Abstract
BACKGROUND/OBJECTIVES
Diet soda (DS) intake (DSI) has been associated with increased cardiometabolic risk, but its specific impact in older adults has not been addressed. Because central obesity increases cardiovascular risk, we examined the relationship between DSI and long-term waist circumference (WC) change (ΔWC) in the bi-ethnic San Antonio Longitudinal Study of Aging (SALSA).
DESIGN
Prospective cohort study.
SETTING
San Antonio, Texas, neighborhoods
PARTICIPANTS
SALSA examined 749 Mexican-American and European-American individuals ≥ 65 years old at baseline (BL: 1992-1996); 79.1% of survivors completed follow-up 1 (FU1) (2000-2001, n=474); 73.4%, FU2 (2001-2003, n=413); and 71.0%, FU3 (2003-2004, n=375). Participants completed a mean of 2.64 follow-up intervals, for 9.41 total follow-up years.
MEASUREMENTS
DSI, WC, height and weight were measured at outset and conclusion of each interval: BL-FU1, FU1-FU2, and FU2-FU3.
RESULTS
Adjusted for initial WC, demographics, physical activity, diabetes, and smoking, mean interval ΔWC (95% confidence interval) for all DS users was almost triple that among non-users: 2.11 (1.45-2.76) vs. 0.77 (0.29-1.23) cm, respectively (p < 0.001). For non-, occasional, and daily DS users, adjusted interval ΔWCs were 0.77 (0.29-1.23), 1.76 (0.96-2.57), and 3.04 (1.82-4.26) cm, respectively (p=0.002 for trend). This translates to ΔWCs of 0.80, 1.83, and 3.16 inches, respectively, for these groups, over the total SALSA follow-up. In sub-analyses stratified separately by key covariates, ΔWC point estimates were consistently higher among DS users.
CONCLUSION
In a striking dose-response relationship, increasing diet soda intake was associated with escalating abdominal obesity, a potential pathway for heightened cardiometabolic risk in this aging population.
Keywords: diet soda, waist circumference, abdominal obesity, non-nutritive sweeteners, artificial sweeteners
INTRODUCTION
Over the past 30 years, mounting concerns over deleterious health impacts of sugar consumption have led to promotion and increased intake of non-nutritive sweeteners 1. During this time, however, the prevalence of obesity has increased dramatically1, and long-term impacts of non-nutritive sweetener (NNS) and diet soda (DS) intake (DSI) on health outcomes remain unclear. While earlier studies focused on weight change, more recent studies have examined relationships between NNS/DSI and cardiometabolic risk. In her 2013 review, Swithers2 summarized results from these studies, some of which have reported either benefits or no adverse effects from NNS/DSI, while others have shown increased cardiometabolic risk. Elevated incidence of overweight/obesity3, hypertension4, metabolic syndrome5-7, diabetes8;9, kidney dysfunction8;10, heart attack11, and hemorrhagic stroke11;12 have all recently been associated with frequent NNS/DSI.
Although human studies have included diverse age groups, most have focused on middle-aged or younger adults, rather than specifically examining the health impacts of frequent DSI on individuals ≥ 65 years old. This gap is important, since cardiometabolic disease burden – and healthcare costs – are highest in this large and growing population segment. Aging-related shifts in body composition contribute to the increased morbidity and mortality experienced by older individuals: waist circumference (WC) – a measure of both total and abdominal adiposity13 – continues to rise throughout the lifespan, despite decreasing muscle mass and body weight in later years14. Aging-related increases in WC are particularly concerning because they reflect disproportionate increases in visceral fat14, which is associated with increased cardiometabolic risk15;16. Thus, elevated WC, a key component of metabolic syndrome, is associated with increased inflammation17, insulin resistance18, incidence of type 2 diabetes17;19;20, cognitive impairment21, cardiovascular disease (CVD)22;23, and mortality13;24;25.
We have therefore prospectively examined the relationship between initial DSI and long-term WC change (ΔWC) within the bi-ethnic cohort of older Mexican-American and European-American individuals in the San Antonio Longitudinal Study of Aging (SALSA).
METHODS
SALSA participants were recruited from the San Antonio Heart Study (SAHS) cohort, a community-based prospective study of cardiovascular risk factors among Mexican Americans and European Americans, conducted in San Antonio, Texas, between 1979 and 1996. SAHS design, sampling, and examination procedures were previously documented 26. All surviving SAHS participants aged 65+ at the time of the SALSA baseline (BL) examination (1992-1996) were invited to participate in SALSA. As previously documented 27, 749 individuals (70.5% of 1062 eligible SAHS survivors) received SALSA BL examinations; 474 (79.1% of 599 BL survivors) returned to follow-up 1 (FU1: 2000-2001). There was no evidence of major attrition bias between the initial SAHS survey and the SALSA baseline examination. Mean BL-FU1 interval was 7.0 (range: 4.4-9.7) years. Differential BL-FU1 intervals, a deliberate feature of the study design, were obtained by re-examining participants in the reverse order in which they were seen at baseline. At follow-up 2 (FU2: 2001-2003), 413 participants (73.4% of 563 BL survivors) were examined; mean FU1-FU2 interval was 1.5 (range: 1.3-2.2) years. At follow-up 3 (FU3: 2003-2004), 375 participants (71.0% of 528 BL survivors) returned, after a mean FU2-FU3 interval of 1.5 (range: 1.0-2.4) years. Among FU3 participants, mean BL-F3 interval was 9.9 (range: 7.4-12.5) years. Among all SALSA participants who returned to at least 1 follow-up, mean total follow-up was 9.41 (range: 4.5-12.5) years.
All examinations, described previously 27, included measurement of fasting plasma glucose values, height, weight, WC, and intake of beverages, including soft drinks. WC (cm) was measured at the level of the umbilicus; body mass index (BMI) was calculated as weight in kilograms (kg), divided by height in meters (m) squared. Leisure-time energy expenditure in kilocalories per week (kcal/wk) was measured using the Minnesota Leisure Time Physical Activity Questionnaire (MLTQ) 28. Presence of diabetes was assessed by 1998 American Diabetes Association criteria, described previously 27. Due to the length of the baseline examination, dietary questionnaires were performed for a subset of 598 individuals (79.8% of BL participants).
