Abstract
The mechanism of the SmI2-mediated reduction of unactivated esters has been studied using a combination of kinetic, radical clocks and reactivity experiments. The kinetic data indicate that all reaction components (SmI2, amine, H2O) are involved in the rate equation and that electron transfer is facilitated by Brønsted base assisted deprotonation of water in the transition state. The use of validated cyclopropyl-containing radical clocks demonstrates that the reaction occurs via fast, reversible first electron transfer, and that the electron transfer from simple Sm(II) complexes to aliphatic esters is rapid. Notably, the mechanistic details presented herein indicate that complexation between SmI2, H2O and amines affords a new class of structurally diverse, thermodynamically powerful reductants for efficient electron transfer to carboxylic acid derivatives as an attractive alternative to the classical hydride-mediated reductions and as a source of acyl-radical equivalents for C=C bond forming processes.
Keywords: electron donors, electron transfer, radicals, reduction, reductive coupling
Samarium(II)-mediated generation of ketyl radicals has been the focus of intense research for more than three decades,[1] and the SmI2-promoted reductions, which enable the synthesis of alcohols under conditions orthogonal to other reagents operating through single- and two-electron pathways,[2,3] are a prominent class of these processes (Figure 1). Until recently, it had been thought that unactivated carboxylic acid derivatives were outside the reducing range of SmI2,[4] which prevented progression of the rich carbonyl chemistry of SmI2 (e.g., reduction, cross-coupling, tandem bond-forming events) to acyl-type radicals generated from carboxylic acid derivatives under mild and chemoselective reaction conditions (Figure 1).
Figure 1.
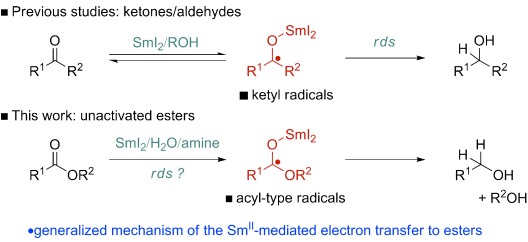
Accepted and proposed mechanism of SmI2-mediated electron transfer to aldehydes, ketones, and carboxylic acid derivatives; rds=rate-determining step.
In 2011, we reported that the SmII reagent produced from SmI2, amine and H2O is capable of reducing unactivated esters via radical intermediates,[5] thus for the first time expanding the carbonyl chemistry of SmI2 beyond ketones and aldehydes.[6] However, the mechanistic details of this process, including the critical role of amine and H2O additives, remained unclear.[6,7] As a better mechanistic understanding of the role of these additives could afford key insights for the development of new reductive processes, including chemoselective reduction of less reactive functional groups, such as nitriles, amides and amino acids, as well as the development of new C=C bond-forming reactions,[8] we initiated a mechanistic investigation into the reduction of unactivated esters using SmI2/amine/H2O. The data described herein show two important features: 1) all reaction components (SmI2, amine, H2O) are involved in the rate equation , and there is a direct correlation between the rate of ester reduction and pKBH+ of amines; 2) the reaction occurs via fast, reversible first electron transfer, and the electron transfer from simple SmII complexes to aliphatic esters is rapid. Importantly, this study sets the stage for the use of SmI2/amine/H2O complexes to generate acyl-type radicals from a plethora of carboxylic acid derivatives.
We started our investigation by conducting a range of kinetic studies (Table 1). tert-Butyl 3-phenylpropanoate (1) was selected as a model substrate, because its rate of reduction is in a convenient range for kinetic studies, and there is ample literature precedent for SmII reduction conditions available for this substrate.[5] Within experimental error, the reduction of 1 in the presence of SmI2/Et3N/H2O was found to be first order in all components of the reaction (Table 1). The rate constant of 1.4±0.1×10 m−3 s−1 was determined for the reduction of 1 under these reaction conditions. Taken together, these results suggest that all reaction components are involved in the rate equation, and that the reduction of 1 is a fast process.
Table 1.
Rate constant and reaction orders for the reduction of 1 using the SmI2/Et3N/H2O system.[a]
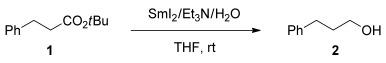 | ||||
|---|---|---|---|---|
| Rate order | ||||
| k[a][m−3 s−1] | Substrate[a] | SmI2[b] | Et3N[c] | H2O[d] |
| 1.4×10 | 0.96±0.10 | 1.09±0.10 | 1.18±0.10 | 0.92±0.10 |
[SmI2]=75 mm; [H2O]=250 mm; [Et3N]=150 mm; [ester]=5–20 mm.
