to the fuel-rich condition by triggering the whitening program, which decreases the abundance of mitochondria to limit lipid oxidation and concomitant oxidative stress, thereby promoting lipid storage and preventing excessive tissue damage.
Where do these findings leave us?
While the study provides novel insights into a whitening program in adipose tissue, the data spawned numerous questions, making us even more aware of how much we don't know. Some questions are definitional in nature, but have important implications. For example, what is mitochondrial remodeling? Most commonly, mitochondrial remodeling is described as changes in the architecture of mitochondria, dictated by mitochondrial fission and fusion processes.25-27 Should this definition be refined? It may be appropriate to have a more general definition for mitochondrial remodeling. For example, changes to mitochondria that affect their metabolic function, which could include upregulation and/or downregulation of mitochondrial proteins (i.e., changes in the mitochondrial proteome), could be a more accurate definition of mitochondrial remodeling. The changes in numerous mitochondrial metabolites along with decreases in citrate synthase activity and upregulation of some genes coding for mitochondria-contained proteins (e.g., Cox7a1), despite downregulation of others (e.g., Sirt3), would be sufficient to fit this criteria.4 Nevertheless, by electron microscopy it appeared that increased fission occurs in adipocytes from obese mice, which leads to the question of whether mitochondrial structure dictates their metabolic function or mitochondrial metabolic function dictates their structure. From the current literature, an argument could be made for both.
Several results of the study fertilize other grounds of thought. Why would the adipocyte upregulate genes such as Cox7a1, considered a heart/muscle-”specific” subunit of cytochrome oxidase, while at the same time downregulating Sirt3 and Pgc1a and activating autophagic programs to diminish mitochondrial abundance? It would appear that increasing Cox7a1 would be more consistent with a browning program rather than a whitening one, and that its induction would be a programmatic conflict of interest. How do we reconcile this response with the whitening program? Excessive consumption of calories has been shown to have the capacity to increase energy utilization via diet-induced thermogenesis (originally coined by Neumann as “luxuskonsumption”)17; hence, it is possible that upregulation of Cox7a1 is an evolutionarily conserved response to promote fuel utilization in the face of nutrient excess. Indeed, interventions that promote a browning of adipose tissue, such as inhibiting autophagy, promote lipid oxidation and have favorable effects on systemic metabolism.28 Could extreme nutrient excess, as occurs with continuous provision of high fat diet, result in a state metabolic confusion evidenced by the initiation of components of both the browning and whitening adipocyte programs? The presence of such incongruities suggest that teasing out the contribution of individual metabolic changes—such as those associated with osmotic stress, Krebs cycle metabolites, as well as interesting genes such as Cox7a1—is a worthwhile undertaking.
Is the whitening program beneficial or deleterious in the context of obesity? This is the ultimate question, one for which we don't yet know the answer. However, I think it is a safe assumption that adipose whitening is part of a much broader program for accommodating a chronically elevated intake of calories. Initially, the physiological response to excessive caloric intake appears to primarily be via hypertrophy of existing white adipocytes with modest recruitment and differentiation of preadipocytes (i.e., adipogenesis)29,30; however, prolonged periods of nutrient excess (in mice) cause large-scale adipocyte death, a robust inflammatory response, and sweeping repopulation of the adipose organ with new adipocytes.16,30 This adipose tissue remodeling process appears to lead to an improved capacity to accommodate lipid and to increase insulin sensitivity.16 Such favorable changes in systemic metabolism may be due to adipocyte hyperplasia, leading to an increased capacity to store excess calories. Indeed, increases in white adipocyte number, such as that coerced in several obesity models in which insulin sensitivity (e.g., adiponectin overexpression31) or mitochondrial function (e.g., modulation of MitoNEET expression32) have been altered, appear to promote the development of an adipose organ with more, smaller adipocytes and results in sustained systemic insulin sensitivity despite morbid obesity. It is currently unclear how the orchestrated program of adipocyte whitening might affect recruitment or hyperplasia of (pre)adipocytes.