Among all SALSA participants, DSI at the beginning, and anthropometric data at beginning and end, of each follow-up interval were available for 364 BL-FU1, 364 FU1-FU2, and 291 FU2-FU3 participants. Participants with these data for ≥ 1 follow-up interval (n=466) were included in these analyses, and contributed 3314 person-years of follow-up by FU1, and 622 and 543 additional person-years by FU2 and FU3, respectively, for a total of 4479 person-years of follow-up. Available WC and BMI data from earlier SAHS baseline and follow-up examinations for SALSA participants were also plotted, along with SALSA data, to display longitudinal WC and BMI trajectories. Anthropometric measurements in SAHS and SALSA followed the same protocols.
To assess DSI, participants were first asked, “How many bottles or cans of soft drinks do you drink per week?” The number of sodas consumed (per day, week, month, or year) was recorded, along with the appropriate time unit. For participants reporting no soda consumption, DSI was set to zero. Soda consumers were asked whether they usually drank sugar-free sodas, regular sodas, or similar amounts of each. For those who drank only DS, DSI was set equal to total soda intake; for those who drank similar amounts of regular and diet sodas, DSI was computed as total soda intake divided by 2; for those who drank only regular sodas, DSI was set to zero. Mean daily DSI was then calculated for each participant. Participants with mean DSI ≥0.05 sodas/day were categorized as DS “users”; participants consuming 0 to 0.05 diet sodas/day, were categorized as “non-users”. All participants were then categorized into one of 3 DSI groups: non-users, occasional users (> 0 but < 1 soda/day), and daily users (≥ 1 soda/day). DSI ≥ 1/day was the threshold selected for the highest consumption category because it represented chronic, ongoing DS exposure, was a meaningful behavioral cut-point, and allowed comparison of SALSA results with those recently published from other observational studies6;9;11;12. SALSA participants’ DS use was newly assessed each time they were examined, and each participant’s DS use status for each of the three follow-up intervals was re-set to equal his or her DS use status at the beginning of that interval. Thus, a participant’s status as a DS user or non-user could vary across intervals.
The key endpoint – change in WC (ΔWC) between the beginning and end of each follow-up interval between consecutive examinations – was then compared across these three initial-DSI categories.
SALSA follow-up response rates were excellent, and ranged from 71.0 to 79.1% of all survivors. The main reason for non-participation in follow-up examinations was death; major health problems, including severe physical impairments, were the second most frequent impediment to participation; remaining causes included out-of-area moves, and loss to follow-up. To assess potential response-rate biases, we compared follow-up drop-out rates by DSI category. Data were censored at the FU3 exam for participants who completed this phase, and at time of last completed exam, or death, for all others. No significant differences in drop-out rates were detected for daily DS users, or for all DS users, compared with non-users: Cox proportional hazard ratios for drop-out prior to FU3, using non-users as the reference group, were 0.924 (p=0.552) for all DS users, and 1.034 (p=0.868) for daily users. The dropout hazard ratio for participants who did not complete the SALSA baseline dietary interview, relative to those who did, was 0.972 (p=0.846).
All SALSA recruitment and study procedures were performed in accordance with the ethical standards of the Institutional Review Board of the University of Texas Health Science Center at San Antonio, and were approved by this Board. All participants gave written informed consent to participate in each study phase.
Analyses were performed using SAS, version 9.2 (SAS Institute, Inc., Cary, NC). Repeated measures generalized estimating equation analysis of covariance was used to compare mean ΔWC and mean change in BMI (ΔBMI), across the 3 DSI categories and follow-up intervals. This analytic approach accounted for the within-subject correlation across intervals while simultaneously accounting for changes in DS intake which occurred over the entire duration of the SALSA follow-up. All interval-change analyses were adjusted for sex, ethnic group, years of education, and residential neighborhood (lower-income barrio, higher-income suburb, or middle-income transitional neighborhood) at the time of SALSA baseline, as well as the following characteristics at the beginning of each follow-up interval: age, WC (or BMI, for ΔBMI), presence of diabetes, kcal/wk of leisure-time activity, smoking status, and length of follow-up interval. Since these covariates are all known to be associated with changes in adiposity measures, potentially misleading unadjusted results were not generated. After excluding observations missing a value for any covariate, fully adjusted models were based on 1076 observations, representing 3706 person-years of follow-up. P values are reported without Bonferroni correction. To account for the correlation between observations from the same participant across follow-up intervals, PROC MIXED was used. Interaction effects between DS use (any versus none) and sex, ethnicity, BMI category, and diabetes status were also tested individually in stratified analyses.
RESULTS
Table 1 compares baseline characteristics of the 384 FU1 participants whose DSI had been ascertained at baseline. DS users did not differ significantly from non-users with respect to age or sex, but had higher education levels, were more likely to live in the suburbs, less likely to smoke or to live in lower-income barrios, and more likely to be European American. Users also tended to have higher leisure-time energy expenditure (kcal/wk), although this difference was not statistically significant.
Table 1.
