Abstract
A growing body of research has documented structural and functional brain development during adolescence, yet little is known about neurochemical changes that occur during this important developmental period. Magnetic resonance spectroscopy (MRS) is a well-developed technology that permits the in vivo quantification of multiple brain neurochemicals relevant to neuronal health and functioning. However, MRS technology has been underused in exploring normative developmental changes during adolescence, and the onset of alcohol and drug use and abuse during this developmental period. This review begins with a brief overview of normative cognitive and neurobiological development during adolescence, followed by an introduction to MRS principles. The subsequent sections provide a comprehensive review of the existing MRS studies of development and cognitive functioning in healthy children and adolescents. The final sections of this article address the potential application of MRS in identifying neurochemical predictors and consequences of alcohol use and abuse in adolescence. MRS studies of adolescent populations hold promise for advancing our understanding of neurobiological risk factors for psychopathology by identifying the biochemical signatures associated with healthy brain development, as well as neurobiological and cognitive correlates of alcohol and substance use and abuse.
Keywords: adolescence, brain development, magnetic resonance spectroscopy, executive function, alcohol use
Significant structural and functional brain development occurs during adolescence, supporting the concurrent development of emotional and cognitive regulation (Casey, Jones, & Hare, 2008; Dahl, 2001; Spear, 2000; Steinberg, 2005). However, brain development during adolescence may also potentiate the occurrence of a variety of forms of psychopathology, including alcohol abuse and dependence (Paus, Keshavan, & Giedd, 2008; Spear, 2000). Adolescent neurological changes have been observed via the use of non-invasive structural and functional magnetic resonance imaging techniques (MRI and fMRI, respectively). Such adolescent neuroimaging research has suggested a developmental trajectory towards increasingly refined neural circuitry and signaling efficiency. Less is known, however, about developmental neurochemical changes occurring during this period, or the impact of these changes on cognition, behavior, or the risk for alcohol addiction. Brain neurochemistry can be explored via magnetic resonance spectroscopy (MRS), a technology that permits non-invasive in vivo detection of multiple brain metabolites relevant to neuronal health and functioning, as well as key neurotransmitter systems such as gamma amino-butyric acid (GABA) and glutamate (Glu). To date, there have been a number of MRS studies exploring normative developmental changes in neurochemistry in the neonatal period, infancy and early childhood, but a paucity of studies focusing on adolescence. There have been significantly more studies conducted using MRS to investigate metabolite abnormalities associated with pediatric psychopathology, including, but not limited to pediatric depression (Gabbay, et al., 2012; Olvera, et al., 2010), bipolar disorder (Davanzo, et al., 2003; Sikoglu, et al., 2013), obsessive-compulsive disorder (Whiteside, Abramowitz, & Port, 2012), generalized anxiety disorder (Strawn, et al., 2013) and attention deficit-hyperactivity disorder (Soliva, et al., 2010; Yeo, Hill, Campbell, Vigil, & Brooks, 2000). In contrast, no MRS data are available that provide insight into the effects of alcohol use and abuse on neurochemistry during adolescence, despite the high prevalence of consumption in this age group (SAMHSA, 2004). Accordingly, the purpose of this review is to 1) provide a brief overview of key cognitive, behavioral, and neurological changes during adolescence; 2) outline the basic principles and methods of MRS; 3) review the existing MRS literature on normative neurochemical development and the relationships between neurochemistry and cognitive functioning in normative development and in adults with alcohol abuse disorders; and 4) present potential applications of MRS in understanding the effects of alcohol consumption and alcohol use disorders on neurochemistry in adolescent populations. Developmental characterization of neurochemistry via MRS during adolescence would help fill in important knowledge gaps and could yield mechanistic hypotheses regarding normative trajectories of adolescent neurochemical development, and importantly, non-normative neurochemical trajectories associated with adolescent alcohol abuse disorders.
Adolescent Development
While structural and functional brain development, along with concurrent cognitive changes, are widely evident and documented during adolescence, less is known about changes in brain chemistry during this age period that may contribute to healthy developmental changes in cognition and behavior, as well as adolescents’ elevated risk for multiple forms of psychopathology (Paus, Keshavan, & Giedd, 2008). Structural, functional and neurochemical changes in the adolescent brain do not occur independently, but rather interact to produce the cognitive and behavioral changes observed during adolescence. Thus, the section below provides a very brief overview of adolescent cognitive and neurobiological development, providing necessary context for the subsequent discussion of MRS findings. More extensive discussions of the many cognitive, behavioral and neurological changes that occur during adolescence are available in multiple existing reviews (e.g. Blakemore & Choudhury, 2006; Casey, et al., 2008; Dahl, 2001; Spear, 2000; Steinberg, 2005, 2010).
Cognitive development in adolescence is predominantly characterized by the maturation of executive functions and the consequent consolidation of collaborative cognitive systems, allowing for flexible thinking, complex problem solving and cognitive control (Hooper, Luciana, Conklin, & Yarger, 2004; Huizinga, Dolan, & van der Molen, 2006; Johnstone, Pleffer, Barry, Clarke, & Smith, 2005; Katya Rubia, et al., 2006; Toga, Thompson, & Sowell, 2006). Adolescents also demonstrate higher levels of sensation-seeking than children or adults, often leading to experimentation with alcohol and drugs, as well as other risky behaviors (Spear, 2000). Adolescence also features interacting and changing relationships between emotion and cognitive control (Cohen-Gilbert & Thomas, 2013; Somerville, Jones, & Casey; Van Leijenhorst, et al., 2011), and elevated risk for multiple forms of psychopathology, including alcohol and substance abuse, mood disorders, eating disorders and conduct disorder (Paus, Keshavan, & Giedd, 2008).
These important cognitive and behavioral changes during adolescence are linked to concurrent structural and functional changes occurring in the adolescent brain (Casey, et al., 2008; Toga, et al., 2006). Structural MRI and histological studies have demonstrated a continuous increase in white matter volume during adolescence, thought to reflect ongoing myelination of axons within the brain (Giedd, et al., 1999; Gogtay, et al., 2004; Sowell, Thompson, Holmes, Jernigan, & Toga, 1999; Sowell, Trauner, Gamst, & Jernigan, 2002; Yakovlev & Lecours, 1967). Diffusion tensor imaging (DTI) data also support the existence of ongoing changes in white matter structural integrity during adolescence (Barnea-Goraly, et al., 2005; Bava, et al., 2010; Liston, et al., 2006; Schmithorst, Wilke, Dardzinski, & Holland, 2002). Higher-order association areas and prefrontal cortex show a peak in grey matter volume in early adolescence, followed by a gradual decline that may reflect continued synaptic pruning (Giedd, et al., 1999; Gogtay, et al., 2004). As a number of brain metabolites, detectable using MRS, have physiological relevance to the expression of structural changes, e.g. choline (Cho) reflective of myelination, N-acetyl-aspartate (NAA) reflective of grey matter integrity, and phosphomonoesters (PME) and phosphodiesters (PDE) reflective of membrane synthesis and breakdown (described in detail below, see also Table 1), integration of measures across structural and neurochemical studies in healthy adolescents and adolescents with psychopathology are warranted.
Table 1.
