Abstract
The magnetic-field-induced alignment of the fibrillar structures present in an aqueous solution of a dipeptide gelator, and the subsequent retention of this alignment upon transformation to a hydrogel upon the addition of CaCl2 or upon a reduction in solution pH is reported. Utilising the switchable nature of the magnetic field coupled with the slow diffusion of CaCl2, it is possible to precisely control the extent of anisotropy across a hydrogel, something that is generally very difficult to do using alternative methods. The approach is readily extended to other compounds that form viscous solutions at high pH. It is expected that this work will greatly expand the utility of such low-molecular-weight gelators (LMWG) in areas where alignment is key.
Keywords: alignment, hydrogel, NMR spectroscopy, peptide, self-assembly
Supramolecular hydrogels are formed by the self-assembly of low-molecular-weight gelators (LMWG) into fibrillar structures, which then entangle or cross-link to form the gel matrix.1 These fascinating materials are currently being investigated for a vast number of applications including wound healing,2 energy harvesting,3 and pollutant capture.4 Functionalised dipeptides, where the N-terminus is substituted with a large hydrophobic group such as naphthalene or fluorenylmethoxycarbonyl, are receiving an increasing amount of interest as LMWG.5 These LMWG generally dissolve to form free-flowing solutions when the pH is adjusted above the pKa of the terminal carboxylic acid.6 Gelation of these solutions can be induced by the addition of salt7 or by lowering the pH of the solution.[6, 8] Given the difficulty in ‘designing’ effective LMWG, there is much interest in how the properties of gels prepared from these known compounds may be controlled and tailored for specific applications.9 Relatively little has been reported on how the relative orientation of the supramolecular structures present in the gels can be controlled, with most studies focusing mainly on the bulk properties of the materials. Aligned materials are of significant interest for optoelectronics,3 and regenerative medicine,10 for example. Zhou et al. recently reported that significant anisotropy in the orientation of fibres formed from naphthalene dipeptides and tripeptides could be obtained using a combination of aromatic–aromatic interfibre interactions and enzymatic hydrogelation.11 With unrelated LMWG, anisotropy in the orientation of the self-assembled fibres has been induced with electric fields,12 chemical gradients,13 magnetic fields14 and shear alignment.15 High-strength magnetic fields can induce anisotropy in supramolecular materials.16 Here, we report that strong magnetic fields induce significant anisotropy in the relative orientations of the structures present in a solution of LWMG, which is retained upon gel formation (Figure 1 a).
Figure 1.
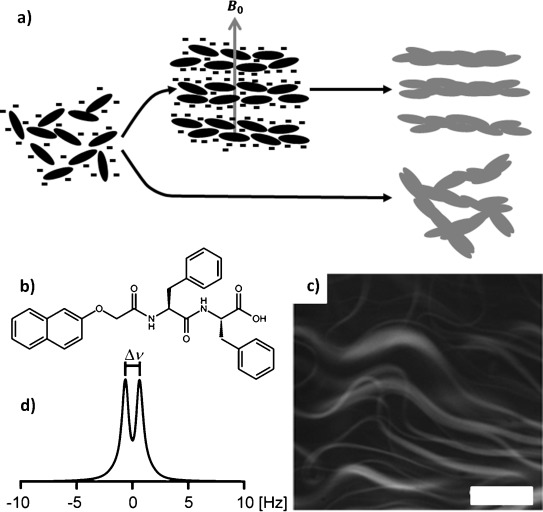
a) NapFF exists in solution at high pH as worm-like micelles that align spontaneously in a strong magnetic field and can subsequently be transformed into gels with aligned fibres upon application of a suitable switch. b) Structure of NapFF. c) Confocal micrograph of a NapFF solution at pD 12.6 (0.5 wt %) showing worm-like micelles, stained with Nile blue. The scale bar represents 100 μm. d) 2H NMR spectrum of a solution of NapFF at pD 12.6 (0.5 wt %), measured in a 9.4 T (400 MHz 1H) NMR spectrometer, |Δν|=1.3 Hz.
We have previously demonstrated that solutions of NapFF (0.5 wt %; Figure 1 b) contain highly anisotropic structures using viscosity measurements, electron microscopy and small-angle X-ray scattering.[7a, 17] Our model is that the carboxylates stabilise worm-like micelles with the core formed from the hydrophobic naphthalene and phenylalanine groups. This data is further confirmed here by confocal microscopy (Figure 1 c), which also suggests that lateral association of the worms takes place.
