Abstract
Aging is the most prominent risk factor contributing to the development of neurodegenerative disorders. In the United States, over 35 million of elderly people suffer from age-related diseases. Aging impairs the self-repair ability of neuronal cells, which undergo progressive deterioration. Once initiated, this process hampers the already limited regenerative power of the central nervous system, making the search for new therapeutic strategies particularly difficult in elderly affected patients. So far, mesenchymal stem cells have proven to be a viable option to ameliorate certain aspects of neurodegeneration, as they possess high proliferative rate and differentiate in vitro into multiple lineages. However, accumulating data have demonstrated that during long-term culture, mesenchymal stem cells undergo spontaneous transformation. Transformed mesenchymal stem cells show typical features of senescence, including the progressive shortening of telomers, which results in cell loss and, as a consequence, hampered regenerative potential. These evidences, in line with those observed in mesenchymal stem cells isolated from old donors, suggest that senescence may represent a limit to mesenchymal stem cells exploitation in therapy, prompting scholars to either find alternative sources of pluripotent cells or to arrest the age-related transformation. In the present review, we summarize findings from recent literature, and critically discuss some of the major hurdles encountered in the search of appropriate sources of mesenchymal stem cells, as well as benefits arising from their use in neurodegenerative diseases. Finally, we provide some insights that may aid in the development of strategies to arrest or, at least, delay the aging of mesenchymal stem cells to improve their therapeutic potential.
Keywords: aging, neurodegenerative disorders, telomere shortening, MSCs, cellular therapy
Introduction
Aging is the most prominent risk factor for the occurrence of neurodegenerative diseases among others, including oxidative stress (Keller et al., 2005; Jain et al., 2011), telomere length (Harris et al., 2006), genetic mutations (Anderton et al., 2002) and head injury (Maiese et al., 2008). In the United States there are over 35 million of people with a mean age of 65 years and even older, that mainly die from age-related diseases (Drago et al., 2011). Aging increases susceptibility of people to environmental stressors, thereby increasing the chance to develop neurodegenerative conditions, most likely because the self-repair ability is compromised and tissues and/or organs undergo a progressive decline (Musumeci et al., 2014a). The aging process is associated with a number of structural, biochemical, functional and neurocognitive changes in the brain. The structural changes include expansion of cerebral ventricles, regional decreases in cerebral volume (Raz et al., 2005), loss of neural circuits and reduced brain plasticity (Burke and Barnes, 2006; Kolb and Gibb, 2011), thinning of the cortex (Shahani et al., 2006), decrease in both of the grey and the white matter volume (Bartzokis, 2011), changes in neuronal morphology (Sowell et al., 2003) and formation of neurofibrillary tangles (Hedden and Gabrieli, 2004; Neill, 2012). Among the age-related biochemical changes there are marked alterations in neurotransmitters and their receptors. Significant decreases in dopamine receptors D1, D2, and D3 (Wang et al., 1998; Kaasinen et al., 2000) and decreasing levels of different serotonin receptors and their transporters such as 5-hydroxytryptamine transporters (5-HTTs) (Chang and Martin, 2009; Chang et al., 2009) have been repeatedly reported. Among the neuropsychological changes, alterations in orientation (Benton et al., 1981) and memory (Hof and Morrison, 2004) are the most common ones. Moreover, many age-related neurodegenerative diseases are characterized by accumulation of disease-specific misfolded proteins in the central nervous system (CNS) (van Ham et al., 2009). These include β-amyloid peptides and tau/phosphorylated tau proteins in Alzheimer's disease (AD), α-synuclein in Parkinson's disease (PD), superoxide dismutase (SOD) in amyotrophic lateral sclerosis (ALS) (Durham et al., 1997), and mutant huntingtin in Huntington's disease (HD) (Scherzinger et al., 1997). The association between age and protein misfolding is not clear yet, but it is probably related to alterations of molecular mechanisms triggered by aging cells, such as telomere shortening, cells shrinkage and decline of quality control over protein synthesis mechanisms (Hung et al., 2010; Thanan et al., 2014) (summarized in Figure 1).
Figure 1.
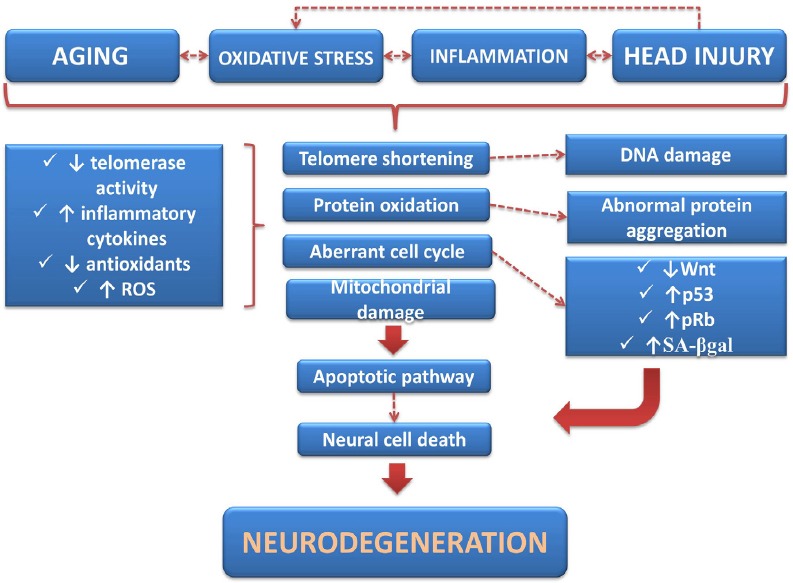
Schematic representation illustrating some of the most common risk factors that contribute to the onset and/or progression of neurodegenerative diseases and the related mechanisms driving the neurodegenerative process.
ROS: Reactive oxygen species; SA-β-gal: se-nescent-associated β-galactosidase.
Telomeres are an evolutionarily conserved repetitive nucleotide sequences (TTAGGG) localized at the end of each chromosome, that are folded into a T loop structure by a protein complex called shelterin (Stewart et al., 2012). Telomeres play four fundamental roles: protecting genetic information from erosion during DNA replication; protecting DNA from damage; serving as a binding site for DNA repair proteins; and providing information about the cell proliferation history (Stewart et al., 2012; Musumeci et al., 2015; Giunta et al., 2015). During each round of DNA replication, telomeres of cultured cells typically lose about 50–200 bp (Allsopp et al., 1992). The telomere length is the sand glass of the cell since it specifies the number of divisions a cell can undergo before it finally dies; thus, it indicates the cell proliferative potential. Telomere shortening leads to the attainment of the so-called ‘Hayflick limit’, which indicates the transition of cells to the state of senescence. Following this step, cells progressively enter a state of crisis, which is accompanied metabolic disturbances that culminate in massive cell death. An enzyme able to maintain telomere length is called telomerase. Telomerase plays a pivotal role in the pathology of aging and cancer by maintaining genome integrity, controlling cell proliferation, and regulating tissue homeostasis. Telomerase is essentially composed of an RNA component, the telomerase RNA or TERC, which serves as a template for telomeric DNA synthesis, and a catalytic subunit, telomerase reverse transcriptase (TERT). The canonical function of TERT is the synthesis of telomeric DNA repeats, and the maintenance of telomere length. However, accumulating evidence indicates that TERT may also exert some fundamental functions that are independent of its enzymatic activity (Verdun and Karlseder, 2007) (please refer to Figure 2). A reduction in telomerase expression contributes to telomere shortening in mitotic cells, while high levels of the enzyme in mesenchymal stem cells (MSCs) contribute to their ‘immortal’ phenotype (Rubtsova et al., 2012). The phenomenon of telomere shortening is closely associated with aging itself, but it has been widely demonstrated that cells can also undergo premature aging due to several factors such as oxidative stress, inflammation and infections, which are able to speed up this process and determine age-related dysfunctions (Hung et al., 2010; Jenny, 2012; Kong et al., 2013; Kota et al., 2015). Therefore, given the involvement of these factors (and in particular of oxidative stress) in the development of neurodegenerative/age-associated diseases, it becomes of primary importance to also gain more insights on the underlying mechanisms triggered by these stressors, as this could serve to improve current therapeutic strategies based on the use of MSCs to treat neurodegenerative conditions.
