Abstract
Intermediate filaments (IFs) are composed of one or more members of a large family of cytoskeletal proteins, whose expression is cell- and tissue type-specific. Their importance in regulating the physiological properties of cells is becoming widely recognized in functions ranging from cell motility to signal transduction. IF proteins assemble into nanoscale biopolymers with unique strain-hardening properties that are related to their roles in regulating the mechanical integrity of cells. Furthermore, mutations in the genes encoding IF proteins cause a wide range of human diseases. Due to the number of different types of IF proteins, we have limited this short review to cover structure and function topics mainly related to the simpler homopolymeric IF networks composed of vimentin, and specifically for diseases, the related muscle-specific desmin IF networks.
Keywords: cell motility, cytoskeleton, intermediate filament, mechanotransduction, signal transduction
Introduction
Intermediate filaments (IFs)2 are composed of one or more members of a large family of mainly cytoskeletal proteins encoded by over 70 genes. These proteins, which typically form 10-nm filaments, are classified into five major types based on their structure and sequence homology. The first four types (I–IV) are cytoplasmic, whereas type V IFs reside in the nucleus. Types I and II are the acidic and neutral-basic keratins, which assemble into heteropolymeric filaments, typically in epithelial cells. In humans, there are 54 different keratins, which are expressed according to cell type and stage of differentiation (1). Type III IFs are composed of homopolymers of vimentin, desmin, peripherin, or glial fibrillary acidic protein (GFAP). Vimentin is typically expressed in fibroblasts, but is also in endothelial cells, the eye lens epithelium, and the dendritic reticulum cells of lymphoid follicles; desmin is the major IF protein of smooth, skeletal, and cardiac muscle; peripherin is found mainly in neurons of the peripheral nervous system; and GFAP is located in astrocytes and glial cells. Type IV IFs include those expressed in the nervous system either as complex heteropolymers such as the neurofilament triplet proteins (NF-L, NF-M, and NF-H) or as homopolymers of α-internexin. Nestin, another Type IV protein, cannot form IFs on its own, but only in association with other IF family members such as vimentin or desmin. Type V IFs include the nuclear lamins (lamin A/C, B1, and B2), which are nucleoskeletal proteins and therefore will also not be discussed here. Another IF protein, filensin, is not classified into any of these five types because of major deviations in the consensus domains of the α-helical rod domain. Filensin is expressed during differentiation of the lens epithelium, and together with phakinin, another IF protein of 47 kDa, it forms heteropolymers resembling a beaded chain (2).
IF proteins comprise anywhere from 0.3 to 85% of total cell protein (3, 4) and are major building blocks of cellular architecture. Recent studies demonstrate that IFs are involved in many cell physiological activities including motility, shape, mechanics, organelle anchorage and distribution, and signal transduction (5–8). Their significance in cell physiology is becoming widely recognized, as more and more human diseases are linked to mutations in cytoskeletal IF genes (9). Due to space limitations, this review focuses on the Type III proteins, vimentin and desmin, which assemble into homopolymers in a variety of differentiated cell types, but during embryogenesis form copolymers in developing myocytes and myofibers (10). Notably, during evolution, the primary amino acid sequences of both of these Type III proteins have changed very little from elasmobranchs to primates. Moreover, certain short sequence motifs that distinguish vimentin from desmin, which are interspersed within extended stretches of sequence identity, have been conserved in both humans and sharks (11).
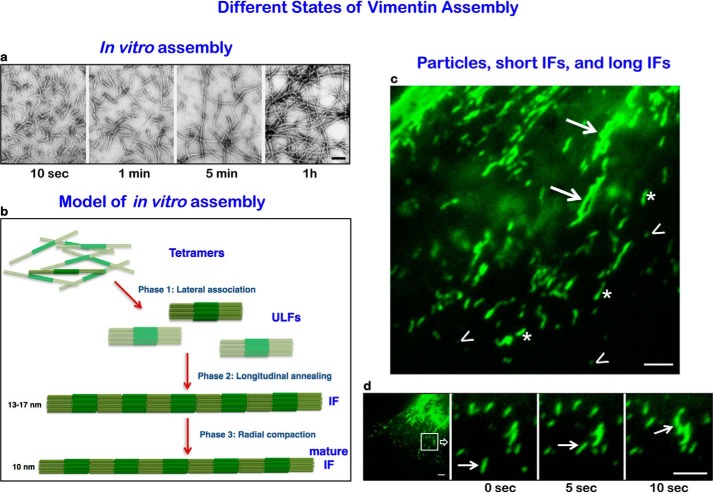
In general, the sequencing of many IF proteins has shown that they consist of a structurally conserved central α-helical rod domain of three sub-helices connected by two linkers, L1 and L12, respectively. The rods are flanked by intrinsically disordered, non-α-helical amino- (“head”) and carboxyl-terminal (“tail”) domains. The rod domains of two IF polypeptide chains align in parallel and in register to form coiled-coil dimers (12). During renaturation from chaotropic agents, dimers associate laterally in an antiparallel and approximately half-staggered fashion to form tetramers. Typically, eight of these tetramers assemble into “unit length” filaments (ULFs), which anneal end to end in an elongation phase to yield non-polar filaments, which are distinctly different from the normally polarized microtubules, and actin filaments. At a certain length, growing filaments radially compact as a final step in the formation of mature IFs of 10-nm cross-sectional diameter (13) (Fig. 1). This basic structure of vimentin and desmin IFs endows them with super-elastic and much more flexible properties when compared with actin and microtubules (14–16). Indeed, it has been shown for desmin IFs and neurofilaments that a single filament can be stretched to more than three times its length before it breaks (17). Furthermore, in rheological experiments, IFs were shown to be flexible at low strain, whereas at high strains, they stiffen and resist breakage (18). It is now understood that this distinct strain-stiffening property makes IFs major factors in regulating the mechanical properties of cells.
FIGURE 1.
Different states of vimentin IF assembly. a, negative-stain electron microscope images of vimentin IF assembly in vitro. Immediately (10 s) after initiating assembly, ULFs form; after 1 min, end-to-end linkages of ULFs form short IFs; after 5 min and 1 h, long mature IFs are assembled (taken from Ref. 2). b, model showing three phases. Phase 1: tetramers assembling laterally into ULF with non-α-helical head and tail domains projecting from the ends of eight tetramers comprising the core coiled-coil region (darker green), Phase 2: end-to-end associations of ULF to form loosely arrayed short IFs. Phase 3: a mature radially compacted short IF. Short IFs can link in tandem to form longer mature IFs. c, total internal reflection fluorescence images of Emerald-tagged vimentin IF assembly states in the lamella/lamellipodial region of a live moving fibroblast. Note non-filamentous particles (arrowheads), short IFs (asterisks), and long IFs (arrows). d, many of the particles and short IFs move rapidly (see arrows pointing to a short IF at 5-s time intervals moving toward and appearing to link with another short IF (arrowhead)). Scale bars, 100 nm (a) and 3 μm (c and d).
