INTRODUCTION
It is well known that exposure to ambient particulate matter (PM) is associated with adverse respiratory and cardiovascular health effects.1-3 The physicochemical composition of PM is complex and several epidemiological studies have found that similar ambient PM concentrations result in different mortality and morbidity in various locations.4-5 These studies suggest that different risk estimates in health by region may result from compositional differences of PM. Human exposure and toxicological studies have also demonstrated that some chemical constituents in PM are associated with adverse health effects.6-9
While these studies are helpful to investigate the association between health effects and specific chemical species of PM, understanding the contribution of multiple components of PM as a mixture to health outcomes is more challenging. To assess the association between ambient exposure to PM as a mixture and health outcomes, it is desirable to use an indicator that can reflect PM as a complex mixture rather than the sum of individual components.
One potential approach to data reduction is Principal Component Analysis (PCA). PCA has been used to reduce the large number of constituents in PM into one or fewer components based on the correlations among the individual constituents in PM.9-12 For example, Wei et al (2009) used PCA to reduce 126 chemical species in PM2.5 in China to 3 components, and then examined the association between these 3 components of PM to changes in oxidative stress.9 In addition to data reduction, PCA can identify compositional patterns that can be used to examine the similarities and differences. Another advantage of PCA is that it can be used to reduce data complexity without loss of original information. Therefore, PCA can be used to explain to what extent PM in a location is different from (or similar to) other locations and which components contribute most to this difference (or similarity).
The objectives of this paper are to describe compositional differences in metals of ambient PM2.5 collected from 8 US counties, and to assess the heterogeneity of ambient PM collected in multiple locations using PCA but is not to identify emission sources of ambient PM that have been reported in many previous studies. Although a source apportionment approach provides useful information such as specific sources in a given location, source apportionment does not provide how PM in multiple locations quantitatively vary as a mixture of constituents.
METHODS
Sampling Locations
Air sample collection from 8 US counties was conducted as part of the US EPA funded Johns Hopkins Particulate Matter Research Center (JHPMRC). Sampling locations were selected by JHPMRC epidemiologists as representing greater or lesser mortality/ morbidity health risk (Figure 1).4
Figure 1.
Map showing locations of the sampling sites and sampling period in the US.
The selected air monitoring sites were located away from large emission sources. All sites were classified as a residential area except the Anoka, MN location. The site designation was defined by the US EPA. Table 1 contains sampling location descriptions.
Table 1.
Description of sampling locations.
| Sacramento CA | Maricopa AZ | Anoka MN | Harris TX | Pinellas FL | Jefferson KY | Allegheny PA | Queens NY | |
|---|---|---|---|---|---|---|---|---|
| Sampling Periods |
Jan–Mar 2008 |
Jun-Jul 2008 |
Sep-Oct 2008 |
Jan-Feb 2009 |
Apr–May 2009 |
Aug-Sep 2009 |
Oct-Nov 2009 |
Nov 2009 – Jan 2010 |
| Coordinates (longitude, latitude) |
38.68327, −121.16443 |
33.48388, −112.14256 |
45.13759, −93.20789 |
29.66996, −95.12848 |
27.98625, −82.78207 |
38.13774, −85.57651 |
40.42065, −79.94591 |
40.73854, −73.82352 |
| County Population a |
1,400,949 | 4,023,132 | 331,582 | 4,070,989 | 909,013 | 721,597 | 1,218,494 | 2,306,712 |
| Area (km2) |
2,577 | 23,890 | 1,156 | 4,605 | 1,575 | 1,033 | 1,929 | 462 |
| EPA Land Use Description |
Residential | Residential | Commercial | Residential | Residential | Residential | Residential | Residential |
Population estimated from US Census Data 2009.
Sample Collection
Sampling was conducted between January 2008 and January 2010. Field investigators set up and maintained the equipment at each monitoring facility during the study period. Weekly (24 h/d for 7days) filter based samples were collected at each site for a duration of 5-6 weeks. PM2.5 samples were collected on pre-weighed 37 mm Teflon filters (PALL Life Sciences, Ann Arbor MI) using the Harvard Impactor at a flow rate of 10 L/min. A field investigator maintained the site daily checking flow rates to ensure adequate sampling quality control. On the 7th day of sampling, filters were removed from the Harvard Impactor and shipped to the JHPMRC laboratory. After post-weights were determined, filters were placed in amber jars and stored under argon at 4 °C until metals analysis by Inductively Coupled Plasma-Mass Spectrometry (ICP-MS).
Metals Analysis
The method described below was adapted from previous studies.13-14 Samples were acid digested using a Mars5 Xpress microwave system (CEM, Matthews NC). Prior to digestion, the polyolefin outer support ring was removed from the Teflo filters. The filter membrane was then transferred to a 7 mL Teflon digestion microwave vessel (CEM, Matthews NC) where it was wetted with 20 μL of ethanol, 60 μL of ultrapure water (Millipore, Billerica MA) and 225 μL of concentrated optima grade nitric acid (Fisher Scientific, Columbia MD). The sample was initially digested using a two-stage ramp-to-temperature method with a maximum temperature of 165 °C and a hold time of 30 min. Following the first digestion, 100 μL of concentrated optima nitric acid and 40 μL of concentrated optima grade hydrofluoric acid (Fisher Scientific, Columbia MD) were added and a second digestion performed according to the same ramp-to-temperature method. At the completion of the second digestion, the Teflon membrane was removed and the sample diluted for metals analysis of the 25 elements listed in Table 2 by ICP-MS. Internal standard, 50 μg/L Li, Ge, Sc, Tb, Bi, Y, In (CPI International, Santa Rosa CA), was added to each sample to monitor for instrument drift over analysis time. For every batch of 21 samples, 3 samples of the NIST standard reference material 1648a Urban Particulate Matter (National Institutes of Standards and Technologies, Rockville MD) and reagent blanks were digested and analyzed for quality control. Total metals analysis was performed using an Agilent 7500ce ICP-MS (Agilent Technologies, Santa Clara CA). The analytical limit of detection (LOD), calculated as 3 times the standard deviation of the lowest detectable calibration standard (1 μg/L), was determined for each metal analyzed and ranged between 0.01 and 1.76 ng/m3 assuming a sampling rate of 10 L/min for 7 days (sampling volume = 6,048 m3). For samples with values that were below the analytical LOD, ½ the LOD was substituted for all statistical analyses.