How could adipocyte whitening fit into, or modify, our current understanding of adipose tissue remodeling? The current collective thought is that, during obesity, adipocytes die due to insufficient perfusion and hypoxia, which is in part caused by extreme increases in adipocyte size and insufficient vasculature.29,33 This is then thought to recruit macrophages to clear the dead cells, with such inflammatory responses contributing to systemic insulin resistance.29,34,35 However, recent studies have challenged this paradigm by showing the absence of hypoxia and even increased oxygen tensions in adipose tissues of obese humans.36-38 Furthermore, oxygen consumption in adipose tissues and adipocytes of obese humans has been demonstrated to be diminished compared with that of lean counterparts,37-39 which could support the concept of adipocyte whitening. The fact that mitochondrial protein abundance is diminished remarkably several weeks prior to the necrotic wave phenomena in mice4,16 would appear to suggest that not only is oxygen consumption by adipocytes unlikely to contribute to hypoxia, but that the ensuing necrotic cell death may be due simply to loss of mitochondrial abundance and lack of a capacity to provide usable energy for adipocyte survival; that is, in adapting to store excess energy, adipocytes might lose the ability to generate ATP for themselves. It would then seem more plausible that the brunt of adipose tissue hypoxia measured in many studies is caused by disproportionate levels of metabolically active macrophages working to clear dead adipocytes (Fig. 3). Should this scenario be correct, then we would expect that preventing adipose tissue whitening would mitigate both adipocyte death and macrophage infiltration in adipose tissue. However, it should be noted that an increase in mitochondrial uncoupling and adipocyte oxygen consumption occurring early (e.g., 3-7 days) after provision of a high fat diet has been associated with higher abundance of HIF1α in adipose tissue, inflammation and insulin resistance,40 suggesting that a more brown-like adipocyte phenotype might not always be beneficial. Future studies in which adipocyte whitening is prevented or coerced could lend insight into whether this process is beneficial or harmful in the context of obesity.
Figure 3.
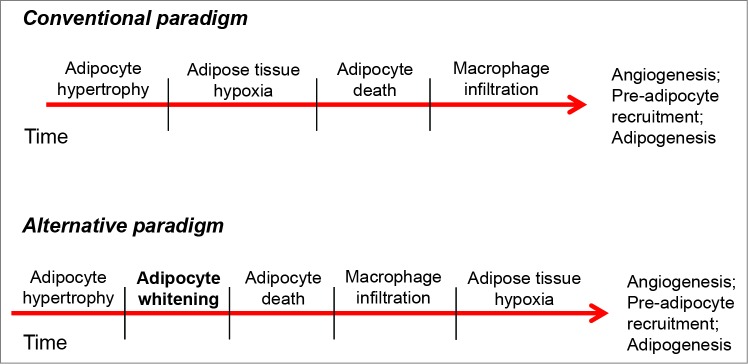
Potential role of adipocyte whitening in the adipose tissue remodeling paradigm. In the conventional view, chronic nutrient excess promotes excessive adipocyte hypertrophy, which contributes to adipose tissue hypoxia, necrotic cell death, and macrophage infiltration. Inclusion of the adipocyte whitening program could change this paradigm. Here, adipocyte hypertrophy is followed in time by decreases in mitochondrial abundance (whitening), which diminishes the capacity of the adipocyte to provide energy for itself. This results in adipocyte necrotic cell death, which is followed by a large influx of inflammatory cells having high metabolic activity. The relatively high rate of oxygen consumption by macrophages leads to adipose tissue hypoxia, which, in turn, is important for signaling for angiogenesis and the recruitment of new pre-adipocytes to aid in tissue remodeling.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
The author acknowledges grant funding by the NIH (GM103492).