Vimentin IF Networks Are Dynamic Components of Cellular Architecture
Vimentin IFs were originally thought to be very stable, i.e. skeletal structures with little subunit exchange. However, studies involving microinjection of fluorophore-tagged vimentin, fluorescence recovery after photobleaching, and photoactivatable GFP probes have demonstrated that the pervasive vimentin IF networks of mammalian cells in fact form highly dynamic linkage elements between the cell surface and the nucleus (19, 20). During various cellular processes such as the cell cycle, cell migration, cell spreading, and cell signaling, vimentin IFs undergo changes in their organization that are functionally significant (8, 21–26). For example, as BHK-21 cells progress from late prophase into metaphase of the cell cycle, vimentin IF organization changes from an elaborate and extensive polymerized network to non-filamentous particles (27). This organizational change requires phosphorylation of vimentin by cyclin-dependent kinase 1 (cdk1), which drives the disassembly of vimentin IFs, a step necessary for its incorporation into daughter cells during mitosis and cytokinesis (26–28). Furthermore, the local disassembly of vimentin IFs in migrating cells is necessary to facilitate the actin-based protrusion of lamellipodia (22). Using various microscopy techniques, three assembly states of vimentin IFs can be recognized in cells: non-filamentous particles, likely representing single or small aggregates of ULFs; short IFs representing end-to-end linkages of ULFs (29); and long or mature IFs (Fig. 1). Particles and short filaments are thought to be precursors to the long vimentin IFs comprising the complex networks present throughout the cytoplasm (21). It has also been shown that subunit exchange can occur at many sites along mature vimentin IFs in an apolar fashion and that the exchangeable form is a tetramer (30). Interestingly, it appears that vimentin IF assembly can be influenced by changes in cellular tension and morphology because various cell types exhibit biphasic changes in vimentin solubility as a function of substrate stiffness (31). Evidence suggests that vimentin particles, as well as short and long IFs, move along microtubule tracks via kinesin and dynein motors. However, the mechanisms linking IF to these motors remain unknown.
Vimentin IFs and Cellular Mechanics
Recent studies have revealed that vimentin IFs are important regulators of the intracellular changes in cytoplasmic mechanics that accompany various physiological activities such as cell contraction, migration, proliferation, and organelle positioning (32). Support for their mechanical roles comes from active micro-rheology and optical magnetic twisting cytometry experiments, which reveal that vimentin IFs are major contributors to the intracellular stiffness of the cytoplasm. In this regard, the cytoplasm of normal fibroblasts expressing vimentin IFs is approximately twice as stiff as fibroblasts that are null for vimentin expression. In contrast, the cortical stiffness in these two cell types is identical as measured by optical magnetic twisting cytometry (32). This contribution of vimentin IFs to cytoplasmic stiffness is thought to help stabilize the positions of organelles, preventing their displacement by random fluctuating cytoplasmic forces. This suggests that vimentin IFs can localize intracellular organelles by tethering (6, 32) (see below). It has also been shown that vimentin-null fibroblasts are more easily deformable than wild-type fibroblasts in response to increasing compressive stress (33, 34). In addition, vimentin IFs enhance the elastic properties of cells, and this response increases as a function of substrate stiffness, suggesting that IF networks can adapt to mechanical changes in their environment, thereby preserving the mechanical integrity of cells (33). Interestingly, in endothelial cells, fluid shear stress causes the rapid redistribution of vimentin IFs at sites distal from the exposed surface (35). Overall, the results obtained to date demonstrate that vimentin IFs are capable of transducing mechanical signals initiated at the cell surface and can further transmit these signals throughout the cytoplasm (36, 37).
Vimentin IFs and the Positioning of Organelles
In addition to modulating cell polarity, the vimentin IF cytoskeletal system also plays an important role in regulating the distribution and organization of organelles within the cytoplasm. For example, mitochondrial motility, distribution, and anchorage are modulated by interactions with vimentin IFs (38). Evidence supporting this comes from studies of vimentin-null fibroblasts in which mitochondrial motility is increased when compared with wild-type cells. This increase in motility reflects, at least in part, a role for vimentin IFs in anchoring and positioning of mitochondria. This latter anchoring function is mediated by vimentin's amino-terminal domain because it has been determined that residues 41–94, when expressed in vimentin-null cells, strongly associate with mitochondria. Vimentin IFs have also been shown to interact with the Golgi complex through binding to the resident Golgi protein, formiminotransferase cyclodeaminase (FTCD), suggesting that they play a role in positioning the Golgi apparatus (39). Additionally, vimentin IFs form an intricate cage surrounding melanosomes that can physically hinder melanosome transport in melanophores (40) (Fig. 2). A vimentin IF cage is also assembled during adipose conversion as vimentin IFs reorganize from an extended network to form a complex cage tightly surrounding lipid droplets (41). Moreover, vimentin IFs are known to accumulate around the nucleus, and perturbations of vimentin IF networks alter the position of the nucleus in both migrating cells and astrocytes (42). These studies emphasize the importance of vimentin IFs in interacting with, stabilizing, and positioning organelles in the cytoplasm and indicate that they can physically alter organelle transport and nuclear positioning (see Fig. 4).
FIGURE 2.
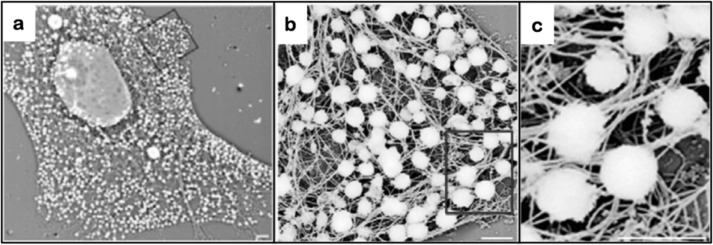
Vimentin IF function in anchoring organelles: melanosomes in Xenopus melanophores. Melanosome association with vimentin IFs is depicted in a whole mount electron micrograph of a melanophore processed to remove microtubules and microfilaments while preserving the IF network. The box in a is seen at higher magnification in b, and the box in b is enlarged in c. Scale bars: 2 μm (a); 1 μm (b and c). Taken from Ref. 40.
FIGURE 4.