Table 2.
Summary of average PM2.5 and metal concentrations at each sampling location.
| Elementa | >LODb (%) | Mean ± S.D. |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| CA (n=6) | AZ (n=6) | MN (n=5) | KY (n=6) | FL (n=6) | TX (n=6) | PA (n=5) | NY (n=5) | ||
| PM2.5 | 100 | 6.86±2.50 | 9.33±1.96 | 7.92±3.02 | 11.27±2.60 | 5.77±1.21 | 8.68±2.98 | 10.32±2.81 | 8.21±2.50 |
| Na | 98 | 427±374 | 405±592 | 5.11±4.86 | 2.78±1.77 | 158±61.2 | 81.6±30.6 | 38.1±21.4 | 53.0±17.2 |
| Ca | 100 | 21.9±6.67 | 205±83.3 | 37.0±11.5 | 29.8±5.89 | 46.7±18.8 | 63.4±30.2 | 51.5±26.2 | 56.3±24.5 |
| Fe | 100 | 36.9±12.2 | 518±158 | 38.4±7.48 | 46.8±5.96 | 23.3±8.17 | 48.0±19.7 | 172±106 | 63.1±18.0 |
| Al | 100 | 25.8±14.5 | 167±103 | 17.5±10.2 | 35.8±15.8 | 29.8±9.95 | 33.5±27.6 | 29.1±15.4 | 21.1±14.5 |
| K | 100 | 63.1±15.0 | 326±87.1 | 42.0±15.9 | 50.9±9.14 | 47.1±13.1 | 66.3±21.3 | 77.8±34.6 | 38.5±9.01 |
| Mg | 100 | 17.0±9.42 | 29.8±30.4 | 7.01±2.93 | 9.38±3.53 | 37.9±14.5 | 18.9±13.6 | 12.2±7.32 | 10.0±3.28 |
| Zn | 100 | 8.97±4.41 | 20.4±6.51 | 7.91±1.89 | 6.90±0.82 | 3.14±1.47 | 11.1±4.97 | 66.2±53.2 | 44.9±30.9 |
| Ti | 96 | 2.16±0.96 | 81.7±65.6 | 4.12±6.25 | 3.20±1.00 | 5.88±6.97 | 7.79±13.8 | 2.99±1.42 | 2.44±0.58 |
| Mn | 100 | 0.82±0.29 | 13.4±4.06 | 1.10±0.22 | 2.50±2.18 | 0.39±0.19 | 1.64±0.63 | 10.7±7.28 | 2.61±1.35 |
| Cu | 100 | 1.72±0.33 | 8.36±2.20 | 1.50±0.24 | 1.92±0.54 | 0.57±0.19 | 3.01±1.49 | 4.98±2.66 | 5.14±1.37 |
| Pb | 100 | 1.23±0.45 | 2.15±0.74 | 9.85±5.01 | 2.21±0.56 | 0.88±0.42 | 1.72±0.57 | 12.1±6.73 | 3.09±1.08 |
| Ni | 76 | 0.05±0.05 | 10.5±4.65 | 0.13±0.12 | 0.03±0.01 | 0.30±0.08 | 1.30±0.82 | 0.53±0.36 | 8.13±4.39 |
| V | 100 | 0.17±0.09 | 2.36±0.74 | 0.14±0.07 | 0.23±0.05 | 1.22±0.24 | 4.21±1.36 | 0.30±0.14 | 2.12±1.08 |
| Cr | 98 | 0.15±0.09 | 3.37±1.08 | 0.55±0.41 | 1.15±0.97 | 0.25±0.07 | 0.86±0.51 | 1.95±1.08 | 0.37±0.14 |
| Sb | 96 | 0.49±0.12 | 1.30±0.95 | 0.61±0.12 | 0.77±0.23 | 0.16±0.06 | 0.51±0.20 | 1.67±0.79 | 3.75±1.38 |
| Mo | 87 | 0.18±0.06 | 1.00±0.35 | 0.18±0.07 | 0.25±0.11 | 0.11±0.07 | 0.88±0.40 | 1.91±1.20 | 1.69±1.00 |
| Sn | 98 | 0.95±0.41 | 1.00±0.36 | 0.70±0.26 | 0.51±0.12 | 0.14±0.06 | 0.79±0.74 | 1.51±0.74 | 0.89±0.22 |
| Se | 82 | 0.02±0.01 | 0.16±0.05 | 0.29±0.10 | 3.92±4.19 | 0.42±0.08 | 0.55±0.24 | 2.81±1.10 | 0.55±0.28 |
| As | 100 | 0.66±0.27 | 0.42±0.15 | 1.24±0.73 | 1.21±0.41 | 0.39±0.18 | 1.39±0.90 | 1.59±0.71 | 0.43±0.07 |
| Co | 42 | 0.02±0.01 | 0.54±0.15 | 0.02±0.01 | 0.02±0.01 | 0.02±0.01 | 0.04±0.02 | 0.05±0.02 | 0.62±0.32 |
| Cd | 76 | 0.05±0.02 | 0.05±0.03 | 0.13±0.05 | 0.01±0.01 | 0.05±0.03 | 0.09±0.03 | 0.49±0.29 | 0.08±0.03 |
| Ag | 0 | 0.21±0.04 | 0.20±0.05 | 0.23±0.06 | 0.23±0.07 | 0.21±0.04 | 0.22±0.07 | 0.20±0.03 | 0.22±0.06 |
| Cs | 22 | 0.02±0.01 | 0.18±0.06 | 0.02±0.01 | 0.01±0.01 | 0.02±0.01 | 0.02±0.01 | 0.07±0.05 | 0.02±0.01 |
| Tl | 8.9 | 0.02±0.01 | 0.02±0.01 | 0.02±0.01 | 0.03±0.02 | 0.02±0.01 | 0.02±0.01 | 0.07±0.05 | 0.02±0.01 |
| Be | 0 | 0.02±0.01 | 0.02±0.01 | 0.02±0.01 | 0.02±0.01 | 0.02±0.01 | 0.02±0.01 | 0.02±0.01 | 0.02±0.01 |
CA: Sacramento County, CA; AZ: Maricopa County, AZ; MN: Anoka County, MN; KY: Jefferson County, KY; FL: Pinellas County, FL; TX: Harris County, TX; PA: Allegheny County, PA; NY: Queens County, NY.