Baseline Characteristics for San Antonio Longitudinal Study of Aging (SALSA) Participants who Returned to the First Follow-Up Exam, by Self-Reported Diet Soda Intake Category at Baseline: Means (± SD) and Percentages
| diet sodas consumed per day: |
||||
|---|---|---|---|---|
| Characteristic | none | >0 and < 1 | ≥ 1 | p for difference |
| n | 255 | 89 | 40 | --- |
| female (%) | 59.2 | 65.2 | 50.0 | 0.260 |
| age (years) | 69.6 (± 3.3) | 69.7 (± 3.7) | 69.0 (± 2.9) | 0.479 |
| Mexican-American (%) | 54.9 | 32.6 | 35.0 | < 0.001 |
| education (years) | 11.1 (± 3.8) | 12.8 (± 3.6) | 12.3 (± 3.8) | < 0.001 |
| suburbs residents (%) | 32.9 | 48.3 | 30.0 | 0.024 |
| barrio residents (%) | 27.8 | 13.5 | 15.0 | < 0.010 |
| currently smoking (%) | 14.5 | 3.4 | 12.5 | 0.019 |
| diet sodas/day | 0.00 (± 0.00) | 0.33 (± 0.24) | 1.54 (± 0.66) | < 0.001 |
| regular sodas/day | 0.30 (± 0.58) | 0.04 (± 0.11) | 0.00 (± 0.00) | < 0.001 |
| total sodas/day | 0.30 (± 0.60) | 0.38 (± 0.26) | 1.54 (± 0.66) | < 0.001 |
| body mass index (BMI: kg/m2) |
28.0 (± 5.1) | 29.0 (± 5.3) | 30.0 (± 5.1) | 0.040 |
| waist (cm) | 98.2 (± 13.4) | 101.8 (± 15.2) | 101.4 (± 12.2) | 0.060 |
| energy expenditure (kcal/week) |
1680 (± 2108) | 1846 (± 2551) | 2205 (± 2885) | 0.395 |
| overweight or obese (%) | 71.8 | 80.7 | 87.5 | 0.043 |
| obese (%) | 27.8 | 34.1 | 45.0 | 0.073 |
| fasting plasma glucose (mg/dL) |
101.0 (± 36.9) | 98.0 (± 33.9) | 106.9 (± 42.6) | 0.534 |
| diabetes (%) | 13.5% | 18.2 | 23.7 | 0.205 |
| intervals per subject | 2.59 (± 0.76) | 2.79 (± 0.55) | 2.63 (± 0.70) | 0.085 |
| time per interval (years) | 3.60 (± 2.81) | 3.47 (± 2.76) | 3.52 (± 2.76) | 0.806 |
| total length of follow-up (years) |
9.35 (± 1.70) | 9.67 (± 1.35) | 9.24 (± 1.74) | 0.222 |
Despite this general pattern of greater socioeconomic advantage and health-promotion behavior, DS users also had significantly higher baseline BMIs than non-users, and tended to have larger WC – a difference which approached significance (p=0.060). Baseline prevalence of overweight/obesity (BMI ≥ 25 kg/m2) was significantly higher (p=0.043) among occasional (80.7%) and daily (87.5%) DS users than among non-users (71.8%). Obesity (BMI ≥ 30 kg/m2) and diabetes prevalence were similarly highest among daily users, lowest among non-users, and intermediate among occasional users, but neither trend was statistically significant (p=0.073 and 0.205, respectively). There were no significant differences in fasting glucose concentrations by DSI category.
Use of regular sodas was relatively infrequent, and was inversely related to DS use; regular soda intake was 0.30, 0.04, and 0.00 cans/bottles per day among non-, occasional, and daily DS users, respectively. Although they consumed no regular sodas, daily DS users consumed significantly more total sodas daily (1.54), compared with occasional DS users (0.38), or non-users (0.34).
In the repeated measures analyses that follow, one observation is included for each follow-up interval for which a participant had measures of both DS consumption at the outset of the interval, and the outcome measure of interest at the beginning and end of the interval. Overall, participants included in these analyses completed an average of 2.64 SALSA follow-up intervals, for a total mean follow-up of 9.41 years. As shown in Table 1, these parameters did not differ significantly by DSI category.
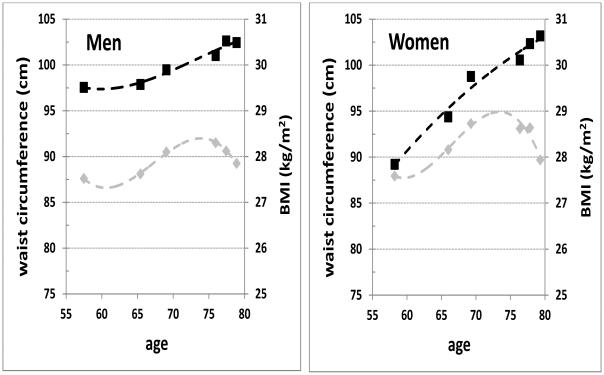
Figure 1 graphically depicts the divergence, with aging, of longitudinal trends in WC and BMI among 375 SALSA participants (146 men and 229 women) who completed their final SALSA follow-up exam (FU3). The first two data points in each panel represent mean anthropometric data (WC and BMI) from participants’ earlier SAHS baseline and follow-up exams; subsequent data points represent means from participants’ SALSA exams (BL through FU3). Among males, after age 65 BMI rose slowly to peak by age 75, then declined rapidly; by contrast, WC increased steadily beyond age 65 to plateau by age 80. Divergence between BMI and WC trajectories was even more striking for women, for whom mean WC at SAHS baseline was considerably lower than that for men, yet increased steadily with time to approximate that of men by SALSA FU3. This divergence is consistent with previous reports of increasing visceral adiposity, with declining muscle mass, in advancing age14.
Figure 1.
Longitudinal change in waist circumference (black squares) and body mass index (BMI: grey diamonds), by sex, from the San Antonio Heart Study baseline exam through the third San Antonio Longitudinal Study of Aging (SALSA) follow-up, for SALSA participants who returned to this last exam. Dashed trend lines represent third-order polynomial fits to the data points.