Physiological significance of MRS metabolites
| 1H MRS | Physiological significance |
| N-acetyl-aspartate (NAA) | Marker of neuronal integrity. Reductions often indicate tissue pathology. |
| Choline (Cho) | Involved in pathways of cellular membrane synthesis and degradation. |
| Creatine + phosphocreatine (tCr) | Markers of cellular energetic state. |
| Myo-Inositol (mI) | Involved in phospholipid metabolism and maintenance of osmotic equilibrium. |
| Glutamate (Glu) | Excitatory neurotransmitter and key molecule in cellular metabolism. Also a precursor for GABA synthesis. |
| Glutamine (Gln) | Precursor for glutamate and plays a role in protein synthesis. |
| GABA | Inhibitory neurotransmitter and key molecule in cellular metabolism. |
| “GLX” | Combination of Glu, Gln, GABA resonances. |
| Lactate (LAC) | By-product of anaerobic metabolism. |
| 31P MRS | |
| Phosphocreatine (PCr) | High-energy phosphate, contributes to the maintenance of β-NTP levels. |
| Nucleotriphosphates: α-, γ-, β-NTP | Level of energy available as ATP in brain (β-NTP). |
| Phosphomonoesters (PME) | Building blocks of membrane phospholipids. |
| Phosphodiesters (PDE) | Major catabolic products of membrane phospholipid degradation. |
| Inorganic Phosphate (Pi) | High-energy phosphate that combines with creatine to form PCr, but also is released from phosphocreatine to synthesize nucleoside triphosphates (ADP to ATP). Chemical shift of inorganic phosphate can be used to calculate intracellular pH. PCr/Pi ratio provides a measure of the energy status in brain, as it is a ratio of the most labile form of high-energy phosphate (PCr) to the ultimate breakdown product of all high-energy phosphate compounds (Pi). |
Functional neuroimaging studies suggest that the brain regions recruited for executive tasks also change significantly during the second decade of life. Studies using fMRI have documented a shift from diffuse to more focal activity and/or greater reliance on the prefrontal cortex (PFC) between late childhood and early adulthood during performance of tasks requiring executive functions (Casey, et al., 1997; Crone, Wendelken, Donahue, van Leijenhorst, & Bunge, 2006; Durston, et al., 2006; Scherf, Sweeney, & Luna, 2006). This shift is thought to reflect improved efficiency in the recruitment of necessary regulatory circuitry, along with reductions in the overall effort necessary to perform executive tasks (Durston, et al., 2006; Luna, Padmanabhan, & O'Hearn, 2010). In addition to changes observed in prefrontal function, developmental changes occur during adolescence in wide-spread circuits including parietal, temporal, cerebellar and thalamic regions. Development of these cortical-subcortical circuits also supports age related improvements in inhibitory control (Rubia, Smith, Taylor, & Brammer, 2007). Functional neuroimaging studies also show evidence that brain circuits involved in reward processing and emotion regulation are differentially activated in adolescence versus in childhood or adulthood (Ernst, et al., 2005; Galvan, et al., 2006; Hare, et al., 2008). It has been suggested that there is a developmental shift towards reliance on more frontal regions during reward processing and decision-making during adolescence (Casey, et al., 2008), supporting improvements in self-regulation and future-oriented decisions. As with structural studies, brain metabolites that can be acquired using MRS can be linked to processes associated with changes in functional brain activation. For example, phosphocreatine (PCr) reflects available energy resources, and beta nucleoside triphosphate (β-NTP) reflects the brain’s available ATP. Neurochemicals measurable via MRS also reflect excitatory and inhibitory neurotransmission: Glu is the brain’s primary excitatory neurotransmitter while GABA reflects inhibitory neurotransmission, both of which are also involved in glucose metabolism. Also potentially impacting functional brain activation, lactate is indicative of anaerobic metabolism when energy substrates are taxed or unavailable due to neurological abnormalities (described in detail below, see also Table 1).
Thus, to extend structural and functional developmental findings, MRS can be employed to assess neuronal health and integrity, bioenergetics, neurotransmission, and cell membrane synthesis and degradation. Use of this technique to identify neurobiological processes operating on a cellular level will extend our understanding of adolescent developmental changes in brain structure and function, as well as cognition, behavior, and risk for psychopathology.
Magnetic Resonance Spectroscopy
Magnetic resonance spectroscopy permits in vivo detection of multiple brain metabolites and some neurotransmitters. MRS data can be acquired using most standard scanners and often spectra can be acquired within the same imaging session as other structural and functional scans. In all forms of MRI, when a sample is placed into a large static magnetic field (B0), each nucleus precesses (or spins) about its axis, with some nuclei aligned with, and some nuclei aligned against the direction of B0. The frequency of this nuclear precessional rotation is unique to the resonant (Larmor) frequency of a specific nucleus at a specific magnetic field strength. When a pulse from a radiofrequency (RF) coil is transmitted at the Larmor frequency for a particular nucleus (e.g. hydrogen), a transient magnetic field (B1) is generated, causing the targeted nuclei to transition to a higher energy state by absorbing the energy from the RF pulse. When the RF pulse is turned off, the nuclei within the sample release their energy in the form of a radiofrequency signal that contains spectral information encoded within it. In MRS, this information-rich signal is detected by the RF coil (acting as a receiver) and subsequently plotted as energy released (signal intensity) as a function of time, and then converted to a series of spectral peaks, whereby signal intensity can be visualized in the frequency domain (Hz), which is independent of magnetic field strength.
Protons (1H) (Figure 1) and phosphorus (31P) (Figure 2) are the two nuclei most commonly studied via MRS, although detection of resonance intensities is also possible for carbon (13C), sodium (23Na), sulfur (33S), fluorine (19F), and lithium (7Li). The use of MRS is limited to these and several other nuclei, based on their physical properties, i.e., possessing a resonance frequency that responds, or aligns, within a magnetic field, and the release of energy after perturbation following application of an RF pulse, all of which make these nuclei MR visible. For a given nucleus (e.g. 1H), chemically distinct groups within a molecule that contain the given nucleus possess minor differences in their local resonant frequencies due to the inhomogeneous and unique distribution of electrons within the molecule (Bovey, 1988). These small differences in resonant frequency, or chemical shift, make it possible to differentiate molecules based on their distinct spectral signatures (e.g., Figure 1 - 1H spectrum, Figure 2 - 31P spectrum). The area of the resonance intensity, or the area under the peak, is proportional to the concentration of molecules that contribute to the resonance, thus allowing for the estimation of the concentration of a particular molecule within the sample.
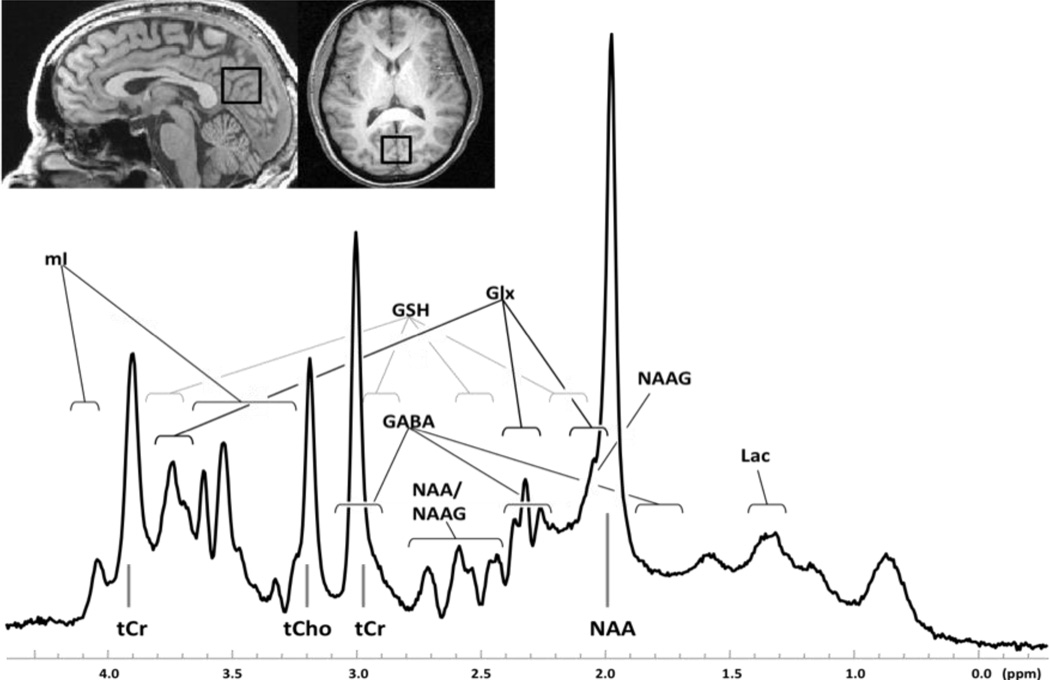
Figure 1. Sample 1H MR spectrum.