We reasoned that the worm-like micelles formed at high pH by NapFF may spontaneously align in high-strength magnetic fields, due to their presumably high diamagnetic anisotropy resulting from their anisotropic shape and abundance of aromatic rings. To probe alignment, we used deuterium (2 H) NMR.18 In a 400 MHz (1H) spectrometer, the deuterium resonance of D2O in a solution of NapFF at high pD exhibits a residual quadrupolar coupling (RQC) (Δν, Figure 1 d), indicating that the worm-like micellar structures present in the solution share a common orientation with respect to the magnetic-field axis. Unlike previous examples, we can therefore gel pre-aligned solutions in a magnetic field, as opposed to having to first disassemble to the monomer state by heating to 90 °C.14b We note that the magnitude of the RQC is temperature- and concentration-dependent (Figures S8 and S9 in the Supporting Information). Solutions at 0.5 wt % sometimes contain birefringent domains. However, at 1.0 wt %, birefringent domains are formed that clearly show alignment after exposure to a magnetic field (Figure S17 in the Supporting Information).
Addition of CaCl2 to solutions of NapFF at high pH results in the formation of stable hydrogels.[7a, 17] This gelation arises as a result of the Ca2+ cross-linking the worm-like structures through formation of salt-bridges between adjacent carboxylate groups.7a When CaCl2 is layered on top of a solution of NapFF in a 5 mm NMR tube and the sample left to stand in a 9.4 T field for the CaCl2 to diffuse to the bottom of the tube, an RQC of D2O is again observed and thus anisotropy in the orientation of the structures in the absence of Ca2+ is retained on gelation. The extent of magnetic-field-induced anisotropy—the magnitude of the RQC of D2O—exhibits a distinct dependence on magnetic field strength, both in solutions of NapFF at high pD and in CaCl2-triggered gels. 2H NMR spectra of D2O in CaCl2-triggered gels prepared at 0, 4.7 and 9.4 T are shown in Figure 2 a, in which the RQCs are 0.2, 0.9 and 1.4 Hz respectively. All spectra were recorded at 9.4 T, confirming that anisotropy is ‘locked in’ upon gel formation. Similar effects are seen with MgCl2 (Figure S14 in the Supporting Information), although this gel had a larger splitting (2.7 versus 1.4 Hz in a similar CaCl2 sample), with apparently a co-existing isotropic phase. Adding NaCl resulted in a slightly increased RQC (Figure S15 in the Supporting Information). This is as expected from our previous data,7a where we showed that MgCl2 can induce gelation, but addition of NaCl resulted in only weak gels being formed.
Figure 2.
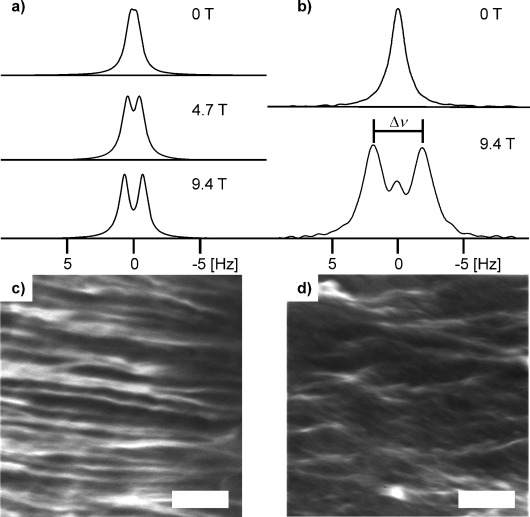
a) 2H NMR spectra of D2O in gels formed in the presence of magnetic fields of varying strengths by the addition of CaCl2 to a solution of NapFF at pD 12.6. All spectra were recorded at 9.4 T. b) 2H NMR spectra of dioxane-d8 in gels formed by the addition of solid GdL to a solution of NapFF at pD 12.6 at magnetic field strength indicated. c) Confocal micrograph of a CaCl2-triggered gel prepared in a 9.4 T magnetic field. d) Confocal micrograph of a CaCl2-triggered gel prepared away from a magnetic field (0 T). The scale bars represent 100 μm.