Figure 2.

Telomerase reverse transcriptase (TERT)'s telomere-dependent and independent functions.
The telomerase is composed of an RNA component, telomerase RNA or telomerase RNA component (TERC), which serves as a template for telomeric DNA synthesis, and a catalytic subunit, TERT. TERT besides its canonical function in telomere elongation has also a role as a transcriptional modula-tor of the Wnt-β-catenin (β-cat) signalling pathway. TERT acts as a cofactor in the β-cat transcription complex; in this complex, TERT interacts with BRG1, a chromatin remodeling factor, to regulate the Wnt/β-cat signalling pathway. TERT is not only acti-vated by the Wnt/β-cat pathway, but β-cat could also be directly regulated by TERT induction, which results in maintenance of telomere length. In the mitochondria, TERT also plays a role in regulating apoptosis in-duced by oxidative damage of mitochondrial DNA (mtDNA). Oxidative stress triggers nu-clear export of TERT to the mitochondria. CEN: Centromere.
A reason why telomeres are the preferred targets of oxidative insult seems to be primarily related to their DNA composition, which tends to be rich in guanine residues (Coluzzi et al., 2014). Indeed, the high incidence of guanine bases promotes the generation of alterations to DNA bases to species called 8-oxoguanine (8-oxoG), which, if not repaired, may lead to single or double strand breaks, mutations or even genomic instability (Grollman et al., 1993). Of interest, genomic instability, oxidative stress and ageing are not to be considered as independent causative factors in telomere shortening, but need to be considered as interconnected phenomena. Consistent with this theory, convergent data has identified an accelerated Wnt/β-catenin cascade activation as a common denominator triggered by these insults. Activation of this pathway reduces MSCs proliferation potential, hampers telomerase activity and drives a cellular shift of MSCs towards a differentiated/senescent phenotype (as elegantly reviewed by Fukada et al., 2014). In the light of these evidences, it is auspicable that strategies aimed at dampening the occurrence of these detrimental events in neurons or to block the Wnt/β-catenin intracellular pathways could have the potential to significantly impact the senescent process, including premature telomere shortening.
Leukocyte Telomere Length (LTL), a Biomarker in Neurodegenerative Disorders
Early neuronal cell death is a feature of neurodegenerative disorders and reduced telomere length has been associated with premature cellular senescence. Studies have shown that reduced telomere length in peripheral blood is associated with the incidence of illnesses associated to the aging phenotypes, such as dementia (Thomas et al., 2008), neurodegenerative disorders such as HD and genetic neurovascular diseases such as ataxia telangiectasia (AT) (Metcalfe et al., 1996; Kota et al., 2015). Since LTL is reflective of global cellular morbidity and mortality, it has been proven that it could be used as a useful tool to screen neurodegenerative disorders (Sahin and DePinho, 2012). It is worth emphasizing, however, that leukocytes include diverse cell populations that play complementary roles in tissue homeostasis and responses to infections and diseases, and the possibility exists that simply monitoring LTL may lead to misleading results. Indeed, the three major classifications of leukocytes are granulocytes, lymphocytes, and monocytes. These populations have different telomere lengths and erosion rates as a result of differences in telomerase activity, proliferation history, and telomere trimming (Stewart et al., 2012). These differences have to be carefully taken into account when considering a possible study of age-related processes in neurodegenerative disorders. However, a recent study has consistently showed that the LTL was reduced in individuals suffering from neurodegenerative disorders as described above, suggesting that the phenomenon of telomere shortening could at least be partly implicated or could contribute to the triggering of pathological pathways activated in these diseases (Kota et al., 2015). In dementia, the reduced telomere length has been attributed to oxidative stress, aberrations in mitochondrial homeostasis, deficient DNA repair mechanisms, and decreased DNA methylation status (von Zglinicki, 2002; Blasco, 2007; Gackowski et al., 2008; Coppedè and Migliore, 2009; van Groen, 2010; Sahin and DePinho, 2012). Nevertheless, the precise mechanism for this attrition needs to be studied further. Interestingly, a review of data from literature concerning the potential use of LTL as a biomarker in AD and PD showed to be inconsistent in both cases, since the number of studies reporting no association between LTL and disease states almost overlapped the ones indicating a correlation between LTL shorthening and neurodegeneration (Eitan et al., 2014). Interestingly enough, a recent study reported an even longer LTL in PD patients, associating short telomeres with reduced risk of PD (Schürks et al., 2014). The reason of these inconsistencies could be dependent on the population number used in these studies, as the low number of patients together with the inter-individual variability may often result in significantly reduced statistical power, and purportedly to unreliable results. It has been shown that variability in LTL in individuals can be induced by different factors such as chronic stress, diet, lifestyle, chronic inflammation state and hormone levels (Liu et al., 2010; Broer et al., 2013). The interaction between these factors and genotype can also play a role in LTL variability (Takata et al., 2012). Certainly, further investigation in this field are needed to clarify the precise role and diagnostic or therapetic potential of LTL in neurodegeneration.
MSCs-based Therapy for Neurodegenerative Disorders
The limited regeneration power of the CNS represents a major challenge for the development of new therapeutic strategies efficacious to promote its functional repair. MSCs have been proposed as a viable therapeutic tool for degenerative disorders as they possess high proliferative ability and they are able to differentiate into multiple lineages (Mobasheri et al., 2014; Musumeci et al., 2014c; Tanna and Sachan, 2014). MSCs can differentiate into neuron-like cells and determine a paracrine effect by modulating the plasticity of damaged host tissues; by secreting neurotrophic and survival-promoting growth factors that inhibit apoptosis and promote neurogenesis, glial scar formation, immunomodulation, angiogenesis and neuronal and glial cell survival; by restoring synaptic transmitter release; by integrating into existing neural and synaptic networks; and by re-establishing functional afferent and efferent connections (Siniscalco et al., 2010; Teixeira et al., 2013; Ooi et al., 2014). In addition, low immunostimulating and high immunosuppressive properties make MSCs a suitable source for cellular therapy (Abumaree et al., 2012; Kwon et al., 2014). Another point in favor to MSCs employment in therapy is that cells can be transplanted directly without any prior genetic modification or reprogramming, and are able to migrate to the tissue injury sites (Amado et al., 2005). MSCs have also been proven to be useful for the treatment of pathologies in which tissue damage is caused by oxidative stress and thus in those pathologies linked to stress-induced telomere shortening and premature aging, where MSCs are likely to be more resistant to oxidative insult than normal somatic cells (Benameur et al., 2015). This feature is particularly important since it makes MSCs an interesting and testable model for the treatment of age-related neurodegenerative disorders.