[SmI2]=50–100 mm; [H2O]=250 mm; [Et3N]=150 mm; [ester]=12.5 mm.
[SmI2]=75 mm; [H2O]=250 mm; [Et3N]=75–250 mm; [ester]=12.5 mm.
[SmI2]=75 mm; [H2O]=75–300 mm; [Et3N]=150 mm; [ester]=12.5 mm. T=23 °C. See the Supporting Information.
To further explore the impact of H2O, the reduction rate of 1 was monitored over a 20-fold concentration range as depicted in Figure 2. In this study, a nonlinear rate dependence on H2O was found. At lower concentrations (up to 300 mm), the rate was found to increase linearly with a slope corresponding to the rate order of one, consistent with saturation behavior (300 mm). However, at higher concentrations (300–1200 mm), the rate decreased dramatically, consistent with substrate displacement from the inner coordination sphere of SmII. In contrast, a linear rate dependence on amine at these concentrations was found. In agreement with previous studies, H2O is expected to show high affinity for SmII and compete for coordination to SmII with the ester substrate.[9] Interestingly, the concentration of H2O at which the decrease in the reaction rate was observed correlates with iodide displacement from the SmII coordination sphere.[10]
Figure 2.
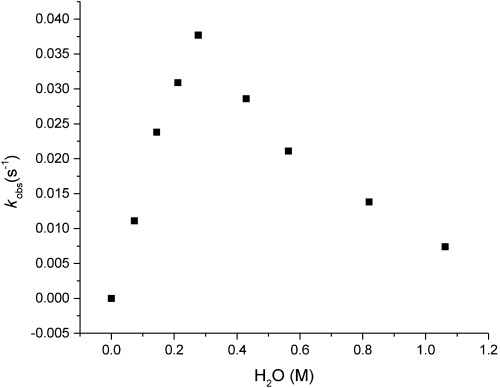
Plot of kobs versus concentration of H2O for the reduction of 1. [H2O]=0.075–1.2 m; [SmI2]=75 mm; [Et3N]=150 mm; [ester]=12.5 mm; T=23 °C.
To further elucidate the role of the amine component, the reduction rate of 1 was measured in the presence of a wide range of amines with varying steric and electronic properties (Table 2). Remarkably, a dramatic change in the reaction rate of over two orders of magnitude was found by simply using different amines for the reduction. Moreover, a good correlation between the reaction rate and basicity of amines was obtained.[11] By plotting log (kobs) versus pKBH+, a linear correlation was found with a slope corresponding to 0.79, which corresponds very well to the value obtained in the reduction of alkyl halides via an outer-sphere mechanism using SmI2/amine/H2O reported by Hilmersson (0.76; for a detailed comparison, see the Supporting Information).[7e] This result strongly suggests that the role of the amine component is independent of the mechanistic pathway (inner- vs. outer-sphere electron transfer) and the relative redox potentials of both classes of substrates. Considering steric properties exerted by these amines, our findings bode well for the chemoselective fine tuning of SmII/amine reductants to specific functional groups.
Table 2.
Determined initial rate in the reduction of 1 using SmI2/amine/H2O versus pKBH+.[a]
| Entry | Amine | vinitial [mM s−1] | pKBH+[b] |
|---|---|---|---|
| 1 | morpholine | 2.4×10−4 | 9.0±0.2 |
| 2 | nBu3N | 3.9×10−5 | 10.0±0.5 |
| 3 | Et3N | 5.0×10−4 | 10.6±0.3 |
| 4 | nBuNH2 | 6.8×10−3 | 10.7±0.1 |
| 5 | pyrrolidine | 8.8×10−3 | 11.3±0.2 |
[SmI2]=75 mm; [H2O]=250 mm; [ester]=12.5 mm; [amine]=150 mm; T=23 °C.
Determined from ACD lab prediction algorithm.
Several additional studies give insight into the electron-transfer steps. 1) The reduction with SmI2/amine/D2O gives the alcohol with >95 % [D]2 incorporation suggesting that anions are protonated in a series of electron transfers. 2) The kinetic isotope effect in the reduction of isopropyl 3-phenylpropanoate using SmI2/Et3N/H2O of 1.5±0.1, parallel runs, and 1.4±0.1, intramolecular competition,[5] indicate that proton transfer is not involved in the rate-determining step.[12] 3) UV/Vis spectrophotometric studies carried out on various SmI2/amine/H2O systems show isosbestic points and absorbance changes upon addition of amines and H2O to SmI2,[7d] which is consistent with the formation of distinct SmII reductants.