Sagittal (left) and axial (right) anatomical images illustrating the placement of a single voxel (2 × 2 × 3cm) in the anterior cingulate cortex of a healthy subject, and the associated 1H spectrum (below). Abbreviations: tCr, total creatine; Gln/Glu, glutamine/glutamate; mI, myo-inositol; tCho, total choline; GABA, gamma amino butyric acid; Glu, glutamate; NAA, N-acetylaspartate.
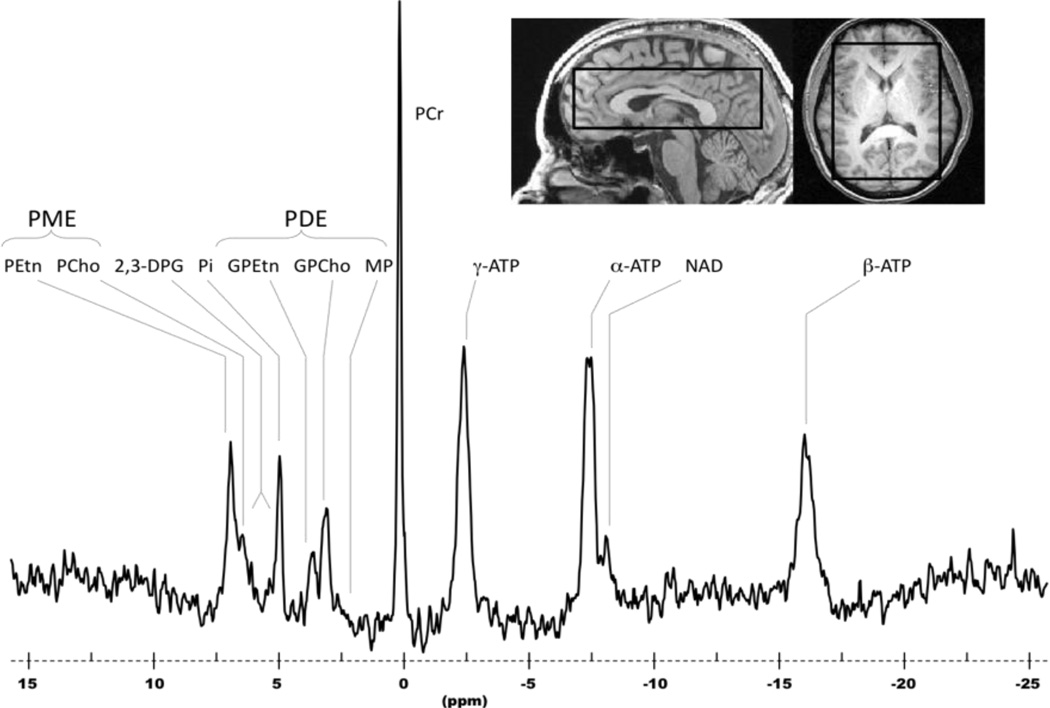
Figure 2. Sample 31P MR spectrum.
Sagittal (left) and axial (right) anatomical images illustrating extracted spectral data from a 3D CSI voxel grid (2.1 × 2.1 × 2.1cm) from a region placed in the anterior cingulate cortex of a healthy subject, and the associated 31P spectrum (below). Abbreviations: PME, phosphomonoesters; PE, phosphoethanolamine; PC, phosphocholine; Pi, inorganic phosphate; PDE, phosphodiesters; GPE, glycerophosphoethanolamine; GPC, glycerophosphocholine; PCr, phosphocreatine; NTP, nucleoside triphosphate.
Nuclei that are capable of producing a resonance signal, and are therefore considered MR visible, differ in detection sensitivity based on their electromagnetic properties and concentrations, which must be high enough (millimolar range) to be detected. Sensitivity, or intensity of the MR signal, can be amplified by increasing the size of the voxel (or volume) sampled and/or by increasing the strength of the magnetic field. While larger voxels have more limited anatomical specificity, the use of stronger magnetic fields is limited by the availability of high field scanners. While a large number of MRS publications have included spectral data acquired at low field (1.5T), more recently, MRS data have been reported at increasingly higher field strengths (3T, 4T, 7T and above) (Mangia, et al., 2006; Mekle, et al., 2009; Tkac, et al., 2001; Tkac, Oz, Adriany, Ugurbil, & Gruetter, 2009). To date, however, published MRS studies in healthy children and adolescents have only been based on data acquired at 4T (one study) or lower field strengths, the majority of which have been acquired at 1.5T. Increases in field strength over the past decade have been particularly valuable for technological advancement of MRS methods, as sensitivity increases linearly with field strength, due in part to greater spectral dispersion, or separation of metabolite peaks, which allows for improved quantification. Although the current trend is to acquire spectral data at relatively short echo times (e.g., 2–20msec) in order to minimize signal loss, in some instances a metabolite-specific echo time is necessary to optimize metabolite detection (e.g., TE=68msec for GABA).
In addition to identifying the nucleus of interest, hypotheses regarding anatomical structures of interest should also be considered when choosing the sequence for spectral acquisition. Spectra are acquired from either single anatomical volumes (single voxel) or from multiple volumes in two- or three-dimensions within the brain, each of which has their own strengths. Multi-dimensional techniques such as chemical shift imaging (CSI) permit the collection of a matrix of spatially-resolved spectral data from a large region of tissue selected using high resolution anatomical MRI. CSI permits the extraction of data from multiple voxels during acquisition, and during post-processing allows for regional examination of metabolite levels (Brown, Kincaid, & Ugurbil, 1982; Wiedermann, et al., 2001). Thus CSI is well suited for exploratory studies, such as those characterizing major metabolites (NAA, Cho, creatine (Cr)) across multiple regions during brain development. Single voxel MRS, on the other hand, permits focus on a small number of discreet brain regions with higher spatial and spectral resolution, which is necessary for regions with increased susceptibility to field inhomogeneties, such as the frontal lobe or the hippocampus. Single voxel acquisition is also necessary when employing specialized acquisition schemes that are optimized for detecting complex metabolites, such as GABA or Glu.
The choice of MRS quantification strategy depends on the nucleus of interest to be examined. The most commonly used commercially available software program employed for the quantification of in vivo proton MR spectra is Linear Combination of Model Spectra (LCModel) (Provencher, 1993, 2001). LCModel fits spectra by comparing in vivo raw spectral data either with in vitro metabolite data collected under identical conditions or with simulated basis sets. Phosphorous spectra are generally fitted in a similar fashion, typically using in-house software that fits spectra in the frequency or time domain using linear and interactive algorithms, similar to those used in LCModel.