Unit : μg/m3 for PM2.5 and ng/m3 for 25 metals.
LOD: Limit of detection
Statistical Analysis
Metal composition data from analysis of weekly samples were pooled and analyzed using Principal Component Analysis (PCA) in order to examine the heterogeneity of ambient PM. Twenty one (21) out of 25 metals were used in the PCA. Ag, Cs, Tl, and Be were excluded from the analysis due to having two thirds or more samples below the LOD. The first step in the PCA was to transform the 21 metal concentrations into dimensionless normalized numbers with a mean of zero (Z-statistic) for each metal to diminish the impact of large differences in variation between the metals. After normalization the standardized numbers have the same order of magnitude for each metal.
All statistical analyses were performed in SAS 9.2 (SAS Inc, Cary NC). Principal components were determined by running Proc Factor using Prin options. Eigenvalues greater than 1.0 were retained in this analysis.10 For each location, weekly scores were determined for each of the retained components. The general equation to determine the standardized principal component score is shown below: 11
Where,
PCSp = the standardized principal components score on principal component p
βmp = the standardized loadings for a measured metal m on principal component p.
Xm = the standardized number from each metal concentration
RESULTS AND DISCUSSION
Summary of PM2.5 and Metal Concentrations
A total of 45 filter samples from 8 counties were used in the analysis (Table 2). Weekly average PM2.5 mass concentrations varied by approximately two fold across these counties, where the highest were Jefferson (11.27 μg/m3) and Allegheny (10.32 μg/m3) and lowest were Sacramento (6.86 μg/m3) and Pinellas (5.77 μg/m3). Metal composition also greatly varied across the counties. The sum of 21 metals in Maricopa was 1,799 ng/m3 while the sum in 7 other counties ranged from 201 to 610 ng/m3. In Maricopa total metal mass concentrations accounted for 19.3% of PM2.5 mass while for the remaining counties total metal mass concentration explained less than 9%.
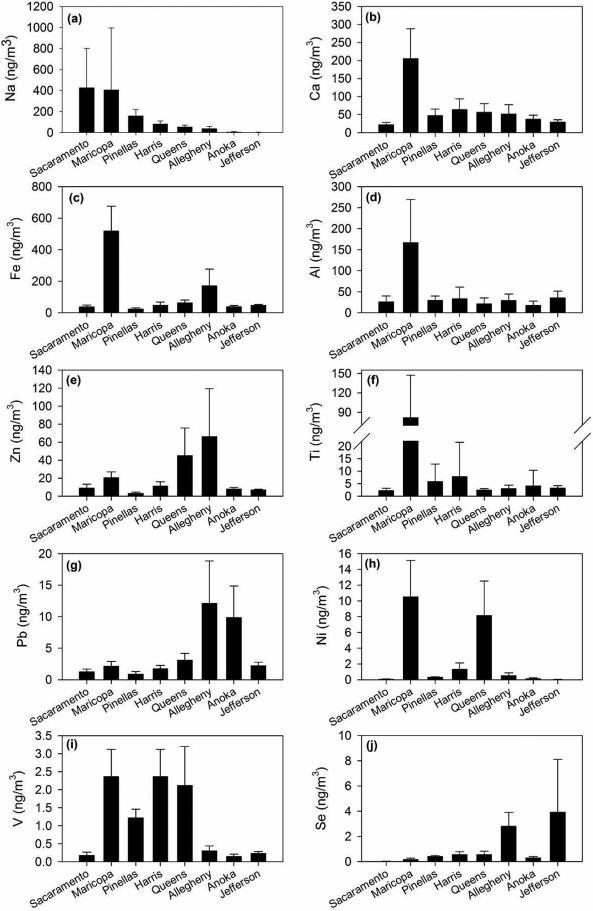
Figure 2 illustrates differences in concentration between sites for selected metals. The metals with average PM 2.5 concentrations greater than 10 ng/m3 for all counties were Ca, Fe, Al, K, and Mg. Within this metal group the distribution of concentrations differed across counties. Average concentrations of Na in Sacramento and Maricopa were greater than 400 ng/m3 while those in Anoka and Jefferson were less than 10 ng/m3 (Figure 2-a). The average concentration of Ca in Maricopa was 3-10 times higher than in the other counties (Figure 2-b). Average concentrations of Fe in Maricopa and Allegheny were also significantly, 3-17 times, higher (Figure 2-c). Aluminum in Maricopa was also 5-8 times higher (Figure 2-d) whereas Zn in Allegheny (29.1 ng/m3) and Queens (21.1 ng/m3) were the highest metal concentration of all counties (Figure 2-e). Metals with concentrations between 1 and 10 ng/m3 included Ti, Mn, Cu, and Pb. Average concentrations of Ti in Maricopa (81.7 ng/m3) were 15-40 times higher than any other counties (Figure 2-f). Average concentrations of Mn in Maricopa and Allegheny were 3-34 times higher. Average concentrations of Pb were approximately 10 ng/m3 in Anoka and Allegheny while those in other counties were less than 3 ng/m3 (Figure 2-g).