Next, we utilized intermolecular competition studies to elucidate the actual productivity difference in the SmI2/amine/H2O-mediated reduction of esters (Table 3). In these experiments, an equimolar amount of two esters was reacted with limiting SmI2 (typically, less than 2 equiv). The relative reactivity values were determined from the product distribution. This method allows to accurately measure the relative reactivity values of SmII-mediated reactions provided that the studied substrates do not participate in alternative reaction pathways.[13] Methyl decanoate was chosen as an arbitrary standard. Remarkably, in the series of eight methyl esters, a reactivity range of over three orders of magnitude was observed, depending on the steric and electronic properties of the α-carbon substituent at the ester group undergoing the reduction (Table 3, entries 1–8). This effect is consistent with both electronic stabilization of ketyl-type radicals (Table 3, entries 1–4) and steric inhibition of coordination to SmII (entries 4–8). Moreover, several substrates with enhanced leaving-group ability compared to the methyl ester were examined (Table 3, entries 9–12). These results further support the importance of electronic effects for the stabilization of the ketyl-type radical intermediates and determining the redox potential of the substrates.[14] Importantly, the data presented in Table 3 indicate high levels of chemoselectivity in the reduction of esters with SmI2/Et3N/H2O.
Table 3.
Steric and electronic influence on the relative rates for the reduction of esters.
| Entry |  |
RV[a] |
|---|---|---|
| 1 | 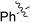 |
>100 |
| 2 | 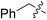 |
9.14 |
| 3 | 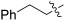 |
4.29 |
| 4 | 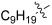 |
1.00 |
| 5 | 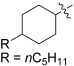 |
0.41 |
| 6 | 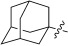 |
0.26 |
| 7 | 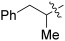 |
0.91 |
| 8 | 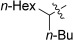 |
0.05 |
| Entry |  |
RV[a] |
|---|---|---|
| 9 | R=OMe | 1.00 |
| 10 | R=OPh | 6.88 |
| 11 | R=Opfp | 9.15 |
| 12 | R=SEt | 5.78 |
Relative reactivity values (RV) determined from product distribution by 1H NMR and/or GC analyses of crude reaction mixtures. All data represent the average of at least two experiments. pfp=pentafluorophenyl.
Evidence for the electronic and steric stabilization of ketyl-type radical intermediates was further substantiated by Hammett and Taft correlation studies (see the Supporting Information). The Hammett correlation study, employing methyl esters of 4-substituted phenylacetic acids,[15] showed a large positive ρ value of 0.43 (R2=0.98), which can be compared with the ρ value of 0.49 for ionization of phenylacetic acids in H2O at 25 °C.[16] The Taft correlation study,[17] obtained by plotting log (kobs) versus ES in a series of aliphatic esters of hydrocinnamic acid showed a large positive slope of 0.97 (R2=0.97). Overall, these results suggest that an anionic intermediate is formed in the transition state of the reaction, and that a conformational change similar in geometry to the ester hydrolysis, tetrahedral intermediate, is taking place in the rate-determining step of the reaction.[18]
Finally, to gain independent evidence on the role of electron-transfer steps, we carried out several studies employing mechanistic probes (Scheme 1 and the Supporting Information). Most importantly, we recognized that implementation of a suitable radical clock should allow the detection of reversible reaction pathways.[19] To this end, the trans-cyclopropane-containing radical clock 3 (approximated unimolecular rate constant kfrag≈3×1011 s−1 at 25 °C)[20] was selected and subjected to the reaction conditions with a limiting amount of SmI2 (Scheme 1). The reaction resulted in rapid cyclopropyl-ring opening to give acyclic ester 4 and alcohol 5 in 94:6 ratio. Cyclopropylcarbinol 6 was not detected in the reaction. Several control experiments were performed (see the Supporting Information). 1) The reaction of 3 with SmI2/H2O (8 equiv, RT, 2 h) resulted in a facile opening to ester 4, with no over-reduction to 5 or 6 observed. 2) The reduction of the methyl ester of cyclopropanecarboxylic acid (approximated unimolecular rate constant kfrag≈9.4×107 s−1 at 25 °C)[20] with excess SmI2/amine/H2O afforded the corresponding acyclic alcohol and cyclopropylcarbinol in 96:4 ratio. This allows to estimate the rate of reduction of ketyl-type radicals with SmII to be comparable to a unimolecular reaction with k of about 108 s−1.[21] 3) The reductive opening of radical clock 3 was not observed with other SmII reagents, including systems with higher redox potential (SmI2/MeOH, SmI2/LiCl, SmI2/HMPA (HMPA=hexamethylphosphoramide), and SmI2/Et3N).[4b] Finally, experiments utilizing chiral probe 7 (Scheme 1) were carried out and demonstrate that enolization did not occur in the process despite basic reaction conditions, whereas control experiments using H218O (Scheme 1 and the Supporting Information) show that the reduction did not proceed via a sequential ester hydrolysis/acid reduction mechanism. Overall, these findings strongly suggest that the reduction of unactivated esters with SmI2/amine/H2O occurs through fast, reversible electron transfer, and, in contrary to the current paradigm,[1,2] show that electron transfer from simple SmI2/H2O complexes to aliphatic esters is rapid.[22]
Scheme 1.
