Abstract
Excessive nutrient intake in obesity triggers the accumulation of various types of immune cells in adipose tissue, particularly visceral adipose tissue (VAT). This can result in chronic inflammation which disrupts insulin effects on adipocytes and muscle cells and culminates in development of insulin resistance. The interplay between immune cells and adipose tissue is a key event for the development of insulin resistance that precedes type 2 diabetes. CD40, a well-documented costimulatory receptor, is required for efficient systemic adaptive immune responses. However, we and other groups recently showed that CD40 unexpectedly ameliorates inflammation in VAT and accordingly attenuates obesity-induced insulin resistance. Specifically, although CD40 is typically considered to play its principal immune roles on B lymphocytes and myeloid cells, we found that CD40+CD8+ T lymphocytes were major contributors to the protective effect. This unexpected inhibitory role of CD40 on CD8+ T cell activation in VAT may reflect unique features of this microenvironment. Additional knowledge gaps include whether CD40 also plays roles in mucosal immunity that control the homeostasis of gut microbiota, and human metabolic diseases. Potential therapeutic approaches, including stimulating CD40 signaling and/or manipulating specific CD40 signaling pathways in the VAT microenvironment, may open new avenues for treatment of obesity-induced insulin resistance, and prevention of type 2 diabetes.
Keywords: CD40, gut microbiota, insulin resistance, mucosal immunity, obesity, T cell
Abbreviations: TNF-α, tumor necrosis factor-α; MCP-1, Monocyte Chemoattractant Protein-1; IL-1β, interleukin-1β; IL-6, interleukin-6; Treg, regulatory T cells; VAT, visceral adipose tissue; PPAR-γ, peroxisome proliferator-activated receptor-γ; Ig, immunoglobulin; Ab, antibody; LFD, low fat diet; HFD, high fat diet; DIO, diet induced obesity; IR, insulin resistance; CD40KO, CD40 deficiency; Mφ, macrophage; UPR, unfolded protein response; Rag1, recombination activating gene 1; HIGM, Hyper-IgM syndrome
Obesity-induced chronic, low-grade inflammation, termed metainflammation, is a major factor leading to the development of insulin resistance (IR) and type 2 diabetes.1,2 Although the underlying mechanisms are still under intensive investigation, it is clear that adipose tissue as a special microenvironment and immune cells as major players, are both essential elements for the initiation and promotion of metainflammation.1-3 It is well known that both innate and adaptive immune cells infiltrate adipose tissue in obesity and promote or regulate IR.2,4-7 Cytokines and chemokines produced by these cells, including TNF-α, MCP-1, IL-1β, and IL-6, are essential inflammation mediators impairing insulin sensitivity in adipocytes and muscle cells.8-11 However, the exact roles these cells and cytokines/chemokines play during the development of inflammation in adipose tissue remain incompletely understood. In addition, once inflammation is present, any changes that disturb the complex interplay of cells and soluble factors may aggravate or attenuate the inflammation. Immune cells and factors are not the only contributors to this inflammation. It is believed that long-term nutrient excess drives chronic inflammation in the visceral adipose tissue (VAT), with potential mechanisms including oxidative stress, endoplasmic reticulum stress, hypoxia, amyloid and lipid deposition, lipotoxicity, and glucotoxicity in adipose tissue.12 Immune cells accumulating in adipose tissue must accommodate to this harsh environment, which might induce alterations in their biological properties. For example, regulatory T (Treg) cells in VAT acquire expression of peroxisome proliferator-activated receptor (PPAR)-γ, the ‘master regulator’ of adipocyte differentiation, which is required for Treg cell function in VAT.13 The uniqueness of the obese VAT environment is also highlighted by the distinctive composition, phenotype, and function of different immune cells, compared to those in lymphoid and other non-lymphoid tissues. Additionally, biased T cell receptor repertoires in CD4+ and CD8+ T cells in VAT in obese mice suggests the existence of potential self-antigens in VAT that induce T cell reactivity.7,14 The crosstalk between immune cells and the adipose tissue environment is a special feature of obesity-associated adipose inflammation.
