Abstract
During recent years different types of adult stem/progenitor cells have been successfully applied for the treatment of many pathologies, including cardiovascular diseases. The regenerative potential of these cells is considered to be due to their high proliferation and differentiation capacities, paracrine activity, and immunologic privilege. However, therapeutic efficacy of the autologous stem/progenitor cells for most clinical applications remains modest, possibly because of the attenuation of their regenerative potential in aged patients with chronic diseases such as cardiovascular diseases and metabolic disorders. In this review we will discuss the risk factors affecting the therapeutic potential of adult stem/progenitor cells as well as the main approaches to mitigating them using the methods of regenerative medicine.
Introduction
Cardiovascular diseases (CVD), particularly coronary artery disease (CAD), are the most frequent causes of mortality worldwide, and along with metabolic pathologies, especially diabetes mellitus type 2 (T2DM), they approach an epidemic status.1,2 An ongoing high frequency of CVD is caused both by the progressive aging of the population and an unhealthy lifestyle associated with risk factors such as obesity, hyperglycemia, hyperlipidemia, and arterial hypertension, which promote early development of atherosclerosis and progression of cardiovascular pathologies.3
Aging is characterized by numerous morphological and functional changes within different tissues and organs. The elasticity of blood vessels declines with age along with an increase in their stiffness, which predetermines the progression of arterial hypertension. As people age, their adipose tissue mass increases, while their muscle volume decreases, leading to the development of insulin resistance, the most important pathogenic factor of T2DM. Aging is also associated with comorbidities, the simultaneous presence of two or more different diseases, often with chronic long-lasting progression. The most frequent age-associated comorbidities confounding each other are CAD and T2DM and obesity, arterial hypertension, and T2DM.4,5
The target affected by the most CVD risk factors is the blood vessel wall. Endothelial dysfunction is considered to be the key pathogenic mechanism of angiopathies associated with CAD and T2DM. It should be noted that endothelial dysfunction develops as a result of the interaction of different risk factors, such as insulin resistance, hyperglycemia, and dyslipidemia. The long-term presence of these factors affects endothelial cells and promotes their apoptosis, which leads to the nitric oxide (NO) production failure. As a consequence, the vasodilatation and anti-aggregation functions of the endothelium are dysregulated along with its ability to inhibit smooth muscle cell proliferation. These factors potentiate atherosclerosis progression, forming the morphological basis of CAD.6
Hyperglycemia is the main pathogenic factor of T2DM, but it also underlies CVD. First, it accelerates the progression of atherosclerosis and macroangiopathies; second, it affects the microvasculature network. This effect is manifested particularly in the disturbance of neovascularization processes in different tissues. In the retina, excessive angiogenesis leads to leaky vessels and diabetic retinopathy, while in the skeletal muscles and myocardium, adaptive angiogenesis is insufficient, which promotes the development of severe ischemia, persistent trophic ulcers, and amputations.7,8
Despite the obvious success in treating CAD and T2DM, all available therapeutic approaches are intended to restrict disease progression. A novel promising approach for the treatment of ischemic diseases is cell-based therapy using autologous stem/progenitor cells.9–14 Several types of cells are involved in the neovascularization processes of ischemic tissues. Endothelial cells; their circulating progenitors (endothelial progenitor cells [EPCs]), which mobilize from the bone marrow as a response to the ischemia and vessel damage; and progenitor cells located within the vessel wall, including multipotent mesenchymal stem/stromal cells (MSCs), interact with each other and contribute to vasculature repair and regeneration.15–17
MSCs, derived from the bone marrow, adipose tissue (ADSCs), or other tissues, are considered a promising tool for cell-based therapy due to their high proliferative and differential potency, ability to stimulate the growth of new blood vessels and nerves, and especially their production of multiple cytokines and growth factors. These cells also secrete plasminogen activators and matrix metalloproteases (MMPs), which actively participate in the remodeling of extracellular matrix (ECM) and the proteolytic release and activation of some growth factors sequestered in the ECM. MSCs serve as a component of the vessel wall in all tissues and play an essential role in the vasculature network development and support in both normal and pathological conditions.18
Many types of stem/progenitor cells, including MSCs, have already been used in clinical trials of cell therapy for ischemic pathologies,12–14,19–22 and their safety and feasibility have been demonstrated, but the clinical effectiveness of these protocols was relatively modest and could not corroborate the promising results of preclinical studies (Table 1). One reason for the insufficient effectiveness of autologous cell therapy may be a lack of understanding about stem/progenitor cells properties in patients with CVD. Most data regarding the regenerative potential of these cells were obtained from cells derived from relatively healthy young donors. However, aging and disease itself may negatively affect stem/progenitor cells and their microenvironment, and impaired stem/progenitor cell functional properties may diminish the effectiveness of autologous cell therapy in aged patients with CAD and metabolic disorders. In this review, we analyze how aging and chronic diseases such as CAD and T2DM affect the properties of stem/progenitor cells.
Table 1.
Meta-Analyses of Autologous Cell Therapy Clinical Trials for the Treatment of Ischemic Diseases
| Disease | Reference | Design | Cell type (method of injection) | Follow-up duration | Outcome |
|---|---|---|---|---|---|
| Peripheral arterial disease | Fadini et al.111 | 37 clinical trials (6 controlled and 31 noncontrolled), n=701 | BMSCs, PB-MNCs mobilized by G-CSF, CD34+ cells (IM or IA) | 6 months | Improvement of ischemia surrogate indexes: |
| - ABI 0.46±0.04 vs. 0.63±0.04 (p=0.011); | |||||
| - TcO2 22.8±2.8 vs. 35.8±2.9, (p=0.0002); | |||||
| - walking capacity 75.7±19.4 vs. 402.3±70.9 m, (p<0.0001); | |||||
| - better ulcer healing (OR 3.54, 95% CI 1.09−11.51, p=0.032); | |||||
| - benefit in amputation rate (OR for amputation 0.09; 95% CI 0.02–0.44, p=0.0005). | |||||
| AMI | Clifford et al.112 | 33 RCTs, n=1765 | BMSCs (IC) | <12 and 12–61 months | No statistically significant changes in the incidence of mortality (RR 0.70, 95% CI 0.40–1.21) or morbidity (re-infarction, hospital re-admission, restenosis, and target vessel revascularization). |
| In short-term follow-up improvement: LVEF (WMD 2.87, 95% CI 2.00–3.73); | |||||
| In long-term follow-up improvement: | |||||
| - WMD 3.75, 95% CI 2.57–4.93; | |||||
| - reduce LVESV and LVEDV and infarct size; | |||||
| - positive correlation between BMSC cell dose and the effect on LVEF | |||||
| Different diseases, including ischemic stroke, AMI, et al. | Lalu et al.113 | 36 clinical trials (8 RCTs), n=1012 | MSCs (IV or IA) | 0–60 months | Safety of the therapy regarding the short-term and long-term adverse effects. |
| There was a significant association between MSCs injection and transient fever (OR 16.82, 95% CI 5.33–53.10). | |||||
| CAD | Fisher et al.114 | 9 RCTs, n=659 | BMSCs (mobilized CD34+ cells) (NOGA system) | 6–12 months | Benefits: |
| - reduced risk of mortality (RR 0.33; 95% CI 0.17 to 0.65; p=0.001); | |||||
| - improvement in angina class (MD- 0.55; 95% CI −1.00 to −0.10; p=0.02) | |||||
| - fewer angina episodes per week (MD −5.21; 95% CI −7.35 to −3.07; p<0.00001); | |||||
| - improved quality of life (p=0.002); | |||||
| - improved exercise/performance (p=0.002); | |||||
| - increase of LVEF (MD 3.47; 95% CI 1.88–5.06, p=0.00002). | |||||
| AMI | Delewi et al.115 | 24 RCTs, n=1624 | BMSCs (IC) | 6–12 months | In 6 month: |
| - improvement of LVEF 2.23% (95% CI 1.00–3.47; p<0.001); | |||||
| - reduction in LVESV −4.81 mL (95% CI −7.86 to −1.76; p<0.001); | |||||
| In 12 month: | |||||
| - improvement of LVEF (11 studies) 3.91% (95% CI 2.56–5.27; p<0.001); | |||||
| - reduction in LVESV −9.41 mL (95% CI −13.64 to −5.17; p<0.001); | |||||
| - decrease in recurrent AMI (RR 0.44, 95% CI 0.24–0.79; p=0.007); | |||||
| - hospital re-admission due to heart failure, unstable angina or chest pain (RR 0.59, 95% CI 0.35–0.98, p=0.04). | |||||
| Critical limb ischemia | Benoit et al.116 | 45 clinical trials(7 RCTs), n=1272 | BMMNCs/PBMNCs (IM) | 1–48 months | Safety: low incidence of adverse events (4.2%), mortality, cancer cases are comparable with the control group. |
| Benefits: | |||||
| - significantly lower amputation rate (OR 0.36, p=0.0004); | |||||
| - some improvement in a variety of functional and surrogate outcomes | |||||