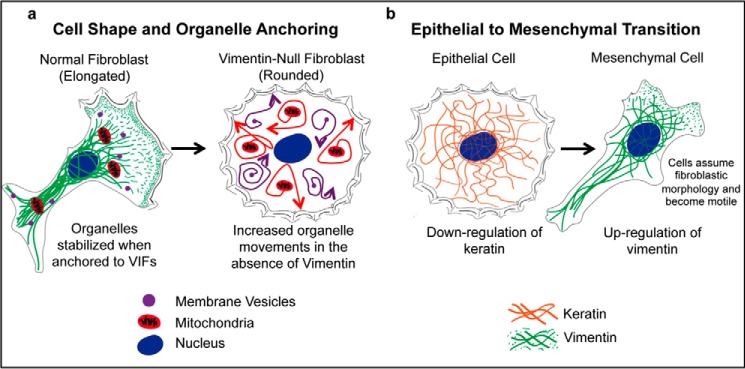
A schematic showing selected roles of vimentin IFs. a, vimentin-null fibroblasts exhibit changes in cell shape relative to WT fibroblasts, and organelle movements increase (arrows; also see Ref. 32). For example, mitochondria and membranous vesicles exhibit significantly increased cytoplasmic movements when vimentin is absent in fibroblasts (32, 38). b, during the EMT transition, keratin is down-regulated, whereas vimentin is up-regulated. These changes in IF expression patterns cause dramatic alterations in cell morphology and motility.
Vimentin IFs Regulate Cell Shape and Motility
Vimentin IFs form an intricate network of complex filamentous structures that extend from the cell membrane to the nucleus (Fig. 3). Studies have shown that these networks of strategically placed vimentin IFs influence cell shape. In neurons, the developmentally regulated replacement of vimentin IFs with Type IV IFs is directly correlated with alterations in cell shape, specifically the outgrowth of neurites or axons (43, 44). Similarly, when vimentin IF networks in fibroblasts are disrupted by expression of a dominant negative mutant, or by silencing with shRNA, the cells transition from a mesenchymal to a rounded epithelial shape (45).
FIGURE 3.
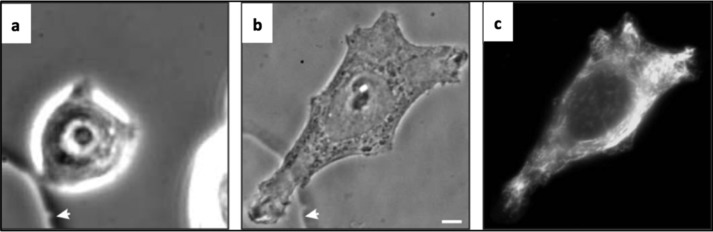
The dramatic impact of vimentin IF assembly in epithelial cells: cell shape and the EMT. a–c, phase contrast image of a living MCF-7 epithelial cell expressing only keratin IF before (a) and 5 h after (b) microinjection of bacterially expressed vimentin, at which time the cell was fixed and processed for indirect immunofluorescence using anti-vimentin (c). The arrows represent a fiducial mark. Scale bars = 10 μm. Taken from Ref. 45.
In moving fibroblasts, large numbers of vimentin IFs surround the nucleus and extend into the trailing edge of the cell. In contrast, the leading edge contains only vimentin particles in the lamellipodium and short IFs within the lamellar region (22). These regional differences in vimentin IF organization are involved in regulating protrusive activity at the cell margin. For example, serum starvation causes fibroblasts to cease movement, and under these conditions, a well formed network of long vimentin IFs extends to all parts of the cell periphery. The addition of serum to starved cells results in the local breakdown of the vimentin IF network and the appearance of short filaments and particles in regions where lamellipodia form (22). The signal transduction cascade linking the growth factors in serum to the initiation of fibroblast motility involves transient activation of Rac1 (46). When photoactivatable Rac1 is turned on locally in serum-deprived cells, a wave of vimentin IF phosphorylation results. This is accompanied by the local conversion of vimentin IFs into short filaments and particles in the immediate region of the activated Rac1 and the subsequent formation of lamellipodia (22). Furthermore, the local disassembly of vimentin IF networks is sufficient to initiate the process of membrane ruffling and lamellipodium formation. This is based upon the finding that the microinjection of a mimetic peptide, which disassembles vimentin IFs into ULFs, is sufficient to locally induce vimentin IF disassembly and the initiation of lamellipodia in serum-starved cells (22). Other studies have shown that vimentin is essential for efficient wound healing both in cultured cells and in animal models (47–49), and vimentin-null mouse fibroblasts exhibit greatly reduced motility, chemotaxis, and the ability to organize collagen fibrils (50). A recent study also provides evidence that vimentin IFs contribute to the formation of the lobopodia that form when intracellular pressure is elevated in cells migrating through complex three-dimensional matrices. In this regard, vimentin IFs are thought to provide linkages between the nucleus (via nesprin-3) and cytoplasmic myosin, which has been postulated to provide the force for moving the nucleus and generating the intracellular pressure (51). Thus vimentin IFs play important roles not only in providing mechanical support, but also in regulating cell motility.
Changes in Vimentin IF Network Composition and the Epithelial-Mesenchymal Transition (EMT)
During embryonic development, the movement of epithelial cells frequently involves a process known as the EMT. Interestingly, the cytoskeletal hallmark of the EMT is the up-regulation of vimentin expression, whereas keratin is down-regulated (52). During this transition, the epithelial cells assume a typical mesenchymal or fibroblastic morphology and become motile (Fig. 4). This EMT program is recapitulated with respect to IF protein composition when cancerous cells become metastatic (53, 54), and importantly, it has been shown that vimentin expression is required for the invasive behavior of prostate and breast cancer cells (55–57). Further evidence that vimentin IFs play a key role in regulating mesenchymal cell shape and motility comes from studies in which vimentin is experimentally reorganized in human foreskin fibroblasts by expressing a dominant negative mutant to disrupt vimentin IF assembly or by down-regulation following silencing of vimentin expression with shRNA. In both cases, the experimental manipulation causes mesenchymal cells to adopt an epithelial shape (45). Conversely, the ectopic expression of vimentin in epithelial cells, either by direct microinjection of vimentin protein or by transfection with vimentin cDNA, induces a transition to a mesenchymal shape (Fig. 3), and this is accompanied by the loss of desmosomes and an increase in focal adhesion dynamics and cell motility (45). With respect to metastasis, vimentin IFs also play an important role in the elongation and stabilization of invadopodia (37, 59). These structures are membrane protrusions rich in matrix metalloproteinases that degrade the basement membrane, enabling the migration of cancerous cells through the extracellular environment (59, 60).
Mutations in Type III IF Genes and Human Disease
The physiological importance of IFs has been dramatically highlighted by the discovery that large numbers of human diseases are associated with mutations in IF genes (9, 61). Because different types of IFs are expressed in a tissue-specific and developmentally regulated fashion, defects can be restricted to specific tissues and/or times during developmental progression. The expression of mutant IF genes in humans causes a variety of diseases such as cataract formation for vimentin (59), myopathies for desmin (60), and Alexander disease for GFAP (62). In the case of Alexander disease, there are phenotypic differences among individuals due to their genetic background. This is supported by the finding that the same mutation, R416W, can result in infantile (0–2 years), juvenile, (2–12 years), or adult (>12 years) onset disease, each of which can also be associated with differences in progression of the disease. The pathogenesis of this disease involves the formation of non-IF-containing structures called Rosenthal fibers, which sequester chaperones and cause activation of stress kinases such as JNK (63).