References
- 1. Williams G, Frühbeck G. Obesity: Science to Practice. Hoboken, NJ: Wiley; 2009. [Google Scholar]
- 2. Ahima RS. Digging deeper into obesity. J Clin Invest 2011; 121:2076-9; PMID:21633174; http://dx.doi.org/ 10.1172/JCI58719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009-2010. NCHS Data Brief 2012; 82:1-8. [PubMed] [Google Scholar]
- 4. Cummins TD, Holden CR, Sansbury BE, Gibb AA, Shah J, Zafar N, Tang Y, Hellmann J, Rai SN, Spite M, et al. . Metabolic remodeling of white adipose tissue in obesity. Am J Physiol Endocrinol Metab 2014; 307:E262-77; PMID:24918202; http://dx.doi.org/ 10.1152/ajpendo.00271.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Collins S, Martin TL, Surwit RS, Robidoux J. Genetic vulnerability to diet-induced obesity in the C57BL6J mouse: physiological and molecular characteristics. Physiol Behav 2004; 81:243-8; PMID:15159170; http://dx.doi.org/ 10.1016/j.physbeh.2004.02.006 [DOI] [PubMed] [Google Scholar]
- 6. Petro AE, Cotter J, Cooper DA, Peters JC, Surwit SJ, Surwit RS. Fat, carbohydrate, and calories in the development of diabetes and obesity in the C57BL6J mouse. Metabolism 2004; 53:454-7; PMID:15045691; http://dx.doi.org/ 10.1016/j.metabol.2003.11.018 [DOI] [PubMed] [Google Scholar]
- 7. Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. Diet-induced type II diabetes in C57BL6J mice. Diabetes 1988; 37:1163-7; PMID:3044882; http://dx.doi.org/ 10.2337/diab.37.9.1163 [DOI] [PubMed] [Google Scholar]
- 8. West DB, Boozer CN, Moody DL, Atkinson RL. Dietary obesity in nine inbred mouse strains. Am J Physiol 1992; 262:R1025-32; PMID:1621856 [DOI] [PubMed] [Google Scholar]
- 9. Sansbury BE, Cummins TD, Tang Y, Hellmann J, Holden CR, Harbeson MA, Chen Y, Patel RP, Spite M, Bhatnagar A, et al. . Overexpression of endothelial nitric oxide synthase prevents diet-induced obesity and regulates adipocyte phenotype. Circulat Res 2012; 111:1176-89; PMID:22896587; http://dx.doi.org/ 10.1161/CIRCRESAHA.112.266395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW, Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003; 112:1796-808; PMID:14679176; http://dx.doi.org/ 10.1172/JCI200319246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 2007; 117:175-84; PMID:17200717; http://dx.doi.org/ 10.1172/JCI29881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes 2007; 56:16-23; PMID:17192460; http://dx.doi.org/ 10.2337/db06-1076 [DOI] [PubMed] [Google Scholar]
- 13.Mathis D. Immunological goings-on in visceral adipose tissue. Cell Metab 2013; 17:851-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, et al. . CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 2009; 15:914-20; PMID:19633658; http://dx.doi.org/ 10.1038/nm.1964 [DOI] [PubMed] [Google Scholar]
- 15. Spite M, Hellmann J, Tang Y, Mathis SP, Kosuri M, Bhatnagar A, Jala VR, Haribabu B. Deficiency of the leukotriene B4 receptor, BLT-1, protects against systemic insulin resistance in diet-induced obesity. J Immunol 2011; 187:1942-9; http://dx.doi.org/ 10.4049/jimmunol.1100196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW, 2nd, DeFuria J, Jick Z, Greenberg AS, Obin MS. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes 2007; 56:2910-8; PMID:17848624; http://dx.doi.org/ 10.2337/db07-0767 [DOI] [PubMed] [Google Scholar]
- 17. Tseng YH, Cypess AM, Kahn CR. Cellular bioenergetics as a target for obesity therapy. Nat Rev Drug Disc 2010; 9:465-82; PMID:20514071; http://dx.doi.org/ 10.1038/nrd3138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Owen OE, Kalhan SC, Hanson RW. The key role of anaplerosis and cataplerosis for citric acid cycle function. J Biol Chem 2002; 277:30409-12; PMID:12087111; http://dx.doi.org/ 10.1074/jbc.R200006200 [DOI] [PubMed] [Google Scholar]
- 19. Regard JB, Sato IT, Coughlin SR. Anatomical profiling of G protein-coupled receptor expression. Cell 2008; 135:561-71; PMID:18984166; http://dx.doi.org/ 10.1016/j.cell.2008.08.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol 2009; 7:99-109; PMID:19148178; http://dx.doi.org/ 10.1038/nrmicro2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schroder K, Tschopp J. The inflammasomes. Cell 2010; 140:821-32; PMID:20303873; http://dx.doi.org/ 10.1016/j.cell.2010.01.040 [DOI] [PubMed] [Google Scholar]