Figure 2.
Average concentrations of selected elements for 8 US counties. Error bars represent standard deviations: (a) Na, (b) Ca, (c) Fe, (d) Al, (e) Zn, (f) Ti, (g) Pb, (h) Ni, (i) V, and (j) Se.
Some metals including Ni, V, and Se also showed important variations in average concentrations between counties. Average concentrations of Ni in Maricopa, Queens, and Harris were above 1 ng/m3 while those in the other counties were less than 0.5 ng/m3 (Figure 2-h). Average concentrations of V were highest in Harris followed by Maricopa, Queens, and Pinellas while those in the remaining four counties were below 0.3 ng/m3 (Figure 2-i). Average concentrations of Se in Jefferson and Allegheny were above 2.5 ng/m3 while those in the remaining counties were below 0.6 ng/m3 (Figure 2-j). The remaining metals did not show large variations between the counties.
PM is a complex mixture and understanding the factors that contribute to this complexity and its human heath significance are important areas of research. Numerous studies have characterized PM composition using an established national monitoring network that provides data on the chemical composition of PM or by using year-long intensive compositional monitoring at the local level. Bell et al (2007) conducted descriptive analyses to examine the spatial and temporal variation of 52 PM constituents in 187 US cities during 2000-2005.15 Kim et al (2000) conducted a 1-year air quality monitoring study in southern California to examine spatial variations of 43 constituents of PM.16 These studies concluded that spatial variations of numerous constituents in PM exist at the local and national level. The findings in these studies are similar to the results observed in this study. PM characterization by individual chemical constituents is useful to explore health effects. However, PM characterization by individual chemical constituents cannot explain the impact of the combined chemical constituents on health outcomes due to the largely unknown interactions among constituents in PM. Clustering constituents of PM can be an approached to explore the impact of combined chemical constituents on health effects. In studies of ambient air pollution, PCA has been used to group constituents of PM in a given location.
Principal Component Analysis
Based on an eigenvalue greater than 1.0, five (5) principal components (PCs) were extracted from the 21 metals included in the dataset. The 5 PCs explained 85% of the total variance in the dataset (Table 3). The first three PCs explained 74% of the original data variance. Principal component 1 (PC-1), the most significant component, is explained by Ca, Fe, Al, K, Mn, Ti, Cu, and Cr. The possible sources for this component are crustal minerals and resuspension road dust. Similar source profiles were observed in previous studies using factor analysis for source apportionment of metals.17-18 PC-2 is characterized by Zn, Pb, Mo, Sn, As, and Cd. These metals can originate from industrial emissions such as smelter and metal production facilities.19 PC-3 explains the variation of Ni, Sb, and Co. The possible sources for this component are a combination of oil combustion and vehicle sources. Nickel is considered an indicator of residual oil combustion20 and Sb originates from brake dust. 17, 20-23 PC-4 is attributed to Na, Se, As. The sign of the component loadings between Na (0.782), Se (−0.638), and As (−0.532) indicates that two different sources may explain this component. The possible sources of PC-4 include marine aerosol and coal power plant emissions. Na is a strong indicator of sea salt particles24 but Se is related to coal power plant emission.25-26 PC-5 is explained by V. This indicates that PC-5 is associated with shipping activities and fuel oil combustion.22, 27-28 In studies of ambient air pollution, factor analysis (FA) or PCA has been used to group contaminants in a given location. FA is typically used to answer questions regarding the relative contribution of different contaminants from identified sources to the PM mixture.28-29 For example, Thurston et al. (2005) demonstrated the association between mortality and source-specific groups of air pollutants.30 However, misclassification or misinterpretation of source contribution is highly likely because designated source-specific pollutants can be emitted from multiple sources, and pollutants are inter-correlated among other measured pollutants.21
Table 3.