Currently, there is a great interest towards the use of MSCs in pioneering therapies aimed at treating chronic and progressive neurodegenerative diseases, which are currently incurable and whose attempts to find disease-modifying therapies have failed, such as AD, PD, ALS and HD. It has been shown that after transplantation into the brain, MSCs promote neuronal growth, decrease apoptosis, reduce the levels of free radicals, stimulate the formation of new synaptic networks from damaged neurons by supporting axonal outgrowth, modulate neuroinflammatory activities and promote proteosomal degradation of ubiquitinated misfolded proteins (Caplan and Dennis, 2006; Mezey, 2007; Uccelli et al., 2011). Through paracrine mechanisms, MSCs are also able to interact with neighbouring damaged host cells and influence their microenvironment, by sharing proteins, RNAs and even mitochondria (Spees et al., 2006; Olson et al., 2012). As a proof-of-concept, Mazzini et al. demonstrated that MSCs can decrease motor neuron cell death through paracrine actions when implanted into the CNS of ALS patients (Mazzini et al., 2003; Boucherie et al., 2009). Recently, the paracrine properties of bone marrow-derived MSCs (BM-MSCs) have been also shown in rat model of AD, suggesting their potential therapeutic role in this disease (Salem et al., 2014). The potential efficacy of human MSCs (hMSCs) has been also confirmed recently, as treatment succeeded to ameliorate some behavioral defects observed in a rodent model of HD, hence demonstrating that xenologous transplantation of hMSCs could be considered a potentially successful approach to counteract neurodegeneration caused by HD, and perhaps other CNS disorders (Hosseini et al., 2014).
MSCs can be readily isolated from various tissues, show high plasticity and are capable to differentiate into many functional cell types (Woodbury et al., 2000; Krampera et al., 2007; Singec et al., 2007). Numerous studies have shown that BM-MSCs can differentiate into cells that display neuronal or even dopaminergic characteristics both in vitro and in vivo (Ni et al., 2010; Zeng et al., 2011). A recent study reported that mouse BM-MSCs provided neuroprotection by secreting a key factor, prosaposin, a molecule capable of rescuing mature neurons from apoptotic death. The secretome of BM-MSCs showed to reduce toxin-induced cell death in cultures of rat pheochromocytoma cells, human ReNcell cortical neurons, and rat cortical primary neurons (Li et al., 2010). Unfortunately, the medical procedure to obtain BM-MSCs from the bone marrow is invasive and definitely painful to patients. Therefore, efforts have been made to find more practical alternatives. Indeed, recently other MSCs sources have gained clinical interest for use in regenerative medicine; and adipose tissue represents one of these sources with a broad spectrum of benefits. Human adipose tissue represents a readily available autologous source of MSCs (Ghasemi and Razavi, 2014). Human adipose tissue-derived MSCs (hAT-MSCs) retain morphological, phenotypic and functional characteristics resembling those of BM-MSCs (Zuk et al., 2002), are stable over long term culture, expand efficiently in vitro and possess multi-lineage differentiation potential (Zuk et al., 2001; Musumeci et al., 2011; Choudhery et al., 2013; Musumeci et al., 2014b). Latest observations suggest that transplantation of hAT-MSCs into the brains of elderly mice improved both locomotor activity and cognitive functions. Transplanted cells rapidly differentiated into neurons and in part, into astrocytes, and produced choline acetyltransferase proteins, restoring acetylcholine levels in thebrain. Moreover, transplantation of hAT-MSCs restored neuronal integrity by stimulating the release of neurotrophic factorsby neighbouring cells (Park et al., 2013). In this regard, an aspect to be considered in MSCs therapies is that it is now well-recognized that many pleiotrophic molecules endowed with neuroprotective potential, including some neuropeptides produced locally by resident glial cells or neurons (i.e., pituitary adenylyl cyclase activating polypeptide and/or vasoactive intestinal peptide), when stimulated by neighbouring cells (i.e., implanted MSCs) may prevent cognitive decline caused by aging (Pirger et al., 2014), facilitate nerve recovery after injury both in the CNS (reviewed by Waschek, 2013) and the periphery (Tamas et al., 2012), stimulate remielination processes and glial regenerative support to neurons (Castorina et al., 2014, 2015) and are even capable to prevent retinal damage and mantain retinal barrier properties (Giunta et al., 2012; Scuderi et al., 2013) or impede oxidative insults (Castorina et al., 2012), a broad spectrum of physiopathological events that, at different degrees, are negatively impacted by senescence. Even more interesting, combinatorial administration of these molecules with MSCs has been suggested to support spinal cord recovery after damage (Fang et al., 2010), inferring on the mutual reciprocity between the two, especially desirable to complement the existing gaps determined by the single therapeutic employment of MSCs in aged patients affected by neurodegenerative disorders.
Another source of MSCs that has captured minor scientific interest is represented by dental pulp stem cells (DPSCs). DPSCs have also been recognized as capable to differentiate into a variety of cell lineages (Zhang et al., 2006; Huang et al., 2009), but more studies are required to better define their potential. Other sources of stem cells are that obtained from human-exfoliated deciduous teeth (SHED), which have been shown to contain multipotent stem cells (Miura et al., 2003). The importance of SHED is that they are derived from a tissue similar to the umbilical cord. Notably, both kinds of DSCs can be induced to differentiate into neuron-like cells and be transplanted in brain injury and/or neurodegenerative disease animal models to conduct neuroregeneration studies (Sakai et al., 2012; Tamaki et al., 2012; Yamagata et al., 2013). Based on these findings, it is plausible to believe that the extracted teeth, considered a common waste product from dental extraction procedures, could be employed in the future to exploit in tissue engineering strategies as a promising substitute of BM-MSCs.
Limits of MSCs-based Therapy
A major issue that has significantly limited the use of MSCs-based therapy is the low yielding of viable MSCs from donor tissue. In fact, in order to harvest sufficient MSCs to procure some clinical benefits cells need to replicate several times in vitro. Unfortunately, a number of studies have demonstrated that MSCs from various animals undergo spontaneous transformation when cultured for long terms, posing a limit to this approach. Indeed, transformed MSCs show some of the features of senescent cells, with a progressive shortening of telomers and consequently, cell death (Ahmadbeigi et al., 2011; Ren et al., 2011; He et al., 2014). Such an aging process occurring in MSCs appears to be tissue-specific and has been shown to be regulated by evolutionarily conserved signaling pathways. More recently, a signaling pathway that has shown to be tightly associated to age-related cellular changes is the Wnt/β-catenin signaling cascade (Decarolis et al., 2008; Hiyama et al., 2010; Stevens et al., 2010). Wnt/β-catenin signaling plays a functional role as a key regulator of self-renewal and differentiation properties in MSCs. Jeoung et al. (2014) found that activation of the Wnt/β-catenin pathway delays the progression of cellular senescence as shown by the decrease in senescence effectors p53 and phospho-retinoblastoma (pRb), lowered senescence-associated β-galactosidase (SA-β-gal) activity, and increased telomerase activity. In contrast, suppression of the Wnt pathway promoted senescence in MSCs (Jeoung et al., 2014). Hoffmeyer et al. (2012) also showed that Wnt/β-catenin pathway is connected and regulates TERT expression through the interaction with Kruppel-like factor 4 (Klf4), a core component of the pluripotency transcriptional network (a schematic representation is depicted in Figure 2). Unfortunately, to date, the mechanism through which the Wnt/β-catenin signaling pathway regulates age-related neurogenic differentiation in MSCs still remains unclear and needs further investigations.