The magnitudes of the RQC of D2O in NapFF solutions at pD 12.6 (Figure 1 d), measured after 40 min equilibration in the magnetic field, were 0.6 and 1.3 Hz at 4.7 and 9.4 T respectively. The RQC at 4.7 T also took longer to reach a steady value (Figure S1 in the Supporting Information). The observed field dependence of the RQC, rather than merely the time to attain a steady RQC, suggests that the worm-like structures formed by the NapFF at high pD interact significantly with one another, such as by forming entanglements, and so the typical model for magnetic alignment of worm-like assemblies (a rigid rod rotating in a continuous medium under the influence of an applied field)19 is apparently not valid here. We note that this magnetic alignment seems to only affect the fibres; the supramolecular packing is unaffected by the magnetic field as shown by the similarity in the IR data for aligned and unaligned samples (Figure S18 in the Supporting Information).
Chemical-shift imaging (CSI), which provides spatially resolved NMR spectra along the length of the sample adjacent to the RF coils,20 reveals that the extent of anisotropy in a gel sample prepared at 4.7 or 9.4 T is uniform throughout this region of the sample (Figure S2 in the Supporting Information). A gel prepared away from the magnetic field exhibits a very small RQC which increases towards the base of the NMR tube, perhaps indicative of a residual CaCl2 concentration gradient leading to a ‘softer’ gel towards the bottom of the tube, which is more able to re-orient in the magnetic field. To determine the sense of the magnetic-field-induced alignment, we turned to confocal microscopy. A sample was prepared in a 9.4 T magnetic field so that the field axis lay perpendicular to the focal plane during imaging. Images of the sample (Figure 2 c) show that the structures present lie distinctly in the focal plane, whereas in a gel prepared away from the field the structures have a discontinuous appearance (Figure 2 d).
It can thus be inferred that the gel fibres align perpendicular to the magnetic field. We hypothesise that the in-plane alignment exhibited by the structures in the gel prepared in the field arises due to a combination of their mutual interactions and possibly a slight lateral component to the magnetic field. In isolation, the structures would be free to adopt any orientation in a plane perpendicular to a single magnetic field axis.21
Self-supporting hydrogels can also be prepared from solutions of NapFF by a decrease in the pH,6 through the hydrolysis of glucono-δ-lactone (GdL),[6, 22] which serves to protonate the terminal carboxylate groups and drive the formation of a cross-linked network. The D2O resonance in gels thus prepared in magnetic fields does not exhibit an RQC; however, splitting is seen on the resonance of 1,4-dioxane-d8 (0.05 vol %), which was added as a hydrophobic probe molecule (see Figure S5 and Figure S10 for further details; the choice of probe is important, as shown in Figures S11 and S12 in the Supporting Information). We attribute these observations to differences in the way D2O and dioxane order around the hydrophobic fibres, resulting in the RQC of D2O being averaged to zero, whereas that of dioxane is not. This points to the potential of 2H NMR to study the solvation of gel fibres, something that is of relevance for many envisaged applications of hydrogels, such as cell culturing.23 Appreciable RQCs are also seen on the dioxane-d8 resonance in solutions of NapFF at high pD and CaCl2-triggered gels (Figures S3 and S4 in the Supporting Information).
GdL was placed as a solid on top of a D2O solution of NapFF/dioxane and the sample placed in the 9.4 T spectrometer field for 60 h to allow the GdL to dissolve, hydrolyse to gluconic acid and diffuse to the base of the NMR tube. The dioxane resonance in this gel exhibits a distinct ‘doublet’ (Δν, Figure 2 b), indicative of significant magnetic-field-induced anisotropy. Spectra taken as the gel formed show how the singlet (isotropic) peak between the doublet peaks grows in as the gluconic acid diffuses down the NMR tube (Figure S5 in the Supporting Information). We attribute this singlet to ‘strain-induced collapse’ as the hydrophobic protonated fibrils pack together, destroying the alignment that they had at high pD to some degree. A gel formed away from the magnetic field shows no anisotropy (Figure 2 b and Figure S6 in the Supporting Information).
Diffusion of a gelation trigger, such as CaCl2 or GdL, through a solution of NapFF is slow and can readily be followed by CSI.24 Figure 3 a shows the diffusion of CaCl2 down through a solution of NapFF, monitored by changes in the RQC of D2O. A CaCl2 diffusion ‘front’ at which the RQC cannot be resolved (at 0 mm, Figure 3), being of appreciable magnitude both above and below this region of the sample, can be detected. 1H spectra taken across the same region of the sample show how the signal from the NapFF present in the solution at high pD is not detectable above the ‘front’ (Figure S7 in the Supporting Information). The ‘front’ can thus be taken to represent the boundary between a solution of NapFF and a Ca2+ cross-linked gel.