Standardized rotated factor loading and communalities from PCA in PM2.5
| Component1 | Component2 | Component3 | Component4 | Component5 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Metal | Loadings | Metal | Loadings | Metal | Loadings | Metal | Loadings | Metal | Loadings |
| K | 0.955 | Cd | 0.942 | Sb | 0.869 | Na | 0.782 | V | 0.761 |
| Fe | 0.952 | Pb | 0.825 | Co | 0.852 | Se | -0.638 | Mg | 0.644 |
| Cr | 0.923 | Zn | 0.804 | Ni | 0.738 | As | -0.562 | Al | 0.364 |
| Ca | 0.880 | Sn | 0.695 | Mo | 0.591 | Mg | 0.427 | Ca | 0.335 |
| Al | 0.862 | Mo | 0.980 | Cu | 0.488 | Pb | -0.172 | Ti | -0.217 |
| Mn | 0.808 | As | 0.578 | Zn | 0.450 | K | 0.168 | Pb | -0.190 |
| Ti | 0.750 | Mn | 0.519 | V | 0.366 | Cr | -0.155 | Se | -0.183 |
| Cu | 0.730 | Sb | 0.374 | As | -0.305 | Al | 0.137 | Mo | 0.169 |
| Ni | 0.627 | Cu | 0.370 | Ca | 0.233 | Mo | -0.136 | Cu | 0.144 |
| Co | 0.446 | Cr | 0.268 | Mg | -0.186 | Co | 0.134 | K | 0.138 |
| Mg | 0.333 | Se | 0.266 | Sn | 0.185 | Fe | 0.123 | Ni | 0.129 |
| Sn | 0.282 | Ti | -0.229 | Fe | 0.177 | Ca | 0.097 | As | 0.091 |
| V | 0.183 | Fe | 0.163 | Mn | 0.160 | Cd | -0.093 | Co | 0.089 |
| Na | 0.163 | V | -0.136 | Se | -0.159 | V | -0.081 | Fe | 0.076 |
| Mo | 0.161 | Ni | -0.104 | Ti | 0.148 | Cu | 0.065 | Cr | 0.051 |
| Zn | 0.102 | Mg | -0.043 | Na | -0.143 | Sb | -0.042 | Sb | 0.041 |
| As | -0.096 | Ca | 0.036 | K | 0.096 | Ni | 0.038 | Na | -0.036 |
| Sb | 0.056 | Co | -0.029 | Al | 0.082 | Ti | 0.031 | Cd | -0.031 |
| Se | 0.053 | K | 0.023 | Cr | 0.062 | Zn | -0.020 | Sn | -0.024 |
| Cd | 0.021 | Na | 0.006 | Pb | -0.058 | Sn | -0.017 | Zn | -0.014 |
| Pb | -0.010 | Al | -0.005 | Cd | -0.034 | Mn | -0.015 | Mn | 0.008 |
| Eigena | 8.51 | Eigen | 4.25 | Eigen | 2.10 | Eigen | 1.20 | Eigen | 1.00 |
| Varb (%) | 42.5 | Var (%) | 21.3 | Var (%) | 10.5 | Var (%) | 5.9 | Var (%) | 5.0 |
Eigen: eigenvalue
Var: variance explained
PCA is typically used to reduce a large number of variables to a few groups in genetic analysis.31-32 Recent air pollution studies have used PCA to qualitatively distinguish regional differences in ambient air quality in multiple locations.33-35 For example, Pires et al (2008) categorized ambient PM into two groups from 10 different monitoring sites in European urban areas. In our study, we applied PCA to our complex compositional dataset to quantitatively distinguish differences in ambient PM between 8 locations based on metal composition data. To identify differences, locations were ranked based on normalized principal component scores. The normalized principal component scores were obtained by transforming the measured concentrations to dimensionless normalized values with a mean of zero (Z-statistics) minimizing the influence of disproportionately high (e.g., Pb in Anoka) and low (e.g., Ni in Jefferson) values.
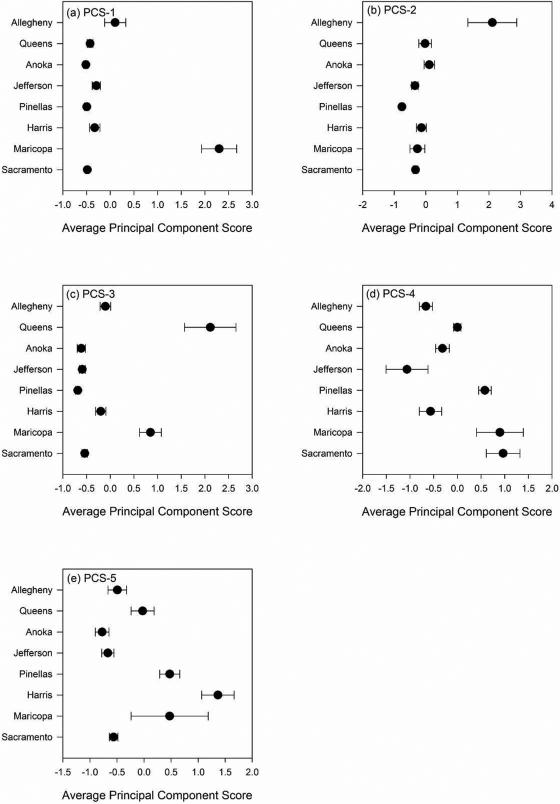
Figure 3 summarizes principal component scores by location. Based on Tukey's test, PC-1 scores (Figure 3-a) can be classified into three clusters. The first cluster consisted only of Maricopa (PC-1 score = 2.30). The second cluster consisted of Harris, Jefferson, and Allegheny (PC-1 scores = −0.33, −0.29, and 0.10 respectively). The third cluster consisted of Sacramento, Pinellas, Anoka, and Queens (PC-1 scores = −0.48, −0.50, −0.52, and −0.43 respectively). The PC-1 score suggests that PM in Maricopa is strongly infuluenced by resuspended dust. However, PM scores in Sacramento, Pinellas, Anoka, and Queens do not appear to be attributed to resuspended dust. These findings are consistent with previous studies.12, 15
Figure 3.