Some MRS studies report absolute values while others report ratios of one metabolite to another. In practice, calculating the absolute values of metabolite concentrations requires knowledge of the T1 and T2 relaxation times of the molecule of interest, the repetition time (TR) and echo time (TE) acquisition parameters, the tissue volume of interest, and the efficiency of signal detection. Calculation of absolute concentrations in LCModel uses a theoretical fitting routine (basis set) that incorporates the spectroscopic imaging parameters (TR, TE) and nuclei-specific relaxation times. However, other factors specific to the volume of interest (partial volume effects or tissue content) and to the MR scanner are not incorporated in these calculations, which make metabolite concentrations very liberal, and perhaps unreliable, estimates of true metabolite levels. Given these difficulties, it is common practice to report relative values of MRS data as metabolite ratios. There remains, however, a significant debate regarding the optimal reference standard for determining metabolite ratios. Metabolite ratios can be calculated using a number of strategies. For example, metabolite peaks can be quantified relative to an external standard, typically a small capsule containing known amounts of metabolites placed near the subject’s head. This method increases scan time, as spectra collected from the in vitro, external standard must be acquired within the same scanning session with the patient in the scanner. Alternatively, reference data can also be acquired from a phantom following removal of the subject from the scanner. However, this method is subject to variations due to differences in coil loading between the subject’s head position and that of the phantom. More recently, the use of an Electric Reference To access In vivo Concentrations (ERETIC) method has been applied for normalizing metabolite levels (Chen, Pavan, Heinzer-Schweizer, Boesiger, & Henning; Heinzer-Schweizer, et al.). This method introduces an additional synthetic reference signal during the spectral acquisition, and is not only sensitive to patient coil loading, but can be quantified independent from in vivo metabolites.
In terms of normalizing to internal standards, the unsuppressed peak arising from water is routinely used to calculate metabolite ratios, but is complicated by the need to discriminate between water in tissue versus water in CSF, which can differ by up to 30–40%. Another common strategy is to use a relatively stable metabolite peak, such as Cr. In proton MRS, the Cr peak represents the concentration of Cr plus PCr, which is typically maintained at constant levels in healthy tissue. However, like water, Cr levels vary between brain regions and tissue types and may also differ between subject populations, complicating interpretations of metabolite ratios when Cr serves as the denominator. Furthermore, because the chemical composition of grey and white matter differ, it is important to assess the tissue content of the sampled brain regions. High resolution anatomical MR images used to define voxels for spectral acquisition allow for tissue segmentation and subsequent calculation of relative metabolite levels in grey versus white matter (Pouwels & Frahm, 1998). Nonetheless, choosing a quantification strategy remains a significant challenge. It is likely that this technological issue will continue to be a major focus of research for the next several years.
While studies utilizing MRS yield an abundance of information about both the structure and chemical composition of tissues, MRS technology is limited by a number of factors. Optimal signal to noise ratio and a homogenous magnetic field are necessary to obtain narrow resonance peaks for quantification, which may require 10 to 45 minutes of scanning, free of motion artifact. As with other MR techniques, subject or patient comfort is critical for minimizing subject motion, which degrades spectral quality. Perhaps the most significant limitation of MRS is low sensitivity. The signal strength of a particular nucleus depends upon its inherent signal intensity and the externally applied magnetic field strength. Sensitivity limitations can be minimized by acquiring spectra at higher field strengths and by applying optimized strategies for spatial localization.
MRS during Healthy Brain Development & Relationships with Cognition
Few studies have been published regarding age-related changes in neurochemical metabolites focused on the first two decades of life. As reviewed in subsequent sections, these studies have used cross-sectional research designs and focus on childhood or the full life span, with little data available regarding neurochemical changes specifically occurring during adolescence. While some data are available regarding the relationships between MRS-visible metabolite concentrations and cognitive functioning in childhood and adolescence, the majority of such studies have been conducted in adults. Regardless, these studies have great value and offer promising new directions for future research in adolescent populations, as brain metabolite levels are predictive of cognitive performance, but also of impairments in cognitive functioning that are associated with psychopathological conditions, such as alcohol use disorders. Accordingly, results from selected studies examining metabolite-cognition relationships in adults are included here in order to elucidate the potential cognitive relevance of developmental changes in MR-visible metabolites.
Development and Cognition: 1H MRS Findings
In vivo 1H MRS permits the detection and quantification of important amino acids that contain proton nuclei, including NAA, cytosolic choline compounds (Cho), Cr, myo-inositol (mI), and lactate (Figure 1). Albeit near the lower limit of detection, Glu, glutamine (Gln), and GABA can also be quantified in the proton spectrum (Behar & Rothman, 2001; Jensen, Frederick, & Renshaw, 2005; Licata, et al., 2009; Petroff, Mattson, & Rothman, 2000; Shulman, Rothman, Behar, & Hyder, 2004). Physiological relevance of proton and phosphorous brain metabolites are provided in Table 1 and developmental 1H MRS findings are summarized in Table 2 and Figure 3.
Table 2.
1H MR Spectroscopic findings in healthy children and adolescents
| Investigators | Sample | MRS | Region(s) of interest | Metabolites/findings |
|---|---|---|---|---|
| van der Knaap et al. (1990) | 1 mo – 16 yrs. mean = 5.9 yrs. (n = 41) no sedation |
SV, 1.5 T 7 × 3 × 3 cm |
paraventricular region, predominantly WM |
|
| Kreis et al. (1993) | 35 wks – 17.8 yrs. (n = 109 scans) healthy (18%) recovered infants (21%) cerebral pathology (61%) chloral hydrate |
SV, 1.5 T 3–8 cm3 young 8–16 cm3 older |
occipital cortex (GM) parieto-occipital (WM) |
|
| Toft et al. (1994) | infants 259 – 295 days mean = 277 days (n = 8) adolescents 10 – 15 yrs. mean = 12.3 yrs. (n = 8) no sedation |
SV, 1.5 T 2 cm3 |
infants: 1 voxel including caudate, putamen, globus pallidus adolescents: 4 voxels occipetal, basal ganglia, temporal, frontal. All voxels included GM and WM. |
|
| Pouwels et al. (1999) | 0 – 18 yrs. (n = 97) healthy (8%) neuropediatric (92%) 18 – 39 yrs. (n = 72) <6 yrs. chloral hydrate |
SV, 2.0 T 8–18 mL 8–18 mL 4–5 mL 4–6 mL 4–6 mL |
parietal GM parieto-occipital WM cerebellum, vermis thalamus basal ganglia |
|
| Choi et al. (2000) | 3 – 14 yrs. mean = 9 yrs. (n = 30) young chloral hydrate |
SV, 1.5 T 1.8 × 2 × 2 cm |
allocortex (hippocampus, parahippocampal) isocortex (medial, frontal and parietal) |
|
| Kadota et al. (2001) | 4 – 88 yrs. mean=45.6 yrs. (n = 90) no sedation |
CSI, 1.5 T 1.125 cm3 |
superior to corpus callosum, 12 voxels: 6 WM, 6 mesial GM, placed bilaterally in anterior, middle and, posterior regions |
|
| Horska et al. (2002) | 3 – 19 yrs. mean = 12.3 yrs. (n = 15) n = 2 nembutal |
CSI, 1.5 T .8cm3 |
frontal, parietal (WM) basal ganglia (GM) thalamus (GM) |
|
| Costa et al. (2002) | 3 – 18 yrs. (n = 37) no sedation |
SV, 1.5T 8cm3 |
parieto-occipital WM cerebellar hemisphere |
|
| Goldstein et al. (2009) | 6 – 18 yrs. (n = 105) no sedation |
CSI, 1.5 T 1.5 × 1.5 × 2 cm |
prefrontal cortex basal ganglia superior temporal cortex inferior parietal cortex centrum semiovale occipetal regions |
|
| Raininko & Mattsson (2010) | 13 – 72 yrs. (n=57) no sedation |
SV, 1.5T 4 × 1.2 × 1.8 cm |
supraventricular WM |
|
| Silveri et al. (2013) | 12–14 yrs, (n = 30) 18–24 yrs. (n = 20) no sedation |
SV, 4T 2 × 2 × 3 cm |
anterior cingulate cortex parieto-occipetal cortex |
|
Abbreviations: SV, single voxel; CSI, chemical shift imaging; WM, white matter; GM, gray matter.