Figure 3.
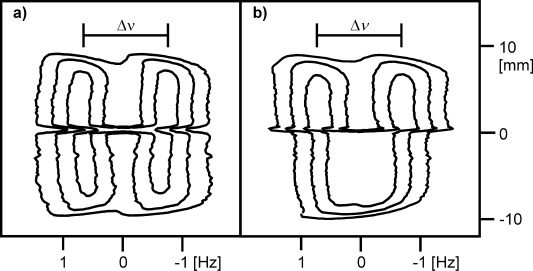
a) 2H image of sample just before removal from magnetic field, shown as a 2D contour plot. The ‘front’ is visible as a discontinuity in the 2H splitting. The vertical axis corresponds to the height above the centre of the NMR radiofrequency coil. b) 2H image of sample after ageing away from magnetic field for two weeks.
It is possible to remove a sample from the magnetic field before the trigger has reached the base of the NMR tube, allowing a gel to be prepared in which the top part of the sample is gelled in the magnetic field while the lower part of the sample is gelled away from the field. This allows us to control the extent of magnetic-field-induced anisotropy spatially across a sample. Figure 3 b shows an image of the same sample from Figure 3 a, removed immediately after NMR imaging and left to gel away from the magnetic field. There is a distinct decrease in the RQC of D2O in the gel, just below the position of the ‘front’ when the sample was removed from the magnetic field. We have thus succeeded in controlling spatially the degree of anisotropy across our sample. The significant splitting observed in the lower portion of the gel (Figure S7f in the Supporting Information), compared to a sample prepared wholly away from the field (Figure S2 in the Supporting Information), is attributable to the slow reorientation of the structures present in solution following removal of the sample from the magnetic field. The ability to control the extent of anisotropy across a gel sample, not just between gels, may prove invaluable to the production of new materials in which the mechanical, transport or electronic properties vary in a spatially controlled fashion.
Finally, we note that our approach to preparing aligned materials is not restricted to NapFF. A prerequisite, however, seems to be the formation of worm-like micelles at high pH17 (Figure S22 in the Supporting Information). Related LMWG that do not form such anisotropic structures cannot be used to form aligned gels (Figure S25 in the Supporting Information). However, where worm-like micelles are formed, the behaviour is analogous to that of NapFF. We expect that this work will greatly expand the utility of LMWGs that form worm-like micelles at high pH, particularly in areas where alignment is key, such as conductive materials and cell culturing.[3, 10, 15]
Experimental Section
NapFF was prepared as described elsewhere.6, 17 All other chemicals were purchased from Sigma–Aldrich and used as received. To prepare a solution of NapFF, solid NapFF was weighed into a 14 mL vial and an appropriate amount of D2O added, followed by standardised NaOD (ca. 1 m(1.2±0.1) equiv). The mixture was then stirred for 24 h to yield a clear, slightly viscous solution with a pD of between 12.1 and 12.6 before being transferred in aliquots (560 μL) to NMR tubes (5 mm) by pipette. The samples were then aged for between 6 and 8 days at (295±3) K before analysis or gel preparation. Analysis by 2H NMR and polarised optical microscopy suggested that the solutions reached a steady state after 5 days (Figures S26 and S27 in the Supporting Information). To prepare CaCl2-triggered gels, CaCl2 solution (0.75 m, 40 μL) was added to the top of the NapFF solution in the NMR tube using a long needle. The sample was then immediately transferred to the NMR spectrometer held at (298±0.5) K, or, for gels prepared away from the magnetic field, a water bath held at (298±0.3) K. After 60 h, the samples prepared in the magnetic field were removed from the spectrometer and placed in the water bath. All samples were left to stand for at least 7 days following CaCl2 addition prior to analysis. The height of the 560 μL solution in the NMR tube (41 mm) is sufficient so that, when first placed in the spectrometer, the sample adjacent to the radiofrequency coils of the spectrometer is completely free from the added CaCl2, which slowly diffuses down the tube into the NMR-active region. Glucono-δ-lactone (GdL)-triggered gels were prepared as for CaCl2-triggered gels, but with solid GdL (3–4 mg) added to the top of the NapFF solution using a Pasteur pipette. Where noted, the NapFF solutions were doped with dioxane-d8 (0.05 vol %, 6 mm) to act as an additional probe for deuterium NMR.