| CAD and congestive heart failure | Fisher et al.117 | 23 RCTs, n=1255 | BMSC (IC or NOGA system) | <12 or ≥12 months | Safety: among 19 trials in which adverse events were reported, adverse events relating to BMSC treatment or procedure occurred in four individuals. |
| Benefits: | |||||
| - reduced incidence of mortality (RR 0.28, 95% CI 0.14–0.53; p=0.0001); | |||||
| - hospital re-admission due to heart failure (RR 0.26, 95% CI 0.07–0.94; p=0.04); | |||||
| - reduction in LVESV (MD −14.64 mL, 95% CI −20.88 ml to −8.39 mL, p<0.00001); | |||||
| - improvement of LVEF (MD 2.62%, 95% CI 0.50%–4.73%, p=0.02); | |||||
| - reduction in NYHA functional class (MD −0.63, 95% CI −1.08 to −0.19, p=0.005); |
RCTs, randomized controlled trials; BMSCs, bone marrow–derived stem cells; BMMNCs, bone marrow mononuclear cells; PBMNCs, peripheral blood mononuclear cells; IM, intramuscularly; IA, intra-arterial; IV, intravenously; IC, intracoronary; ABI, ankle brachial index; TCO2, transcutaneous oxygen tension; G-CSF granulocyte colony-stimulating factor; AMI, acute myocardial infarction; CAD, coronary artery disease; NYHA, New York Heart Association class; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volumes; LVEDV, left ventricular end-diastolic volume; CI, confidence interval; OR, ratio of the odds; RR, relative risk; WMD, weighted mean difference; MD, mean difference.
Influence of Aging on Stem/Progenitor Cells
Notion of aging in stem/progenitor cell compartment
Stem/progenitor cells mediate lifelong physiological renewal and regeneration of tissues. Attenuated regeneration potential of aged organisms might be caused by age-associated changes of stem/progenitor cells activity. Both intrinsic and extrinsic mechanisms as well as cell response to systemic signals are involved in the normal and pathological aging of stem/progenitor cells, including MSCs.23,24 Inhibition of stem/progenitor cell functional activity might be mediated by telomere shortening and decreased telomerase activity,25 decline of the proliferation potency, weakening of the antioxidant protection system and/or presence of oxidative stress,26 irreversible protein modification, and accumulated damage to the DNA repair system and methylation pattern.27
It should be emphasized that substantial differences exist between cellular senescence and organismal aging. Aging can be defined as “the sum of primary restrictions in regenerative mechanisms of multicellular organisms.”28 This definition highlights the involvement of stem/progenitor cells in cell replenishment and thus in influencing lifespan.28 A wide spectrum of age-associated pathologies exist, including atherosclerosis, CAD, stroke, oncological diseases, psychiatry disorders, and so forth.
Cellular senescence is the equivalent of replicative senescence, and it can be defined as “an essentially irreversible arrest of cell division,” which underscores the changes in both function and replicative capacity of senescent cells. The senescent cell becomes a major actor of the aging process, among others, by acquiring a senescence-associated secretory phenotype.29
Aging markers in stem/progenitor cells
Influence of aging on stem/progenitor cells is well studied for MSCs. Bone marrow-derived MSCs from aged donors were shown to have worse proliferation and differentiation capacity28,30–33 and were less effective for tissue repair after ischemic injury; for example, in an animal model of myocardial infarction.33 In contrast to bone marrow-derived MSCs, the number of ADSCs in fat tissue, as evaluated by flow cytometry, does not decrease with age.34,35 However, their clonogenic and proliferation capacity declines36–40 as does the differentiation potential34,36,41 and the production of vascular endothelial growth factor (VEGF).42
Using the appropriate age markers is important for the evaluation of aging impact to the properties of stem/progenitor cells. One marker is the relative telomere length, which indicates the number of cell divisions. Telomere shortening is considered to be the main causal mechanism for replicative cell senescence and age-associated telomere damage, and the diminution of the telomere “capping” function and associated p53 activation have emerged as prime instigators of tissue stem/progenitor cells functional decline.25,43 In poorly differentiated cells, telomere length is maintained due to the high activity of telomerase. Telomerase also has some telomere-independent functions. Telomerase activity was shown to be the highest in stem and tumor cells and was detected to a certain degree in many kinds of progenitor cells.37,44 Its activity is repressed as stem cells start to differentiate.25,37 Age-associated telomere shortening in MSCs has been shown.28,30 According to our data, relative telomere length decreases with age in both murine and human ADSCs.45,46 Interestingly, telomeres in “aged” stem/progenitor cells are still longer than telomeres in somatic cells from the same tissues,25,27 which could be explained by the lower proliferation activity of stem/progenitor cells or by special mechanisms of telomere-shortening prevention.
Age-dependent decrease of stem/progenitor cell proliferation activity is considered to be related to higher expression of cell cycle inhibitors like p16INK4a, p21, p53, and p19ARF or inhibition of their degradation.28,47 In accord with telomere shortening in ADSCs from aged patients and old mice, we observed a decrease in proliferation activity of ADSCs with age as well as fewer actively proliferating cells.45,46
Because stem/progenitor cells persist in tissues throughout life, albeit mostly in a quiescent state, they experience age-related long-term exposure to genotoxic insults from both endogenous and exogenous sources. Accordingly, accumulation of DNA damage in aged stem/progenitor cells has been noted in several studies. The DNA damage response pathways necessary for stabilizing the genomic integrity may have reduced activity in stem cells with age. Accumulation of DNA damage in aged stem cells could cause stem cell senescence or apoptosis and alterations in stem cell self-renewal and differentiation.24
Oxidative stress and its regulators play an important role in aging of stem/progenitor cells. Increasing production of reactive oxygen species (ROS) and weakening of the antioxidant protective system in cells promote oxidative damage and lead to the loss of redox control, which affects ROS-regulated biological processes such as growth, proliferation, migration, apoptosis, differentiation, and so forth.28 Different factors like hypoxia inducible factor-1 alpha (HIF-1α), ataxia telangiectasia mutated (ATM) protein, Bmi-1, and FoxO family factors may change ROS level in the cells.27 In bone marrow–derived MSCs obtained from aged patients, the activity of superoxide scavenger (superoxide dismutase) is decreased and the levels of ROS, NO, and oxidized and glycosylated proteins are increased.30 Supporting the hypothesis that ROS generation may promote stem cell aging, studies of aged human stem/progenitor cells, including MSCs, neural stem cells, and others, have found that excessive cellular ROS concentrations lead to abnormal proliferation, malignancy, and compromised stem cell self-renewal and differentiation capacity.24 The role of ROS in aging-related changes of ADSC properties was also demonstrated.48
A direct relationship between mitochondrial dysfunction and aging has been suggested by many studies,49 and an age-related deficit of mitochondrial function leading to respiratory chain dysfunction in stem/progenitor cells is actively being investigated. These effects are considered to result largely from an accumulation of mutations in mitochondrial DNA damaged by elevated ROS or other mechanisms.24,49 Aside from primary mitochondrial lesions (mitochondrial DNA mutations), secondary alterations in mitochondrial function driven by age-related cellular and metabolic changes may also contribute to the aging process.49
Age-related changes of stem/progenitor cells paracrine functions
Paracrine activity of stem/progenitor cells, including MSCs, changes with age. Interleukin (IL)-6 secretion by bone marrow–derived MSCs co-cultured with T cells decreased in aged patients.32 According to our data, the angiogenic potential of ADSCs is significantly impaired during aging. We have shown that ADSCs isolated from old mice (18 months), along with expressing age markers (shorter telomeres, higher rate of apoptotic cells, less proliferative capacity, enhanced oxidative damage), have an impaired ability to stimulate blood vessel growth on in vitro and in vivo models of angiogenesis compared to ADSCs from young animals (1–2 months).45 Similar results were obtained with human ADSCs, including in patients with cardiovascular pathologies.46 We demonstrated that the mechanisms of age-associated decline of ADSC angiogenic activity include decreased production of key pro-angiogenic factors such as VEGF, placental growth factor (PlGF), hepatocyte growth factor (HGF), angiopoetin-1, and angiogenin.46 It should be noted that age-associated differences in the expression of pro-angiogenic factor genes were not found,46 indicating that posttranscriptional mechanisms, such as regulation by microRNA,38,50 age-associated protein misfolding, and so forth, could underlie the decreased secretion of angiogenic factors by ADSCs from aged patients.