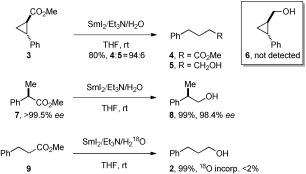
Studies designed to investigate the mechanism of reduction of unactivated esters using SmI2/Et3N/H2O.
A mechanism that best fits the kinetic and reactivity studies presented herein features the following steps (Scheme 2):[24] 1) Formation of the reactive complex between SmI2, H2O, and amine, in which one or more molecules of H2O and amine are coordinated to the SmII center.[23] Within this complex, one molecule of amine participates in partial deprotonation of H2O, resulting in a formal negative charge at oxygen and an overall increase of the redox potential of the SmII reductant in the transition state; 2) reversible ester coordination, protonation and first electron transfer steps; 3) rate-limiting second electron-transfer step; 4) inner-sphere electron-transfer process that is inhibited by large concentrations of H2O and facilitated by Brønsted basic amines; and 5) rate-determining step that can be fine-tuned by steric and electronic properties of the ester substrate. The formation of a partial negative charge at oxygen was further supported by our finding that under optimized reaction conditions, SmI2/NaOH/H2O[25] reduces aliphatic esters in high yield. From a practical point of view,[26] the pKBH+-dependent elongation of the hydrogen bond from H2O in SmI2/amine/H2O complexes can have a profound impact on the chemoselectivity of electron transfer to carboxylic acid derivatives.
Scheme 2.
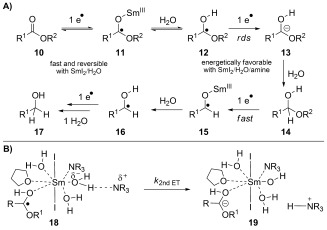
A) Proposed mechanism. B) Final steps of the reduction of esters using SmI2/Et3N/H2O.
In summary, we have presented a series of kinetic and reactivity experiments that probe the mechanism of the reduction of unactivated esters by using SmI2/amine/H2O. Our data are consistent with the formation of distinct SmII reductants by complexation between SmII, amine, and H2O. The ester reduction appears to proceed after deprotonation of a molecule of H2O by amine and to involve a reversible first electron-transfer step. Most crucially, our results demonstrate that a set of new SmII reductants that can be fine-tuned by the pKBH+ of the amine component is now available for challenging electron-transfer reactions to carboxylic acid derivatives. Equally importantly, this work shows that the major role of additives (e.g., H2O, amine/H2O) is to stabilize the ketyl intermediates. We fully expect that these findings will serve as a foundation to enable the development of new electron-transfer reactions. Work in this direction using SmII systems is ongoing in our laboratories, and these results will be reported shortly.
Acknowledgments
We acknowledge the EPSRC and GSK for financial support.
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/chem.201400295.