Influence of MSCs Donor Age on Cellular Therapy
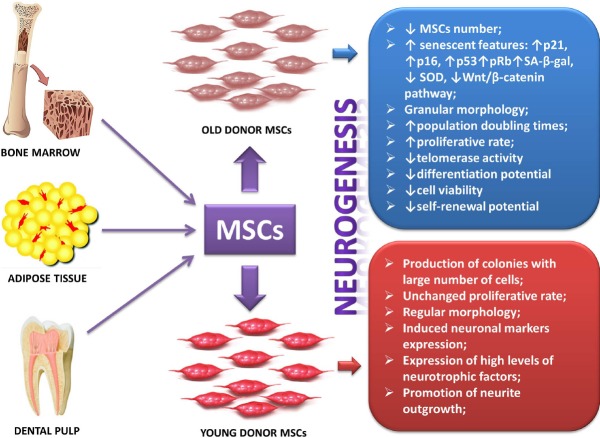
It has been assumed that aging is presumably linked to diminished organ repair capacity due to reduced functionality of MSCs. For this reason, it should be taken into account that the effectiveness of MSCs-based therapies are highly influenced by donor age. There are several studies supporting this concept. It was observed that progressively aging murine BM-MSCs exhibit a decline in MSCs number, proliferation, differentiation, angiogenic and wound healing properties, along with enhanced apoptotic and senescent features (Kretlow et al., 2008; Choudhery et al., 2012a, b). In a study using adipose tissue-derived mesenchymal stem cells (AT-MSCs) from both young and old donors, it was observed that both were able to form colonies, but AT-MSC from younger donors produce more colonies containing larger numbers of cells and increased proliferative rate than those obtained from older donors (Alt et al., 2012). Moreover, AT-MSCs obtained from aged donors displayed increased senescent features, as indicated by the greater expression levels of p16 and p21 genes, which have been indicated as markers of senescence (Stolzing et al., 2008). In the latter study, the expression of SA-β-gal was measured and it was also found at higher levels in aged AT-MSCs cultures, while SOD activity was decreased (Stolzing et al., 2008). It was further identified that MSCs from elderly donors became more granular and developed a more flat and larger morphology at passages 5–6, indicating the appearance of typical morphological signs of replicative senescence (Khan et al., 2009). Recently, in a study conducted on hBM-MSCs from young and old donors used to differentiate and promote neurite outgrowth from dorsal root ganglia neurons (DRGn), Brohlin and coworkers observed that treatment of hBM-MSCs with growth factors induced protein expression of the glial cell marker S100 in cultures from young but not old donors. However, exogenous administration of growth factors enhanced the levels of brain-derived neurotrophic factor (BDNF) and of vascular endothelial growth factor (VEGF) transcripts in both donor cell groups and partly recovered stemness properties of MSCs from elderly, supporting the hypothesis stated above. Finally, in the same study it was demonstrated that MSCs co-cultured with DRGn significantly enhanced total neurite length only when obtained from young but not old donors. Moreover, MSCs from young donors maintained their proliferation rate while those from the old ones showed increased population doubling times (Brohlin et al., 2012). These observations suggest that MSCs isolated from either young or old donors may benefit of a combinatorial approach to retain, at least in part, their regenerating properties on neurons. Nevertheless, to date MSCs from young donors are still to be considered the first choice MSCs source to use for CNS repair (Figure 3).
Figure 3.
Influence of donor age on neurogenic potential of mesenchymal stem cells (MSCs).
Representation of the most relevant differences between MSCs isolated from old and young donors. SA-β-gal: Senescent-associated β-galactosidase; SOD: superoxidedismutase.
Conclusions
The fact that MSCs can be conveniently obtained from different accessible tissues (such as bone marrow, blood, adipose and dental tissue) and demonstrate neuroprotective effects, immunomodulatory properties and self-migratory activity, makes them an attractive therapeutic tool for potential application in neurodegenerative disorders. However, there are some critical points that still need to be clarified before MSC-based therapy can be adopted in clinical practice. These include the reduced stemness properties of MSCs isolated from elderly or caused by long-term expansion in vitro, which could result in reduced efficacy for regenerative cellular therapy. The complex pathways involved in neurodegenerative disorders, should be evaluated with care, in the attempt to extend the current understanding of the pathogenesis of these diseases and identifying targets for intervention. To be suitable for use inneuroregenerative therapy, the mechanisms that govern the self renewal capacity of MSCs should be characterized in depth. For this purpose, it is proposed that scientific effort should focus more on finding the appropriate microenvironment (culture conditions) that more likely will allow to yield sufficient number of functional MSCs. As previously discussed, amolecular mechanism worthy of attention could be represented by the Wnt/β-catenin signaling pathway, whose involvement in triggering the shift of MSCs towards a senescent phenotype appears to be clear. These findings, together with the evidences obtained with combinatorial approaches using neuroprotective agents, support the idea that trophic molecules, including some neuropeptides, may elicit a regulatory function on the Wnt signaling cascade, which in turn, could be the key element in controlling MSCs senescence (Jeoung et al., 2014). A similar effect could be achieved by targeting the Wnt/β-catenin directly with Wnt analogues. Alternatively, another critical mechanism to target could be telomere regulation, but this strategy has already reach general consensus, since studies on the mechanisms controlling telomere status and regulation in these cells have progressively gained importance in the last years. In fact, strategies to prevent telomere loss or to increase telomere length of MSCs may prevent or delay degeneration and hence the onset of symptoms in neurodegenerative disorders, improving the results of MSCs-based therapetic approaches. Finally, a further and reasonable method to expand MSCs validity in therapy could be represented by banking younger adipose tissue for later use. Preservation of MSCs at a younger age, when their biological utility is maximal, could provide a usable source of functional MSCs with full regenerative potential for future applications in regenerative medicine.
Footnotes
Funding: This study was in part supported by the Department of Biomedical Sciences and Biotechnologies, Medical School, University of Catania, Italy (National Grant. PON 01_00110).
Conflicts of Interest: The authors declare that they have no competing interests.