Apart from the angiogenic growth factor effects, ECM remodeling is crucial for successful angiogenesis. ECM remodeling and direction of cell migration for vessel wall formation are regulated by multiple factors such as urokinase (uPA) and its receptor (uPAR), plasminogen activator inhibitor-1 (PAI-1), MMPs, and so forth. Analyzing the expression of factors involved in ECM remodeling and vascular cell migration and invasion, we found that mRNA levels of uPA, uPAR, and PAI-1 as well as uPAR surface expression along with the activation of MMP-2 and MMP-9 were higher in ADSCs from aged patients with CAD.46,51 These results are consistent with those we previously obtained from ADSCs from young and old mice.45 The findings could reflect the adaptive reaction of stromal cells to the age-associated ECM changes within the blood vessel wall52 and increasing levels of pro-inflammatory factors and ROS. Also considering the regulatory role of the uPA system in growth factor–induced endothelial cell migration and invasion and stimulation of angiogenesis in ischemic tissues,53 we can speculate that its activation in ADSCs from aged patients might be a compensatory response to the reduction of pro-angiogenic factor secretion.
Aging as a risk factor for autologous cell therapy
Given the total evidence, aging could essentially affect the properties of stem/progenitors cells, thereby diminishing the effectiveness of autologous cell therapy. Testing of cell material before use may be required along with developing effective approaches for pretreatment or modification of stem/progenitor cells from aged patients to enhance their therapeutic potential. Some of these approaches will be discussed below.
Since MSC are considered to be the components of vessel wall and take part in its repair after injury, cellular modifications due to aging can be an important pathogenic factor of age-related diseases such as atherosclerosis, diabetes, and arterial hypertension.11 Aging is also associated with EPC dysfunction that further negatively affects the neovascularization and angiogenesis in tissues.54
We can conclude that organismal aging is a complex process in which resident stem/progenitor cells are involved both as a cell reservoir for the repair and regeneration of tissues and as targets exposed to the multiple local and systemic stimuli of the aged organism. These changes should be considered during the development of cell-based therapy using different types of autologous stem/progenitor cells.
Influence of Chronic Diseases on Stem/Progenitor Cells
Stem/progenitor cell quantity and characteristics in the presence of chronic diseases
During recent years a substantial amount of data have shown that chronic pathologies, including CAD and T2DM, affect the properties of stem/progenitor cells. It should be noted that widespread risk factors associated with CAD (age, dyslipidemia, obesity, smoking, arterial hypertension, glucose intolerance) may have an impact on the decrease in number and/or functional activity of stem and progenitor cells.16,55–57 Among the pathologies affecting the functionality of stem and progenitor cells, autoimmune diseases, such as systemic sclerosis and systemic lupus, may be included.58
Numerous studies showed that the number, proliferation activity, ability for adhesion, migration, and angiogenic properties of EPCs are significantly impaired in patients with CAD55,59–61 and metabolic disorders including obesity and T2DM.62–65 Van Ark et al.66 demonstrated that in patients with T2DM both EPCs and circulating angiogenic cells levels were reduced and the ratio between EPCs and smooth muscle progenitor cells was disturbed, which may translate into reduced vascular repair capacity, thereby promoting macrovascular disease in T2DM.
According to the results of several studies the number of MSCs is unlikely to be decreased in patients with cardiovascular diseases, but their regenerative potential may be attenuated. Thus, Harris et al.35 showed that the number of ADSCs obtained from 50 patients with different vascular pathologies was relatively consistent independent of age and comorbidities like obesity, T2DM, and so forth. Similar results were obtained by Madonna et al.57 in 42 patients with different grades of cardiovascular risk. However, ADSCs isolated from adipose tissue of patients with T2DM were shown to have lower proliferation activity and were less responsive to pro-angiogenic stimuli such as hypoxia.67 In patients with obesity, impaired differentiation potential of ADSCs and a decline in their ability to stimulate blood vessel growth were observed.68 In a study performed by Vecellio et al.,69 MSCs obtained from the heart tissue of patients with T2DM were characterized by a reduced proliferation rate, diminished phosphorylation at histone H3 serine 10 (H3S10P), decreased differentiation potential, and premature cellular senescence compared to the control group.
Changes of stem/progenitor cell properties in patients with CAD and T2DM: evidence from ADSCs
We analyzed how ADSC properties are changed in patients with CAD and T2DM.70 We showed that ADSCs from the patients with CAD (n=32) and CAD+T2DM (n=28) had similar morphology and immunophenotype, preserved adipogenic and osteogenic differentiation potency, and higher proliferation activity, but shorter telomeres compared to ADSCs from control patients without established chronic pathologies (n=19). These findings might reflect the depletion of progenitor cell compartment in the presence of chronic pathologies such as CAD and T2DM.
Analyzing ADSCs as a tool for therapeutic angiogenesis we found that the angiogenic potential of ADSCs obtained from patients with CAD and with CAD+T2DM was decreased compared to patients without established cardiovascular pathologies independent of such factors as age and sex. We also did not see any statistically significant evidence that T2DM had an additional impact on the decline of ADSC angiogenic activity when combined with CAD. Since a paracrine mechanism is considered to be the main regulator of the beneficial effects of ADSCs, we examined the ability of ADSC to secrete some key angiogenic and anti-apoptotic growth factors. Interestingly, we revealed significantly higher production of some pro-angiogenic factors by ADSCs: VEGF and HGF for patients with CAD and HGF and PlGF for patients with CAD+T2DM, whereas the angiogenic activity of all products secreted by ADSCs from patients with both CAD and CAD+T2DM was significantly decreased compared to the control group. Absence of an elevation in the VEGF level in ADSC-conditioned medium from patients with CAD+T2DM may be associated with hyperglycemia. A high glucose level reduces endothelial nitric oxide synthase (eNOS) and stimulates inducible NOS (iNOS) expression that can inhibit HIF-1a gene expression. The activity of HIF-1a gene expression in its turn directly mediates VEGF gene expression. Moreover, by reducing eNOS the high glucose level inhibits the production of NO in endothelial and vascular smooth muscle cells, which is known as a downstream mediator of several angiogenic factors including angiopoietin-1.71,72
The process of angiogenesis depends on the intricate balance between angiogenic and angiostatic factors. We speculated that the main cause of impaired angiogenic potential of ADSCs obtained from patients with CAD and CAD+T2DM was the increased level of the angiogenesis inhibitors. Among others we analyzed production of endostatin and thrombospondin-1 (THBS1) by ADSCs and showed that THBS-1 gene expression is significantly increased in ADSCs from patients with CAD and CAD+T2DM, but we could not confirm this finding on the protein level. However, statistical analysis revealed negative correlation between mRNA THBS-1 expression and angiogenic activity of summary products secreted by ADSCs from patients with CAD and CAD+T2DM. It allows us to assume that some indirect mechanisms of THSP-1 participation in the angiogenic effects of ADSCs could be discussed.