Note: Subjects in all studies were healthy, normally developing individuals except where noted otherwise. Findings reported in this table reflect healthy subjects only.
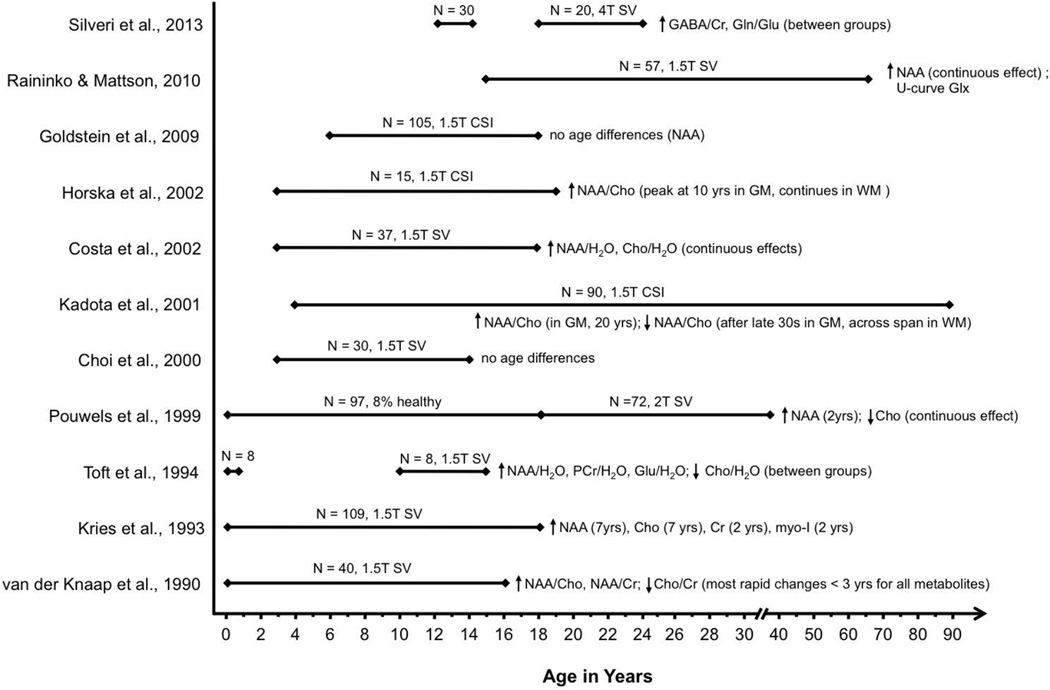
Figure 3. Summary of normative developmental 1H MRS studies.
Age spans investigated across all available 1H MRS studies of normative development. Ages in parentheses indicate plateau of age effects.
NAA contributes the largest signal in the proton spectrum at a chemical shift of 2.009 ppm. NAA is primarily found in mature neurons and has been viewed as an indicator of neuronal integrity (Birken & Oldendorf, 1989; Demougeot, Marie, Giroud, & Beley, 2004; Inglese, et al., 2008; Moffett, Ross, Arun, Madhavarao, & Namboodiri, 2007; Pouwels & Frahm, 1997; Sullivan, et al., 2001). Across the published developmental 1H MRS studies, in vivo levels of NAA have most often been reported to increase with age, whether reported as a concentration, or as a ratio relative to Cho, Cr or the unsuppressed water signal (Costa, Lacerda, Garcia Otaduy, Cerri, & Da Costa Leite, 2002; Grachev & Apkarian, 2000; Horska, et al., 2002; Hüppi, et al., 1991; Kadota, Horinouchi, & Kuroda, 2001; Kreis, Ernst, & Ross, 1993; Pouwels, et al., 1999; van der Knaap, et al., 1990). NAA levels have been reported to increase most rapidly within the first few years of life, and plateau anywhere from 2 to 30 years of age depending on the region and tissue type studied. Two published studies, however, have failed to find any significant associations between NAA and age in samples spanning from 3 to 14 years (Choi, Ko, Lee, Lee, & Suh, 2000) and from 6 to 18 years (Goldstein, et al., 2009), using moderate to large sample sizes. NAA has been most widely accepted as a biomarker of neuronal integrity, but may also index neuronal density. Thus, early NAA increases with age may reflect the rapid changes in brain tissue volume occurring early in life. However, production of NAA also is related to energy metabolism, suggesting that developmental increases in NAA may also result from the global increases in glucose metabolism associated with brain maturation (Chugani, 1998).
In adults, NAA concentrations in a left occipito-parietal voxel have been found to correlate positively with a composite measure of neuropsychological performance (Jung, Yeo, Chiulli, Sibbitt Jr, & Brooks, 2000) and with full-scale IQ, as measured by the Wechsler Adult Intelligence Scale, 3rd edition (WAIS-III) (Jung, et al., 2005). A later study using CSI reported both a positive relationship between NAA values in right posterior grey matter and performance IQ and a negative correlation between NAA in right anterior grey matter and verbal IQ (Jung, et al., 2009). Pfleiderer and colleagues (Pfleiderer, et al., 2004), however, found a positive relationship between vocabulary scores and NAA in the left dorsolateral prefrontal cortex, though this effect only reached significance in women. Studies exploring metabolite-cognition relationships in healthy elderly populations have revealed positive relationships between NAA and NAA/Cr ratios and performance on tests of executive function, attention, processing speed and memory (Charlton, McIntyre, Howe, Morris, & Markus, 2007; Ferguson, et al., 2002; Pfefferbaum, Adalsteinsson, Spielman, Sullivan, & Lim, 1999; Ross, Sachdev, Wen, Valenzuela, & Brodaty, 2005; Valenzuela, et al., 2000). Several studies have also reported associations between NAA and cognitive functioning within normative pediatric samples. In a sample of healthy 10 to18 year-olds, (Gimenez, et al., 2004) positive correlations were reported between measures of NAA/Cho in left hippocampus and measures of both verbal and non-verbal learning and memory. Similarly, (Yeo, et al., 2000) reported positive relationships between NAA and performance on a 2-back memory task in a sample of 7 to 12 year-olds. This study, however, found no relationship between NAA concentrations and a second memory measure - the Visual Temporal Order Memory Test. In contrast with earlier research (Jung, et al., 1999), neither of these studies found a significant relationship between metabolite measures and general intelligence. In a third developmental study, a large sample of healthy individuals between 6 and 18 years of age were examined (Ozturk, et al., 2009). Along with increased NAA/Cr with age in both parietal grey matter and white matter, this study reported a relationship between the NAA/Cr ratio in left frontal white matter and right-handed scores on the Perdue pegboard task of dexterity, while the same metabolite ratio in the right frontal white matter correlated with scores on a bead memory task. In a similarly aged sample (6 – 18 years), Goldstein and colleagues (2009) found a positive relationship between NAA levels and performance on a battery of visual-spatial construction tasks (Goldstein, et al., 2009). Taken together, these studies suggest both an increase in neural integrity with age, as indexed by NAA, and a positive relationship between NAA concentrations and cognitive functioning in both children and adults.