Soon after the first desmin mutations causing myopathies were discovered in 1998 (64, 65), it became clear that a large percentage of patients presenting with dilated cardiomyopathy have mutations in the desmin gene (66). Typically, the hallmark of “desminopathies” is the formation of massive desmin aggregates within myofibers (for review, see Ref. 67). It should also be noted that desmin aggregation is observed in desmin-related myopathies that are not associated with mutations in desmin, but rather with mutations in its chaperone, αB-crystallin (68). The biochemical investigation of the first reported mutation, which was missing seven amino acids (i.e. a heptad repeat) in coil 1B, revealed that the mutant desmin would not form IFs either after cDNA transfection into Type III-free cultured cells or in vitro when the recombinant protein was tested for assembly according to standard conditions (69). As more desmin mutations were identified, 14 of them were systematically analyzed for their ability to form IFs (70). Most of these mutations arrested formation at one of the intermediate stages in the IF assembly process, e.g. the protein formed ULFs but did not longitudinally anneal; or subunits started to assemble longitudinally but then would open up to form large sheets. Although some mutants formed apparently normal desmin IF, closer examination revealed that they incorporated more subunits per cross-section than wild-type desmin (69). In addition, they exhibited different mechanical properties as determined by single filament manipulation with atomic force microscopy (71) or by macro-rheology (72). The latter measurements involved desmin variants with point mutations in the tail domain, and they revealed that for some mutations, the filaments significantly lost their ability to strain stiffen. These studies further revealed that the tail domain is responsible for strain stiffening because the tailless variant, although able to form apparently normal filaments and filament networks, did not exhibit any sign of strain stiffening in response to mechanical stress.
A number of human neurological diseases involve both Type III and Type IV IF proteins, which in many cases form abnormal aggregates of fully polymerized IF in nerve cell bodies and along axons (64–70). One example is the rare neurodegenerative disease, giant axonal neuropathy (GAN). GAN is an early onset recessive disease caused by mutations in the GAN gene, which encodes gigaxonin, an E3 ligase adaptor protein thought to target IF proteins for degradation via the ubiquitin-proteasome pathway (4, 73). The disease is unusual among neurodegenerative diseases in that IFs in non-neuronal tissues also form aggregates, including vimentin IFs in patient fibroblasts.
In contrast to the human diseases, early studies of Type III IF gene knock-out mice were somewhat misleading as they did not cause embryonic or early postnatal lethality and they appeared to develop and reproduce normally (74). However, over the years since the vimentin knock-out mouse was introduced, much more careful studies have revealed numerous abnormalities and deficiencies. For example, in the cerebellum, Bergmann glia and Purkinje cells exhibit morphological defects, whereas behavioral studies show motor coordination deficits in the absence of vimentin (75). These mice also exhibit impaired wound healing (47), defects in steroid production (76), and severe defects in diapedesis (77). Defects in the vascular endothelium were also detected in vimentin-null mice with respect to their ability to dilate mesenteric resistance arteries in response to blood flow (78). Similarly, although desmin-null mice are viable and muscle differentiation takes place, defects have been reported in skeletal, cardiac, and smooth muscle tissue (79, 80). For example, the desmin-null mouse model is deficient in endurance exercise performance when compared with wild-type mice as monitored by treadmill tests (81).
The Regulation of Vimentin and Desmin IF Assembly
The regulation of vimentin IF assembly, structure, and function involves reversible post-translational modifications (PTMs). Although there are a few examples of PTMs within the highly α-helical central rod domain, the majority of these covalent modifications reside in the non-α-helical head and tail domains. Known PTMs include phosphorylation, glycosylation, ubiquitylation, sumoylation, acetylation, farnesylation, transamidation, and ADP-ribosylation (82, 83). The most extensively studied PTM is phosphorylation. During mitosis in baby hamster kidney (BHK) cells, for example, there is a transient phosphorylation of vimentin accompanied by a dramatic reorganization and change in the state of vimentin IF assembly (84). Vimentin is hyper-phosphorylated at serine 55 by cdk1, which drives disassembly into non-filamentous IF particles likely to be ULFs, which are distributed into daughter cells for subsequent dephosphorylation and reassembly into vimentin IF networks (25, 27). Phosphorylation of this site within vimentin's head domain during mitosis is consistent with its known importance in the assembly of IFs (26, 28, 85–87). Additionally, phosphorylation of vimentin at serine 71 by Rho kinase causes the inhibition of IF formation in vitro (86). To achieve total disassembly of the vimentin IF network during mitosis, another IF protein, nestin, is also required (88).
Phosphorylation may also play a role in disassembling IF polymers into smaller subunits that can be processed by the ubiquitin-proteasome system. In fasting animals, for example, increased muscle breakdown (atrophy) is a physiological response to provide nutrients for survival. It has been shown that an early event in this process is the phosphorylation of three serines within the head domain of desmin. This phosphorylation results in the disassembly of desmin IFs, interaction of the resulting subunits with the TRIM32 ubiquitin ligase, and eventual degradation by the ubiquitin-proteasome system (89). Once the muscle IF system is dismantled, then thin filaments, Z-bands, and other components of the myofibril are turned over to provide energy for the fasting animal.
Vimentin IFs Link the Cell Surface with the Nucleus
IFs are structural elements capable of connecting the exterior of the cell with the interior of the nucleus. In mesenchymal cells, vimentin IFs interact with the extracellular matrix via integrins (90). The vimentin IF network spans the cytoplasm and connects to the nucleus through interactions with the linker of nucleoskeleton and cytoskeleton (LINC) complex. This complex consists of nuclear membrane-associated SUN domain proteins linked to the Type V IF proteins, the nuclear lamins, and KASH domain proteins that connect to cytoskeletal IFs through interactions with plectin and nesprin 3 (91, 92). These connections, along with the global distribution of IFs, make the system an ideal candidate for transmitting and regulating information flow. It is known, for example, that different inactive kinases can bind to vimentin and that when these kinases are activated, they phosphorylate their respective IF partner and then translocate to other regions of the cell (93). Vimentin and various other IFs have been shown to interact with the regulatory 14-3-3 proteins (94–97). When vimentin is phosphorylated in its head domain (amino acids 1–96), it binds the 14-3-3 protein in a Raf-1·14-3-3 complex, causing the Raf-1 to be released, and subsequently decreases Raf-1 kinase activity (94). There are many other effector proteins that are thought to interact dynamically with vimentin IFs including adaptors, receptors, kinases, and other effectors (58, 98, 99). Therefore it is now obvious that vimentin IF networks play important roles in signal transduction in mammalian cells.