As for the factors involved in ECM remodeling, we found that the mRNA level of PAI-1 as well as its secretion by ADSCs was significantly increased in groups of patients with CAD and CAD+T2DM. PAI-1 is one of the primary regulators of the fibrinolytic system, and it has a crucial effect on cell migration and adhesion. A high level of PAI-1 was linked with a high risk of CAD, diabetes, and obesity. PAI-1 can both promote and inhibit vascular remodeling, but its role in angiogenesis and tissue regeneration is still controversial. The balance between these two mechanisms may depend on a disease state. Plasma PAI-1 is closely correlated with such factors as hypoxia, glucose-related signaling molecules, inflammatory cytokines, triacylglycerol, and insulin.73–75 Acosta et al.76 showed that ADSCs obtained from patients with T2DM have less fibrinolytic ability because they secrete more PAI-1 and less tissue activator of plasminogen and D-dimer. This situation led to the development of microthrombotic complications when these cells were used to treat critical low limb ischemia. PAI-1 actively interacted with uPA, an important factor of extracellular proteolysis that not only specifically cleaves plasminogen and converts it into plasmin, causing activation of different MMPs, but also initiates intracellular signaling upon binding to its receptor on the cell surface. It therefore plays multiple roles in vascular remodeling and angiogenesis. We propose that PAI-1 produced by ADSCs exerts anti-angiogenic effects also through the inhibition of uPA.
Importantly, we demonstrated that by neutralizing only one factor in ADSC-conditioned medium—PAI-1—we could partially restore the angiogenic activity of ADSCs obtained from patients with chronic diseases. Our data are corroborated by the results obtained by Tashiro et al.77 who have shown that PAI-1 inhibition in vivo under ischemic conditions increases the activity of pro-angiogenic factors such as VEGF-A and fibroblast growth factor (FGF)-2 and leads to the stimulation of angiogenesis and improved restoration of tissue perfusion. However, MMP-9 deficiency and VEGF-A blockade reversed the PAI-1 inhibitor-mediated neovascularization within the ischemic niche. Some experts have suggested that a PAI-1 blockade and an increase in MMP-9 gene expression under ischemic conditions could be promising molecular approach in the therapeutic angiogenesis.77,78 It should be noted that we did not find significant increase in the MMP-9 mRNA level in ADSCs from patients with cardiovascular pathologies in our study. Therefore, we assume that high expression of PAI-1 and insufficient activity of MMP-9 and, for diabetic patients, VEGF may be one of the mechanisms of impaired angiogenic potential of ADSCs from patients with CAD and CAD+T2DM.
Hyperglycemia affects both specialized and stem/progenitor cells
One of the main adverse factors for cells from patients with T2DM is hyperglycemia. Several studies have shown that long-term exposure of vascular cells to a high level of glucose causes dysfunction and promotes apoptosis.79 We have previously found that endothelial cells cultured in hyperglycemia modeling conditions (25 mM of glucose) have an impaired ability for VEGF- and serum-induced migration as well as forming capillary-like structures on Matrigel in vitro. To reveal the possible mechanisms of these effects we analyzed the expression of VEGF receptors (VEGFR) on endothelial cells because this growth factor specifically induced adaptive angiogenesis in ischemic tissues. We observed fivefold decrease in VEGFR1 expression and more than twofold decrease in VEGFR2 expression in the endothelial cells cultured in hyperglycemic conditions compared to the standard conditions.80
We also analyzed the influence of hyperglycemia on progenitor cells localized in the perivascular niche, such as MSCs. Culturing of ADSCs in high glucose (25 mM) medium didn't affect their proliferation activity, viability, or migratory properties, but the ability of the total secreted products of these cells to stimulate capillary-like tube formation in vitro was significantly decreased. We evaluated the transcriptome of ADSCs cultured in the standard conditions or in the presence of high glucose (25 mM) and found the most significant changes in gene expression of ephrin receptors, vitronectin, and plexin domain 1 contained protein (decreased in hyperglycemic conditions) and leptin, tumor necrosis factor α (TNFα), plasminogen, and angiopoetin-like factor 3 (increased in hyperglycemic conditions). However, we failed to find any significant changes in gene expression of key angiogenesis-related factors and their receptors associated with high-glucose culturing. To explain the decreased angiogenic activity of ADSCs cultured in hyperglycemic conditions, we focused on the elevated level of plasminogen in these cells. It is known that high expression of plasminogen in tumors is associated with activation of MMPs that perform cleave plasminogen to generate angiostatin, an angiogenesis inhibitor. This mechanism might also be realized in ADSCs, impairing their angiogenic potential. A combination of increased expression of TNFα and angiopoetin-like factor 3 also could affect ADSC angiogenic activity because these factors stimulate apoptosis of endothelial cells. Taken together, long-term incubation of vascular cells in hyperglycemic conditions affect both endothelial cells and ADSC functional properties, which can be one of the reasons for insufficient adaptive angiogenesis in patients with diabetes.81
It should be emphasized that EPC number and functional properties are also significantly changed in patients with metabolic disorders.82,83 EPCs play a very important role in the repair of damaged endothelium and the revascularization of ischemic tissues. T2DM has been shown to affect EPC mobilization from the bone marrow, resulting in a lower amount of EPCs in circulation and a distortion of angiogenesis and normal repair processes in injured tissues.84 This promotes the progression of ischemic diseases and adversely affects the prognosis of patients with T2DM.
No consensus exists about the EPC phenotype for the identification of these cells. In clinical practice, the most frequently used markers are CD133+/CD34+, CD133+/CD34+/VEGF-R2+, CD133+/VEGF-R2+, CD34+/VEGF-R2+.85–87 However, some investigators have demonstrated that the subpopulation identified as CD45−VEGFR2+(KDR)CD34+ cells consists of mostly leukocyte progenitors, particularly monocytes.88,89 Contradictory data about the immunophenotype of EPCs is explained by the substantial overlap of surface markers between the endothelial and hematopoetic progenitors and differences in the protocols and flow cytometry analysis.
Given the association of both CAD and T2DM with endothelial dysfunction and tissue ischemia, EPCs might serve as biomarkers of severity and prognosis in patients with such pathologies. We analyzed total populations of CD34+ cells, including hematopoetic stem/progenitor cells as well as EPCs in the peripheral blood of patients with CAD and with CAD+T2DM. We found that EPC numbers were significantly higher in the patients with CAD only compared to the healthy control age- and sex-matched subjects. This increase could be caused by the stimulated mobilization of these cells from bone marrow in response to tissue ischemia. But in patients with CAD+T2DM, such an elevation was not observed, suggesting the possible distortion of EPC mobilization in T2DM.
Analyzing EPC numbers based on the severity of T2DM, we found that in patients with compensated and subcompensated diabetes, the level of EPCs was similar to the normal level, but patients with decompensated diabetes and comparable severity of CAD had significantly lower numbers of these cells, which could indicate impaired mobilization of EPCs from bone marrow. We showed that EPC numbers were negatively correlated with glucose levels.58 Similar data were obtained by Churdchomjan et al.84
Altogether ample evidence exists that chronic diseases such as CAD and T2DM severely affect not only the fully terminated endothelial cells, but also stem/progenitor cells involved in vascular remodeling in normal and pathological conditions. Further investigation of the influence of the mechanisms of chronic diseases on the quantitative and functional characteristics of stem/progenitor cells is important for the development of effective methods for diagnosis and correction of micro- and macroangiopathies.
Perspective of Personalized Approaches to Autologous Cell Therapy
Taking into account the results of numerous studies discussed, it is important to know the changes of patient's stem/progenitor cells to develop the methods of individual pretreatment of autologous cell material to improve its therapeutic potential.90,91
Hypoxic preconditioning is one of the most accessible and widespread approaches to treat stem/progenitor cells before transplantation to enhance their resistance to the ischemic stimuli and stimulate production of angiogenic factors in these cells. Many studies have shown that hypoxia stimulates angiogenic properties of bone marrow–derived MSCs and ADSC.45,92–97 Thus, culture of bone marrow–derived MSC in hypoxic conditions before transplantation improved the survival of these cells in damaged tissues as well as their ability to stimulate blood vessel growth.98
In several studies, including our work, even short-term (2–3 days) exposure of ADSCs to low oxygen levels (1%–5%) stimulated their proliferation and angiogenic activity.93–97,99 Moreover, we found that after hypoxic preconditioning the balance between pro- and anti-angiogenic factors was shifted to the more pro-angiogenic profile in ADSCs obtained from both young and aged donors. The preconditioning enhances the ability of ADSCs to stimulate angiogenesis and stabilize the newly forming vessels.45,94 Despite the many advantages of using hypoxic preconditioning as a pretreatment procedure for autologous stem/progenitor cells, length of hypoxia, oxygen level, and number of hypoxia/reperfusion cycles vary from study to study, so the optimal pattern of hypoxic preconditioning remains open for discussion.