CD40 and CD154 (CD40 ligand)
CD40 is a key costimulatory receptor mediating interactions between antigen presenting cells (APC) and T cells. In addition to its constitutive expression on APC such as myeloid cells and B lymphocytes, CD40 expression can be induced on eosinophils, T cells, adipocytes, fibroblasts, epithelial cells and others, under specific conditions.15-17 The most common source of the natural ligand for CD40, CD154 (previously known as CD40L or gp39), is induced expression on activated CD4+ T cells. However, CD154 expression can also be induced upon activation on CD8+ T cells, mast cells, basophils, eosinophils, and platelets.17 Humans with mutations in the cd154 gene leading to deficiencies in expression or CD40 binding, such as those found in X-linked Hyper-IgM syndrome (HIGM), have lower basal serum levels of IgG, IgA, and IgE isotypes of antibody (Ab) due to impaired immunoglobulin isotype switching, and are susceptible to opportunistic bacterial infections.18,19 CD40 stimulation of APC is also important to development of T cell-mediated immunity to intracellular pathogens.20 Mice deficient in CD154 or CD40 (CD40KO) recapitulate HIGM.21,22 CD40 can also be expressed on a subset of hyper-activated CD4+ T cells, which was reported to exacerbate pathogenesis in animal models of autoimmune insulin-dependent type 1 diabetes and collagen-induced arthritis.15,16 Given the important role of CD40 in immune responses, blockade or stimulation of CD154-CD40 signaling are strategies being broadly explored for the treatment of various human diseases.23
Potential Roles of CD154 in Obesity and Insulin Resistance
As a critical costimulator in immune responses, it was reasonable to assume that deficiency in CD40 signaling would have the effect of attenuating inflammation in obese VAT. A clinical study showed that obese and diabetic individuals have higher levels of active soluble CD154 in the circulation than lean healthy subjects, and CD40 mRNA levels in white adipose tissue positively correlate with body mass index.24 Genetic deficiency of CD154 in mice of the C57Bl/6J (B6) genetic background attenuates the development of diet-induced obesity (DIO) and hepatic steatosis, and results in improved systemic insulin sensitivity. Immune cell infiltration in VAT is also reduced.25 Another report showed that CD154 deficiency in B6 mice results in a favorable metabolic phenotype and attenuated inflammation in VAT when mice are fed a low fat diet (LFD), but interestingly, not when they consume a high fat diet (HFD). Typical measures of energy metabolism of these mice when fed a LFD—including food intake, heat production, and respiratory exchange ratio—are not altered from those of CD154-sufficient mice. Reduced MCP-1 production in VAT of the CD154-deficient mice is attributed to attenuated inflammation, and it was hypothesized that consuming a HFD might overwhelm the anti-inflammatory capacity in CD154-deficient mice.26 In contrast to these reports, deficiency of CD154 in mice of the Balb/c genetic background induces severe hepatic steatosis due to an altered unfolded protein response (UPR), when fed a diet rich in olive oil. Therefore, CD154 plays roles in hepatic steatosis by modulating the UPR in hepatocytes.27 The variation in results found between these studies may be at least in part attributable to the use of genetically distinct mouse strains and/or experimental approaches.
The Role of CD40 in Obesity and IR
Although findings with CD154-deficient mice may appear to support the expected prediction that CD40 signaling promotes inflammation in adipose tissue and therefore aggravates IR, results from CD40KO mice surprisingly gave the opposite result. It was first reported by Guo et al.28 that CD40KO mice exhibit severe liver steatosis, IR, glucose intolerance, and aggravated inflammation in adipose tissue. This result seems incompatible with the well-documented costimulatory effect of CD40 in immune responses. However, 3 recently published reports, including one from our lab, show similar results. Thus, these reports from multiple labs together establish a protective role for CD40 in obesity-associated IR, and inflammation in adipose tissue. The revelation of this novel role for CD40 in metabolic diseases now raises intriguing questions about the nature of the molecular mechanisms by which CD40 ameliorates the development of obesity and IR, a role which may be unique to the VAT environment.