References
- [1a].Procter DJ, Flowers RA. :307. Organic Synthesis Using Samarium Diiodide: A Practical Guide20102II, T. Skrydstrup,, RSC Publishing, Cambridge,; Recent reviews on SmI: [Google Scholar]
- [1b].Molander GA, Harris CR. Chem. Rev. 96 doi: 10.1021/cr950019y. [DOI] [PubMed] [Google Scholar]
- [1c].Kagan HB. Tetrahedron. 1996;59 [Google Scholar]
- [1d].Edmonds DJ, Johnston D, Procter DJ. Chem. Rev. 2003;104 doi: 10.1021/cr030017a. [DOI] [PubMed] [Google Scholar]
- [1e].Nicolaou KC, Ellery SP, Chen JS. Angew. Chem. 2004;121 doi: 10.1002/anie.200902151. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2009;48 [Google Scholar]
- [1f].Szostak M, Procter DJ. Angew. Chem. 2009;123 [Google Scholar]; Angew. Chem. Int. Ed. 2011;50 [Google Scholar]
- [1g].Szostak M, Spain M, Procter DJ. Chem. Soc. Rev. 2011;42 doi: 10.1039/c3cs60223k. [DOI] [PubMed] [Google Scholar]
- [1h].Berndt M, Gross S, Hölemann A, Reissig HU. Synlett. 2013 [Google Scholar]
- [1i].Beemelmanns C, Reissig HU. Chem. Soc. Rev. 2011;40 doi: 10.1039/c0cs00116c. [DOI] [PubMed] [Google Scholar]
- [2].p. 2771. Reviews on metal-mediated radical reactions:
- [2a].Trost BM, Fleming I. Comprehensive Organic Synthesis. New York: Pergamon Press; [Google Scholar]
- [2b].Gansäuer A, Bluhm H. Chem. Rev. 1991;100 doi: 10.1021/cr9902648. [DOI] [PubMed] [Google Scholar]
- [2c].Szostak M, Procter DJ. Angew. Chem. 2000;124 [Google Scholar]; Angew. Chem. Int. Ed. 2012;51 [Google Scholar]
- [2d].Gansäuer A. Radicals in Synthesis I and II, Vol. 263–264. Heidelberg: Springer; 2012. ; An excellent review on reductive umpolung: [Google Scholar]
- [2e].Streuff J. Synthesis. 2013;45 [Google Scholar]
- [3].p. 6128.
- [3a].Hudlicky M. Reductions in Organic Chemistry. Chichester: Ellis Horwood; [Google Scholar]
- [3b].Seyden-Penne J. Reductions by Alumino and Borohydrides in Organic Synthesis. New York: Wiley; [Google Scholar]
- [3c].Andersson PG, Munslow IJ. Modern Reduction Methods. Weinheim: Wiley-VCH; 1997. [Google Scholar]
- [3d].Addis D, Das S, Junge K, Beller M. Angew. Chem. 2008;123 doi: 10.1002/anie.201100145. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2011;50 [Google Scholar]
- [4].p. 943. Selected mechanistic studies on SmIIreductants.
- [4a].Curran DP, Fevig TL, Jasperse CM, Totleben MJ. Synlett [Google Scholar]
- [4b].Dahlén A, Hilmersson G. Eur. J. Inorg. Chem [Google Scholar]
- [4c].Hansen AM, Lindsay KB, Antharjanam PKS, Karaffa J, Daasbjerg K, Flowers RA, II, Skrydstrup T. J. Am. Chem. Soc. 2004;128 doi: 10.1021/ja060553v. . [DOI] [PubMed] [Google Scholar]
- [4d].Amiel-Levy M, Hoz S. J. Am. Chem. Soc. 2006;131 doi: 10.1021/ja9013997. [DOI] [PubMed] [Google Scholar]
- [4e].Berndt M, Hölemann A, Niermann A, Bentz C, Zimmer R, Reissig HU. Eur. J. Org. Chem. 2009 [Google Scholar]
- [4f].Kefalidis CE, Perrin L, Maron L. Eur. J. Inorg. Chem. doi: 10.1021/ic402837n. [DOI] [PubMed] [Google Scholar]
- [4g].Szostak M, Spain M, Choquette KA, Flowers RA, II, Procter DJ. J. Am. Chem. Soc. 2013;135 doi: 10.1021/ja4078864. ; For a recent elegant application in the synthesis of natural products, see: [DOI] [PubMed] [Google Scholar]
- [4h].Beemelmanns C, Reissig HU. Angew. Chem. 2013;122 doi: 10.1002/anie.201003320. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2010;49 ; For selected applications: [Google Scholar]