For some purposes stem/progenitor cells could be initially differentiated ex vivo using the specific induction medium100 or sorted by the specific markers like CD34.101 Several growth factors and chemokines could be added to the culture medium of stem/progenitor cells, such as basic FGF (bFGF), epidermal growth factor, TNFα, insulin-growth factor-1, bone morphogenetic protein-2, and so forth, to enhance the viability, proliferation, migratory properties, and therapeutic potential of these cells.101,102
Another approach includes modulation of intracellular signal cascades, for example, by pretreating stem/progenitor cells with statins that activate the Akt/eNOS pathway within the cells103 or with protein kinase p38 inhibitor,104 or by culturing the cells in the presence of melatonin to increase their viability and proliferation activity and stimulate angiogenic factor production and angiogenic potential after transplantation to the ischemic kidney parenchyma.105 Lutolf et al.106 described a novel approach to restore the regenerative potential of aged stem/progenitor muscle cells: p38 inhibition combined with bioengineered modeling of the cell microenvironment.106
Sun et al.31 have demonstrated that MSCs from old animals could have their capability for renewal and osteogenic potential restored by being cultured on ECM produced by the cells of young donors. Similarly, EPCs isolated from old rat peripheral blood recover their functions in vitro and in vivo after being cultured in the presence of young animal's serum.36
One of the most promising tools for stem/progenitor cell pretreatment is genetic modification of these cells. Through the use of different vectors, various genetic constructions could be efficiently inserted to ADSCs. ADSCs modified by VEGF and HGF produced significantly higher amount of these factors and had enhanced angiogenic activity.36,107,108 Modification of stem/progenitor cells with other factors improved their ability for homing (stromal derived factor-1, CXCR4), increased viability (Akt, Bcl-2, heat shock protein-20, hemoxygenase-1, bFGF), and stimulated paracrine function (angiogenin, angiopoetin-1, IL-18-binding protein, TNFα receptors 1 and 2).57,91 Several factors could be combined for modification; for example, using MMP-3 tissue inhibitor109 or telomerase110 with VEGF to modify MSCs obtained from aged donors allowed significant improvement of cell regenerative potential.
Conclusions
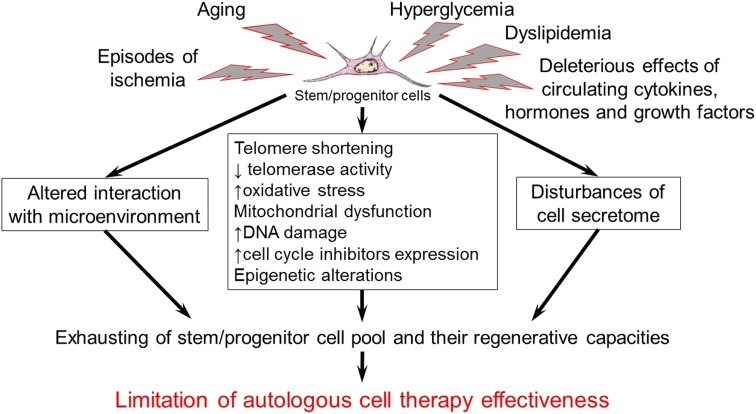
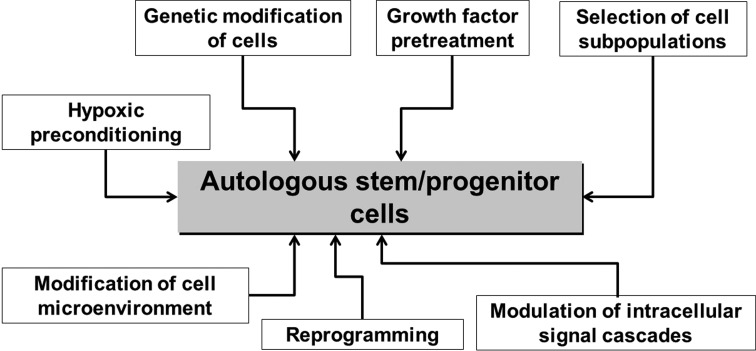
Aging and chronic diseases including CVD and diabetes substantially affect stem/progenitor cells of adult organism (Fig. 1). Such conditions could restrict the effectiveness of autologous cell therapy in aged patients with CAD, lower limb ischemia, T2DM and other chronic pathologies, although these patients are some of the most obvious candidates for cell therapy. These findings also indicate the necessity of careful testing of autologous cell material before use as well as developing effective approaches for pretreatment or modification of stem/progenitor cells from aged patients with multiple comorbidities (Fig. 2) to enhance therapeutic potential and stimulate endogenous regenerative processes.
FIG. 1.
Aging and chronic diseases affect adult stem/progenitor properties and may cause low effectiveness of autologous cell therapy.
FIG. 2.
Variety of approaches for pretreatment or modification to enhance the therapeutic potential of stem/progenitor cells from aged patients with multiple comorbidities.
Abbreviations Used
- ADSC
adipose-derived stem cell
- bFGF
basic fibroblast growth factor
- CAD
coronary artery disease
- CVD
cardiovascular disease
- ECM
extracellular matrix
- eNOS
endothelial nitric oxide synthase
- EPC
endothelial progenitor cell
- FGF
fibroblast growth factor
- HGF
hepatocyte growth factor
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- MMP
matrix metalloprotease
- MSC
mesenchymal stem/stromal cell
- NO
nitric oxide
- PAI
plasminogen activator inhibitor
- PlGF
placental growth factor
- ROS
reactive oxygen species
- T2DM
type 2 diabetes mellitus
- TNFα
tumor necrosis factor
- uPA
urokinase
- uPAR
urokinase receptor
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor
Acknowledgments
This work was supported by the European Union Seventh Framework Program (FP7/2007–2013) [grant agreement no. 241558] (SICA-HF); the Russian Ministry of Science and Education within the FTP “R&D in priority fields of the S&T complex of Russia 2007–2012” [no. 02.527.11.0007, no. 02.740.11.0307]; the Russian Foundation of Basic Research [no. 14-04-00637a] and the grant of the Russian Ministry of Education and Science [no. 14.607.21.0045]. The authors would like to thank Prof. I.O. Golubev and Dr. V.I. Makunin (Trauma and Orthopedic Department of the Faculty of Medicine, MSU) for acquiring adipose tissue samples. We acknowledge George V. Sharonov for help in FACS data acquisition and analysis (FACS core facility, Faculty of Medicine, Moscow State University). We thank medical students E.E. Starostina and E.V. Gluhanyuk for their participation in data collection and the staff of Russian Cardiology Research and Production Center and the Faculty of Medicine of Lomonosov Moscow State University for their assistance. The authors express their gratitude to Reviewers and Editor for their valuable and critical comments.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Belenkov YuN, Mareev VYu, Ageev FT. Chronic heart failure. Selected lectures on cardiology [In Russian]. GEOTAR-Media: Moscow, 2006 [Google Scholar]
- 2.Yancy C, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:e240–e327 [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. WHO global InfoBase, 2008. Available at: http://www.who.int/infobase (accessed October21, 2013)
- 4.American Diabetes Association; National Heart, Lung and Blood Institute; Juvenile Diabetes Foundation International; National Institute of Diabetes and Digestive and Kidney Disease; American Heart Association. Diabetes mellitus: a major risk factor for cardiovascular disease. Circulation. 1999;100:1132–1133 [DOI] [PubMed] [Google Scholar]
- 5.Kotseva K, Wood D, De Backer G, et al. EUROASPIRE III: a survey on the lifestyle, risk factors and use of cardioprotective drug therapies in coronary patients from 22 European countries. Eur J Cardiovasc Prev Rehabil. 2009;16:121–137 [DOI] [PubMed] [Google Scholar]
- 6.Ageev FT. Role of endothelial dysfunction in the development and progression of cardiovascular disease [In Russian]. J Heart Failure. 2003;4:22–24 [Google Scholar]
- 7.Balabolkin MI, Klebanova EM, Kreminskaya VM. Treatment of diabetes mellitus and its complications. Medicine: Moscow, Russia; pp. 304–414; 2005 [Google Scholar]
- 8.Spinetti G, Kraenkel N, Emanueli C, et al. Diabetes and vessel wall remodelling: from mechanistic insights to regenerative therapies. Cardiovasc Res. 2008;78:265–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valina C, Pinkernell K, Song YH, et al. Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. Eur Heart J. 2007;28:2667–2677 [DOI] [PubMed] [Google Scholar]
- 10.Madonna R, De Caterina R. Adipose tissue: a new source for cardiovascular repair. J Cardiovasc Med (Hagerstown). 2010;11:71–80 [DOI] [PubMed] [Google Scholar]