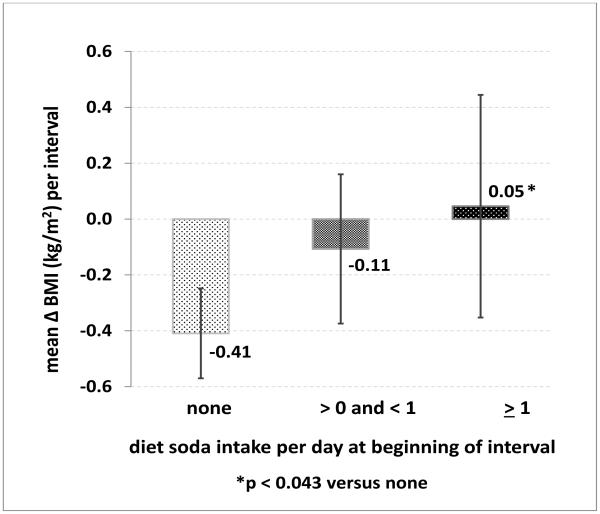
Among all SALSA participants who returned to one or more follow-up exams, adjusted net interval change in BMI (ΔBMI) was minimal (Figure 2), yet varied by DSI category. Point estimates for ΔBMI (95% CI) were lowest for DS non-users [−0.41 (−0.57 to −0.25) kg/m2], intermediate among occasional users [−0.11 (−0.38 to 0.16) kg/m2], and highest for daily users [0.05 (−0.35 to 0.45) kg/m2; p=0.043 for daily vs. non-users; p=0.049 for trend]. Non-users thus experienced minimal BMI loss, while DS users experienced no significant change in BMI.
Figure 2.
Mean change in body mass index (kg/m2 (95% confidence interval)) per follow-up interval, by diet soda consumption category at the beginning of the interval, adjusted for sex, age, ethnicity, education, neighborhood, beginning body mass index, leisure physical activity level, diabetes, smoking status, and length of interval.
By contrast, WC gains (ΔWC) occurred across all DSI categories, but were dramatically higher for DS users than non-users, despite adjustment for initial WC, age, diabetes status, leisure-time physical activity, smoking status, demographic factors, and follow-up length. Adjusted interval ΔWC (95% CI) was 2.11 (1.45-2.76) cm for all DS users combined – both daily and occasional – versus 0.77 (0.29-1.23) cm for non-users (p < 0.001 for difference from users). Mean ΔWC among all users was thus almost 3 times that among non-users.
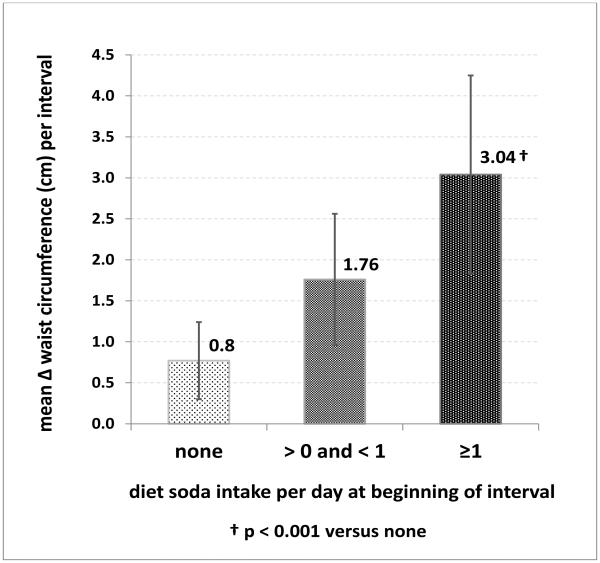
When DSI was further subdivided into occasional or daily use, a striking, positive dose-response relationship (p=0.002 for trend) emerged between DSI and WC gain (Figure 3): mean adjusted ΔWC (95% CI) for non-users, occasional, and daily users were 0.77 (0.29-1.23), 1.76 (0.96-2.57), and 3.04 (1.82-4.26) cm per interval, respectively. Thus, interval ΔWC among daily users was nearly 4 times that among non-users (p=0.001). This would translate into cumulative adjusted ΔWCs of 0.80, 1.83, and 3.16 inches for non-users, occasional, and daily users, respectively, over the total SALSA follow-up.
Figure 3.
Mean change in waist circumference (cm (95% confidence interval)) per follow-up interval, by diet soda consumption category at the beginning of the interval, adjusted for sex, age, ethnicity, education, neighborhood, beginning waist circumference, leisure physical activity level, diabetes, smoking status, and length of interval.
By contrast, no consistent relationship was observed between regular soda use and mean ΔWC (95% CI), which was highest among non-users [1.93 (1.44-2.42) cm], lowest among occasional users [0.37 (−0.31 to 1.05) cm; p=0.001 for difference from non-users], and intermediate for daily users [1.68 (0.36-2.99) cm] of regular soda.
Table 2 compares ΔWC for all DS users versus non-users, stratified separately by sex, ethnic group, BMI category, and diabetes status at the beginning of each follow-up interval. In these comparisons, point estimates for ΔWC were higher for DS users than for non-users within all examined strata. Differences in ΔWC between users and non-users were pronounced and significant for men, European Americans, participants with BMIs ≥30 kg/m2, and participants without diabetes; ΔWC differences approached significance for participants with diabetes (p=0.051).
Table 2.