Acknowledgments
We thank Unilever for a Case Award (M.W.) and the EPSRC for funding a DTA (M.W.). We thank the EPSRC for funding (EP/C005643/1 and EP/K039687/1).
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/chem.201405500.
References
- [1].pp. 3133–3160.
- [1a].Terech P, Weiss RG. Chem. Rev. 97 doi: 10.1021/cr9700282. [DOI] [PubMed] [Google Scholar]
- [1b].de Loos M, Feringa BL, van Esch JH. Eur. J. Org. Chem. 1997;2005 [Google Scholar]
- [2].Boekhoven J, Stupp SI. Adv. Mater. 2014;26:1642–1659. doi: 10.1002/adma.201304606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Babu SS, Praveen VK, Ajayaghosh A. Chem. Rev. 2014;114:1973–2129. doi: 10.1021/cr400195e. [DOI] [PubMed] [Google Scholar]
- [4].Kiyonaka S, Sugiyasu K, Shinkai S, Hamachi I. J. Am. Chem. Soc. 2002;124:10954–10955. doi: 10.1021/ja027277e. [DOI] [PubMed] [Google Scholar]
- [5].pp. 1671–1682.
- [5a].Fichman G, Gazit E. Acta Biomater. 10 doi: 10.1016/j.actbio.2013.08.013. [DOI] [PubMed] [Google Scholar]
- [5b].Adams DJ. Macromol. Biosci. 2014;11 doi: 10.1002/mabi.201000316. [DOI] [PubMed] [Google Scholar]
- [6].Chen L, Revel S, Morris K, Serpell LC, Adams DJ. Langmuir. 2010;26:13466–13471. doi: 10.1021/la102059x. [DOI] [PubMed] [Google Scholar]
- [7].pp. 12071–12073.
- [7a].Chen L, Pont G, Morris K, Lotze G, Squires A, Serpell LC, Adams DJ. Chem. Commun. 47 doi: 10.1039/c1cc15474e. [DOI] [PubMed] [Google Scholar]
- [7b].Roy S, Javid N, Frederix PWJM, Lamprou DA, Urquhart AJ, Hunt NT, Halling PJ, Ulijn RV. Chem. Eur. J. 2011;18 doi: 10.1002/chem.201201217. [DOI] [PubMed] [Google Scholar]
- [8].Jayawarna V, Ali M, Jowitt TA, Miller AF, Saiani A, Gough JE, Ulijn RV. Adv. Mater. 2006;18:611–614. [Google Scholar]
- [9].Raeburn J, Zamith Cardoso A, Adams DJ. Chem. Soc. Rev. 2013;42:5143–5156. doi: 10.1039/c3cs60030k. [DOI] [PubMed] [Google Scholar]
- [10].Matson JB, Stupp SI. Chem. Commun. 2012;48:26–33. doi: 10.1039/c1cc15551b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhou J, Du X, Gao Y, Shi J, Xu B. J. Am. Chem. Soc. 2014;136:2970–2973. doi: 10.1021/ja4127399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yoshio M, Shoji Y, Tochigi Y, Nishikawa Y, Kato T. J. Am. Chem. Soc. 2009;131:6763–6767. doi: 10.1021/ja8093718. [DOI] [PubMed] [Google Scholar]
- [13].Ziemecka I, Koper GJM, Olive AGL, van Esch JH. Soft Matter. 2013;9:1556–1561. [Google Scholar]
- [14].pp. 2108–2112.
- [14a].Shklyarevskiy IO, Jonkheijm P, Christianen PCM, Schenning APHJ, Del Guerzo A, Desvergne J-P, Meijer EW, Maan JC. Langmuir. 21 doi: 10.1021/la047166o. [DOI] [PubMed] [Google Scholar]
- [14b].Löwik DWPM, Shklyarevskiy IO, Ruizendaal L, Christianen PCM, Maan JC, van Hest JCM. Adv. Mater. 2005;19 [Google Scholar]
- [15].Zhang S, Greenfield MA, Mata A, Palmer LC, Bitton R, Mantei JR, Aparicio C, de La Cruz MO, Stupp SI. Nat. Mater. 2010;9:594–601. doi: 10.1038/nmat2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].pp. 16101–16104.