References
- Abumaree M, Al Jumah M, Pace RA, Kalionis B. Immunosuppressive properties of mesenchymal stem cells. Stem Cell Rev. 2012;8:375–392. doi: 10.1007/s12015-011-9312-0. [DOI] [PubMed] [Google Scholar]
- Ahmadbeigi N, Shafiee A, Seyedjafari E, Gheisari Y, Vassei M, Amanpour S, Amini S, Bagherizadeh I, Soleimani M. Early spontaneous immortalization and loss of plasticity of rabbit bone marrow mesenchymal stem cells. Cell Prolif. 2011;44:67–74. doi: 10.1111/j.1365-2184.2010.00731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allsopp RC, Vaziri H, Patterson C, Goldstein S, Younglai EV, Futcher AB, Greider CW, Harley CB. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci U S A. 1992;89:10114–10118. doi: 10.1073/pnas.89.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt EU, Senst C, Murthy SN, Slakey DP, Dupin CL, Chaffin AE, Kadowitz PJ, Izadpanah R. Aging alters tissue resident mesenchymal stem cell properties. Stem Cell Res. 2012;8:215–225. doi: 10.1016/j.scr.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Amado LC, Saliaris AP, Schuleri KH, St John M, Xie JS, Cattaneo S, Durand DJ, Fitton T, Kuang JQ, Stewart G, Lehrke S, Baumgartner WW, Martin BJ, Heldman AW, Hare JM. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci U S A. 2005;102:11474–11479. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderton BH. Ageing of the brain. Mech Ageing Dev. 2002;123:811–817. doi: 10.1016/s0047-6374(01)00426-2. [DOI] [PubMed] [Google Scholar]
- Bartzokis G. Neuroglialpharmacology: white matter pathophysiologies and psychiatric treatments. Front Biosci (Landmark Ed) 2011;16:2695–2733. doi: 10.2741/3881. [DOI] [PubMed] [Google Scholar]
- Benameur L, Charif N, Li Y, Stoltz JF, de Isla N. Toward an understanding of mechanism of aging-induced oxidative stress in human mesenchymal stem cells. Biomed Mater Eng. 2015;25:41–46. doi: 10.3233/BME-141247. [DOI] [PubMed] [Google Scholar]
- Benton AL, Eslinger PJ, Damasio AR. Normative observations on neuropsychological test performances in old age. J Clin Neuropsychol. 1981;3:33–42. doi: 10.1080/01688638108403111. [DOI] [PubMed] [Google Scholar]
- Blasco MA. The epigenetic regulation of mammalian telomeres. Nat Rev Genet. 2007;8:299–309. doi: 10.1038/nrg2047. [DOI] [PubMed] [Google Scholar]
- Boucherie C, Schäfer S, Lavand’homme P, Maloteaux JM, Hermans E. Chimerization of astroglial population in the lumbar spinal cord after mesenchymal stem cell transplantation prolongs survival in a rat model of amyotrophic lateral sclerosis. J Neurosci Res. 2009;87:2034–2046. doi: 10.1002/jnr.22038. [DOI] [PubMed] [Google Scholar]
- Broer L, Codd V, Nyholt DR, Deelen J, Mangino M, Willemsen G, Albrecht E, Amin N, Beekman M, de Geus EJ, Henders A, Nelson CP, Steves CJ, Wright MJ, de Craen AJ, Isaacs A, Matthews M, Moayyeri A, Montgomery GW, Oostra BA, et al. Meta-analysis of telomere length in 19,713 subjects reveals high heritability, stronger maternal inheritance and a paternal age effect. Eur J Hum Genet. 2013;21:1163–1168. doi: 10.1038/ejhg.2012.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brohlin M, Kingham PJ, Novikova LN, Novikov LN, Wiberg M. Aging effect on neurotrophic activity of human mesenchymal stem cells. PLoS One. 2012;7:e45052. doi: 10.1371/journal.pone.0045052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat Rev Neurosci. 2006;7:30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- Castorina A, Giunta S, Scuderi S, D’Agata V. Involvement of PACAP/ADNP signaling in the resistance to cell death in malignant peripheral nerve sheath tumor (MPNST) cells. J Mol Neurosci. 2012;48:674–683. doi: 10.1007/s12031-012-9755-z. [DOI] [PubMed] [Google Scholar]
- Castorina A, Scuderi S, D’Amico AG, Drago F, D’Agata V. PACAP and VIP increase the expression of myelin-related proteins in rat schwannoma cells: involvement of PAC1/VPAC2 receptor-mediated activation of PI3K/Akt signaling pathways. Exp Cell Res. 2014;322:108–121. doi: 10.1016/j.yexcr.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Castorina A, Waschek JA, Marzagalli R, Cardile V, Drago F. PACAP interacts with PAC1 receptors to induce tissue plasminogen activator (tPA) expression and activity in Schwann cell-like cultures. PLos One. 2015;10:e0117799. doi: 10.1371/journal.pone.0117799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Jiang CS, Ernst T. Effects of age and sex on brain glutamate and other metabolites. Magn Reson Imaging. 2009;27:142–145. doi: 10.1016/j.mri.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Q, Martin LJ. Glycinergic innervation of motoneurons is deficient in amyotrophic lateral sclerosis mice: a quantitative confocal analysis. Am J Pathol. 2009;174:574–585. doi: 10.2353/ajpath.2009.080557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhery MS, Khan M, Mahmood R, Mohsin S, Akhtar S, Ali F, Khan SN, Riazuddin S. Mesenchymal stem cells conditioned with glucose depletion augments their ability to repair-infarcted myocardium. J Cell Mol Med. 2012a;16:2518–2529. doi: 10.1111/j.1582-4934.2012.01568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhery MS, Khan M, Mahmood R, Mehmood A, Khan SN, Riazuddin S. Bone marrow derived mesenchymal stem cells from aged mice have reduced wound healing, angiogenesis, proliferation and anti-apoptosis capabilities. Cell Biol Int. 2012b;36:747–753. doi: 10.1042/CBI20110183. [DOI] [PubMed] [Google Scholar]
- Choudhery MS, Badowski M, Muise A, Harris DT. Comparison of human adipose and cord tissue derived mesenchymal stem cells. Cytotherapy. 2013;15:330–343. doi: 10.1016/j.jcyt.2012.11.010. [DOI] [PubMed] [Google Scholar]
- Coluzzi E, Colamartino M, Cozzi R, Leone S, Meneghini C, O’Callaghan N, Sgura A. Oxidative stress induces persistent telomeric DNA damage responsible for nuclear morphology change in mammalian cells. PLoS One. 2014;9:e110963. doi: 10.1371/journal.pone.0110963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppedè F, Migliore L. DNA damage and repair in Alzheimer’s disease. Curr Alzheimer Res. 2009;6:36–47. doi: 10.2174/156720509787313970. [DOI] [PubMed] [Google Scholar]
- DeCarolis NA, Wharton KA, Jr, Eisch AJ. Which way does the Wnt blow? Exploring the duality of canonical Wnt signaling on cellular aging. Bioessays. 2008;30:102–106. doi: 10.1002/bies.20709. [DOI] [PubMed] [Google Scholar]
- Drago V, Babiloni C, Bartrés-Faz D, Caroli A, Bosch B, Hensch T, Didic M, Klafki HW, Pievani M, Jovicich J, Venturi L, Spitzer P, Vecchio F, Schoenknecht P, Wiltfang J, Redolfi A, Forloni G, Blin O, Irving E, Davis C, Hårdemark HG, Frisoni GB. Disease tracking markers for Alzheimer's disease at the prodromal (MCI) stage. J Alzheimers Dis. 2011;26:159–199. doi: 10.3233/JAD-2011-0043. [DOI] [PubMed] [Google Scholar]
- Durham HD, Roy J, Dong L, Figlewicz DA. Aggregation of mutant Cu/Zn superoxide dismutase proteins in a culture model of ALS. J Neuropathol Exp Neurol. 1997;56:523–530. doi: 10.1097/00005072-199705000-00008. [DOI] [PubMed] [Google Scholar]