Average principal component (PC) scores for 8 US counties. Closed circles represent average PC scores. Error bars represents standard deviations: (a) average PC score 1, (b) average PC score 2, (c) average PC score 3, (d) average PC score 4, and (e) average PC score 5.
Average PC-2 scores indicated two clusters, with Allegheny (PC-2 score = 2.11) significantly different from the other locations (Figure 3-b). The significance of PC-2 scores between Allegheny and the rest of the counties may result from the specific industrial facilities. It is known that steel factories and smelting industry are major industrial sources in Allegheny.19, 25
Figure 3-c also shows that the average PC-3 scores distinguish the 8 locations into 3 clusters with cluster 1 consisting of only Queens, which had the highest PC-3 score (2.11). Maricopa (PC-3 score = 0.85) comprised the second cluster, and the remainder of the locations comprised the third cluster. The highest average PC-3 score in Queens may be related to oil combustion for residential heating and vehicle emission. 20, 22 It is surprising that the average PC-3 score in Maricopa is the second highest. This suggests that PM in Maricopa may be affected by other undetermined emission sources in addition to fuel combustion.
Unlike scores from PC-1 through PC-3 which indicated a maximum of 3 clusters, 4 clusters were observed within the PC-4 scores (Figure 3-d). A distinct difference was found between cluster 1 consisting of Sacramento (PC-4 score = 0.97) and Maricopa (PC-4 score = 0.90) and cluster 2 containing Harris (PC-4 score = −0.57), Jefferson (PC-4 score = −1.06), and Allegheny (PC-4 score = −0.66). Another cluster (3) consisted of Pinellas (PC-4 score=0.58) and Queens (PC-4 score= 0.00). Anoka (PC score = −0.31) was also considered as an independent cluster (4) different from the other three clusters. The three clusters may indicate that the PC-4 scores may be influenced by 4 different emission sources. The first cluster is likely associated with crustal sources while cluster 2 may result from utility generation process such as electricity.25,26 The third cluster suggests the impact of marine aerosol24 and the cluster 4 may result from a combination of these sources or undetermined sources. Figure 3-e shows that the average PC-5 scores defined two clusters: cluster 1 consisted of Harris (PC-5 score=1.36), Pinellas (PC-5 score=0.48), and Maricopa (PC-5 score=0.47). Cluster 2 consisted of the remaining 5 counties with PC-5 scores ranging from −0.03 to −0.78. Cluster 1 may represent combination of vessel shipping activities and mineral dust but cluster 2 is associated with unidentified sources.
These computed PC scores suggest a qualitative and quantitative rank order of differences. For example for PC-1 (Figure 3-a), the magnitude of the absolute difference between Maricopa and Anoka (2.82) as compared to that of Pinellas and Anoka (0.02) suggests that the overall composition of PM is very different between the former and similar between the latter. It should be noted that the highest PC score in a given location does not imply that PM in this location is more toxic than PM in another location that has a lower PC score. Unlike FA, PCA can include both positive and negative values on component loadings and component scores. Expanding the scale to include negative scores allows us to evaluate broad range of differences in metal composition between locations. Identifying these clusters of PM components may be used to help explain differences in PM toxicity or health effects varied across different locations.
A limitation of this study was that we were able to visit each location only one time. Another limitation is the relatively small number of filter samples collected at each location. The sample size recommended for PCA is preferably 50 plus the number of variables of interest. However, work done by Henry et al (1984) showed that the minimum number of samples needed to obtain a statistically stable PCA results require N > 30 + (S+3)/2; where S is the number of variables of interest. Under this definition, the dataset used in this study (N=45) is larger than the minimum acceptable criteria (N=42) as defined by Henry et al.36
CONCLUSIONS
In this study we characterized metal concentrations in ambient PM2.5 collected from 8 US counties for a time period of 5-6 weeks at each location. Each location showed a unique metals profile and the percent contribution of each metal to the total mass differed widely by location as expected. Metal composition in fine particles for weekly samples collected in eight US counties has been analyzed using PCA to evaluate the heterogeneity of PM as a metal mixture. PCA showed that 5 principal components explained 85% of the total variance. The average standardized PC scores representing compositional differences in PM significantly differed between the counties. The results in this study showed that systematic comparison using principal component analysis can be used to evaluate differences in metal composition across location.
IMPLICATIONS.
Previous studies have demonstrated associations between health effects and particulate matter (PM) using a single component or a combination of few components. Other studies have shown constituents of PM can vary greatly by location and that these differences may explain why the health effects associated with PM exposure are different by location. However, a single or a combination of a few components cannot represent PM as a whole. To address the need for evaluating PM as a complex mixture, we demonstrated the utility of principal component analysis to assess heterogeneity of PM.
ACKNOWLEDGEMENTS
This work was supported in part by EPA's grant # RD83241701, NIEHS's grant # P30 ES003819, P30 ES00319, and R01 ES019560. ICP-MS analysis was supported in part by the Maryland Cigarette Restitution Fund Program at the Johns Hopkins Bloomberg School of Public Health. We would like to thank all local agencies and managers who made this work possible: Ken Lashbrook and John Ching (SMAQMD), Randy Redman (MAQD), Ben Davis (ADEQ), Rick Strassman (MPCA), Earle Wright and Marc Wooten (TCEQ), Thomas Stringfellow (PDEM), Larry Garrison (LMAPCD), Darrell Stern (ACHD), David Wheeler and Mike Christopherson (NYDEC). Although the research described in this manuscript has been funded wholly or in part by the United States Environmental Protection Agency through grant/cooperative agreement # RD83241701 to Dr. Patrick Breysse, it has not been subjected to the Agency's required peer and policy review and therefore does not necessarily reflect the views of the Agency and no official endorsement should be inferred.