We observed for some time that CD40KO mice tend to be larger than their littermate controls. Recently, we decided to investigate the underlying cause of this phenotype, using the well-characterized HFD-induced obesity mouse model.29 Interestingly, CD40KO mice gain more body weight than control mice, largely due to more visceral fat deposition on the HFD. These mice also exhibit exacerbated IR and evidence for dysregulation of both carbohydrate and lipid metabolism.29 Consistent with the previous report,28 aggravated local inflammation in VAT was found, particularly a significant increase in the presence of macrophages (Mφ) and CD8+ T cells. To our surprise, we found that CD40 expressed on CD8+ T cells, but not B cells, CD4+ T cells, myeloid cells, or non-immune cells plays major protective effects in decreasing inflammation in the VAT and development of IR. Consistently, CD40-deficient CD8+ T cells in VAT produce more proinflammatory cytokines and chemokines, which is critical for immune cell infiltration or proliferation and IR induction in DIO CD40KO mice. We thus propose that CD40 may play a special role in the microenvironment of obese VAT through regulating CD8+ T cell function.
A comparable phenotype for CD40KO mice was observed by Wolf et al.30 Consistent with our report, they also found that CD40KO T cells but not B cells transfer the dysmetabolism and VAT inflammation phenotype in Rag1 deficient mice, indicating a protective role of CD40 on T cells in the metabolic syndrome. Interestingly, activation of CD40 with the agonistic mAb FGK45 abrogates further weight gain, improves insulin sensitivity and dampens adipose tissue inflammation, by reducing pro-inflammatory cytokines produced by T cells after CD40 activation. Together, the findings from our labs raise the possibility of potential therapeutic approaches to combat VAT inflammation and IR via targeting pathways regulated by CD40 in CD8+ T cells. It is noteworthy that although systemic usage of CD40-stimulating Ab improves IR,30 activation of CD40 signaling in the whole body may cause global side effects.31 The proper approach to CD40 as a therapeutic target might be local administration of CD40-binding Ab in adipose tissue. In addition, Abs targeting different epitopes of CD40 generate distinct biological effects31 and different CD40 signaling pathways play distinct roles in CD40-mediated biological functions.32,33 For example, agonistic anti-CD40 Abs do not mimic the natural ligand CD154 in stimulating CD40-mediated IL-6 production by B cells.34 Therefore, exploring Abs targeting different CD40 epitopes, or reagents specifically stimulating or blocking certain signaling pathways of CD40 in adipose tissue may be the best approach to using CD40 as a therapeutic target to modulate DIO and/or IR. In addition to the reports discussed above, another study showed that although increased body weight gain occurs in a short period in CD40KO mice fed a HFD, these mice also display worsened IR due to severe inflammation in adipose tissue.35
The reason for the different phenotypes in CD154 vs. CD40-deficient mice in response to HFD feeding is not clear. It was reported that CD154-mediated signaling in T cells acts as a co-stimulator to enhance T cell activation.36 As the expression profiles of CD40 and CD154 are different,17 and CD154 can function through distinct receptors additional to CD40, such as Mac-1/CD11b,37 it is possible that the distinct phenotypes observed may result from overlapping but different physiologic alterations in mice lacking CD154 vs. CD40. More studies are required to delineate the specific roles of CD154 and CD40 in metabolic disease.
The Role of CD40 in the Microenvironment of Adipose Tissue
Although there is marked infiltration of several immune cell types in VAT in CD40KO mice, CD8+ T cells proved to be the most critical factor for inducing inflammation in the VAT and systemic IR.29 We observed that CD40 expression on these T cells is only seen in the VAT. Interestingly, CD40 expressed by CD8+ T cells plays an inhibitory role, as WT CD8+ T cells isolated from VAT produce less pro-inflammatory cytokines/chemokines than CD40KO CD8+ T cells. Furthermore, simultaneous stimulation of CD8+ T cells with anti-CD3+anti-CD28 Abs and CD40-specific agonist Abs partially inhibits this cytokine/chemokine production.29 Therefore, CD40 expressed on CD8+ T cells in the VAT suppresses CD8+ T cell activation.