- Eitan E, Hutchison ER, Mattson MP. Telomere shortening in neurological disorders: an abundance of unanswered questions. Trends Neurosci. 2014;37:256–263. doi: 10.1016/j.tins.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang KM, Chen JK, Hung SC, Chen MC, Wu YT, Wu TJ, Lin HI, Chen CH, Cheng H, Yang CS, Tzeng SF. Effects of combinatorial treatment with pituitary adenylate cyclase activating peptide and human mesenchymal stem cells on spinal cord tissue repair. PLoS One. 2010;5:e15299. doi: 10.1371/journal.pone.0015299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukada S, Ma Y, Uezumi A. Adult stem cell andmesenchymalprogenitortheoriesofaging. Front Cell Dev Biol. 2014;2:1–9. doi: 10.3389/fcell.2014.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gackowski D, Rozalski R, Siomek A, Dziaman T, Nicpon K, Klimarczyk M, Araszkiewicz A, Olinski R. Oxidative stress and oxidative DNA damage is characteristic for mixed Alzheimer disease/vascular dementia. J Neurol Sci. 2008;266:57–62. doi: 10.1016/j.jns.2007.08.041. [DOI] [PubMed] [Google Scholar]
- Ghasemi N, Razavi S. Transdifferentiation potential of adipose-derived stem cells into neural lineage and their application. J Histol Histopathol. 2014;1:12. [Google Scholar]
- Giunta S, Castorina A, Bucolo C, Magro G, Drago F, D’Agata V. Early changes in pituitary adenylate cyclase-activating peptide, vasoactive intestinal peptide and related receptors expression in retina of streptozotocin-induced diabetic rats. Peptides. 2012;37:32–39. doi: 10.1016/j.peptides.2012.06.004. [DOI] [PubMed] [Google Scholar]
- Giunta S, Castorina A, Marzagalli R, Szychlinska MA, Pichler K, Mobasheri A, Musumeci G. Ameliorative effects of PACAP against cartilage degeneration. Morphological, immunohistochemical and biochemical evidence from in vivo and in vitro models of rat osteoarthritis. Int J Mol Sci. 2015;16:5922–5944. doi: 10.3390/ijms16035922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grollman AP, Moriya M. Mutagenesis by 8-oxoguanine: an enemywithin. Trends Genet. 1993;9:246–249. doi: 10.1016/0168-9525(93)90089-z. [DOI] [PubMed] [Google Scholar]
- Harris SE, Deary IJ, MacIntyre A, Lamb KJ, Radhakrishnan K, Starr JM, Whalley LJ, Shiels PG. The association between telomere length, physical health, cognitive ageing, and mortality in non-demented older people. Neurosci Lett. 2006;406:260–264. doi: 10.1016/j.neulet.2006.07.055. [DOI] [PubMed] [Google Scholar]
- He L, Zheng Y, Wan Y, Song J. A shorter telomere is the key factor in preventing cultured human mesenchymal stem cells from senescence escape. Histochem Cell Biol. 2014;142:257–267. doi: 10.1007/s00418-014-1210-5. [DOI] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JD. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci. 2004;5:87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- Hiyama A, Sakai D, Risbud MV, Tanaka M, Arai F, Abe K, Mochida J. Enhancement of intervertebral disc cell senescence by WNT/β-catenin signaling-induced matrix metalloproteinase expression. Arthritis Rheum. 2010;62:3036–3047. doi: 10.1002/art.27599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hof PR, Morrison JH. The aging brain: morphomolecular senescence of cortical circuits. Trends Neurosci. 2004;27:607–613. doi: 10.1016/j.tins.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Hoffmeyer K, Raggioli A, Rudloff S, Anton R, Hierholzer A, Del Valle I, Hein K, Vogt R, Kemler R. Wnt/β-catenin signaling regulates telomerase in stem cells and cancer cells. Science. 2012;336:1549–1554. doi: 10.1126/science.1218370. [DOI] [PubMed] [Google Scholar]
- Hosseini M, Moghadas M, Edalatmanesh MA, Hashemzadeh MR. Xenotransplantation of human adipose derived mesenchymal stem cells in a rodent model of Huntington's disease: motor and non-motor outcomes. Neurol Res. 2015;37:309–319. doi: 10.1179/1743132814Y.0000000456. [DOI] [PubMed] [Google Scholar]
- Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009;88:792–806. doi: 10.1177/0022034509340867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung CW, Chen YC, Hsieh WL, Chiou SH, Kao CL. Ageing and neurodegenerative diseases. Ageing Res Rev. 2010;9:S36–46. doi: 10.1016/j.arr.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Jain A, Mal J, Mehndiratta V, Chander R, Patra SK. Study of oxidative stress in vitiligo. Indian J Clin Biochem. 2011;26:78–81. doi: 10.1007/s12291-010-0045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenny NS. Inflammation in aging: cause, effect, or both? Discov Med. 2012;13:451–460. [PubMed] [Google Scholar]
- Jeoung JY, Nam HY, Kwak J, Jin HJ, Lee HJ, Lee BW, Baek JH, Eom JS, Chang EJ, Shin DM, Choi SJ, Kim SW. A decline in Wnt3a signaling is necessary for mesenchymal stem cells to proceed to replicative senescence. Stem Cells Dev. 2015;24:973–982. doi: 10.1089/scd.2014.0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaasinen V, Vilkman H, Hietala J, Någren K, Helenius H, Olsson H, Farde L, Rinne J. Age-related dopamine D2/D3 receptor loss in extrastriatal regions of the human brain. Neurobiol Aging. 2000;21:683–688. doi: 10.1016/s0197-4580(00)00149-4. [DOI] [PubMed] [Google Scholar]
- Keller JN, Schmitt FA, Scheff SW, Ding Q, Chen Q, Butterfield DA, Markesbery WR. Evidence of increased oxidative damage in subjects with mild cognitive impairment. Neurology. 2005;64:1152–1156. doi: 10.1212/01.WNL.0000156156.13641.BA. [DOI] [PubMed] [Google Scholar]
- Khan WS, Adesida AB, Tew SR, Andrew JG, Hardingham TE. The epitope characterisation and the osteogenic differentiation potential of human fat pad-derived stem cells is maintained with ageing in later life. Injury. 2009;40:150–157. doi: 10.1016/j.injury.2008.05.029. [DOI] [PubMed] [Google Scholar]
- Kolb B, Gibb R. Brain plasticity and behaviour in the developing brain. J Can Acad Child Adolesc Psychiatry. 2011;20:265–276. [PMC free article] [PubMed] [Google Scholar]
- Kong CM, Lee XW, Wang X. Telomere shortening in human diseases. FEBS J. 2013;280:3180–3193. doi: 10.1111/febs.12326. [DOI] [PubMed] [Google Scholar]
- Kota LN, Bharath S, Purushottam M, Moily NS, Sivakumar PT, Varghese M, Pal PK, Jain S. Reduced telomere length in neurodegenerative disorders may suggest shared biology. J Neuropsychiatry Clin Neurosci. 2015;27:e92–96. doi: 10.1176/appi.neuropsych.13100240. [DOI] [PubMed] [Google Scholar]