A Look into the Future
In this brief review, we have attempted to provide an overview of the current status of IF research using vimentin and desmin as examples. Despite a recent surge of interest in IFs, they still remain the least studied and the least understood of all of the cytoskeletal systems. The coordinated use of cell biological, biochemical, biophysical, and computational techniques will be required to gain insights into the precise structures and functions of this large cytoskeletal protein family. These combined approaches will lead to new insights into the specific roles of IFs in a wide range of functions including their roles in mediating cytoskeletal cross-talk. In support of the latter, IFs are known to interact extensively with microtubules and their associated motors, dynein and kinesin, as well as actin and myosin, but little is known about the protein-protein interactions involved. Also, given the number of IF subtypes, there are undoubtedly a large number of IF-associated proteins; however, few such IF-associated proteins have been identified and rigorously studied, perhaps with the exception of plectin. In addition, there is a significant amount of information suggesting roles for IFs in signal transduction, including their involvement in mechano-signaling in cells. However, there is very little information available reflecting on the specific roles of IFs in these cellular processes. Finally, the scaffolding functions of cytoplasmic IF networks need to be defined in the context of their reported associations with many cellular structures, including the nucleus, cell membranes, and organelles such as mitochondria and lipid droplets.
This work was supported by National Institutes of Health Grant PO1GM096971 from the NIGMS and Hannah's Hope Fund (to R. D. G.). This work was also supported by German Research Foundation (DFG) Grants HE 1853/9-2 (FOR 1228) and HE1853/11-1 (to H. H.). This is the second article in the Thematic Minireview series “The State of the Cytoskeleton in 2015.” The authors declare that they have no conflicts of interest with the contents of this article.
- IF
- intermediate filament
- GFAP
- glial fibrillary acidic protein
- NF
- neurofilament
- ULF
- unit length filaments
- EMT
- epithelial to mesenchymal transition
- GAN
- giant axonal neuropathy
- PTM
- post-translational modification.
References
- 1. Moll R., Divo M., Langbein L. (2008) The human keratins: biology and pathology. Histochem. Cell Biol. 129, 705–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Herrmann H., Aebi U. (2004) Intermediate filaments: molecular structure, assembly mechanism, and integration into functionally distinct intracellular Scaffolds. Annu. Rev. Biochem. 73, 749–789 [DOI] [PubMed] [Google Scholar]
- 3. Fuchs E., Coulombe P. A. (1992) Of mice and men: genetic skin diseases of keratin. Cell 69, 899–902 [DOI] [PubMed] [Google Scholar]
- 4. Mahammad S., Murthy S. N., Didonna A., Grin B., Israeli E., Perrot R., Bomont P., Julien J. P., Kuczmarski E., Opal P., Goldman R. D. (2013) Giant axonal neuropathy-associated gigaxonin mutations impair intermediate filament protein degradation. J. Clin. Invest. 123, 1964–1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chernoivanenko I. S., Matveeva E. A., Gelfand V. I., Goldman R. D., Minin A. A. (2015) Mitochondrial membrane potential is regulated by vimentin intermediate filaments. FASEB J. 29, 820–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guo M., Ehrlicher A. J., Jensen M. H., Renz M., Moore J. R., Goldman R. D., Lippincott-Schwartz J., Mackintosh F. C., Weitz D. A. (2014) Probing the stochastic, motor-driven properties of the cytoplasm using force spectrum microscopy. Cell 158, 822–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Helfand B. T., Mendez M. G., Pugh J., Delsert C., Goldman R. D. (2003) A role for intermediate filaments in determining and maintaining the shape of nerve cells. Mol. Biol. Cell 14, 5069–5081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yoon M., Moir R. D., Prahlad V., Goldman R. D. (1998) Motile properties of vimentin intermediate filament networks in living cells. J. Cell Biol. 143, 147–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Omary M. B. (2009) “IF-pathies”: a broad spectrum of intermediate filament-associated diseases. J. Clin. Invest. 119, 1756–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gard D. L., Lazarides E. (1980) The synthesis and distribution of desmin and vimentin during myogenesis in vitro. Cell 19, 263–275 [DOI] [PubMed] [Google Scholar]
- 11. Schaffeld M., Herrmann H., Schultess J., Markl J. (2001) Vimentin and desmin of a cartilaginous fish, the shark Scyliorhinus stellaris: sequence, expression patterns and in vitro assembly. Eur. J. Cell Biol. 80, 692–702 [DOI] [PubMed] [Google Scholar]
- 12. Geisler N., Weber K. (1982) The amino acid sequence of chicken muscle desmin provides a common structural model for intermediate filament proteins. EMBO J. 1, 1649–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Engel A., Eichner R., Aebi U. (1985) Polymorphism of reconstituted human epidermal keratin filaments: determination of their mass-per-length and width by scanning transmission electron microscopy (STEM). J. Ultrastruct. Res. 90, 323–335 [DOI] [PubMed] [Google Scholar]
- 14. Fudge D. S., Gardner K. H., Forsyth V. T., Riekel C., Gosline J. M. (2003) The mechanical properties of hydrated intermediate filaments: insights from hagfish slime threads. Biophys. J. 85, 2015–2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guzmán C., Jeney S., Kreplak L., Kasas S., Kulik A. J., Aebi U., Forró L. (2006) Exploring the mechanical properties of single vimentin intermediate filaments by atomic force microscopy. J. Mol. Biol. 360, 623–630 [DOI] [PubMed] [Google Scholar]
- 16. Kreplak L., Bär H., Leterrier J. F., Herrmann H., Aebi U. (2005) Exploring the mechanical behavior of single intermediate filaments. J. Mol. Biol. 354, 569–577 [DOI] [PubMed] [Google Scholar]
- 17. Kreplak L., Herrmann H., Aebi U. (2008) Tensile properties of single desmin intermediate filaments. Biophys. J. 94, 2790–2799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Janmey P. A., Euteneuer U., Traub P., Schliwa M. (1991) Viscoelastic properties of vimentin compared with other filamentous biopolymer networks. J. Cell Biol. 113, 155–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ho C. L., Martys J. L., Mikhailov A., Gundersen G. G., Liem R. K. (1998) Novel features of intermediate filament dynamics revealed by green fluorescent protein chimeras. J. Cell Sci. 111, 1767–1778 [DOI] [PubMed] [Google Scholar]