REFERENCES
- 1.Pope CA, Dockery DW. Health effects of fine particulate air pollution: Lines that connect. J Air Waste Manage. 2006;56(6):709–742. doi: 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- 2.Dockery DW, Pope CA, Xu XP, Spengler JD, Ware JH, Fay ME, Ferris BG, Speizer FE. An Association between Air-Pollution and Mortality in 6 United-States Cities. New England Journal of Medicine. 1993;329(24):1753–1759. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- 3.Pope CA. Mortality effects of longer term exposures to fine particulate air pollution: Review of recent epidemiological evidence. Inhalation Toxicology. 2007;19:33–38. doi: 10.1080/08958370701492961. [DOI] [PubMed] [Google Scholar]
- 4.Bell ML, Ebisu K, Peng RD, Walker J, Samet JM, Zeger SL, Dominici F. Seasonal and Regional Short-term Effects of Fine Particles on Hospital Admissions in 202 US Counties, 1999-2005. Am J Epidemiol. 2008;168(11):1301–1310. doi: 10.1093/aje/kwn252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pope CA, Ezzati M, Dockery DW. Fine-Particulate Air Pollution and Life Expectancy in the United States. New England Journal of Medicine. 2009;360(4):376–386. doi: 10.1056/NEJMsa0805646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bell ML, Ebisu K, Peng RD, Samet JM, Dominici F. Hospital Admissions and Chemical Composition of Fine Particle Air Pollution. Am J Resp Crit Care. 2009;179(12):1115–1120. doi: 10.1164/rccm.200808-1240OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dominici F, Peng RD, Ebisu K, Zeger SL, Samet JM, Bell ML. Does the effect of PM10 on mortality depend on PM nickel and vanadium content? A reanalysis of the NMMAPS data. Environ Health Persp. 2007;115(12):1701–1703. doi: 10.1289/ehp.10737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng RD, Bell ML, Geyh AS, McDermott A, Zeger SL, Samet JM, Dominici F. Emergency Admissions for Cardiovascular and Respiratory Diseases and the Chemical Composition of Fine Particle Air Pollution. Environ Health Persp. 2009;117(6):957–963. doi: 10.1289/ehp.0800185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei YJ, Han IK, Shao M, Hu M, Zhang JF, Tang XY. PM2.5 Constituents and Oxidative DNA Damage in Humans. Environmental Science & Technology. 2009;43(13):4757–4762. doi: 10.1021/es803337c. [DOI] [PubMed] [Google Scholar]
- 10.Hopke PK, Ito K, Mar T, Christensen WF, Eatough DJ, Henry RC, Kim E, Laden F, Lall R, Larson TV, Liu H, Neas L, Pinto J, Stolzel M, Suh H, Paatero P, Thurston GD. PM source apportionment and health effects: 1. Intercomparison of source apportionment results. J Expo Sci Env Epid. 2006;16(3):275–286. doi: 10.1038/sj.jea.7500458. [DOI] [PubMed] [Google Scholar]
- 11.Thurston GD, Spengler JD. A Quantitative Assessment of Source Contributions to Inhalable Particulate Matter Pollution in Metropolitan Boston. Atmospheric Environment. 1985;19(1):9–25. [Google Scholar]
- 12.Mar TF, Ito K, Koenig JQ, Larson TV, Eatough DJ, Henry RC, Kim E, Laden F, Lall R, Neas L, Stolzel M, Paatero P, Hopke PK, Thurston GD. PM source apportionment and health effects. 3. Investigation of inter-method variations in associations between estimated source contributions Of PM2.5 and daily mortality in Phoenix, AZ. J Expo Sci Env Epid. 2006;16(4):311–320. doi: 10.1038/sj.jea.7500465. [DOI] [PubMed] [Google Scholar]
- 13.Kinney PL, Chillrud SN, Ross JM, Ramstrom S, Spengler JD. Personal exposures to PM2.5 and black carbon among NYC youth: Influences of temporal and spatial factors. Epidemiology. 2002;13(4):S100–S100. [Google Scholar]
- 14.Chillrud SN, Geyh A, Ross J, Wallace S, Ramstrom S, Spengler JD, Breysse P, Kinney PL. Comparing World Trade Center into related exposures of particulate bound metals to levels measured in the NYC teach study. Epidemiology. 2002;13(4):S122–S123. [Google Scholar]
- 15.Bell ML, Dominici F, Ebisu K, Zeger SL, Samet JM. Spatial and temporal variation in PM2.5 chemical composition in the United States for health effects studies. Environ Health Persp. 2007;115(7):989–995. doi: 10.1289/ehp.9621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim BM, Teffera S, Zeldin MD. Characterization of PM2.5 and PM10 in the South Coast Air Basin of southern California: Part 1 - Spatial variations. J Air Waste Manage. 2000;50(12):2034–2044. [PubMed] [Google Scholar]
- 17.Alleman LY, Lamaison L, Perdrix E, Robache A, Galloo JC. PM10 metal concentrations and source identification using positive matrix factorization and wind sectoring in a French industrial zone. Atmospheric Research. 2010;96(4):612–625. [Google Scholar]
- 18.Moreno T, Querol X, Alastuey A, Viana M, Salvador P, de la Campa AS, Artinano B, de la Rosa J, Gibbons W. Variations in atmospheric PM trace metal content in Spanish towns: Illustrating the chemical complexity of the inorganic urban aerosol cocktail. Atmospheric Environment. 2006;40(35):6791–6803. [Google Scholar]