- [4i].Masson G, Py S, Vallée Y. Angew. Chem. 2010;114 doi: 10.1002/1521-3773(20020517)41:10<1772::aid-anie1772>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2002;41 [Google Scholar]
- [4j].Masson G, Cividino P, Py S, Vallée Y. Angew. Chem. 2002;115 doi: 10.1002/anie.200250480. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2003;42 [Google Scholar]
- [4k].Jensen CM, Lindsay KB, Taaning RH, Karaffa J, Hansen AM, Skrydstrup T. J. Am. Chem. Soc. 2003;127 doi: 10.1021/ja050420u. ; For recent studies on the mechanism of other SET-mediated reactions, see: [DOI] [PubMed] [Google Scholar]
- [4l].Findlay NJ, Park SR, Schoenebeck F, Cahard E, Zhou S, Berlouis LEA, Spicer MD, Tuttle T, Murphy JA. J. Am. Chem. Soc. 2005;132 doi: 10.1021/ja107703n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4m].Cahard E, Schoenebeck F, Garnier J, Cutulic SPY, Zhou S, Murphy JA. Angew. Chem. 2010;124 doi: 10.1002/anie.201200084. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2012;51 [Google Scholar]
- [4n].Doni E, Mondal B, O’Sullivan S, Tuttle T, Murphy JA. J. Am. Chem. Soc. 2012;135 doi: 10.1021/ja4050168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4o].Doni E, O′Sullivan S, Murphy JA. Angew. Chem. 2013;125 doi: 10.1002/anie.201208066. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2013;52 [Google Scholar]
- [4p].Daasbjerg K, Svith H, Grimme S, Gerenkamp M, Mück-Lichtenfeld C, Gansäuer A, Barchuk A, Keller F. Angew. Chem. 2013;118 doi: 10.1002/anie.200504176. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2006;45 [Google Scholar]
- [4q].Paradas M, Campaña AG, Jiménez T, Robles R, Oltra JE, Buñuel E, Justicia J, Cárdenas DJ, Cuerva JM. J. Am. Chem. Soc. 2006;132 doi: 10.1021/ja105670h. [DOI] [PubMed] [Google Scholar]
- [4r].Gansäuer A, Behlendorf M, Cangönül A, Kube C, Cuerva JM, Friedrich J, van Gastel M. Angew. Chem. 2010;124 doi: 10.1002/anie.201107556. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2012;51 [Google Scholar]
- [4s].Gansäuer A, Klatte M, Brändle GM, Friedrich J. Angew. Chem. 2012;124 doi: 10.1002/anie.201202818. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2012;51 [Google Scholar]
- [5].Szostak M, Spain M, Procter DJ. Chem. Commun. 2011;47:10254. doi: 10.1039/c1cc14014k. [DOI] [PubMed] [Google Scholar]
- [6].p. 1136. Recent studies on chemoselective reductions using SmII.
- [6a].Duffy LA, Matsubara H, Procter DJ. J. Am. Chem. Soc. 130 doi: 10.1021/ja078137d. [DOI] [PubMed] [Google Scholar]
- [6b].Guazzelli G, De Grazia S, Collins KD, Matsubara H, Spain M, Procter DJ. J. Am. Chem. Soc. 2008;131 doi: 10.1021/ja901715d. ; See also: [DOI] [PubMed] [Google Scholar]
- [6c].Szostak M, Spain M, Procter DJ. Nat. Protoc. 2009;7 doi: 10.1038/nprot.2012.034. [DOI] [PubMed] [Google Scholar]
- [6d].Szostak M, Spain M, Procter DJ. Org. Lett. 2012;14 doi: 10.1021/ol203361k. [DOI] [PubMed] [Google Scholar]
- [6e].Szostak M, Collins KD, Fazakerley NJ, Spain M, Procter DJ. Org. Biomol. Chem. 2012;10 doi: 10.1039/c2ob00017b. [DOI] [PubMed] [Google Scholar]
- [6f].Szostak M, Sautier B, Spain M, Behlendorf M, Procter DJ. Angew. Chem. 2012;125 doi: 10.1002/anie.201306484. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2013;52 ; For a study on Tm(II) see: [Google Scholar]