Mean Adjusted Interval Change in Waist Circumference (cm (95% CI)) by Diet Soda Intake Category, and Number of Person-Years (PY) of Follow-up Represented in Each Subgroup
| stratum | diet soda intake category: |
difference | p for difference |
p for inter- actions |
|
|---|---|---|---|---|---|
| none | any | ||||
| overall | 0.77 (0.29 to 1.23) |
2.11 (1.45 to 2.76) |
1.34 (0.47 to 2.19) |
< 0.001 | --- |
| PY: | 2405 | 1301 | |||
| men | 0.29 (−0.47 to 1.05) |
2.31 (1.30 to 3.32) |
2.02 (0.74 to 3.30) |
0.002 | 0.154 |
| PY: | 955 | 526 | |||
| women | 1.09 (0.47 to 1.71) |
1.92 (1.05 to 2.79) |
0.83 (−0.27 to 1.93) |
0.139 | |
| PY: | 1450 | 774 | |||
| Mexican- American |
0.76 (0.07 to 1.46) |
1.71 (0.67 to 2.75) |
0.95 (−0.35 to 2.24) |
0.150 | 0.439 |
| PY: | 1299 | 517 | |||
| European- American |
0.80 (0.10 to 1.49) |
2.40 (1.55 to 3.25) |
1.60 (0.49 to 2.71) |
0.005 | |
| PY: | 1106 | 784 | |||
| BMI <25 | 1.70 (0.68 to 2.72) |
1.92 (0.10 to 3.74) |
0.22 (−2.00 to 2.44) |
0.833 | |
| PY: | 623 | 205 | |||
| 25 ≤BMI <30 | 1.19 (0.55 to 1.84) |
2.24 (1.38 to 3.10) |
1.05 (−0.08 to 2.17) |
0.067 | <0.001 1 |
| PY: | 1076 | 575 | |||
| BMI ≥30 | −0.53 (−1.68 to 0.62) |
1.53 (0.19 to 2.87) |
2.06 (0.20 to 3.93) |
0.031 | <0.001 2 |
| PY: | 701 | 512 | |||
| diabetic | −0.93 (−2.45 to 0.60) |
1.24 (−0.21 to 2.68) |
2.17 (−0.01 to 4.33) |
0.051 | 0.641 |
| PY: | 345 | 317 | |||
| non-diabetic | 1.15 (0.66 to 1.64) |
2.30 (1.55 to 3.05) |
1.15 (0.20-2.09) |
0.018 | |
| PY: | 1990 | 954 | |||
Among men, mean adjusted ΔWC (95% CI) was dramatically higher in DS users [2.31 (1.30-3.32) cm] than in non-users [0.29 (−0.47 to 1.05) cm] (p=0.002 for difference). Among women, differences in ΔWC were less dramatic and were not statistically significant; nonetheless, among women, point estimates for mean adjusted ΔWC were 75% higher in DS users than in non-users, and – in data not shown – point estimates for ΔWC in non-users, and in occasional and daily DS users increased monotonically in women, from 1.2 (0.54-1.85) cm to 2.1 (0.92-3.24) cm and 2.2 (0.21-4.18) cm, respectively. Thus, although our study was not powered to detect statistically significant differences in ΔWC between DS users and non-users within all participant subgroups, the point estimate for ΔWC in women who were daily DS users was almost double that of non-users. The ΔWC patterns observed in women were therefore congruent with those observed in men, and we were unable to detect a statistically significant difference, by sex (p=0.154), in the association between DS use and ΔWC.
BMI category had a major moderating effect on the association between DSI and ΔWC. Interval differences in ΔWC between DS users and non-users were negligible (0.22 cm) among participants with initial BMIs < 25 kg/m2, intermediate and approaching significance (1.05 cm, p=0.067) among participants with BMIs ≥ 25 and < 30 kg/m2, and significant (2.06 cm, p=0.031) for those with BMIs ≥ 30 kg/m2.
DISCUSSION
Among individuals in a bi-ethnic cohort of Mexican Americans and European Americans aged 65+ years at baseline, we observed a striking, positive dose-response relationship between initial diet soda intake and subsequent long-term increases in waist circumference, over a mean total follow-up of almost a decade. Over the course of this time, mean interval waist gain among all DS users – including both daily and occasional users – was almost 3 times that among non-users. Among daily users, interval ΔWC was almost 4 times that among non-users. These differences were adjusted for demographic and socioeconomic factors, and initial WC, diabetes status, leisure-time physical activity, smoking status, and length of follow-up.
Table 2 displayed the results of sensitivity analyses we performed to compare ΔWCs within ethnic, sex, BMI, and diabetes strata. In each of the 9 subgroup comparisons we performed, point estimates for ΔWC were higher for DS users than for non-users – and were in fact strikingly higher for DS users in all but one stratum: those with BMIs < 25 kg/m2, among whom they were only slightly higher in users. But for overweight users, ΔWC was double that in non-users, and this gap was further doubled among obese individuals, who had already demonstrated heightened vulnerability to weight gain. (A similar phenomenon has been observed in female rats: greater NNS-related weight and adiposity gains occurred among the obesity-prone29.) This is particularly concerning because obese individuals may be highly motivated to use DS to control weight, yet obese users had the worst outcomes in our study.
These results are consistent with findings from other studies, in both humans and animals, in which frequent use of DS and/or non-nutritively sweetened foods or beverages has been associated prospectively with increased body mass index3 and metabolic dysregulation2, and increased incidence of overweight and obesity3, metabolic syndrome5;6, diabetes8;9, and cardiovascular events11;12. Our results suggest one potential pathway – increased abdominal adiposity – through which daily DS consumption might be linked to the increased cardiometabolic risk observed in some of these studies. Waist-gain differentials on the same scale as those we have observed between daily DS users (ΔWC = 3.04 cm) and non-users (ΔWC = 0.77 cm) during a single follow-up interval have, for example, been associated with higher incidence of hyperinsulinemia, metabolic syndrome, elevated blood pressure, and diabetes30;31.
Clinical Relevance for our Aging Population
Adult waist circumferences have increased substantially in the U.S. during the past quarter century32;33. If frequent DS consumption is in fact causally related to the increasing central obesity observed among daily users in our study, the clinical relevance of this association could be substantial. Over the past 20 years, abdominal adiposity has been prospectively associated with increased risk of an array of adverse health outcomes15;16;34;35, including increased incidence of coronary heart disease and cardiovascular events36; albuminuria in women37; depression38; cognitive decline in men39; and increased mortality due to cancer24;40 cardiovascular disease24, and all causes24;40;41. Recommendations for clinical practice have therefore included the measurement of WC, in conjunction with BMI, as part of an individual’s medical evaluation41;42. According to these guidelines, WC measurement can be useful in identifying individuals with excess cardiometabolic risk: both among those with BMIs ≥ 25.0 and < 35 kg/m2, and among normal-weight individuals, for whom elevated WC may offer early warning of hidden cardiometabolic risk42.