The role of CD40 expressed on CD8+ T cells in T cell-mediated adaptive immunity has been the topic of a number of previous investigations, but mixed conclusions have resulted from these studies. Using an HY transgenic mouse model, it was reported that CD40 expression on CD8+ T cells is required for memory T cell differentiation and function.38 Recent studies also showed that intrinsic CD40 signaling is important for optimizing CD8+ T cell responses during influenza infection, and for rescuing exhausted CD8+ T cells.39-41 CD40 signaling also promotes optimal CD8+ effector T cell responses and blocks the differentiation of antigen specific inducible Treg cells in a skin transplant model.42 In contrast, other studies indicate that CD40 signaling in CD8+ T cells is not required for the development and function of memory T cells in viral or bacterial infection models.43,44 These disparate conclusions could be due to the different mouse models used and/or distinct aspects of the specific immune responses observed.
Our findings that CD40 signaling in CD8+ T cells in adipose tissue inhibits their function adds new information, albeit new complexity, to this question. However, the unique microenvironment of the VAT must be considered, as it could broadly affect the biology of CD8+ T cells. It was reported that interactions between adipocytes and CD8+ T cells are crucial for CD8+ T cells to mediate Mφ migration and activation, and both CD4+ and CD8+ T cells in the VAT show a biased TCR repertoire,6,7,14 highlighting the unique immune environment of the VAT. We propose that the metabolism of CD8+ T cells might be altered in this “high lipid” environment, and continued expression and activation of CD40 might lead to exhaustion of CD8+ T cells. Although there are still many questions to answer, our results indicate that local tissue environmental factors could be a critical regulator of the role of CD40 during T cell functions. From this standpoint, usage of anti-CD40 Ab locally in the adipose tissue is more desirable and could avoid unnecessary side effects.
Multiple Mechanisms of CD40 Function in DIO and Metabolism
Because CD40 deficiency affects immune responses in multiple ways, other mechanisms that contribute to the aggravated IR and inflammation in adipose tissue cannot be excluded. It is now well-appreciated that the gut flora is a major modulator of nutrient metabolism and mucosal immunity is critical to maintain the homeostasis of healthy gut bacteria.45,46 CD40 deficiency in mice results in impaired Th17 cell differentiation, which plays important roles in mucosal immune homeostasis, due to reduced IL-6 production.47,48 In addition, systemic IgA is considerably reduced in CD40KO mice, although interestingly, the total amount of mucosal IgA is only modestly affected, suggesting that innate immune stimulation of B cells in the gut compensates for the lack of CD40 signaling.18,19,21,22,49 Our preliminary studies suggest that the composition of the gut flora in CD40KO mice differs from that of their littermate controls (ZY, GAB and J. Kirby, unpublished data). Thus, how CD40 impacts the homeostasis of the gut flora and how this in turn impacts metabolic disease needs further investigation. Adipocytes can also express CD40, produce chemokines, and promote macrophage M1 polarization upon CD154 stimulation.50 Although we demonstrated that CD40 expression on CD8+ T cells plays major roles in CD40-mediated protective effects on IR, the cumulative effects of multiple mechanisms are likely to contribute to regulation of diet-associated metabolic homeostasis in vivo (Fig. 1).
Figure 1.
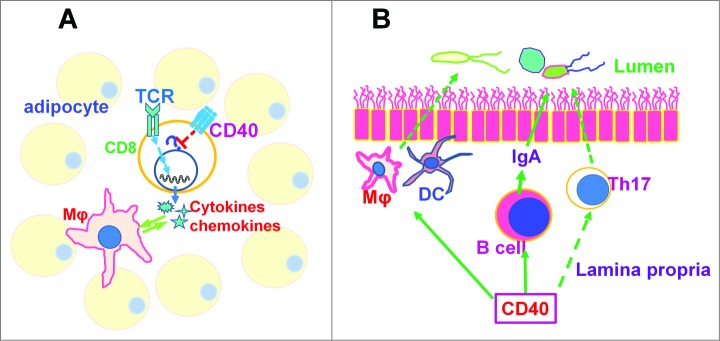
Potential mechanisms by which CD40 regulates obesity and insulin resistance. (A) In adipose tissue, CD40 expression on CD8+ T cells suppresses TCR-induced production of cytokines and chemokines, which are critical factors for Mφ recruitment and activation. How CD40 signaling results in T cell hypo-responsiveness is not yet clear. (B) CD40 is required for Th17 cell differentiation, IgA production by B cell and also for Mφ and DC function in vivo, which are important for mucosal immunity. Deficiency of CD40 might impair mucosal immunity and thereby disrupt the homeostasis of gut flora, affecting nutrient metabolism. Mφ, macrophage. DC, dendritic cell.