- 11.Williams AR, Hare JM. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res. 2011;109:923–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsiao LC, Carr C, Chang KC, et al. Review article: stem cell-based therapy for ischemic heart disease. Cell Transplant. 2013;22:663–675 [DOI] [PubMed] [Google Scholar]
- 13.Mathiasen AB, Haack-Sørensen M, Jørgensen E, et al. Autotransplantation of mesenchymal stromal cells from bone-marrow to heart in patients with severe stable coronary artery disease and refractory angina—final 3-year follow-up. Int J Cardiol. 2013;170:246–251 [DOI] [PubMed] [Google Scholar]
- 14.Bura A, Planat-Benard V, Bourin P, et al. Phase I trial: the use of autologous cultured adipose-derived stroma/stem cells to treat patients with non-revascularizable critical limb ischemia. Cytotherapy. 2014;16:245–257 [DOI] [PubMed] [Google Scholar]
- 15.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hill J, Zalos G, Halcox JP. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600 [DOI] [PubMed] [Google Scholar]
- 17.Siddique A, Shantsila E, Lip GY, et al. Endothelial progenitor cells: what use for the cardiologist? J Angiogenes Res. 2010;2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nombela-Arrieta C, Ritz J, Silberstein LE. The elusive nature and function of mesenchymal stem cells. Nat Rev Mol Cell Biol. 2011;12:126–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Germani A, Di Campli C, Pompilio G. Regenerative therapy in peripheral artery disease. Cardiovasc Ther. 2009;27:289–304 [DOI] [PubMed] [Google Scholar]
- 20.Jezierska-Woźniak K, Mystkowska D, Tutas A, et al. Stem cells as therapy for cardiac disease—a review. Folia Histochem Cytobiol. 2011;49:13–25 [DOI] [PubMed] [Google Scholar]
- 21.Mizuno H, Tobita M, Uysal C. Concise review: adipose-derived stem cells as a novel tool for future regenerative medicine. Stem Cells. 2012;30:804–810 [DOI] [PubMed] [Google Scholar]
- 22.Gimble JM, Bunnell BA, Frazier T, et al. Adipose derived stromal/stem cells: a primer. Organogenesis. 2013;9:3–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rando TA. Stem cells, ageing and the quest for immortality. Nature. 2006;441:1080–1086 [DOI] [PubMed] [Google Scholar]
- 24.Oh J, Lee YD, Wager AJ. Stem cell aging: mechanisms, regulators and therapeutic opportunities. Nat Med. 2014;20:870–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flores I, Blasco MA. The role of telomeres and telomerase in stem cell aging. FEBS Lett. 2010;584:3826–3830 [DOI] [PubMed] [Google Scholar]
- 26.Dasgupta J, Kar S, Liu R, et al. Reactive oxygen species control senescence-associated matrix metalloproteinase-1 through c-Jun-N-terminal kinase. J Cell Physiol. 2010;225:52–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang C, Jurk D, Maddick M, et al. DNA damage response and cellular senescence in tissues of aging mice. Aging Cell. 2009;8:311–323 [DOI] [PubMed] [Google Scholar]
- 28.Sethe S, Scutt A, Stolzing A. Aging of mesenchymal stem cells. Ageing Res Rev. 2006;5:91–116 [DOI] [PubMed] [Google Scholar]
- 29.Campisi J, Robert L. Cell senescence: role in aging and age-related diseases. Interdiscip Top Gerontol. 2014;39:45–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stolzing A, Jones E, McGonagle D, et al. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008;129:163–173 [DOI] [PubMed] [Google Scholar]
- 31.Sun Y, Li W, Lu Z, et al. Rescuing replication and osteogenesis of aged mesenchymal stem cells by exposure to a young extracellular matrix. FASEB J. 2011;25:1474–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siegel G, Kluba T, Hermanutz-Klein U, et al. Phenotype, donor age and gender affect function of human bone marrow-derived mesenchymal stromal cells. BMC Med. 2013;11:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fan M, Chen W, Liu W, et al. The effect of age on the efficacy of human mesenchymal stem cell transplantation after a myocardial infarction. Rejuvenation Res. 2010;13:429–438 [DOI] [PubMed] [Google Scholar]
- 34.de Girolamo L, Lopa S, Arrigoni E, et al. Human adipose-derived stem cells isolated from young and elderly women: their differentiation potential and scaffold interaction during in vitro osteoblastic differentiation. Cytotherapy. 2009;11:793–803 [DOI] [PubMed] [Google Scholar]
- 35.Harris LJ, Zhang P, Abdollahi H, et al. Availability of adipose-derived stem cells in patients undergoing vascular surgical procedures. J Surg Res. 2010;163:e105–e112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu M, Kohan E, Bradley J, et al. The effect of age on osteogenic, adipogenic and proliferative potential of female adipose-derived stem cells. J Tissue Eng Regen Med. 2009;3:290–301 [DOI] [PubMed] [Google Scholar]
- 37.Huang SC, Wu TC, Yu HC, et al. Mechanical strain modulates age-related changes in the proliferation and differentiation of mouse adipose-derived stromal cells. BMC Cell Biol. 2010;11:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alt EU, Senst C, Murthy SN, et al. Aging alters tissue resident mesenchymal stem cell properties. Stem Cell Res. 2012;8:215–225 [DOI] [PubMed] [Google Scholar]
- 39.van Harmelen V, Skurk T, Rohrig K, et al. Effect of BMI and age on adipose tissue cellularity and differentiation capacity in women. Int J Obes Relat Metab Disord. 2003;27:889–895 [DOI] [PubMed] [Google Scholar]
- 40.Schipper BM, Marra KG, Zhang W, et al. Regional anatomic and age effects on cell function of human adipose-derived stem cells. Ann Plast Surg. 2008;60:538–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khan WS, Adesida AB, Tew SR, et al. The epitope characterisation and the osteogenic differentiation potential of human fat pad-derived stem cells is maintained with ageing in later life. Injury. 2009;40:150–157 [DOI] [PubMed] [Google Scholar]
- 42.El-Ftesi S, Chang EI, Longaker MT, et al. Aging and diabetes impair the neovascular potential of adipose-derived stromal cells. Plast Reconstr Surg. 2009;123:475–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sahin E, Depinho RA. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature. 2010;464:520–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin CS, Xin ZC, Deng CH, et al. Defining adipose tissue-derived stem cells in tissue and in culture. Histol Histopathol. 2010;25:807–815 [DOI] [PubMed] [Google Scholar]
- 45.Efimenko A, Starostina E, Kalinina N, et al. Angiogenic properties of aged adipose derived mesenchymal stem cells after hypoxic conditioning. J Translat Med. 2011;9:10–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Efimenko A, Dzhoyashvili NA, Kalinina NI, et al. Adipose-derived stromal cells (ADSC) from aged patients with coronary artery disease keep MSC properties but exhibit age markers and have an impaired angiogenic potential. Stem Cells Translat Med. 2014;3:32–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Melzer D, Frayling TM, Murray A, et al. A common variant of the p16(INK4a) genetic region is associated with physical function in older people. Mech Ageing Dev. 2007;128:370–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Barros S, Dehez S, Arnaud E, et al. Aging-related decrease of human ASC angiogenic potential is reversed by hypoxia preconditioning through ROS production. Mol Ther. 2013;21:399–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bratic A, Larsson NGG. The role of mitochondria in aging. J Clin Invest. 2013;123:951–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pandey AC, Semon JA, Kaushal D, et al. MicroRNA profiling reveals age-dependent differential expression of nuclear factor κB and mitogen-activated protein kinase in adipose and bone marrow-derived human mesenchymal stem cells. Stem Cell Res Ther. 2011;2:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Efimenko A, Starostina EE, Kalinina NI, et al. Age effects on angiogenic properties of adipose tissue mesenchymal stem cells. Cell Transpl Tissue Eng. 2011;6:48–57 [Google Scholar]