The largest Cho signal resonates at 3.22 ppm and reflects a number of choline-containing compounds, including free choline, phosphorylcholine, and glycerophosphocholine (PC and GPC, respectively) (Barker, et al., 1994). Choline-containing compounds (Cho) are involved in pathways of cellular membrane synthesis and degradation (Miller, 1991; Pouwels & Frahm, 1998), with most of the choline in the brain being membrane-bound as phosphatidylcholine, which is MR invisible (Miller, 1991). Declines in measurable Cho are consistent with accelerated myelination, as NMR visible choline residues become incorporated into MR invisible macromolecules associated with myelin production. Developmental findings in childhood and adolescence with regard to Cho are inconsistent, with some studies reporting an age-related decrease in Cho (Pouwels, et al., 1999; Toft, Christiansen, Pryds, Lou, & Henriksen, 1994; van der Knaap, et al., 1990), others reporting an age-related increase in Cho (Costa, et al., 2002; Kreis, et al., 1993), and some claiming no significant relationship between Cho levels and age (Raininko & Mattsson, 2010). These disparate results may be partly accounted for by differences between studies in voxel size and location. Looking at the ratio of NAA/Cho, a number of studies have found age-related increases, which may reflect both increases in NAA and decreases in Cho (Horska, et al., 2002; Kadota, et al., 2001; van der Knaap, et al., 1990). Not all studies, however, report developmental changes in this metric (Choi, et al., 2000; Costa, et al., 2002; Gimenez, et al., 2004). MRS studies of developmental delay in children have reported reduced NAA and elevated Cho levels, thought to reflect delayed myelination (Fayed & Modrego, 2005). Thus, simultaneous changes in NAA and Cho may reflect maturational processes critical to normative cognitive development. Consistent with these findings, in adults, Cho concentrations in a temporal voxel, were found to be negatively correlated with performance on visual memory and digit span tasks (Buckley, et al., 1994). In contrast, however, a positive relationship between vocabulary scores and Cho in the left dorsolateral prefrontal cortex (DLPFC) has been reported (Pfleiderer, et al., 2004). The conflicting results and lack of replication of MRS studies examining both developmental changes in measurable Cho and relationships between Cho concentrations and cognition make it difficult to draw firm conclusions regarding the roll of this metabolite in typical neurological and cognitive development at this time.
Singlet Cr resonances at 3.03 ppm and 3.93 ppm arise from protons in Cr and phosphorylated Cr (PCr). Cr plays a major role in brain energy metabolism, acting both as an energy buffer, by maintaining constant brain adenosine triphosphate (ATP) levels through the creatine kinase reaction, and by distributing energy (via mitochondria) within the brain (Kemp, 2000; Miller, 1991). There is some evidence that developmental changes in Cr levels plateau by the second year of life (Kreis, et al., 1993; Pouwels, et al., 1999), with the exception of a study documenting higher Cr levels when comparing infants to adolescents (Toft, et al., 1994). Infants tested in this study, however, were less than one year old and thus the results do not preclude the possibility that the Cr changes observed between infancy and adolescence occurred by two years of age. In healthy adults, a positive relationship has been observed between temporal Cr levels and visual memory span performance (Buckley, et al., 1994). In a sample of 7 to 12 year-olds, a similarly positive relationship was observed between Cr and 2-back memory task performance but not between Cr concentrations and a temporal order memory task (Yeo, et al., 2000). In elderly populations, however, Cr concentrations were found to correlate positively with increasing age and negatively with memory and executive function (Charlton, et al., 2007; Ferguson, et al., 2002; Valenzuela, et al., 2000). Thus, Cr increases may index metabolic changes both in the first two years of life and in old age. Furthermore, Cr concentrations show differing relationships with cognitive skills at different stages of development, suggesting a changing significance of this metabolic marker with age.
The most pronounced mI resonance occurs at 3.54 ppm. mI is involved in the synthesis and turnover of phospholipid membranes, maintenance of osmotic equilibrium, and is a phosphorylated derivative in the synthesis of secondary messengers (Berridge & Irvine, 1989; Brand, Richter-Landsberg, & Leibfritz, 1993; Downes & Macphee, 1990; Kim, McGrath, & Silverstone, 2005; Moore, et al., 1999; Wolfson, et al., 2000). mI concentrations have been reported to increase with age until reaching a plateau by age 2 (Kreis, et al., 1993). However, increases in mI have also been detected in early adulthood (Grachev & Apkarian, 2000) and across the lifespan (Raininko & Mattsson, 2010). Concentrations of mI, as measured via MRS, are yet to be linked to cognitive performance in pediatric or young adult populations. However, increases in the mI/Cr ratio have been observed in cases of mild cognitive impairment and Alzheimer’s disease in aging populations (Catani, et al., 2001; Kantarci, et al., 2002).
Lactate, or lactic acid, increases significantly when the brain is deprived of oxygen, or anaerobic respiration increases. Thus, lactate is an important metabolic marker. The lactate resonance is a doublet at 1.33 ppm, which is obscured by a resonance arising from lipids (Auer, Gossl, Schirmer, & Czisch, 2001; Behar, Rothman, Spencer, & Petroff, 1994), but can be reliably resolved when a long time to echo (TE) is employed (Behar, et al., 1994). To date, no age or cognition-related changes have been associated with lactate concentrations in healthy children or adolescents. An age related increase in lactate has, however, been reported in a young adult sample (19–31 years), when data were averaged across multiple voxels (Grachev & Apkarian, 2000).
Gln, Glu, and GABA all play multiple critical roles in neurological functioning. Gln serves as a precursor for Glu, which is a major excitatory neurotransmitter found in all brain cell types, with the highest concentrations typically observed in neurons. GABA is the major inhibitory neurotransmitter found in the mammalian brain (McCormick, 1989). Gln, Glu and GABA also play important roles in glucose metabolism, neuronal energetics and ammonia detoxification (Behar & Rothman, 2001; Patel, et al., 2005). While brain Glu is present at much higher concentrations than GABA, only a small fraction of Glu participates in neurotransmission. These metabolites each consist of multiple peaks that have strong spectral overlap, and thus are obscured by peaks of higher concentrations, such as Cr. For instance, GABA resonates at three chemical shift positions, triplet peaks at 2.31 and 3.01 ppm and a quintet at 1.91pm. Glu also consists of a number of multiplet peaks, although the majority of the Glu signal is observed at 2.35 ppm. Since there is considerable overlap of Gln and Glu, predominantly around 3.75 ppm, a combined "GLX" resonance intensity is often reported particularly when spectra are acquired at low field strength (1.5 Tesla). For example, higher GLX concentrations have been reported to predict inferior global cognitive functioning in patients with Type 1 diabetes (Lyoo, et al., 2009). Recently, increasing availability of high field MR scanners and specialized editing techniques have enhanced MR visibility of these metabolites by facilitating separation of resonances, allowing more accurate quantification of individual Glu, Gln and GABA resonances (Jensen, et al., 2009; Keltner, Wald, Frederick, & Renshaw, 1997; Mescher, Merkle, Kirsch, Garwood, & Gruetter, 1998; Weber, Trabesinger, Duc, Meier, & Boesiger, 1997). One such study in found increased GABA in the frontal eye fields (FEF) predicted diminished impact of distractors on directed visual saccades in healthy adults (Sumner, Edden, Bompas, Evans, & Singh). Furthermore, in an all-male adult sample, higher GABA concentrations in DLPFC were found to correlate with lower self-report based scores of rash impulsivity (Frederic Boy, et al., 2011). Higher Glu in medial prefrontal cortex was found to be associated with poorer performance on a continuous performance task (CPT) in an adult sample including both the healthy siblings of patients with schizophrenia and healthy control subjects (Purdon, Valiakalayil, Hanstock, Seres, & Tibbo, 2008). In a study of young adults, GABA, Glu and Gln were all found to be higher in a 21–31 year-old group when compared to a 19–20 year-old group (Grachev & Apkarian, 2000). Our preliminary study (Silveri, et al., 2013) provides the first in vivo human brain evidence of lower GABA/Cr in adolescence relative to adulthood, measured at 4T, in 30 healthy adolescents relative to 20 emerging adults in the anterior cingulate cortex (ACC), but not in a control region (parieto-occipital cortex). Higher ACC GABA significantly predicted better accuracy on the NoGo trials of a Go NoGo task and lower overall BIS trait impulsivity. Given this developmental increase in ACC GABA observed during adolescence, and the predictive nature of brain GABA levels on response inhibition, motor control and impulsivity (F. Boy, et al., 2010; Frederic Boy, et al., 2011; Silveri, et al., 2013), further characterization of brain metabolites and potential links to alcohol abuse disorders in adolescence is warranted.