- 20. Martys J. L., Ho C. L., Liem R. K., Gundersen G. G. (1999) Intermediate filaments in motion: observations of intermediate filaments in cells using green fluorescent protein-vimentin. Mol. Biol. Cell 10, 1289–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Helfand B. T., Chang L., Goldman R. D. (2003) The dynamic and motile properties of intermediate filaments. Annu. Rev. Cell Dev. Biol. 19, 445–467 [DOI] [PubMed] [Google Scholar]
- 22. Helfand B. T., Mendez M. G., Murthy S. N., Shumaker D. K., Grin B., Mahammad S., Aebi U., Wedig T., Wu Y. I., Hahn K. M., Inagaki M., Herrmann H., Goldman R. D. (2011) Vimentin organization modulates the formation of lamellipodia. Mol. Biol. Cell 22, 1274–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ben-Ze'ev A. (1984) Differential control of cytokeratins and vimentin synthesis by cell-cell contact and cell spreading in cultured epithelial cells. J. Cell Biol. 99, 1424–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ivaska J., Pallari H. M., Nevo J., Eriksson J. E. (2007) Novel functions of vimentin in cell adhesion, migration, and signaling. Exp. Cell Res. 313, 2050–2062 [DOI] [PubMed] [Google Scholar]
- 25. Yamaguchi T., Goto H., Yokoyama T., Silljé H., Hanisch A., Uldschmid A., Takai Y., Oguri T., Nigg E. A., Inagaki M. (2005) Phosphorylation by Cdk1 induces Plk1-mediated vimentin phosphorylation during mitosis. J. Cell Biol. 171, 431–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yasui Y., Goto H., Matsui S., Manser E., Lim L., Nagata K.-i., Inagaki M. (2001) Protein kinases required for segregation of vimentin filaments in mitotic process. Oncogene 20, 2868–2876 [DOI] [PubMed] [Google Scholar]
- 27. Chou Y. H., Bischoff J. R., Beach D., Goldman R. D. (1990) Intermediate filament reorganization during mitosis is mediated by p34cdc2 phosphorylation of vimentin. Cell 62, 1063–1071 [DOI] [PubMed] [Google Scholar]
- 28. Inagaki M., Nishi Y., Nishizawa K., Matsuyama M., Sato C. (1987) Site-specific phosphorylation induces disassembly of vimentin filaments in vitro. Nature 328, 649–652 [DOI] [PubMed] [Google Scholar]
- 29. Robert A., Herrmann H., Davidson M. W., Gelfand V. I. (2014) Microtubule-dependent transport of vimentin filament precursors is regulated by actin and by the concerted action of Rho- and p21-activated kinases. FASEB J. 28, 2879–2890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nöding B., Herrmann H., Köster S. (2014) Direct observation of subunit exchange along mature vimentin intermediate filaments. Biophys. J. 107, 2923–2931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Murray M. E., Mendez M. G., Janmey P. A. (2014) Substrate stiffness regulates solubility of cellular vimentin. Mol. Biol. Cell 25, 87–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guo M., Ehrlicher A. J., Mahammad S., Fabich H., Jensen M. H., Moore J. R., Fredberg J. J., Goldman R. D., Weitz D. A. (2013) The role of vimentin intermediate filaments in cortical and cytoplasmic mechanics. Biophys. J. 105, 1562–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mendez M. G, Restle D., Janmey P. A. (2014) Vimentin enhances cell elastic behavior and protects against compressive stress. Biophys. J. 107, 314–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ofek G., Wiltz D. C., Athanasiou K. A. (2009) Contribution of the cytoskeleton to the compressive properties and recovery behavior of single cells. Biophys. J. 97, 1873–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Helmke B. P., Goldman R. D., Davies P. F. (2000) Rapid displacement of vimentin intermediate filaments in living endothelial cells exposed to flow. Circ. Res. 86, 745–752 [DOI] [PubMed] [Google Scholar]
- 36. Conway D. E., Breckenridge M. T., Hinde E., Gratton E., Chen C. S., Schwartz M. A. (2013) Fluid shear stress on endothelial cells modulates mechanical tension across VE-cadherin and PECAM-1. Curr. Biol. 23, 1024–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schnittler H. J., Schmandra T., Drenckhahn D. (1998) Correlation of endothelial vimentin content with hemodynamic parameters. Histochem. Cell Biol. 110, 161–167 [DOI] [PubMed] [Google Scholar]
- 38. Nekrasova O. E., Mendez M. G., Chernoivanenko I. S., Tyurin-Kuzmin P. A., Kuczmarski E. R., Gelfand V. I., Goldman R. D., Minin A. A. (2011) Vimentin intermediate filaments modulate the motility of mitochondria. Mol. Biol. Cell 22, 2282–2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gao Y., Sztul E. (2001) A novel interaction of the Golgi complex with the vimentin intermediate filament cytoskeleton. J. Cell Biol. 152, 877–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chang L., Barlan K., Chou Y. H., Grin B., Lakonishok M., Serpinskaya A. S., Shumaker D. K., Herrmann H., Gelfand V. I., Goldman R. D. (2009) The dynamic properties of intermediate filaments during organelle transport. J. Cell Sci. 122, 2914–2923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Franke W. W., Hergt M., Grund C. (1987) Rearrangement of the vimentin cytoskeleton during adipose conversion: formation of an intermediate filament cage around lipid globules. Cell 49, 131–141 [DOI] [PubMed] [Google Scholar]
- 42. Dupin I., Sakamoto Y., Etienne-Manneville S. (2011) Cytoplasmic intermediate filaments mediate actin-driven positioning of the nucleus. J. Cell Sci. 124, 865–872 [DOI] [PubMed] [Google Scholar]
- 43. Shea T. B., Beermann M. L., Fischer I. (1993) Transient requirement for vimentin in neuritogenesis: intracellular delivery of anti-vimentin antibodies and antisense oligonucleotides inhibit neurite initiation but not elongation of existing neurites in neuroblastoma. J. Neurosci. Res. 36, 66–76 [DOI] [PubMed] [Google Scholar]
- 44. Cochard P., Paulin D. (1984) Initial expression of neurofilaments and vimentin in the central and peripheral nervous system of the mouse embryo in vivo. J. Neurosci. 4, 2080–2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mendez M. G., Kojima S., Goldman R. D. (2010) Vimentin induces changes in cell shape, motility, and adhesion during the epithelial to mesenchymal transition. FASEB J. 24, 1838–1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ridley A. J., Paterson H. F., Johnston C. L., Diekmann D., Hall A. (1992) The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 70, 401–410 [DOI] [PubMed] [Google Scholar]