- 52.Wang M, Monticone RE, Lakatta EG. Arterial aging: a journey into subclinical arterial disease. Curr Opin Nephrol Hypertens. 2010;19:201–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Traktuev DO, Tsokolaeva ZI, Shevelev AA, et al. Urokinase gene transfer augments angiogenesis in ischemic skeletal and myocardial muscle. Mol Ther. 2007;15:1939–1946 [DOI] [PubMed] [Google Scholar]
- 54.Hoenig MR, Bianchi C, Rosenzweig A, et al. Decreased vascular repair and neovascularization with ageing: mechanisms and clinical relevance with an emphasis on hypoxia-inducible factor-1. Curr Mol Med. 2008;8:754–767 [DOI] [PubMed] [Google Scholar]
- 55.Vasa M, Fichtlscherer S, Aicher A, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–E7 [DOI] [PubMed] [Google Scholar]
- 56.Choi JH, Hur J, Yoon CH. Augmentation of therapeutic angiogenesis using genetically modified human endothelial progenitor cells with altered glycogen synthase kinase-3beta activity. J Biol Chem 2004;279:4943–4948 [DOI] [PubMed] [Google Scholar]
- 57.Madonna R, Renna FV, Cellini C, et al. Age-dependent impairment of number and angiogenic potential of adipose tissue-derived progenitor cells. Eur J Clin Invest. 2011;41:126–133 [DOI] [PubMed] [Google Scholar]
- 58.Takahashi M, Izawa A, Ishigatsubo Y, et al. Therapeutic neovascularization by implantation of autologous mononuclear cells for patients with connective tissue diseases. Curr Pharm Des. 2009;15:2778–2783 [DOI] [PubMed] [Google Scholar]
- 59.Eizawa T, Ikeda U, Murakami Y, et al. Decrease in circulating endothelial progenitor cells in patients with stable coronary artery disease. Heart. 2004;90:685–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kochegura TN, Akopyan ZhA, Sharonov GV, et al. The influence of concomitant type 2 diabetes mellitus on the number of circulating progenitor cells in patients with ischemic cardiomyopathy. Diabetes Mellitus. 2011;3:36–43 [Google Scholar]
- 61.Bozdag-Turan I, Turan RG, Paranskaya L, et al. Correlation between the functional impairment of bone marrow-derived circulating progenitor cells and the extend of coronary artery disease. J Transl Med. 2012;10:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Loomans CJ, de Koning EJ, Staal FJ, et al. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes. 2004;53:195–199 [DOI] [PubMed] [Google Scholar]
- 63.Chen JZ, Zhang FR, Tao QM, et al. Number and activity of endothelial progenitor cells from peripheral blood in patients with hypercholesterolaemia. Clin Sci (Lond). 2004;107:273–280 [DOI] [PubMed] [Google Scholar]
- 64.MacEneaney OJ, Kushner EJ, Westby CM, et al. Endothelial progenitor cell function, apoptosis, and telomere length in overweight/obese humans. Obesity. 2010;18:1677–1682 [DOI] [PubMed] [Google Scholar]
- 65.Drela E, Stankowska K, Kulwas A, et al. Endothelial progenitor cells in diabetic foot syndrome. Adv Clin Exp Med. 2012;21:249–254 [PubMed] [Google Scholar]
- 66.van Ark J, Moser J, Lexis CP, et al. Type 2 diabetes mellitus is associated with an imbalance in circulating endothelial and smooth muscle progenitor cell numbers. Diabetologia. 2012;55:2501–2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gu JH, Lee JS, Kim DW, et al. Neovascular potential of adipose-derived stromal cells (ASCs) from diabetic patients. Wound Repair Regen. 2012;20:243–252 [DOI] [PubMed] [Google Scholar]
- 68.Oñate B, Vilahur G, Ferrer-Lorente R, et al. The subcutaneous adipose tissue reservoir of functionally active stem cells is reduced in obese patients. FASEB J. 2012;26:4327–4336 [DOI] [PubMed] [Google Scholar]
- 69.Vecellio M, Spallotta F, Nanni S, et al. The histone acetylase activator pentadecylidenemalonate 1b rescues proliferation and differentiation in human cardiac mesenchymal cells of type 2 diabetic patients. Diabetes. 2014;63:2132–2147 [DOI] [PubMed] [Google Scholar]
- 70.Dzhoyashvili NA, Efimenko A, Akchurin RS, et al. Angiogenic properties of multipotent mesenchymal stromal cells of adipose tissue of patients with coronary heart disease. Russian J Cardiol. 2013;5:27–34 [Google Scholar]
- 71.Babaei S, Teichert-Kuliszewska K, Zhang Q, et al. Angiogenic actions of angiopoietin-1 require endothelium-derived nitric oxide. Am J Pathol. 2003;162:1927–1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kolluru GK, Bir SC, Kevil CG. Endothelial dysfunction and diabetes: effects on angiogenesis, vascular remodeling, and wound healing. Int J Vasc Med. 2012;2012:918267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dua MM, Miyama N, Azuma J, et al. Hyperglycemia modulates plasminogen activator inhibitor-1 expression and aortic diameter in experimental aortic aneurysm disease. Surgery. 2010;148:429–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gabriely I, Yang XM, Cases JA, et al. Hyperglycemia induces PAI-1 gene expression in adipose tissue by activation of the hexosamine biosynthetic pathway. Atherosclerosis. 2002;160:115–122 [DOI] [PubMed] [Google Scholar]
- 75.Weiss TW, Seljeflot I, Hjerkinn EM, et al. Adipose tissue pro-inflammatory gene expression is associated with cardiovascular disease. Int J Clin Pract. 2011;65:939–944 [DOI] [PubMed] [Google Scholar]
- 76.Acosta L, Hmadcha A, Escacena N, et al. Adipose mesenchymal stromal cells isolated from type 2 diabetic patients display reduced fibrinolytic activity. Diabetes. 2013;62:4266–4269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tashiro Y, Nishida C, Sato-Kusubata K, et al. Inhibition of PAI-1 induces neutrophil-driven neoangiogenesis and promotes tissue regeneration via production of angiocrine factors in mice. Blood. 2012;119:6382–6393 [DOI] [PubMed] [Google Scholar]
- 78.Stefansson S, McMahon GA, Petitclerc E, et al. Plasminogen activator inhibitor-1 controls bone marrow-derived cells therapeutic effect through MMP9 signaling: role in physiological and pathological wound healing. Curr Pharm Des. 2003;9:1545–156412871067 [Google Scholar]
- 79.Simonavicius N, Ashenden M, Weverwijk A, et al. Pericytes promote selective vessel regression to regulate vascular patterning. Blood. 2012;120:1516–1527 [DOI] [PubMed] [Google Scholar]
- 80.Kalinina NI, Akopyan ZhA, Pakhomova EA, et al. Effects of hyperglycemia on functional state of human umbilical vein endothelial cells in vitro. Dokl Biol Sci. 2009;426:210–212 [DOI] [PubMed] [Google Scholar]
- 81.Akopyan ZhA, Sharonov GV, Kochegura TN, et al. The influence of high glucose concentration on the ability of mesenchymal stromal cells to stimulate blood vessel growth. Diabetes Mellitus. 2011;2:32–36 [Google Scholar]
- 82.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–353 [DOI] [PubMed] [Google Scholar]
- 83.Chen YH, Lin SJ, Lin FY. High glucose impairs early and late endothelial progenitor cells by modifying nitric oxide-related but not oxidative stress-mediated mechanisms. Diabetes. 2007;56:1559–1568 [DOI] [PubMed] [Google Scholar]