- Krampera M, Marconi S, Pasini A, Galiè M, Rigotti G, Mosna F, Tinelli M, Lovato L, Anghileri E, Andreini A, Pizzolo G, Sbarbati A, Bonetti B. Induction of neural-like differentiation in human mesenchymal stem cells derived from bone marrow fat, spleen and thymus. Bone. 2007;40:382–390. doi: 10.1016/j.bone.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Kretlow JD, Jin YQ, Liu W, Zhang WJ, Hong TH, Zhou G, Baggett LS, Mikos AG, Cao Y. Donor age and cell passage affects differentiation potential of murine bone marrow-derived stem cells. BMC Cell Biol. 2008;9:60. doi: 10.1186/1471-2121-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon MS, Noh MY, Oh KW, Cho KA, Kang BY, Kim KS, Kim YS, Kim SH. The immunomodulatory effects of human mesenchymal stem cells on peripheral blood mononuclear cells in ALS patients. J Neurochem. 2014 doi: 10.1111/jnc.12814. doi: 10.1111/jnc.12814. [DOI] [PubMed] [Google Scholar]
- Li N, Sarojini H, An J, Wang E. Prosaposin in the secretome of marrow stroma-derived neural progenitor cells protects neural cells from apoptotic death. J Neurochem. 2010;112:1527–1538. doi: 10.1111/j.1471-4159.2009.06565.x. [DOI] [PubMed] [Google Scholar]
- Liu JP, Chen SM, Cong YS, Nicholls C, Zhou SF, Tao ZZ, Li H. Regulation of telomerase activity by apparently opposing elements. Ageing Res Rev. 2010;9:245–256. doi: 10.1016/j.arr.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Maiese K. Triple play: promoting neurovascular longevity with nicotinamide, WNT, and erythropoietin in diabetes mellitus. Biomed Pharmacother. 2008;62:218–232. doi: 10.1016/j.biopha.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzini L, Fagioli F, Boccaletti R, Mareschi K, Oliveri G, Olivieri C, Pastore I, Marasso R, Madon E. Stem cell therapy in amyotrophic lateral sclerosis: a methodological approach in humans. Amyotroph Lateral Scler Other Motor Neuron Disord. 2003;4:158–161. doi: 10.1080/14660820310014653. [DOI] [PubMed] [Google Scholar]
- Metcalfe JA, Parkhill J, Campbell L, Stacey M, Biggs P, Byrd PJ, Taylor AM. Accelerated telomere shortening in ataxia telangiectasia. Nat Genet. 1996;13:350–353. doi: 10.1038/ng0796-350. [DOI] [PubMed] [Google Scholar]
- Mezey E. Bone marrow-derived stem cells in neurological diseases: stones or masons? Regen Med. 2007;2:37–49. doi: 10.2217/17460751.2.1.37. [DOI] [PubMed] [Google Scholar]
- Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100:5807–5812. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobasheri A, Gauthaman K, Musumeci G, Batt ME. Chondrocyte and mesenchymal stem cell-based therapies for cartilage repair in osteoarthritis and related orthopaedic conditions. Maturitas. 2014;78:188–198. doi: 10.1016/j.maturitas.2014.04.017. [DOI] [PubMed] [Google Scholar]
- Musumeci G, Lo Furno D, Loreto C, Giuffrida R, Caggia S, Leonardi R, Cardile V. Mesenchymal stem cells from adipose tissue which have been differentiated into chondrocytes in three-dimensional culture express lubricin. Exp Biol Med. 2011;236:1333–1341. doi: 10.1258/ebm.2011.011183. [DOI] [PubMed] [Google Scholar]
- Musumeci G, Castrogiovanni P, Trovato FM, Imbesi R, Giunta S, Szychlinska MA, Loreto C, Castorina S, Mobasheri A. Physical activity ameliorates cartilage degeneration in a rat model of aging: A study on lubricin expression. Scand J Med Sci Sports. 2014a;25:e222–230. doi: 10.1111/sms.12290. [DOI] [PubMed] [Google Scholar]
- Musumeci G, Mobasheri A, Trovato FM, Szychlinska MA, Graziano ACE, Lo Furno D, Avola R, Mangano S, Giuffrida R, Cardile R. Biosynthesis of collagen I, II, RUNX2 and lubricin at different time points of chondrogenic differentiation in a 3D in vitro model of human mesenchymal stem cells derived from adipose tissue. Acta Histochem. 2014b;116:1407–1417. doi: 10.1016/j.acthis.2014.09.008. [DOI] [PubMed] [Google Scholar]
- Musumeci G, Castrogiovanni P, Leonardi R, Trovato FM, Szychlinska MA, Di Giunta A, Loreto C, Castorina S. Kneeosteoarthritis. New perspectives for articular cartilage repair treatment through tissue engineering. A contemporary review. World J Orthop. 2014c;5:80–88. doi: 10.5312/wjo.v5.i2.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musumeci G, Szychlinska MA, Mobasheri A. Age-related degeneration of articular cartilage in the pathogenesis of osteoarthritis: molecular markers of senescent chondrocytes. Histol Histopathol. 2015;30:1–12. doi: 10.14670/HH-30.1. [DOI] [PubMed] [Google Scholar]
- Neill D. Should Alzheimer's disease be equated with human brain ageing? A maladaptive interaction between brain evolution and senescence. Ageing Res Rev. 2012;11:104–122. doi: 10.1016/j.arr.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Ni WF, Yin LH, Lu J, Xu HZ, Chi YL, Wu JB, Zhang N. In vitro neural differentiation of bone marrow stromal cells induced by cocultured olfactory ensheathing cells. Neurosci Lett. 2010;475:99–103. doi: 10.1016/j.neulet.2010.03.056. [DOI] [PubMed] [Google Scholar]
- Olson SD, Kambal A, Pollock K, Mitchell GM, Stewart H, Kalomoiris S, Cary W, Nacey C, Pepper K, Nolta JA. Examination of mesenchymal stem cell-mediated RNAi transfer to Huntington's disease affected neuronal cells for reduction of huntingtin. Mol Cell Neurosci. 2012;49:271–281. doi: 10.1016/j.mcn.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi YY, Dheen ST, Sam Wah Tay S. Paracrine effects of mesenchymal stem cells-conditioned medium on microglial cytokines expression and nitric oxide production. Neuroimmunomodulation in press. 2014 doi: 10.1159/000365483. [DOI] [PubMed] [Google Scholar]
- Park D, Yang G, Bae DK, Lee SH, Yang YH, Kyung J, Kim D, Choi EK, Choi KC, Kim SU, Kang SK, Ra JC, Kim YB. Human adipose tissue-derived mesenchymal stem cells improve cognitive function and physical activity in ageing mice. J Neurosci Res. 2013;91:660–670. doi: 10.1002/jnr.23182. [DOI] [PubMed] [Google Scholar]
- Pirger Z, Naskar S, László Z, Kemenes G, Reglődi D4, Kemenes I. Reversal of age-related learning deficiency by the vertebrate PACAP and IGF-1 in a novel invertebrate model of aging: the pond snail (Lymnaeastagnalis) J Gerontol A Biol Sci Med Sci. 2014;69:1331–1338. doi: 10.1093/gerona/glu068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Ren Z, Wang J, Zhu W, Guan Y, Zou C, Chen Z, Zhang YA. Spontaneous transformation of adult mesenchymal stem cells from cynomolgus macaques in vitro. Exp Cell Res. 2011;317:2950–2957. doi: 10.1016/j.yexcr.2011.09.008. [DOI] [PubMed] [Google Scholar]
- Rubtsova MP, Vasilkova DP, Malyavko AN, Naraikina YV, Zvereva MI, Dontsova OA. Telomere lengthening and other functions of telomerase. Acta Naturae. 2012;4:44–61. [PMC free article] [PubMed] [Google Scholar]
- Sahin E, DePinho RA. Axis of ageing: telomeres, p53 and mitochondria. Nat Rev Mol Cell Biol. 2012;13:397–404. doi: 10.1038/nrm3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Yamamoto A, Matsubara K, Nakamura S, Naruse M, Yamagata M, Sakamoto K, Tauchi R, Wakao N, Imagama S, Hibi H, Kadomatsu K, Ishiguro N, Ueda M. Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J Clin Invest. 2012;122:80–90. doi: 10.1172/JCI59251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem AM, Ahmed HH, Atta HM, Ghazy MA, Aglan HA. Potential of bone marrow mesenchymal stem cells in management of Alzheimer's disease in female rats. Cell Biol Int. 2014;38:1367–1383. doi: 10.1002/cbin.10331. [DOI] [PubMed] [Google Scholar]