Future Perspectives
The unanticipated finding that CD40 protects mice against obesity and metabolic dysregulation is of considerable interest, and opens new areas of inquiry and raises many new questions. In particular, that CD40 expression on CD8+ T cells plays a major role during this process is quite unexpected and intriguing. The potential mechanisms of (1) How CD40 signaling impacts CD8+ T cell function in the VAT, (2) How immune cells interact with this special microenvironment, and (3) The nature of the roles played by mucosal immunity and gut microbiota, await further investigation. As various human CD40 polymorphisms are associated with human diseases,32 whether and how they affect obesity, IR and other metabolic diseases in human populations should be clarified. Finally, multiple signaling pathways can be activated by CD40.33 Activation or blockade of certain signaling pathways could act more precisely than generally activating or blocking all CD40 signaling pathways. The promising therapeutic effects of CD40-specific antibody30,31 and the potential to stimulate some but not all signaling pathways downstream of CD40 should be well addressed and may raise new opportunities for management of and intervention in metabolic disorders.
Funding
This work was supported by a Senior Research Career Scientist Award from the Dept. of Veterans Affairs (GAB) and a Biological Sciences Funding Program pilot award from The University of Iowa (GAB). ZY received support from NIH fellowship 5T32AI007260.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Johnson AM1, Olefsky JM. The origins and drivers of insulin resistance. Cell 2013; 152:673-84; PMID:23415219; http://dx.doi.org/ 10.1016/j.cell.2013.01.041 [DOI] [PubMed] [Google Scholar]
- 2. Mathis D. Immunological goings-on in visceral adipose tissue. Cell Metab 2013; 17:851-9; PMID:23747244; http://dx.doi.org/ 10.1016/j.cmet.2013.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol 2011; 11:85-97; PMID:21252989; http://dx.doi.org/ 10.1038/nri2921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW, Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003; 112:1796-1808; PMID:14679176; http://dx.doi.org/ 10.1172/JCI200319246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 2003; 112:1821-30; PMID:14679177; http://dx.doi.org/ 10.1172/JCI200319451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 2009; 15: 914-20; PMID:19633658; http://dx.doi.org/ 10.1038/nm.1964 [DOI] [PubMed] [Google Scholar]
- 7. Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med 2009; 15:921-9; PMID:19633657; http://dx.doi.org/ 10.1038/nm.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hotamisligil GS1, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 1993; 259:87-91; PMID:7678183; http://dx.doi.org/ 10.1126/science.7678183 [DOI] [PubMed] [Google Scholar]
- 9. Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest 2006; 116:1494-1505; PMID:16691291; http://dx.doi.org/ 10.1172/JCI26498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, Brickey WJ, Ting JP. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol 2011; 12:408-15; PMID:21478880; http://dx.doi.org/ 10.1038/ni.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001; 286:327-34; PMID:11466099; http://dx.doi.org/ 10.1001/jama.286.3.327 [DOI] [PubMed] [Google Scholar]
- 12. Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol 2011; 29:415-45; PMID:21219177; http://dx.doi.org/ 10.1146/annurev-immunol-031210-101322 [DOI] [PubMed] [Google Scholar]