- 47. Eckes B., Colucci-Guyon E., Smola H., Nodder S., Babinet C., Krieg T., Martin P. (2000) Impaired wound healing in embryonic and adult mice lacking vimentin. J. Cell Sci. 113, 2455–2462 [DOI] [PubMed] [Google Scholar]
- 48. Rogel M. R., Soni P. N., Troken J. R., Sitikov A., Trejo H. E., Ridge K. M. (2011) Vimentin is sufficient and required for wound repair and remodeling in alveolar epithelial cells. FASEB J. 25, 3873–3883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Menko A. S., Bleaken B. M., Libowitz A. A., Zhang L., Stepp M. A., Walker J. L. (2014) A central role for vimentin in regulating repair function during healing of the lens epithelium. Mol. Biol. Cell 25, 776–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Eckes B., Dogic D., Colucci-Guyon E., Wang N., Maniotis A., Ingber D., Merckling A., Langa F., Aumailley M., Delouvée A., Koteliansky V., Babinet C., Krieg T. (1998) Impaired mechanical stability, migration and contractile capacity in vimentin-deficient fibroblasts. J. Cell Sci. 111, 1897–1907 [DOI] [PubMed] [Google Scholar]
- 51. Petrie R. J., Koo H., Yamada K. M. (2014) Generation of compartmentalized pressure by a nuclear piston governs cell motility in a 3D matrix. Science 345, 1062–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kokkinos M. I., Wafai R., Wong M. K., Newgreen D. F., Thompson E. W., Waltham M. (2007) Vimentin and epithelial-mesenchymal transition in human breast cancer: observations in vitro and in vivo. Cells Tissues Organs 185, 191–203 [DOI] [PubMed] [Google Scholar]
- 53. Satelli A., Li S. (2011) Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell. Mol. Life Sci. 68, 3033–3046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Thiery J. P. (2002) Epithelial-mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2, 442–454 [DOI] [PubMed] [Google Scholar]
- 55. Wei J., Xu G., Wu M., Zhang Y., Li Q., Liu P., Zhu T., Song A., Zhao L., Han Z., Chen G., Wang S., Meng L., Zhou J., Lu Y., Wang S., Ma D. (2008) Overexpression of vimentin contributes to prostate cancer invasion and metastasis via Src regulation. Anticancer Res. 28, 327–334 [PubMed] [Google Scholar]
- 56. Zhu Q. S., Rosenblatt K., Huang K. L., Lahat G., Brobey R., Bolshakov S., Nguyen T., Ding Z., Belousov R., Bill K., Luo X., Lazar A., Dicker A., Mills G. B., Hung M. C., Lev D. (2011) Vimentin is a novel AKT1 target mediating motility and invasion. Oncogene 30, 457–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Vuoriluoto K., Haugen H., Kiviluoto S., Mpindi J. P., Nevo J., Gjerdrum C., Tiron C., Lorens J. B., Ivaska J. (2011) Vimentin regulates EMT induction by Slug and oncogenic H-Ras and migration by governing Axl expression in breast cancer. Oncogene 30, 1436–1448 [DOI] [PubMed] [Google Scholar]
- 58. Eriksson J. E., Dechat T., Grin B., Helfand B., Mendez M., Pallari H. M., Goldman R. D. (2009) Introducing intermediate filaments: from discovery to disease. J. Clin. Invest. 119, 1763–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Schoumacher M., Goldman R. D., Louvard D., Vignjevic D. M. (2010) Actin, microtubules, and vimentin intermediate filaments cooperate for elongation of invadopodia. J. Cell Biol. 189, 541–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Martin K. H., Hayes K. E., Walk E. L., Ammer A. G., Markwell S. M., Weed S. A. (2012) Quantitative measurement of invadopodia-mediated extracellular matrix proteolysis in single and multicellular contexts. J. Vis. Exp. e4119, 10.3791/4119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Omary M. B., Coulombe P. A., McLean W. H. (2004) Intermediate filament proteins and their associated diseases. N. Engl. J. Med. 351, 2087–2100 [DOI] [PubMed] [Google Scholar]
- 62. Brenner M., Johnson A. B., Boespflug-Tanguy O., Rodriguez D., Goldman J. E., Messing A. (2001) Mutations in GFAP, encoding glial fibrillary acidic protein, are associated with Alexander disease. Nat. Genet. 27, 117–120 [DOI] [PubMed] [Google Scholar]
- 63. Quinlan R. A., Brenner M., Goldman J. E., Messing A. (2007) GFAP and its role in Alexander disease. Exp. Cell Res. 313, 2077–2087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Goldfarb L. G., Park K. Y., Cervenáková L., Gorokhova S., Lee H. S., Vasconcelos O., Nagle J. W., Semino-Mora C., Sivakumar K., Dalakas M. C. (1998) Missense mutations in desmin associated with familial cardiac and skeletal myopathy. Nat. Genet. 19, 402–403 [DOI] [PubMed] [Google Scholar]
- 65. Muñoz-Mármol A. M., Strasser G., Isamat M., Coulombe P. A., Yang Y., Roca X., Vela E., Mate J. L., Coll J., Fernández-Figueras M. T., Navas-Palacios J. J., Ariza A., Fuchs E. (1998) A dysfunctional desmin mutation in a patient with severe generalized myopathy. Proc. Natl. Acad. Sci. U.S.A. 95, 11312–11317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Taylor M. R., Slavov D., Ku L., Di Lenarda A., Sinagra G., Carniel E., Haubold K., Boucek M. M., Ferguson D., Graw S. L., Zhu X., Cavanaugh J., Sucharov C. C., Long C. S., Bristow M. R., Lavori P., Mestroni L., Familial Cardiomyopathy Registry, and BEST (Beta-Blocker Evaluation of Survival Trial) DNA Bank (2007) Prevalence of desmin mutations in dilated cardiomyopathy. Circulation 115, 1244–1251 [DOI] [PubMed] [Google Scholar]
- 67. Clemen C. S., Herrmann H., Strelkov S. V., Schröder R. (2013) Desminopathies: pathology and mechanisms. Acta Neuropathol. 125, 47–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Perng M. D, Wen S. F, van den IJssel P., Prescott A. R., Quinlan R. A. (2004) Desmin aggregate formation by R120G αB-crystallin is caused by altered filament interactions and is dependent upon network status in cells. Mol. Biol. Cell 15, 2335–2346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bär H., Mücke N., Ringler P., Müller S. A., Kreplak L., Katus H. A., Aebi U., Herrmann H. (2006) Impact of disease mutations on the desmin filament assembly process. J. Mol. Biol. 360, 1031–1042 [DOI] [PubMed] [Google Scholar]
- 70. Bär H., Mücke N., Kostareva A., Sjöberg G., Aebi U., Herrmann H. (2005) Severe muscle disease-causing desmin mutations interfere with in vitro filament assembly at distinct stages. Proc. Natl. Acad. Sci. U.S.A. 102, 15099–15104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kreplak L., Bär H. (2009) Severe myopathy mutations modify the nanomechanics of desmin intermediate filaments. J. Mol. Biol. 385, 1043–1051 [DOI] [PubMed] [Google Scholar]
- 72. Bär H., Schopferer M., Sharma S., Hochstein B., Mücke N., Herrmann H., Willenbacher N. (2010) Mutations in desmin's carboxy-terminal “tail” domain severely modify filament and network mechanics. J. Mol. Biol. 397, 1188–1198 [DOI] [PubMed] [Google Scholar]