- Scherzinger E, Lurz R, Turmaine M, Mangiarini L, Hollenbach B, Hasenbank R, Bates GP, Davies SW, Lehrach H, Wanker EE. Huntingtin-encoded polyglutamine expansions form amyloid-like protein aggregates in vitro and in vivo. Cell. 1997;90:549–558. doi: 10.1016/s0092-8674(00)80514-0. [DOI] [PubMed] [Google Scholar]
- Schürks M, Buring J, Dushkes R, Gaziano JM, Zee RY, Kurth T. Telomere length and Parkinson's disease in men: a nested case-control study. Eur J Neurol. 2014;21:93–99. doi: 10.1111/ene.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuderi S, D’Amico AG, Castorina A, Imbesi R, Carnazza ML, D’Agata V. Ameliorative effect of PACAP and VIP against increased permeability in a model of outer blood retinal barrier dysfunction. Peptides. 2013;39:119–124. doi: 10.1016/j.peptides.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Shahani N, Subramaniam S, Wolf T, Tackenberg C, Brandt R. Tau aggregation and progressive neuronal degeneration in the absence of changes in spine density and morphology after targeted expression of Alzheimer's disease-relevant tau constructs in organotypic hippocampal slices. J Neurosci. 2006;26:6103–6114. doi: 10.1523/JNEUROSCI.4245-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singec I, Jandial R, Crain A, Nikkhah G, Snyder EY. The leading edge of stem cell therapeutics. Annu Rev Med. 2007;58:313–328. doi: 10.1146/annurev.med.58.070605.115252. [DOI] [PubMed] [Google Scholar]
- Siniscalco D, Giordano C, Galderisi U, Luongo L, Alessio N, Di Bernardo G, de Novellis V, Rossi F, Maione S. Intra-brain microinjection of human mesenchymal stem cells decreases allodynia in neuropathic mice. Cell Mol Life Sci. 2010;67:655–669. doi: 10.1007/s00018-009-0202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Spees JL, Olson SD, Whitney MJ, Prockop DJ. Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci U S A. 2006;103:1283–1288. doi: 10.1073/pnas.0510511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JR, Miranda-Carboni GA, Singer MA, Brugger SM, Lyons KM, Lane TF. Wnt10b deficiency results in age-dependent loss of bone mass and progressive reduction of mesenchymal progenitor cells. J Bone Miner Res. 2010;25:2138–2147. doi: 10.1002/jbmr.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JA, Chaiken MF, Wang F, Price CM. Maintaining the end: roles of telomere proteins in end-protection, telomere replication and length regulation. Mutat Res. 2012;730:12–19. doi: 10.1016/j.mrfmmm.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolzing A, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008;129:163–173. doi: 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Takata Y, Kikukawa M, Hanyu H, Koyama S, Shimizu S, Umahara T, Sakurai H, Iwamoto T, Ohyashiki K, Ohyashiki JH. Association between ApoE phenotypes and telomere erosion in Alzheimer’s disease. J Gerontol A Biol Sci Med Sci. 2012;67:330–335. doi: 10.1093/gerona/glr185. [DOI] [PubMed] [Google Scholar]
- Tamaki Y, Nakahara T, Ishikawa H, Sato S. In vitro analysis of mesenchymal stem cells derived from human teeth and bone marrow. Odontology. 2013;101:121–132. doi: 10.1007/s10266-012-0075-0. [DOI] [PubMed] [Google Scholar]
- Tamas A, Reglodi D, Farkas O, Kovesdi E, Pal J, Povlishock JT, Schwarcz A, Czeiter E, Szanto Z, Doczi T, Buki A, Bukovics P. Effect of PACAP in central and peripheral nerve injuries. Int J Mol Sci. 2012;13:8430–8448. doi: 10.3390/ijms13078430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanna T, Sachan V. Mesenchymal stem cells: potential in treatment of neurodegenerative diseases. Curr Stem Cell Res Ther. 2014;9:513–521. doi: 10.2174/1574888x09666140923101110. [DOI] [PubMed] [Google Scholar]
- Teixeira FG, Carvalho MM, Sousa N, Salgado AJ. Mesenchymal stem cells secretome: a new paradigm for central nervous system regeneration? Cell Mol Life Sci. 2013;70:3871–3882. doi: 10.1007/s00018-013-1290-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanan R, Oikawa S, Hiraku Y, Ohnishi S, Ma N, Pinlaor S, Yongvanit P, Kawanishi S, Murata M. Oxidative stress and its significant roles in neurodegenerative diseases and cancer. Int J Mol Sci. 2014;16:193–217. doi: 10.3390/ijms16010193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, O’ Callaghan NJ, Fenech M. Telomere length in white blood cells, buccal cells and brain tissue and its variation with ageing and Alzheimer's disease. Mech Ageing Dev. 2008;129:183–190. doi: 10.1016/j.mad.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Uccelli A, Laroni A, Freedman MS. Mesenchymal stem cells for the treatment of multiple sclerosis and other neurological diseases. Lancet Neurol. 2011;10:649–656. doi: 10.1016/S1474-4422(11)70121-1. [DOI] [PubMed] [Google Scholar]
- van Groen T. Epigenetics of Aging. New York, Springer: Tollefsbol TO; 2010. DNA methylation and Alzheimer's disease. [Google Scholar]
- van Ham TJ, Breitling R, Swertz MA, Nollen EA. Neurodegenerative diseases: Lessons from genome-wide screens in small model organisms. EMBO Mol Med. 2009;1:360–370. doi: 10.1002/emmm.200900051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdun RE, Karlseder J. Replication and protection of telomeres. Nature. 2007;447:924–931. doi: 10.1038/nature05976. [DOI] [PubMed] [Google Scholar]
- von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27:339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chan GL, Holden JE, Dobko T, Mak E, Schulzer M, Huser JM, Snow BJ, Ruth TJ, Calne DB, Stoessl AJ. Age-dependent decline of dopamine D1 receptors in human brain: a PET study. Synapse. 1998;30:56–61. doi: 10.1002/(SICI)1098-2396(199809)30:1<56::AID-SYN7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Waschek JA. VIP and PACAP: neuropeptide modulators of CNS inflammation, injury, and repair. Br J Pharmacol. 2013;169:512–523. doi: 10.1111/bph.12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61:364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Yamamoto A, Kako E, Kaneko N, Matsubara K, Sakai K, Sawamoto K, Ueda M. Human dental pulp-derived stem cells protect against hypoxic-ischemic brain injury in neonatal mice. Stroke. 2013;44:551–554. doi: 10.1161/STROKEAHA.112.676759. [DOI] [PubMed] [Google Scholar]
- Zeng R, Wang LW, Hu ZB, Guo WT, Wei JS, Lin H, Sun X, Chen LX, Yang LJ. Differentiation of human bone marrow mesenchymal stem cells into neuron-like cells in vitro. Spine. 2011;36:997–1005. doi: 10.1097/BRS.0b013e3181eab764. [DOI] [PubMed] [Google Scholar]
- Zhang W, Walboomers XF, Shi S, Fan M, Jansen JA. Multilineage differentiation potential of stem cells derived from human dental pulp after cryopreservation. Tissue Eng. 2006;12:2813–2823. doi: 10.1089/ten.2006.12.2813. [DOI] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multi-lineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. MolBiol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]