- 13. Cipolletta D, Feuerer M, Li A, Kamei N, Lee J, Shoelson SE, Benoist C, Mathis D. PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature 2012; 486:549-53; PMID:22722857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang H, Youm YH, Vandanmagsar B, Ravussin A, Gimble JM, Greenway F, Stephens JM, Mynatt RL, Dixit VD. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J Immunol 2010; 185:1836-45; PMID:20581149; http://dx.doi.org/ 10.4049/jimmunol.1000021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wagner DH, Jr, Vaitaitis G, Sanderson R, Poulin M, Dobbs C, Haskins K. Expression of CD40 identifies a unique pathogenic T cell population in type 1 diabetes. Proc Natl Acad Sci U S A 2002; 99:3782-7; PMID:11891296; http://dx.doi.org/ 10.1073/pnas.052247099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Munroe ME, Bishop GA. A costimulatory function for T cell CD40. J Immunol 2007; 178:671-82; PMID:17202327; http://dx.doi.org/ 10.4049/jimmunol.178.2.671 [DOI] [PubMed] [Google Scholar]
- 17. Graham JP, Arcipowski KM, Bishop GA. Differential B-lymphocyte regulation by CD40 and its viral mimic, latent membrane protein 1. Immunol Rev 2010; 237:226-48; PMID:20727039; http://dx.doi.org/ 10.1111/j.1600-065X.2010.00932.x [DOI] [PubMed] [Google Scholar]
- 18. Callard E, Armitage RJ, Fanslow WC, Spriggs MK. CD40L and its role in X-linked hyper-IgM syndrome. Immunol Today 1993; 14: 559-64; PMID:7506037; http://dx.doi.org/ 10.1016/0167-5699(93)90188-Q [DOI] [PubMed] [Google Scholar]
- 19. DiSanto JP, Bonnefoy JY, Gauchat JF, Fischer A, de Saint Basile G. CD40L mutations in X-linked immunodeficiency with hyper-IgM. Nature 1993; 361:541-3; PMID:8094231; http://dx.doi.org/ 10.1038/361541a0 [DOI] [PubMed] [Google Scholar]
- 20. Andrade RM, Wessendarp M, Gubbels MJ, Striepen B, Subauste CS. CD40 induces macrophage anti-Toxoplasma gondii activity by triggering autophagy-dependent fusion of pathogen-containing vacuoles and lysosomes. J Clin Invest 2006; 116:2366-77; PMID:16955139; http://dx.doi.org/ 10.1172/JCI28796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Renshaw BR, Fanslow WC, 3rd, Armitage RJ, Campbell KA, Liggitt D, Wright B, Davison BL, Maliszewski CR. Humoral immune responses in CD40 ligand-deficient mice. J Exp Med 1994; 180:1889-900; PMID:7964465; http://dx.doi.org/ 10.1084/jem.180.5.1889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kawabe T, Naka T, Yoshida K, Tanaka T, Fujiwara H, Suematsu S, Yoshida N, Kishimoto T, Kikutani H. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity 1994; 1:167-78; PMID:7534202; http://dx.doi.org/ 10.1016/1074-7613(94)90095-7 [DOI] [PubMed] [Google Scholar]
- 23. Khong A, Nelson DJ, Nowak AK, Lake RA, Robinson BW. The use of agonistic anti-CD40 therapy in treatments for cancer. Int Rev Immunol 2012; 31:246-66; PMID:22804570; http://dx.doi.org/ 10.3109/08830185.2012.698338 [DOI] [PubMed] [Google Scholar]
- 24. Unek IT, Bayraktar F, Solmaz D, Ellidokuz H, Sisman AR, Yuksel F, Yesil S. The levels of soluble CD40 ligand and C-reactive protein in normal weight, overweight and obese people. Clin Med Res 2010; 8:89-95; PMID:20660932; http://dx.doi.org/ 10.3121/cmr.2010.889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Poggi M, Engel D, Christ A, Beckers L, Wijnands E, Boon L, Driessen A, Cleutjens J, Weber C, Gerdes N, et al. CD40L deficiency ameliorates adipose tissue inflammation and metabolic manifestations of obesity in mice. Arterioscler Thromb Vasc Biol 2011; 31:2251-60; PMID:21817098; http://dx.doi.org/ 10.1161/ATVBAHA.111.231357 [DOI] [PubMed] [Google Scholar]