- 22. Giordano A, Murano I, Mondini E, Perugini J, Smorlesi A, Severi I, Barazzoni R, Scherer PE, Cinti S. Obese adipocytes show ultrastructural features of stressed cells and die of pyroptosis. J Lipid Res 2013; 54:2423-36; PMID:23836106; http://dx.doi.org/ 10.1194/jlr.M038638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yu BL, Zhao SP, Hu JR. Cholesterol imbalance in adipocytes: a possible mechanism of adipocytes dysfunction in obesity. Obes Rev 2010; 11:560-7; PMID:20025694; http://dx.doi.org/ 10.1111/j.1467-789X.2009.00699.x [DOI] [PubMed] [Google Scholar]
- 24. Wernstedt Asterholm I, Tao C, Morley TS, Wang QA, Delgado-Lopez F, Wang ZV, Scherer PE. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab 2014; 20:103-18; PMID:24930973; http://dx.doi.org/ 10.1016/j.cmet.2014.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Karbowski M, Youle RJ. Dynamics of mitochondrial morphology in healthy cells and during apoptosis. Cell Death Differ 2003; 10:870-80; PMID:12867994; http://dx.doi.org/ 10.1038/sj.cdd.4401260 [DOI] [PubMed] [Google Scholar]
- 26. Cheung EC, McBride HM, Slack RS. Mitochondrial dynamics in the regulation of neuronal cell death. Apoptosis: An Int J Programmed Cell Death 2007; 12:979-92; PMID:17453163; http://dx.doi.org/ 10.1007/s10495-007-0745-5 [DOI] [PubMed] [Google Scholar]
- 27. Galloway CA, Yoon Y. Mitochondrial morphology in metabolic diseases. Antioxidants Redox Signaling 2013; 19:415-30; PMID:22793999; http://dx.doi.org/ 10.1089/ars.2012.4779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Goldman S, Baerga R, Zhao Y, Komatsu M, Jin S. Adipose-specific deletion of autophagy-related gene 7 (atg7) in mice reveals a role in adipogenesis. Proc Natl Acad Sci U S A 2009; 106:19860-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest 2011; 121:2094-101; PMID:21633177; http://dx.doi.org/ 10.1172/JCI45887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med 2013; 19:1338-44; PMID:23995282; http://dx.doi.org/ 10.1038/nm.3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest 2007; 117:2621-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kusminski CM, Holland WL, Sun K, Park J, Spurgin SB, Lin Y, Askew GR, Simcox JA, McClain DA, Li C, et al. MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nat Med 2012; 18:1539-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Trayhurn P. Hypoxia and adipocyte physiology: implications for adipose tissue dysfunction in obesity. Ann Rev Nutr 2014; 34:207-36; PMID:24819450; http://dx.doi.org/ 10.1146/annurev-nutr-071812-161156 [DOI] [PubMed] [Google Scholar]
- 34. Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, et al. . Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 2003; 112:1821-30; PMID:14679177; http://dx.doi.org/ 10.1172/JCI200319451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. British J Nutr 2004; 92:347-55; PMID:15469638; http://dx.doi.org/ 10.1079/BJN20041213 [DOI] [PubMed] [Google Scholar]
- 36. Goossens GH, Bizzarri A, Venteclef N, Essers Y, Cleutjens JP, Konings E, Jocken JW, Cajlakovic M, Ribitsch V, Clement K, et al. . Increased adipose tissue oxygen tension in obese compared with lean men is accompanied by insulin resistance, impaired adipose tissue capillarization, and inflammation. Circulation 2011; 124:67-76; PMID:21670228; http://dx.doi.org/ 10.1161/CIRCULATIONAHA.111.027813 [DOI] [PubMed] [Google Scholar]
- 37. Hodson L, Humphreys SM, Karpe F, Frayn KN. Metabolic signatures of human adipose tissue hypoxia in obesity. Diabetes 2013; 62:1417-25; PMID:23274888; http://dx.doi.org/ 10.2337/db12-1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hodson L. Adipose tissue oxygenation: effects on metabolic function. Adipocyte 2014; 3:75-80; PMID:24575375; http://dx.doi.org/ 10.4161/adip.27114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yin X, Lanza IR, Swain JM, Sarr MG, Nair KS, Jensen MD. Adipocyte mitochondrial function is reduced in human obesity independent of fat cell size. J Clin Endocrinol Metab 2014; 99:E209-16; PMID:24276464; http://dx.doi.org/ 10.1210/jc.2013-3042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee YS, Kim JW, Osborne O, Oh da Y, Sasik R, Schenk S, Chen A, Chung H, Murphy A, Watkins SM, et al. Increased adipocyte O2 consumption triggers HIF-1alpha, causing inflammation and insulin resistance in obesity. Cell 2014; 157:1339-52. [DOI] [PMC free article] [PubMed] [Google Scholar]