- [6g].Szostak M, Spain M, Procter DJ. Angew. Chem. 2013;125 doi: 10.1002/anie.201303178. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2013;52 [Google Scholar]
- [6h]. J. Org. Chem201479 M. Szostak, M. Spain, D. J. Procter, , DOI. [DOI] [PubMed]
- [6i]. Org. Lett201416 M. Szostak, B. Sautier, M. Spain, D. J. Procter, , DOI: [DOI] [PubMed]
- [6j].Szostak M, Spain M, Eberhart AJ, Procter DJ. J. Am. Chem. Soc. 2013;136 doi: 10.1021/ja412578t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6k].Szostak M, Sautier B, Procter DJ. Org. Lett. 2014;16 doi: 10.1021/ol403340j. [DOI] [PubMed] [Google Scholar]
- [6l].Szostak M, Sautier B, Procter DJ. Chem. Commun. 2014;50 doi: 10.1039/c3cc48932a. [DOI] [PubMed] [Google Scholar]
- [7].p. 949. Other selected studies on SmI22/amine/H O:
- [7a].Cabri W, Candiani I, Colombo M, Franzoi L, Bedeschi A. Tetrahedron Lett. 36 [Google Scholar]
- [7b].Dahlén A, Hilmersson G. Tetrahedron Lett. 1995;43 [Google Scholar]
- [7c].Dahlén A, Hilmersson G. Chem. Eur. J. 2002;9 [Google Scholar]
- [7d].Dahlén A, Hilmersson G, Knettle BW, Flowers RA., II J. Org. Chem. 2003;68 doi: 10.1021/jo034173t. . [DOI] [PubMed] [Google Scholar]
- [7e].Dahlén A, Hilmersson G. J. Am. Chem. Soc. 2003;127 doi: 10.1021/ja043323u. [DOI] [PubMed] [Google Scholar]
- [7f].Dahlén A, Nilsson Å, Hilmersson G. J. Org. Chem. 2005;71 doi: 10.1021/jo052268k. [DOI] [PubMed] [Google Scholar]
- [7g].Ankner T, Hilmersson G. Org. Lett. 2006;11 doi: 10.1021/ol802243d. [DOI] [PubMed] [Google Scholar]
- [7h].Ankner T, Hilmersson G. Tetrahedron. 2009;65 [Google Scholar]
- [7i].Ankner T, Stålsmeden AS, Hilmersson G. Chem. Commun. 2009;49 doi: 10.1039/c3cc41642a. [DOI] [PubMed] [Google Scholar]
- [8].p. 15467.
- [8a].Parmar D, Duffy LA, Sadasivam DV, Matsubara H, Bradley PA, Flowers RA, II, Procter DJ. J. Am. Chem. Soc. 131 doi: 10.1021/ja906396u. . [DOI] [PubMed] [Google Scholar]
- [8b].Collins KD, Oliveira JM, Guazzelli G, Sautier B, De Grazia S, Matsubara H, Helliwell M, Procter DJ. Chem. Eur. J. 2009;16 doi: 10.1002/chem.201000632. [DOI] [PubMed] [Google Scholar]
- [8c].Parmar D, Price K, Spain M, Matsubara H, Bradley PA, Procter DJ. J. Am. Chem. Soc. 2010;133 doi: 10.1021/ja1114908. [DOI] [PubMed] [Google Scholar]
- [8d].Parmar D, Matsubara H, Price K, Spain M, Procter DJ. J. Am. Chem. Soc. 2011;134 doi: 10.1021/ja3047975. [DOI] [PubMed] [Google Scholar]
- [8e].Sautier B, Lyons SE, Webb MR, Procter DJ. Org. Lett. 2012;14 doi: 10.1021/ol2029367. ; see also ref. [4g] and ref. [6f] [DOI] [PubMed] [Google Scholar]
- [9].p. 261.
- [9a].Yacovan A, Bilkis I, Hoz S. J. Am. Chem. Soc. 118 [Google Scholar]
- [9b].Prasad E, Flowers RA., II J. Am. Chem. Soc. 1996;127 doi: 10.1021/ja056352t. . [DOI] [PubMed] [Google Scholar]
- [9c].Tarnopolsky A, Hoz S. J. Am. Chem. Soc. 2005;129 doi: 10.1021/ja0686662. [DOI] [PubMed] [Google Scholar]
- [9d].Upadhyay SK, Hoz S. J. Org. Chem. 2007;76 doi: 10.1021/jo1023206. [DOI] [PubMed] [Google Scholar]
- [10].Sadasivam DV, Teprovich JA, Jr, Procter DJ, Flowers RA., II Org. Lett. 2010;12:4140. doi: 10.1021/ol101722c. , . [DOI] [PubMed] [Google Scholar]
- [11].p. 997.