We observed dramatically increased ΔWC in daily DS users, despite their stable BMIs. Based on evidence from other studies, this divergence suggests that abdominal fat levels – and visceral fat, specifically – increased with frequent DSI because a) aging-related increases in WC reflect increasing abdominal fat – even in the absence of weight change42; b) elevated WC in individuals of similar BMI levels is associated with increased visceral fat 13; and c) aging-related increases in abdominal fat tend to reflect disproportionately greater increases in visceral fat, compared with subcutaneous fat 14. Thus, for these older DS users, increasing abdominal girth is of particular concern because it is associated with disproportionate increases in visceral fat 14;30, which in turn is associated with increased cardiometabolic risk15;16. Even small increases in abdominal obesity, similar to those observed in daily DS users in SALSA, have been associated with significant increases in cardiometabolic risk factor levels41.
In some studies, abdominal adiposity has outperformed BMI in identifying older individuals at increased cardiometabolic risk15;30. Central adiposity has been associated with elevated glucose concentrations14; dyslipidemia14; elevated C reactive protein43; loss of physical function among individuals with metabolic syndrome44; incidence of depression among men45; and incidence of coronary heart disease46-48 and CVD events48. Among older individuals, and individuals with coronary artery disease, central obesity has also been associated with dramatically increased risk of future CVD events15;30 and mortality15;16;30.
Our results are of particular concern because approximately half of SALSA’s participants are Mexican American, and thus members of the fastest-growing segment of the older U.S. population49. Along with other U.S. ethnic minorities, Mexican Americans have experienced increased levels of abdominal obesity33 and cardiometabolic risk – including increased diabetes incidence and mortality due to cardiovascular disease50. Health-conscious older Mexican American adults might therefore use DS or other non-nutritively sweetened beverages in an attempt to lower their metabolic and cardiovascular risk. If this is the case, our results suggest that such behavior might put them in double jeopardy.
For this reason, dietary counseling for older individuals would ideally include the promotion of unsweetened coffee and tea, mineral water – either unsweetened, or lightly sweetened with 100% fruit juice – or simply water, as alternatives to highly sweetened beverages. Such alternatives would provide increased hydration and intake of natural antioxidants, while decreasing intake of diet beverages, which are intensely sweet and – like their sugar-sweetened counterparts – have been associated with significantly increased incidence of cardiometabolic disease and other health problems2-12.
Strengths and Limitations
The number of SALSA participants included in these analyses is relatively modest (n=466); our results, however, are based upon 3706 person-years of follow-up. SALSA participants were 65+ years old at baseline; the degree to which younger individuals would experience the same results is unknown. Whether DSI exacerbated the WC gains observed in SALSA participants is unclear; our analyses include adjustment for anthropometric measures and other characteristics at the outset of each follow-up interval, but participants’ decisions to use DS may have been driven by other factors – including family history and/or perceived personal weight-gain/health-risk trajectories – which increased ΔWC, yet were not captured in our analyses. Complete dietary intake data are not available for SALSA participants; these results are thus unadjusted for caloric intake. Nonetheless, our findings of increased ΔWC are consistent with reports from other observational studies of increased cardiometabolic risk among daily DS users, even after adjustment for total caloric intake. Each participant’s status as a DS user or non-user was reset at the beginning of each follow-up interval, and thus could change across intervals. Across all intervals, however, approximately 80% of daily DS users at the beginning of the interval remained DS users at the outset of the next follow-up period, and 82% of non-users at the outset of each follow-up period remained non-users at the outset of the subsequent follow-up period. SALSA, a prospective community-based study of older individuals, had several important strengths: multiple follow-up examinations over almost a decade of follow-up; high response rates among survivors within each follow-up interval; representation of individuals from a wide range of socioeconomic environments; and equal representation of European Americans and Mexican Americans, who comprise a major and increasing component of 65+ year-olds in our nation.
Conclusion
We observed a striking, positive dose-response relationship between increasing diet soda intake and escalating abdominal obesity, which represents a potential pathway for future heightened cardiometabolic risk in this vulnerable population. Together with emerging reports from other animal and human studies, these results raise concerns about the safety of chronic diet soda consumption by older individuals, especially those already at increased cardiometabolic risk.
Acknowledgments
Funding sources and related paper presentations:
The SALSA study was supported by National Institute on Aging (NIA) Grant 1-R01-AG10444 (SALSA), NIA Grant 1-R01-AG16518 (Disablement in an Aging, Bi-ethnic Cohort), National Institute of Diabetes and Digestive and Kidney Diseases Grant 1-K25 DK075092, and National Center for Research Resources Grant M01-RR01346 (Fredrick C. Bartter General Clinical Research Center).
These analyses were supported by CTSA grant (UL1RR025767).
Footnotes
The results of earlier, preliminary analyses were presented at the 71st Scientific Sessions of the American Diabetes Association.