- [16a].Michot LJ, Bihannic I, Maddi S, Funari SS, Baravian C, Levitz P, Davidson P. Proc. Natl. Acad. Sci. USA. 103 doi: 10.1073/pnas.0605201103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16b].Tiburu EK, Moton DM, Lorigan GA. Biochim. Biophys. Acta Biomembranes. 2006;1512 doi: 10.1016/s0005-2736(01)00320-0. [DOI] [PubMed] [Google Scholar]
- [16c].Hill RJA, Sedman VL, Allen S, Williams P, Paoli M, Adler-Abramovich L, Gazit E, Eaves L, Tendler SJB. Adv. Mater. 2001;19 [Google Scholar]
- [16d].Raja SO, Dasgupta AK. Chem. Phys. Lett. 2007;554 [Google Scholar]
- [16e].Enozawa H, Hasegawa M, Isomura E, Nishinaga T, Kato T, Yamato M, Kimura T, Iyoda M. ChemPhysChem. 2012;10 doi: 10.1002/cphc.200900545. [DOI] [PubMed] [Google Scholar]
- [16f].Rikken RSM, Kerkenaar HHM, Nolte RJM, Maan JC, van Hest JCM, Christianen PCM, Wilson DA. Chem. Commun. 2009;50 doi: 10.1039/c3cc47483f. [DOI] [PubMed] [Google Scholar]
- [16g].Takazawa K, Inoue J-i, Mitsuishi K. Nanoscale. 2014;6 doi: 10.1039/c3nr06760b. [DOI] [PubMed] [Google Scholar]
- [16h].Matsumoto K, Kimura F, Tsukui S, Kimura T. Cryst. Growth Des. 2014;11 [Google Scholar]
- [16i].Shklyarevskiy IO, Jonkheijm P, Stutzmann N, Wasserberg D, Wondergem HJ, Christianen PCM, Schenning APHJ, de Leeuw DM, Tomović Ž, Wu J, Müllen K, Maan JC. J. Am. Chem. Soc. 2011;127 doi: 10.1021/ja054694t. [DOI] [PubMed] [Google Scholar]
- [16j].Shklyarevskiy IO, Jonkheijm P, Christianen PCM, Schenning APHJ, Meijer EW, Henze O, Kilbinger AFM, Feast WJ, Del Guerzo A, Desvergne J-P, Maan JC. J. Am. Chem. Soc. 2005;127 doi: 10.1021/ja0431096. [DOI] [PubMed] [Google Scholar]
- [16k].Gielen JC, Ver Heyen A, Klyatskaya S, Vanderlinden W, Höger S, Maan JC, De Feyter S, Christianen PCM. J. Am. Chem. Soc. 2005;131 doi: 10.1021/ja904816m. [DOI] [PubMed] [Google Scholar]
- [16l].Alam TM, McIntyre SK. Langmuir. 2009;24 doi: 10.1021/la801681n. [DOI] [PubMed] [Google Scholar]
- [16m].Liebi M, van Rhee PG, Christianen PCM, Kohlbrecher J, Fischer P, Walde P, Windhab EJ. Langmuir. 2008;29 doi: 10.1021/la3050785. [DOI] [PubMed] [Google Scholar]
- [17].Chen L, McDonald TO, Adams DJ. RSC Adv. 2013;3:8714–8720. [Google Scholar]
- [18].Delville A, Grandjean J, Laszlo P. J. Phys. Chem. 1991;95:1383–1392. [Google Scholar]
- [19].Rapp A, Ermolaev K, Fung BM. J. Phys. Chem. B. 1999;103:1705–1711. [Google Scholar]
- [20].Trigo-Mouriño P, Merle C, Koos MRM, Luy B, Gil RR. Chem. Eur. J. 2013;19:7013–7019. doi: 10.1002/chem.201300254. [DOI] [PubMed] [Google Scholar]
- [21].Kimura T, Yoshino M. Langmuir. 2005;21:4805–4808. doi: 10.1021/la050182g. [DOI] [PubMed] [Google Scholar]
- [22].Adams DJ, Butler MF, Frith WJ, Kirkland M, Mullen L, Sanderson P. Soft Matter. 2009;5:1856–1862. [Google Scholar]
- [23].Lee P, Lin R, Moon J, Lee L. Biomed. Microdevices. 2006;8:35–41. doi: 10.1007/s10544-006-6380-z. [DOI] [PubMed] [Google Scholar]
- [24].Östlund Å, Bernin D, Nordstierna L, Nydén M. J. Colloid Interface Sci. 2010;344:238–240. doi: 10.1016/j.jcis.2009.12.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