In a young adult sample (19–31 years), Grachev & Apkerian (2000) detected elevated NAA in females relative to males in sensorimotor cortex and higher lactate in males relative to females in the dorsolateral prefrontal cortex. To date, no published studies explore sex differences in 1H metabolites in healthy children or adolescents. Using 1H MRS to explore neurochemical changes paralleling the sexual differentiation that occurs in the neuro-endocrine system during puberty may prove to be a particularly fruitful area of study. Increased sexual dimorphism during this period may also result in divergent cognition-metabolite relationships in men and women. Exploring such relationships may provide key insights into sex differences in emotion and reward processing that emerge during this period.
Development and Cognition: 31P MRS Findings
In vivo 31P MRS permits the detection of high-energy phosphate metabolites and constituents of membrane synthesis, which reflect cellular bioenergetic state and cell membrane integrity and function, respectively (Table 1). Phospholipid metabolites associated with high-energy intracellular metabolism that are detectable include PCr, inorganic phosphate (Pi), and β-NTP, primarily reflecting adenosine triphosphate (ATP) in the brain. Components of cell membranes detectable with 31P MRS include PME and PDE (see Figure 2 for a sample 31P spectrum). For 31P MRS, the chemical shift of each metabolite is referenced relative to PCr, which is used as an internal standard. Physiological relevance of each phosphorous metabolite is listed in Table 1 and developmental 31P MRS studies are summarized in Table 3 and Figure 4.
Table 3.
31P MR Spectroscopic findings in healthy children and adolescent
| Investigators | Sample | MRS | Region(s) of interest | Metabolites/findings |
|---|---|---|---|---|
| Boesch et al. (1989) | 33 wks – 6 yrs. (n = 40, 48 exams) healthy (25%) cerebral pathology (75%) chloral hydrate |
SV, 2.35 T | bilateral fronto-temporal regions |
|
| van der Knaap et al. (1990) | 1 mo – 16 yrs. mean=5.9 yrs. (n = 41) no sedation |
SV, 1.5T | paraventricular region, predominantly WM |
|
| Moss & Talagala (1997) | mean = 13.4 yrs. (n = 29) sedation not specified |
CSI, 1.5 T 3.5cm3 |
mesial frontal lobe mesial occipital lobe |
|
| Hanaoka et al. (1998) | 4 mo – 13 yrs. (n = 37) sedation not specified |
SV, 2.0 T 60–90cm3 40–60cm3 |
bilateral fronto-parietal cerebrum bilateral cerebellar hemispheres |
|
| Goldstein et al. (2009) | 6 – 18 yrs. (n = 105) no sedation |
CSI, 1.5 T 3 × 4.5 × 3 cm |
prefrontal cortex basal ganglia superior temporal cortex inferior parietal cortex centrum semiovale occipetal regions |
|
Abbreviations: SV, single voxel; CSI, chemical shift imaging; WM, white matter; GM, gray matter.
Note: Subjects in all studies were healthy, normally developing individuals except where noted otherwise. Findings reported in this table reflect healthy subjects only.
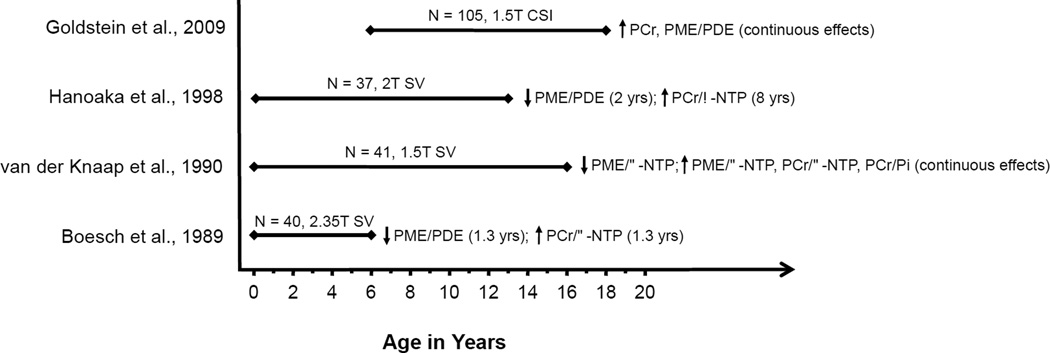
Figure 4. Summary of normative developmental 31P MRS studies.
Age spans investigated across all available 31P MRS studies of normative development. Ages in parentheses indicate plateau of age effects.
High-energy PCr serves as a buffer for maintenance of β-NTP levels and shuttles energy from sites of production to sites of utilization (Bessman & Geiger, 1981; Wallimann, Wyss, Brdiczka, Nicolay, & Eppenberger, 1992). Thus, availability of PCr stimulates the production of β-NTP via conversion to Cr and high-energy phosphate (Pi) (Wallimann, et al., 1992). This process results in a reduction of PCr levels, while the levels of ADP and Pi increase to support maintenance of β-NTP levels (Gyulai, Roth, Leigh, & Chance, 1985). The ratio of PCr relative to Pi, therefore, is thought to reflect phosphorylation potential, or available energy within a cell (Nioka, et al., 1990). The chemical shift of Pi can also be used to calculate internal pH level, which serves to modulate synaptic transmission and plasticity, and neuronal excitability, and can aid in the discrimination between diseased and healthy tissue (de Graaf, 2002). In general, the ratio of PCr to both β-NTP and γ-NTP increase most rapidly within the first few years of life and then increase more gradually, reaching a plateau around age eight (Boesch, Gruetter, Martin, Duc, & Wuthrich, 1989; Hanaoka, Takashima, & Morooka, 1998; van der Knaap, et al., 1990). More recent work, however, suggests that while PCr values may level off in late childhood and early adolescence, with the positive relationship between PCr and age once again becoming significant between 12 and 18 years of age (Goldstein, et al., 2009). Therefore, it has been posited that increases in cellular energy stores enable synaptic remodeling – an energetically demanding process. Goldstein and colleagues (2009) also reported positive relationships between PCr levels and age-related improvements in composite measures of language abilities, visuospatial construction and memory, but not executive functioning. In contrast to these findings in adolescents, Voltz and colleagues (Volz, et al., 1998) found a negative relationship between energy cycling, indicated as PCr/ATP, and executive function performance measured via the Wisconsin Card Sorting Test, in healthy adults. Taken together, these findings suggest a changing relationship between energy cycling and executive functioning skills, with developmental changes likely reflecting a change over to increased neuronal efficiency (Casey, et al., 2008; Durston, et al., 2006), which requires fewer energy resources, such as PCr and β-NTP, for the individual to perform at a higher level. This represents a maturational change from the more diffuse activation typically observed during performance of higher order cognitive functions during childhood and early adolescence, where PCr levels are not directly coupled to executive functioning abilities.