REFERENCES
- 1.Yang Q. Gain weight by "going diet?" Artificial sweeteners and the neurobiology of sugar cravings: Neuroscience 2010. Yale J Biol Med. 2010;83:101–108. [PMC free article] [PubMed] [Google Scholar]
- 2.Swithers SE. Artificial sweeteners produce the counterintuitive effect of inducing metabolic derangements. Trends Endocrinol Metab. 2013 doi: 10.1016/j.tem.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fowler SP, Williams K, Resendez RG, et al. Fueling the obesity epidemic? Artificially sweetened beverage use and long-term weight gain. Obesity (Silver Spring) 2008;16:1894–1900. doi: 10.1038/oby.2008.284. [DOI] [PubMed] [Google Scholar]
- 4.Cohen L, Curhan G, Forman J. Association of sweetened beverage intake with incident hypertension. J Gen Intern Med. 2012;27:1127–1134. doi: 10.1007/s11606-012-2069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lutsey PL, Steffen LM, Stevens J. Dietary intake and the development of the metabolic syndrome: the Atherosclerosis Risk in Communities study. Circulation. 2008;117:754–761. doi: 10.1161/CIRCULATIONAHA.107.716159. [DOI] [PubMed] [Google Scholar]
- 6.Dhingra R, Sullivan L, Jacques PF, et al. Soft Drink Consumption and Risk of Developing Cardiometabolic Risk Factors and the Metabolic Syndrome in Middle-Aged Adults in the Community. Circulation. 2007 doi: 10.1161/CIRCULATIONAHA.107.689935. [DOI] [PubMed] [Google Scholar]
- 7.Duffey KJ, Steffen LM, Van HL, et al. Dietary patterns matter: diet beverages and cardiometabolic risks in the longitudinal Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Clin Nutr. 2012;95:909–915. doi: 10.3945/ajcn.111.026682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fagherazzi G, Vilier A, Saes SD, et al. Consumption of artificially and sugar-sweetened beverages and incident type 2 diabetes in the Etude Epidemiologique aupres des femmes de la Mutuelle Generale de l'Education Nationale-European Prospective Investigation into Cancer and Nutrition cohort. Am J Clin Nutr. 2013;97:517–523. doi: 10.3945/ajcn.112.050997. [DOI] [PubMed] [Google Scholar]
- 9.Nettleton JA, Steffen LM, Ni H, et al. Dietary patterns and risk of incident type 2 diabetes in the Multi-Ethnic Study of Atherosclerosis (MESA) Diabetes Care. 2008;31:1777–1782. doi: 10.2337/dc08-0760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin J, Curhan GC. Associations of sugar and artificially sweetened soda with albuminuria and kidney function decline in women. Clin J Am Soc Nephrol. 2011;6:160–166. doi: 10.2215/CJN.03260410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gardener H, Rundek T, Markert M, et al. Diet Soft Drink Consumption is Associated with an Increased Risk of Vascular Events in the Northern Manhattan Study. J Gen Intern Med. 2012 doi: 10.1007/s11606-011-1968-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernstein AM, de KL, Flint AJ, et al. Soda consumption and the risk of stroke in men and women. Am J Clin Nutr. 2012;95:1190–1199. doi: 10.3945/ajcn.111.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Despres JP. Body fat distribution and risk of cardiovascular disease: an update. Circulation. 2012;126:1301–1313. doi: 10.1161/CIRCULATIONAHA.111.067264. [DOI] [PubMed] [Google Scholar]
- 14.Kuk JL, Saunders TJ, Davidson LE, et al. Age-related changes in total and regional fat distribution. Ageing Res Rev. 2009;8:339–348. doi: 10.1016/j.arr.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Chang SH, Beason TS, Hunleth JM, et al. A systematic review of body fat distribution and mortality in older people. Maturitas. 2012;72:175–191. doi: 10.1016/j.maturitas.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oliveros E, Somers VK, Sochor O, et al. The concept of normal weight obesity. Prog Cardiovasc Dis. 2014;56:426–433. doi: 10.1016/j.pcad.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Luft VC, Schmidt MI, Pankow JS, et al. Chronic inflammation role in the obesity-diabetes association: a case-cohort study. Diabetol Metab Syndr. 2013;5:31. doi: 10.1186/1758-5996-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karter AJ, Mayer-Davis EJ, Selby JV, et al. Insulin sensitivity and abdominal obesity in African-American, Hispanic, and non-Hispanic white men and women. The Insulin Resistance and Atherosclerosis Study. Diabetes. 1996;45:1547–1555. doi: 10.2337/diab.45.11.1547. [DOI] [PubMed] [Google Scholar]
- 19.Bray GA, Jablonski KA, Fujimoto WY, et al. Relation of central adiposity and body mass index to the development of diabetes in the Diabetes Prevention Program. Am J Clin Nutr. 2008;87:1212–1218. doi: 10.1093/ajcn/87.5.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biggs ML, Mukamal KJ, Luchsinger JA, et al. Association between adiposity in midlife and older age and risk of diabetes in older adults. JAMA. 2010;303:2504–2512. doi: 10.1001/jama.2010.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kerwin DR, Gaussoin SA, Chlebowski RT, et al. Interaction between body mass index and central adiposity and risk of incident cognitive impairment and dementia: results from the Women's Health Initiative Memory Study. J Am Geriatr Soc. 2011;59:107–112. doi: 10.1111/j.1532-5415.2010.03219.x. [DOI] [PubMed] [Google Scholar]
- 22.Canoy D, Cairns BJ, Balkwill A, et al. Coronary heart disease incidence in women by waist circumference within categories of body mass index. Eur J Prev Cardiol. 2013 doi: 10.1177/2047487313492631. [DOI] [PubMed] [Google Scholar]
- 23.Li TY, Rana JS, Manson JE, et al. Obesity as compared with physical activity in predicting risk of coronary heart disease in women. Circulation. 2006;113:499–506. doi: 10.1161/CIRCULATIONAHA.105.574087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pischon T, Boeing H, Hoffmann K, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008;359:2105–2120. doi: 10.1056/NEJMoa0801891. [DOI] [PubMed] [Google Scholar]
- 25.Teucher B, Rohrmann S, Kaaks R. Obesity: focus on all-cause mortality and cancer. Maturitas. 2010;65:112–116. doi: 10.1016/j.maturitas.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 26.Hazuda HP, Haffner SM, Stern MP, et al. Effects of acculturation and socioeconomic status on obesity and diabetes in Mexican Americans. The San Antonio Heart Study. Am J Epidemiol. 1988;128:1289–1301. doi: 10.1093/oxfordjournals.aje.a115082. [DOI] [PubMed] [Google Scholar]