- [11a].Graton J, Laurence C, Berthelot M, Le Questel JY, Besseau F, Raczynska ED. J. Chem. Soc. Perkin Trans. 2 [Google Scholar]
- [11b].Graton J, Berthelot M, Laurence C. J. Chem. Soc. Perkin Trans. 2 [Google Scholar]
- [11c].Graton J, Besseau F, Berthelot M, Raczynska ED, Laurence C. Can. J. Chem. 1999;80 [Google Scholar]
- [12].Simmons EM, Hartwig JF. Angew. Chem. 124:3120. [Google Scholar]; Angew. Chem. Int. Ed. 2012;51 [Google Scholar]
- [13].Taaning RH, Lindsay KB, Schiøtt B, Daasbjerg K, Skrydstrup T. J. Am. Chem. Soc. 2009;131:10253. doi: 10.1021/ja903401y. [DOI] [PubMed] [Google Scholar]
- [14].p. 1709. Reduction potential of thioesters is lower than that of corresponding esters: EORESR=−3.0 V vs. SCE; =−2.6 V vs. SCE;
- [14a].Weïwer M, Olivero S, Duñach E. Tetrahedron. 61 [Google Scholar]
- [14b].Belotti D, Cossy J, Pete JP, Portella C. J. Org. Chem. 2005;51 [Google Scholar]
- [15].Jaffé HH. Chem. Rev. 1953;53:191. [Google Scholar]
- [16]. 4-Substituted benzyl alcohols undergo reductive cleavage of benzyl heteroatom bonds. See Ref. [7h]
- [17].Hansch C, Leo A, Taft RW. Chem. Rev. 1991;91:165. [Google Scholar]
- [18].Wu YD, Houk KN. J. Am. Chem. Soc. 1992;114:1656. [Google Scholar]
- [19a].Curran DP, Porter NA, Giese B. Stereochemistry of Radical Reactions. Weinheim: Wiley-VCH; [Google Scholar]
- [19b].Renaud P, Sibi M. Radicals in Organic Synthesis. Weinheim: Wiley-VCH; 1996. [Google Scholar]
- [19c].Chatgilialoglu C, Studer A. Encyclopedia of Radicals in Chemistry, Biology and Materials. Wiley-Blackwell; 2001. [Google Scholar]
- [20].p. 1151.
- [20a].Newcomb M. Tetrahedron. 49 ; Tanko has amply demonstrated that the rate of radical anion radical clocks is similar to that of the corresponding neutral radicals: [Google Scholar]
- [20b].Stevenson JP, Jackson WF, Tanko JM. J. Am. Chem. Soc. 1993;124 doi: 10.1021/ja0041831. [DOI] [PubMed] [Google Scholar]
- [20c].Tanko JM, Gillmore JG, Friedline R, Chahma M. J. Org. Chem. 2002;70 [Google Scholar]
- [20d].Tanko JM, Li X, Chahma M, Jackson WF, Spencer JN. J. Am. Chem. Soc. 2005;129 doi: 10.1021/ja063857q. [DOI] [PubMed] [Google Scholar]
- [20e].Chahma M, Li X, Phillips JP, Schwartz P, Brammer LE, Wang Y, Tanko JM. J. Phys. Chem. A. 2007;109 [Google Scholar]
- [21].Hasegawa E, Curran DP. Tetrahedron Lett. 1993;34:1717. ≈2.3×10 s at 25 °C) produced the reduction and cyclization products in approximately 1:1 ratio (see the Supporting Information). See: In agreement with this observation, the reaction of a substituted 5-hexenyl radical clock (approximated unimolecular rate constant k5−exo5−1[19] [Google Scholar]
- [22].Szostak M, Spain M, Parmar D, Procter DJ. Chem. Commun. 2012;48:330. doi: 10.1039/c1cc14252f. [DOI] [PubMed] [Google Scholar]
- [23]. Reagent stoichiometry studies indicate that 1:2:3 ratio of SmI22/amine/H O is required for the ester reduction (see the Supporting Information)
- [24]. Under pseudo-first order conditions, the observed rate law is consistent with conclusions described in the manuscript.
- [25].p. 893.
- [25a].Kamochi Y, Kudo T. Chem. Lett [Google Scholar]
- [25b].Kamochi Y, Kudo T. Tetrahedron Lett. 1991;32 [Google Scholar]
- [25c].Kamochi Y, Kudo T. Bull. Chem. Soc. Jpn. 1991;65 [Google Scholar]
- [26].Szostak M, Spain M, Procter DJ. J. Org. Chem. 2012;77:3049. doi: 10.1021/jo300135v. A practical method for the preparation of SmI2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