- 26. Wolf D, Jehle F, Ortiz Rodriguez A, Dufner B, Hoppe N, Colberg C, Lozhkin A, Bassler N, Rupprecht B, Wiedemann A, et al. CD40L deficiency attenuates diet-induced adipose tissue inflammation by impairing immune cell accumulation and production of pathogenic IgG-antibodies. PLoS One 2012; 7:e33026; PMID:22412980; http://dx.doi.org/ 10.1371/journal.pone.0033026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Villeneuve J, Lepreux S, Mulot A, Bérard AM, Higa-Nishiyama A, Costet P, De Ledinghen V, Bioulac-Sage P, Balabaud C, Nurden AT, et al. A protective role for CD154 in hepatic steatosis in mice. Hepatology 2010; 52:1968-79; PMID:21064031; http://dx.doi.org/ 10.1002/hep.23935 [DOI] [PubMed] [Google Scholar]
- 28. Guo CA, Kogan S, Amano SU, Wang M, Dagdeviren S, Friedline RH, Aouadi M, Kim JK, Czech MP. CD40 deficiency in mice exacerbates obesity-induced adipose tissue inflammation, hepatic steatosis and insulin resistance. Am J Physiol Endocrinol Metab 2013; 304:E951-63; PMID:23482447; http://dx.doi.org/ 10.1152/ajpendo.00514.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yi Z, Stunz LL, Bishop GA. CD40-mediated maintenance of immune homeostasis in the adipose tissue microenvironment. Diabetes 2014; 63:2751-60; PMID:24647739; http://dx.doi.org/ 10.2337/db13-1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wolf D, Jehle F, Anto Michel N, Bukosza EN, Rivera J, Chen YC, Hoppe N, Dufner B, Ortiz Rodriguez A, Colberg C, et al. Co-Inhibitory Suppression of T Cell Activation by CD40 Protects from Obesity and Adipose Tissue Inflammation in Mice. Circulation 2014; 129:2414-25; PMID:24664276; http://dx.doi.org/ 10.1161/CIRCULATIONAHA.113.008055 [DOI] [PubMed] [Google Scholar]
- 31. Law CL, Grewal IS. Therapeutic interventions targeting CD40L (CD154) and CD40: the opportunities and challenges. Adv Exp Med Biol 2009; 647:8-36; PMID:19760064; http://dx.doi.org/ 10.1007/978-0-387-89520-8_2 [DOI] [PubMed] [Google Scholar]
- 32. Bishop GA. The many faces of CD40: multiple roles in normal immunity and disease. Semin Immunol 2009; 21:255-6; PMID:19713124; http://dx.doi.org/ 10.1016/j.smim.2009.08.002 [DOI] [PubMed] [Google Scholar]
- 33. Bishop GA, Moore CR, Xie P, Stunz LL, Kraus ZJ. TRAF proteins in CD40 signaling. Adv Exp Med Biol 2007; 597:131-51; PMID:17633023; http://dx.doi.org/ 10.1007/978-0-387-70630-6_11 [DOI] [PubMed] [Google Scholar]
- 34. Baccam M, Bishop GA. Membrane-bound CD154, but not CD40-specific antibody, mediates NF-kappaB-independent IL-6 production in B cells. Eur J Immunol. 1999;29:3855-66; PMID:10601993; http://dx.doi.org/ 10.1002/(SICI)1521-4141(199912)29:12%3c3855::AID-IMMU3855%3e3.0.CO;2-S [DOI] [PubMed] [Google Scholar]
- 35. Chatzigeorgiou A, Seijkens T, Zarzycka B, Engel D, Poggi M, van den Berg S, van den Berg S, Soehnlein O, Winkels H, Beckers L, et al. Blocking CD40-TRAF6 signaling is a therapeutic target in obesity-associated insulin resistance. Proc Natl Acad Sci U S A 2014; 111:2686-91; PMID:24492375; http://dx.doi.org/ 10.1073/pnas.1400419111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. El Fakhry Y, Alturaihi H, Diallo D, Merhi Y, Mourad W. Critical role of lipid rafts in CD154-mediated T cell signaling. Eur J Immunol 2010; 40:770-9; PMID:20039299; http://dx.doi.org/ 10.1002/eji.200939646 [DOI] [PubMed] [Google Scholar]
- 37. Zirlik A, Maier C, Gerdes N, MacFarlane L, Soosairajah J, Bavendiek U, Ahrens I, Ernst S, Bassler N, Missiou A, et al. CD40L mediates inflammation independently of CD40 by interaction with Mac-1. Circulation 2007; 115:1571-80; PMID:17372166; http://dx.doi.org/ 10.1161/CIRCULATIONAHA.106.683201 [DOI] [PubMed] [Google Scholar]