- 27.Wang CP, Hazuda HP. Better glycemic control is associated with maintenance of lower-extremity function over time in Mexican American and European American older adults with diabetes. Diabetes Care. 2011;34:268–273. doi: 10.2337/dc10-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richardson MT, Leon AS, Jacobs DR, Jr., et al. Comprehensive evaluation of the Minnesota Leisure Time Physical Activity Questionnaire. J Clin Epidemiol. 1994;47:271–281. doi: 10.1016/0895-4356(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 29.Swithers SE, Sample CH, Davidson TL. Adverse effects of high-intensity sweeteners on energy intake and weight control in male and obesity-prone female rats. Behav Neurosci. 2013;127:262–274. doi: 10.1037/a0031717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balkau B, Picard P, Vol S, et al. Consequences of change in waist circumference on cardiometabolic risk factors over 9 years: Data from an Epidemiological Study on the Insulin Resistance Syndrome (DESIR) Diabetes Care. 2007;30:1901–1903. doi: 10.2337/dc06-2542. [DOI] [PubMed] [Google Scholar]
- 31.Gautier A, Roussel R, Ducluzeau PH, et al. Increases in waist circumference and weight as predictors of type 2 diabetes in individuals with impaired fasting glucose: influence of baseline BMI: data from the DESIR study. Diabetes Care. 2010;33:1850–1852. doi: 10.2337/dc10-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li C, Ford ES, McGuire LC, et al. Increasing trends in waist circumference and abdominal obesity among US adults. Obesity (Silver Spring) 2007;15:216–224. doi: 10.1038/oby.2007.505. [DOI] [PubMed] [Google Scholar]
- 33.Ford ES, Li C, Zhao G, et al. Trends in obesity and abdominal obesity among adults in the United States from 1999-2008. Int J Obes (Lond) 2011;35:736–743. doi: 10.1038/ijo.2010.186. [DOI] [PubMed] [Google Scholar]
- 34.Amato MC, Guarnotta V, Giordano C. Body composition assessment for the definition of cardiometabolic risk. J Endocrinol Invest. 2013;36:537–543. doi: 10.3275/8943. [DOI] [PubMed] [Google Scholar]
- 35.Thomas GN, Ho SY, Lam KS, et al. Impact of obesity and body fat distribution on cardiovascular risk factors in Hong Kong Chinese. Obes Res. 2004;12:1805–1813. doi: 10.1038/oby.2004.224. [DOI] [PubMed] [Google Scholar]
- 36.Canoy D. Coronary heart disease and body fat distribution. Curr Atheroscler Rep. 2010;12:125–133. doi: 10.1007/s11883-010-0092-9. [DOI] [PubMed] [Google Scholar]
- 37.Nam GE, Han K, Park YG, et al. Abdominal obesity is associated with albuminuria in women: the 2011 Korea National Health and Nutrition Examination Survey. J Womens Health (Larchmt ) 2014;23:267–274. doi: 10.1089/jwh.2013.4497. [DOI] [PubMed] [Google Scholar]
- 38.Zhao G, Ford ES, Li C, et al. Waist circumference, abdominal obesity, and depression among overweight and obese U.S. adults: National Health and Nutrition Examination Survey 2005-2006. BMC Psychiatry. 2011;11:130. doi: 10.1186/1471-244X-11-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanaya AM, Lindquist K, Harris TB, et al. Total and regional adiposity and cognitive change in older adults: The Health, Aging and Body Composition (ABC) study. Arch Neurol. 2009;66:329–335. doi: 10.1001/archneurol.2008.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang C, Rexrode KM, van Dam RM, et al. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: sixteen years of follow-up in US women. Circulation. 2008;117:1658–1667. doi: 10.1161/CIRCULATIONAHA.107.739714. [DOI] [PubMed] [Google Scholar]
- 41.Cerhan JR, Moore SC, Jacobs EJ, et al. A pooled analysis of waist circumference and mortality in 650,000 adults. Mayo Clin Proc. 2014;89:335–345. doi: 10.1016/j.mayocp.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. National Institutes of Health Obes Res. 1998;6(Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 43.Snijder MB, Visser M, Dekker JM, et al. Low subcutaneous thigh fat is a risk factor for unfavourable glucose and lipid levels, independently of high abdominal fat. The Health ABC Study. Diabetologia. 2005;48:301–308. doi: 10.1007/s00125-004-1637-7. [DOI] [PubMed] [Google Scholar]
- 44.Nakajima K, Yamaoka H, Morita K, et al. Elderly people with low body weight may have subtle low-grade inflammation. Obesity (Silver Spring) 2009;17:803–808. doi: 10.1038/oby.2008.596. [DOI] [PubMed] [Google Scholar]
- 45.Beavers KM, Hsu FC, Houston DK, et al. The role of metabolic syndrome, adiposity, and inflammation in physical performance in the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2013;68:617–623. doi: 10.1093/gerona/gls213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Canoy D, Boekholdt SM, Wareham N, et al. Body fat distribution and risk of coronary heart disease in men and women in the European Prospective Investigation Into Cancer and Nutrition in Norfolk cohort: a population-based prospective study. Circulation. 2007;116:2933–2943. doi: 10.1161/CIRCULATIONAHA.106.673756. [DOI] [PubMed] [Google Scholar]
- 47.Vogelzangs N, Kritchevsky SB, Beekman AT, et al. Obesity and onset of significant depressive symptoms: results from a prospective community-based cohort study of older men and women. J Clin Psychiatry. 2010;71:391–399. doi: 10.4088/JCP.08m04743blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rexrode KM, Carey VJ, Hennekens CH, et al. Abdominal adiposity and coronary heart disease in women. JAMA. 1998;280:1843–1848. doi: 10.1001/jama.280.21.1843. [DOI] [PubMed] [Google Scholar]
- 49.U.S.Administration on Aging . A Statistical Profile of Hispanic Older Americans Aged 65+ U.S. Administration on Aging, U.S. Department of Health and Human Services; 1-16-0010. [Google Scholar]
- 50.Hunt KJ, Resendez RG, Williams K, et al. All-cause and cardiovascular mortality among Mexican-American and non-Hispanic White older participants in the San Antonio Heart Study- evidence against the "Hispanic paradox". Am J Epidemiol. 2003;158:1048–1057. doi: 10.1093/aje/kwg249. [DOI] [PubMed] [Google Scholar]