- 84.Churdchomjan W, Kheolamai P, Manochantr S, et al. Comparison of endothelial progenitor cell function in type 2 diabetes with good and poor glycemic control. BMC Endocr Disord. 2010;10:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Asahara T, Murohara T, Sullivan A. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967 [DOI] [PubMed] [Google Scholar]
- 86.Yoder MC, Mead LE, Prater D, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fisher JC. Endothelial progenitor cells. In: Cellular Diagnostics: Basics, Methods and Clinical Applications of Flow Cytometry. Sack U, Tárnok A, Rothe G. (eds). Karger: Basel; pp. 305–316; 2009 [Google Scholar]
- 88.Pujol BF, Lucibello FC, Gehling UM. Endothelial-like cells derived from human CD14 positive monocytes. Differentiation. 2000;65:287–300 [DOI] [PubMed] [Google Scholar]
- 89.Rookmaaker MB, Vergeer M, van Zonneveld AJ, et al. Endothelial progenitor cells: mainly derived from the monocyte/macrophage-containing CD34− mononuclear cell population and only in part from the hematopoietic stem cell-containing CD34+ mononuclear cell population. Circulation. 2003;108:e150. [DOI] [PubMed] [Google Scholar]
- 90.Springer ML. A balancing act: therapeutic approaches for the modulation of angiogenesis. Curr Opin Investig Drugs. 2006;7:243–250 [PubMed] [Google Scholar]
- 91.Herrmann JL, Abarbanell AM, Weil BR, et al. Optimizing stem cell function for the treatment of ischemic heart disease. J Surg Res. 2011;166:138–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Das R, Jahr H, van Osch GJ, et al. The role of hypoxia in bone marrow-derived mesenchymal stem cells: considerations for regenerative medicine approaches. Tissue Eng Part B Rev. 2010;16:159–168 [DOI] [PubMed] [Google Scholar]
- 93.Buravkova LB, Grinakovskaya OS, Andreeva ER, et al. Characteristics of human lipoaspirate-isolated mesenchymal stromal cells cultivated under a lower oxygen tension. Cytology. 2009;51:5–10 [PubMed] [Google Scholar]
- 94.Efimenko AYu, Starostina EE, Rubina KA, et al. Viability and angiogenic activity of mesenchymal stromal cells from adipose tissue and bone marrow in hypoxia and inflammation in vitro [In Russian]. Tsitologiia. 2010;52:144–154 [PubMed] [Google Scholar]
- 95.Kalinina NI, Efimenko AYu, Starostina EE, et al. Hypoxia as the main activator of angiogenesis and fatty tissue growth. Russian J Physiol. 2009;95:283–289 [PubMed] [Google Scholar]
- 96.Rehman J, Traktuev D, Li J, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–1298 [DOI] [PubMed] [Google Scholar]
- 97.Thangarajah H, Vial IN, Chang E, et al. IFATS collection: adipose stromal cells adopt a proangiogenic phenotype under the influence of hypoxia. Stem Cells. 2009;27:266–274 [DOI] [PubMed] [Google Scholar]
- 98.Hu X, Yu SP, Fraser JL, et al. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thorac Cardiovasc Surg. 2008;135:799–808 [DOI] [PubMed] [Google Scholar]
- 99.Rubina KA, Kalinina NI, Efimenko AYu, et al. Adipose stromal cells stimulate angiogenesis via promoting progenitor cell differentiation, secretion of angiogenic factors, and enhancing vessel maturation. Tissue Eng Part A. 2009;15:2039–2050 [DOI] [PubMed] [Google Scholar]
- 100.Rubin JP, DeFail A, Rajendran N, et al. Encapsulation of adipogenic factors to promote differentiation of adipose-derived stem cells. J Drug Target. 2009;17:207–215 [DOI] [PubMed] [Google Scholar]
- 101.Suga H, Matsumoto D, Eto H, et al. Functional implications of CD34 expression in human adipose-derived stem/progenitor cells. Stem Cells Dev. 2009;18:1201–1210 [DOI] [PubMed] [Google Scholar]
- 102.Hebert TL, Wu X, Yu G, et al. Culture effects of epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF) on cryopreserved human adipose-derived stromal/stem cell proliferation and adipogenesis. J Tissue Eng Regen Med. 2009;3:553–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dimmeler S, Aicher A, Vasa M, et al. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest. 2001;108:391–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Seeger FH, Haendeler J, Walter DH, et al. p38 mitogen-activated protein kinase downregulates endothelial progenitor cells. Circulation. 2005;111:1184–1191 [DOI] [PubMed] [Google Scholar]
- 105.Mias C, Trouche E, Seguelas MH, et al. Ex vivo pretreatment with melatonin improves survival, proangiogenic/mitogenic activity, and efficiency of mesenchymal stem cells injected into ischemic kidney. Stem Cells. 2008;26:1749–1757 [DOI] [PubMed] [Google Scholar]
- 106.Lutolf MP, Gilbert PM, Blau HM. Designing materials to direct stem-cell fate. Nature. 2009;462:433–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shevchenko EK, Makarevich PI, Tsokolaeva ZI, et al. Effective transduction of human adipose stromal cells by a recombinant adeno-associated virus. Cell Transpl Tissue Eng. 2010;5:60–64 [Google Scholar]
- 108.Shevchenko EK, Makarevich PI, Tsokolaeva ZI, et al. Transplantation of modified human adipose derived stromal cells expressing VEGF165 results in more efficient angiogenic response in ischemic skeletal muscle. J Transl Med. 2013;11:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yao J, Jiang SL, Liu W, et al. Tissue inhibitor of matrix metalloproteinase-3 or vascular endothelial growth factor transfection of aged human mesenchymal stem cells enhances cell therapy after myocardial infarction. Rejuvenation Res. 2012;15:495–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tang H, Xiang Y, Jiang X, et al. Dual expression of hTERT and VEGF prolongs life span and enhances angiogenic ability of aged BMSCs. Biochem Biophys Res Commun. 2013;440:502–508 [DOI] [PubMed] [Google Scholar]
- 111.Fadini GP, Agostini C, Avogaro A. Autologous stem cell therapy for peripheral arterial disease meta-analysis and systematic review of the literature. Atherosclerosis. 2010;209:10–17 [DOI] [PubMed] [Google Scholar]
- 112.Clifford DM, Fisher SA, Brunskill SJ, et al. Stem cell treatment for acute myocardial infarction. Cochrane Database Syst Rev. 2012;2:CD006536. [DOI] [PubMed] [Google Scholar]
- 113.Lalu MM, McIntyre L, Pugliese C, et al. Canadian Critical Care Trials Group Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS One. 2012;7:e47559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fisher SA, Dorée C, Brunskill SJ, et al. Bone marrow stem cell treatment for ischemic heart disease in patients with no option of revascularization: a systematic review and meta-analysis. PLoS One. 2013;8:e64669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Delewi R, Andriessen A, Tijssen JG, et al. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: a meta-analysis of randomised controlled clinical trials. Heart. 2013;99:225–232 [DOI] [PubMed] [Google Scholar]
- 116.Benoit E, O'Donnell TF, Patel AN. Safety and efficacy of autologous cell therapy in critical limb ischemia: a systematic review. Cell Transplant. 2013;22:545–562 [DOI] [PubMed] [Google Scholar]
- 117.Fisher SA, Brunskill SJ, Doree C, et al. Stem cell therapy for chronic ischemic heart disease and congestive heart failure. Cochrane Database Syst Rev. 2014;4:CD007888. [DOI] [PubMed] [Google Scholar]
References
Cite this article as: Efimenko AY, Kochegura TN, Akopyan ZA, Parfyonova YV (2015) Autologous stem cell therapy: how aging and chronic diseases affect stem and progenitor cells, BioResearch Open Access 4:1, 26–38, DOI: 10.1089/biores.2014.0042.