- 38. Bourgeois C, Rocha B, Tanchot C. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science 2002; 297:2060-3; PMID:12242444; http://dx.doi.org/ 10.1126/science.1072615 [DOI] [PubMed] [Google Scholar]
- 39. Johnson S, Zhan Y, Sutherland RM, Mount AM, Bedoui S, Brady JL, Carrington EM, Brown LE, Belz GT, Heath WR, et al. Selected Toll-like receptor ligands and viruses promote helper-independent cytotoxic T cell priming by upregulating CD40L on dendritic cells. Immunity 2009; 30:218-27; PMID:19200758; http://dx.doi.org/ 10.1016/j.immuni.2008.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Seah SG, Brady JL, Carrington EM, Ng WC, Sutherland RM, Hancock MS, La Gruta NL, Brown LE, Turner SJ, Lew AM, et al. Influenza-induced, helper-independent CD8+ T cell responses use CD40 costimulation at the late phase of the primary response. J Leukoc Biol 2013; 93:145-54; PMID:23108101; http://dx.doi.org/ 10.1189/jlb.0612266 [DOI] [PubMed] [Google Scholar]
- 41. Bhadra R, Gigley JP, Khan IA. Cutting edge: CD40-CD40L pathway plays a critical CD8-intrinsic and -extrinsic role during rescue of exhausted CD8 T cells. J. Immunol 2011; 187:4421-5; PMID:21949017; http://dx.doi.org/ 10.4049/jimmunol.1102319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Martin S, Pahari S, Sudan R, Saha B. CD40 signaling in CD8+CD40+ T cells turns on contra-T regulatory cell functions. J. Immunol 2010; 184:5510-8; PMID:20400702; http://dx.doi.org/ 10.4049/jimmunol.0902762 [DOI] [PubMed] [Google Scholar]
- 43. Sun JC, Bevan MJ. Cutting edge: long-lived CD8 memory and protective immunity in the absence of CD40 expression on CD8 T cells. J Immunol 2004; 172:3385-9; PMID:15004136; http://dx.doi.org/ 10.4049/jimmunol.172.6.3385 [DOI] [PubMed] [Google Scholar]
- 44. Lee BO, Hartson L, Randall TD. CD40-deficient, influenza-specific CD8 memory T cells develop and function normally in a CD40-sufficient environment. J Exp Med 2003; 198:1759-64; PMID:14657225; http://dx.doi.org/ 10.1084/jem.20031440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008; 57:1470-81; PMID:18305141; http://dx.doi.org/ 10.2337/db07-1403 [DOI] [PubMed] [Google Scholar]
- 46. Cerf-Bensussan N1, Gaboriau-Routhiau V. The immune system and the gut microbiota: friends or foes? Nat Rev Immunol 2010; 10:735-44; PMID:20865020; http://dx.doi.org/ 10.1038/nri2850 [DOI] [PubMed] [Google Scholar]
- 47. Perona-Wright G, Jenkins SJ, O'Connor RA, Zienkiewicz D, McSorley HJ, Maizels RM, Anderton SM, MacDonald AS. A pivotal role for CD40-mediated IL-6 production by dendritic cells during IL-17 induction in vivo. J Immunol 2009; 182:2808-15; PMID:19234175; http://dx.doi.org/ 10.4049/jimmunol.0803553 [DOI] [PubMed] [Google Scholar]
- 48. Iezzi G, Sonderegger I, Ampenberger F, Schmitz N, Marsland BJ, Kopf M. CD40-CD40L cross-talk integrates strong antigenic signals and microbial stimuli to induce development of IL-17-producing CD4+ T cells. Proc Natl Acad Sci U S A 2009; 106:876-81; PMID:19136631; http://dx.doi.org/ 10.1073/pnas.0810769106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bergqvist P, Stensson A, Lycke NY, Bemark M. T cell-independent IgA class switch recombination is restricted to the GALT and occurs prior to manifest germinal center formation. J Immunol 2010; 184:3545-53; PMID:20207993; http://dx.doi.org/ 10.4049/jimmunol.0901895 [DOI] [PubMed] [Google Scholar]
- 50. Chatzigeorgiou A, Phieler J, Gebler J, Bornstein SR, Chavakis T. CD40L stimulates the crosstalk between adipocytes and inflammatory cells. Horm Metab Res 2013; 45:741-7; PMID:23918687; http://dx.doi.org/ 10.1055/s-0033-1348221 [DOI] [PubMed] [Google Scholar]