- 19.Martello DV, Pekney NJ, Anderson RR, Davidson CI, Hopke PK, Kim E, Christensen WF, Mangelson NF, Eatough DJ. Apportionment of ambient primary and secondary fine particulate matter at the Pittsburgh National Energy Laboratory particulate matter characterization site using positive matrix factorization and a potential source contributions function analysis. J Air Waste Manage. 2008;58(3):357–368. doi: 10.3155/1047-3289.58.3.357. [DOI] [PubMed] [Google Scholar]
- 20.Lippmann M, Peltier R, Lippmann M. Seasonal and Spatial Distributions of Nickel in New York City Ambient Air. Epidemiology. 2008;19(6):S317–S317. [Google Scholar]
- 21.Grahame T, Hidy GM. Pinnacles and pitfalls for source apportionment of potential health effects from airborne particle exposure. Inhalation Toxicology. 2007;19(9):727–744. doi: 10.1080/08958370701399687. [DOI] [PubMed] [Google Scholar]
- 22.Lippmann M. Semi-continuous speciation analyses for ambient air particulate matter: An urgent need for health effects studies. J Expo Sci Env Epid. 2009;19(3):235–247. doi: 10.1038/jes.2008.65. [DOI] [PubMed] [Google Scholar]
- 23.Lippmann M, Ito K, Hwang JS, Maciejczyk P, Chen LC. Cardiovascular effects of nickel in ambient air. Environ Health Persp. 2006;114(11):1662–1669. doi: 10.1289/ehp.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engel-Cox JA, Weber SA. Compilation and assessment of recent positive matrix factorization and UNMIX receptor model studies on fine particulate matter source apportionment for the eastern United States. J Air Waste Manage. 2007;57(11):1307–1316. doi: 10.3155/1047-3289.57.11.1307. [DOI] [PubMed] [Google Scholar]
- 25.Rutter AP, Snyder DG, Schauer JJ, Deminter J, Shelton B. Sensitivity and Bias of Molecular Marker-Based Aerosol Source Apportionment Models to Small Contributions of Coal Combustion Soot. Environmental Science & Technology. 2009;43(20):7770–7777. doi: 10.1021/es901280p. [DOI] [PubMed] [Google Scholar]
- 26.Chow JC, Watson JG. Review of PM2.5 and PM10 apportionment for fossil fuel combustion and other sources by the chemical mass balance receptor model. Energ Fuel. 2002;16(2):222–260. [Google Scholar]
- 27.Pandolfi M, Gonzalez-Castanedo Y, Alastuey A, de la Rosa JD, Mantilla E, de la Campa AS, Querol X, Pey J, Amato F, Moreno T. Source apportionment of PM(10) and PM(2.5) at multiple sites in the strait of Gibraltar by PMF: impact of shipping emissions. Environ Sci Pollut R. 2011;18(2):260–269. doi: 10.1007/s11356-010-0373-4. [DOI] [PubMed] [Google Scholar]
- 28.Querol X, Viana M, Alastuey A, Amato F, Moreno T, Castillo S, Pey J, de la Rosa J, de la Campa AS, Artinano B, Salvador P, Dos Santos SG, Fernandez-Patier R, Moreno-Grau S, Negral L, Minguillon MC, Monfort E, Gil JI, Inza A, Ortega LA, Santamaria JM, Zabalza J. Source origin of trace elements in PM from regional background, urban and industrial sites of Spain. Atmospheric Environment. 2007;41(34):7219–7231. [Google Scholar]
- 29.Lee JH, Hopke PK. Apportioning sources of PM2.5 in St. Louis, MO using speciation trends network data. Atmospheric Environment. 2006;40:S360–S377. [Google Scholar]
- 30.Thurston GD, Ito K, Mar T, Christensen WF, Eatough DJ, Henry RC, Kim E, Laden F, Lall R, Larson TV, Liu H, Neas L, Pinto J, Stolzel M, Suh H, Hopke PK. Workgroup report: Workshop on source apportionment of particulate matter health effects -Intercomparison of results and implications. Environ Health Persp. 2005;113(12):1768–1774. doi: 10.1289/ehp.7989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reich D, Price AL, Patterson N. Principal component analysis of genetic data. Nat Genet. 2008;40(5):491–492. doi: 10.1038/ng0508-491. [DOI] [PubMed] [Google Scholar]
- 32.Kang HM, Sul JH, Service SK, Zaitlen NA, Kong SY, Freimer NB, Sabatti C, Eskin E. Variance component model to account for sample structure in genome-wide association studies. Nat Genet. 2010;42(4):348–U110. doi: 10.1038/ng.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baker J. A cluster analysis of long range air transport pathways and associated pollutant concentrations within the UK. Atmospheric Environment. 2010;44(4):563–571. [Google Scholar]
- 34.Ragosta M, Caggiano R, Macchiato M, Sabia S, Trippetta S. Trace elements in daily collected aerosol: Level characterization and source identification in a four-year study. Atmospheric Research. 2008;89(1-2):206–217. [Google Scholar]
- 35.Pires JCM, Sousa SIV, Pereira MC, Alvim-Ferraz MCM, Martins FG. Management of air quality monitoring using principal component and cluster analysis - Part II: CO, NO2 and O-3. Atmospheric Environment. 2008;42(6):1261–1274. [Google Scholar]
- 36.Henry RC, Lewis CW, Hopke PK, Williamson HJ. Review of Receptor Model Fundamentals. Atmospheric Environment. 1984;18(8):1507–1515. [Google Scholar]