- 73. Bomont P., Cavalier L., Blondeau F., Ben Hamida C., Belal S., Tazir M., Demir E., Topaloglu H., Korinthenberg R., Tüysüz B., Landrieu P., Hentati F., Koenig M. (2000) The gene encoding gigaxonin, a new member of the cytoskeletal BTB/kelch repeat family, is mutated in giant axonal neuropathy. Nat. Genet. 26, 370–374 [DOI] [PubMed] [Google Scholar]
- 74. Colucci-Guyon E., Portier M. M., Dunia I., Paulin D., Pournin S., Babinet C. (1994) Mice lacking vimentin develop and reproduce without an obvious phenotype. Cell 79, 679–694 [DOI] [PubMed] [Google Scholar]
- 75. Colucci-Guyon E., Giménez Y. R. M., Maurice T., Babinet C., Privat A. (1999) Cerebellar defect and impaired motor coordination in mice lacking vimentin. Glia 25, 33–43 [PubMed] [Google Scholar]
- 76. Shen W. J., Zaidi S. K., Patel S., Cortez Y., Ueno M., Azhar R., Azhar S., Kraemer F. B. (2012) Ablation of vimentin results in defective steroidogenesis. Endocrinology 153, 3249–3257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Nieminen M., Henttinen T., Merinen M., Marttila-Ichihara F., Eriksson J. E., Jalkanen S. (2006) Vimentin function in lymphocyte adhesion and transcellular migration. Nat. Cell Biol. 8, 156–162 [DOI] [PubMed] [Google Scholar]
- 78. Henrion D., Terzi F., Matrougui K., Duriez M., Boulanger C. M., Colucci-Guyon E., Babinet C., Briand P., Friedlander G., Poitevin P., Lévy B. I. (1997) Impaired flow-induced dilation in mesenteric resistance arteries from mice lacking vimentin. J. Clin. Invest. 100, 2909–2914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Li Z., Colucci-Guyon E., Pinçon-Raymond M., Mericskay M., Pournin S., Paulin D., Babinet C. (1996) Cardiovascular lesions and skeletal myopathy in mice lacking desmin. Dev. Biol. 175, 362–366 [DOI] [PubMed] [Google Scholar]
- 80. Milner D. J., Weitzer G., Tran D., Bradley A., Capetanaki Y. (1996) Disruption of muscle architecture and myocardial degeneration in mice lacking desmin. J. Cell Biol. 134, 1255–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Haubold K. W., Allen D. L., Capetanaki Y., Leinwand L. A. (2003) Loss of desmin leads to impaired voluntary wheel running and treadmill exercise performance. J. Appl. Physiol. 95, 1617–1622 [DOI] [PubMed] [Google Scholar]
- 82. Snider N. T., Omary M. B. (2014) Post-translational modifications of intermediate filament proteins: mechanisms and functions. Nat. Rev. Mol. Cell Biol. 15, 163–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hyder C. L., Pallari H. M., Kochin V., Eriksson J. E. (2008) Providing cellular signposts: post-translational modifications of intermediate filaments. FEBS Lett. 582, 2140–2148 [DOI] [PubMed] [Google Scholar]
- 84. Chou Y. H., Rosevear E., Goldman R. D. (1989) Phosphorylation and disassembly of intermediate filaments in mitotic cells. Proc. Natl. Acad. Sci. U.S.A. 86, 1885–1889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Beuttenmüller M., Chen M., Janetzko A., Kühn S., Traub P. (1994) Structural elements of the amino-terminal head domain of vimentin essential for intermediate filament formation in vivo and in vitro. Exp. Cell Res. 213, 128–142 [DOI] [PubMed] [Google Scholar]
- 86. Goto H., Kosako H., Tanabe K., Yanagida M., Sakurai M., Amano M., Kaibuchi K., Inagaki M. (1998) Phosphorylation of vimentin by Rho-associated kinase at a unique amino-terminal site that is specifically phosphorylated during cytokinesis. J. Biol. Chem. 273, 11728–11736 [DOI] [PubMed] [Google Scholar]
- 87. Takai Y., Ogawara M., Tomono Y., Moritoh C., Imajoh-Ohmi S., Tsutsumi O., Taketani Y., Inagaki M. (1996) Mitosis-specific phosphorylation of vimentin by protein kinase C coupled with reorganization of intracellular membranes. J. Cell Biol. 133, 141–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Chou Y. H., Khuon S., Herrmann H., Goldman R. D. (2003) Nestin promotes the phosphorylation-dependent disassembly of vimentin intermediate filaments during mitosis. Mol. Biol. Cell 14, 1468–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Cohen S., Zhai B., Gygi S. P., Goldberg A. L. (2012) Ubiquitylation by Trim32 causes coupled loss of desmin, Z-bands, and thin filaments in muscle atrophy. J. Cell Biol. 198, 575–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Bhattacharya R., Gonzalez A. M., Debiase P. J., Trejo H. E., Goldman R. D., Flitney F. W., Jones J. C. (2009) Recruitment of vimentin to the cell surface by β3 integrin and plectin mediates adhesion strength. J. Cell Sci. 122, 1390–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ketema M., Kreft M., Secades P., Janssen H., Sonnenberg A. (2013) Nesprin-3 connects plectin and vimentin to the nuclear envelope of Sertoli cells but is not required for Sertoli cell function in spermatogenesis. Mol. Biol. Cell 24, 2454–2466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Razafsky D., Hodzic D. (2009) Bringing KASH under the SUN: the many faces of nucleo-cytoskeletal connections. J. Cell Biol. 186, 461–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Sin W. C., Chen X. Q., Leung T., Lim L. (1998) RhoA-binding kinase α translocation is facilitated by the collapse of the vimentin intermediate filament network. Mol. Cell. Biol. 18, 6325–6339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Tzivion G., Luo Z. J., Avruch J. (2000) Calyculin A-induced vimentin phosphorylation sequesters 14-3-3 and displaces other 14-3-3 partners in vivo. J. Biol. Chem. 275, 29772–29778 [DOI] [PubMed] [Google Scholar]
- 95. Li H., Guo Y., Teng J., Ding M., Yu A. C., Chen J. (2006) 14-3-3γ affects dynamics and integrity of glial filaments by binding to phosphorylated GFAP. J. Cell Sci. 119, 4452–4461 [DOI] [PubMed] [Google Scholar]
- 96. Ku N. O., Liao J., Omary M. B. (1998) Phosphorylation of human keratin 18 serine 33 regulates binding to 14-3-3 proteins. EMBO J. 17, 1892–1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Liao J., Omary M. B. (1996) 14-3-3 proteins associate with phosphorylated simple epithelial keratins during cell cycle progression and act as a solubility cofactor. J. Cell Biol. 133, 345–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Chang L., Goldman R. D. (2004) Intermediate filaments mediate cytoskeletal crosstalk. Nat. Rev. Mol. Cell Biol. 5, 601–613 [DOI] [PubMed] [Google Scholar]
- 99. Coulombe P. A., Wong P. (2004) Cytoplasmic intermediate filaments revealed as dynamic and multipurpose scaffolds. Nat. Cell Biol. 6, 699–706 [DOI] [PubMed] [Google Scholar]