Components of cell membranes detectable with 31P MRS include PME and PDE. The PME resonance arises primarily from phospholipid membrane precursors (Pettegrew, et al., 1991), while the PDE resonance arises from the presence of phospholipid catabolites. Therefore, the PME peak reflects membrane synthesis and the PDE peak reflects products of membrane breakdown while the ratio of PME/PDE indexes membrane phospholipid turnover. At high field (4.0 Tesla and higher), subpeaks within the PME peak can be further quantified into phosphoethanolamine (PE) and phosphocholine (PC) anabolites, and in the PDE peak, glycerophosphoethanolamine (GPE) and glycerolphosphocholine (GPC) catabolites, allowing more precise quantification of metabolites and reflecting concurrent changes in the biophysical state of membrane phospholipids. A decline in the ratio of PME/PDE has been observed with age, until approximately two years of age, in both the cerebrum and the cerebellum (Boesch, et al., 1989; Hanaoka, et al., 1998; van der Knaap, et al., 1990). Significant reductions in PME/PDE with age have also observed between childhood and adolescence (Goldstein, et al., 2009). While these age-related changes in the ratio of PME/PDE may reflect changing rates of membrane synthesis and degradation, likely associated with developmental profiles of pruning and myelination, decreases in PME may also be due in part to the incorporation of membrane precursors into larger membrane macromolecules (e.g., synaptic receptors comprised of large protein structures), which are largely MR invisible.
From the limited data on sex differences in 31P metabolites during childhood and adolescence, males do not appear to differ from females in terms of overall age-related changes in metabolite levels. However, sex differences in the regional distributions of metabolites have been reported during this age period in a single study (Moss & Talagala, 1997). Higher β-NTP has been observed in frontal versus occipital regions in males, whereas females exhibit higher β-NTP in the occipital region relative to frontal regions. Furthermore, males demonstrate higher PDE levels in the occipital region than the frontal region (Moss & Talagala, 1997). Sex differences in 31P metabolites may therefore reflect the well-documented sex differences in structural and functional brain changes during childhood and adolescence, as well as the cognitive differences between males and females. For example, the more rapid increase in white matter in males versus females and an earlier peak in grey matter volume in females versus males may be reflected in differential neurometabolite profiles. Thus, sex differences in the timing of structural brain changes may result in variable energy demands and rates of membrane turnover in various brain regions, affecting the concentrations of β-NTP and PDE, respectively. Further research is needed to explore this possibility.
MRS in Alcohol and Substance Abuse and Dependence
Risk-taking is a key feature of adolescence, which includes experimentation with alcohol and drugs. Prevalence of alcohol use increases from 2.6% at age 12 to 67.5% at age 21 (SAMHSA, 2004). Quantity of alcohol consumed likewise increases during adolescence, sometimes reaching binge-like levels. A study of of risk-taking behaviors among U.S. teenagers found that 25.5% of all students had drunk more than 5 drinks on one occasion (i.e., a binge episode) during the previous month (Eaton, et al., 2006). Both heavy episodic use and early initiation of alcohol use are associated with higher rates of abuse and dependence (Grant & Dawson, 1997; Nigg, et al., 2006). It is clear that the onset of drinking during adolescence raises a serious public health concern, as it increases dangerous risk-taking behaviors already potentiated by adolescent brain development and can have a negative impact on neurological development during an important period of brain reorganization.
Cognitive alterations are frequently reported in association with alcohol abuse and dependence in adult populations (Oscar-Berman, 1990; Oscar-Berman, 2000; Oscar-Berman & Marinkovic, 2003; Parsons & Nixon, 1998). Accordingly, MR studies have documented a wide variety of associated neurobiological changes, which highlight the frontal lobe and hippocampus as being the most compromised by chronic alcohol use in adult cohorts (Harris, et al., 2008; Marlene Oscar-Berman & Marinkovic, 2007; Paulus, Tapert, Pulido, & Schuckit, 2006; Pfefferbaum, Sullivan, Mathalon, & Lim, 1997; Sullivan & Pfefferbaum, 2005). More recently, a growing body of literature has likewise demonstrated alterations in brain structure and function in frontal networks and the hippocampus, in adolescents and young adults with alcohol use disorders (DeBellis, et al., 2000; Tapert, et al., 2001; Tapert, Pulido, Paulus, Schuckit, & Burke, 2004; Tapert, Schweinsburg, et al., 2004) and those reporting binge alcohol consumption (McQueeny, et al., 2009; Schweinsburg, McQueeny, Nagel, Eyler, & Tapert, 2010; Squeglia, Schweinsburg, Pulido, & Tapert, 2011), as well as those who have not yet begun to drink alcohol, but who are family history positive for alcoholism (Hill, et al., 2009; Silveri, Rogowska, McCaffrey, & Yurgelun-Todd, 2011; Spadoni, Norman, Schweinsburg, & Tapert, 2008).
While no studies to date have used MRS to investigate the consequences of alcohol use on neurochemistry during adolescence, numerous MRS studies conducted in adult cohorts have demonstrated abnormalities in brain metabolites associated with alcohol use, abuse and dependence. In the majority of these published MRS studies, which have largely been confined to recently detoxified alcoholic adult males (>35 years old), substantial evidence for reductions in NAA and Cho, mostly in frontal lobe regions of interest, has been reported (for a comprehensive review and table of MRS studies in alcohol abusing and dependent populations, see Meyerhoff, Durazzo, & Ende, 2011). There is less evidence of abnormalities in Cr and mI associated with chronic alcohol use, although when reported, decreased Cr likely reflects altered cell bioenergetics and elevated mI reflects proliferation of glial cells (Meyerhoff, et al., 2011). Alterations in metabolite levels have been observed during acute abstinence, with recovery of metabolite levels increasing with length of alcohol abstinence in dependent populations that exhibit physiologic withdrawal (Meyerhoff, et al., 2011). Importantly, recovery of brain metabolites is predictive of restoration of cognitive functioning, underscoring the dual utility of MRS, to capture the impact of alcohol use on brain chemistry, but also to identify neurochemical correlates associated with recovery.
There have been fewer MRS studies that have quantified GABA and Glu metabolite levels in heavy alcohol-using adult populations, which is extremely relevant given that these compounds are central targets of alcohol action, and that heavy alcohol use, dependence and withdrawal have been shown to be associated with neurobiological alterations in GABAergic and glutamatergic systems (Krupitsky, et al., 2007; Krystal, Petrakis, Limoncelli, et al., 2003; Krystal, Petrakis, Mason, Trevisan, & D'Souza, 2003; Krystal, et al., 2006; Tsai & Coyle, 1998). Of the limited studies available, relative to healthy comparison subjects, detoxified alcoholics exhibited significantly lower occipital GABA levels, which was negatively correlated with impaired verbal recall (Behar, et al., 1999). More recent studies have reported no significant group differences in ACC or occipital GABA in alcohol dependent patients studied early in abstinence (Abe, et al., 2012; Mason, et al., 2006; Mon, Durazzo, & Meyerhoff), although smoking history emerged as an important mediator of metabolite changes (Mason, et al., 2006). In a study of young adult social alcohol drinkers, a 13 ± 8% reduction in occipital GABA was observed after an acute alcohol infusion, which is consistent with facilitation of the GABAA receptor by alcohol (Gomez, et al., 2011).
In stark contrast, only one study to date has used MRS to explore the neurochemical predictors of risk for substance abuse in adolescents. Moss and colleagues used 31P spectroscopic imaging to study a sample of peripubertal children (mean age of 12.5 years) at varying degrees of risk for developing a substance use disorder (Moss, Talagala, & Kirisci, 1997). Subjects in this study were split into three groups: 1) adolescents with a paternal history of substance use disorder and a personal diagnosis of disruptive behavior disorder, 2) adolescents with a paternal history of substance use disorder but no personal history of disruptive behavior disorder and, 3) adolescents with no paternal history and no disruptive disorders - a healthy comparison group. The first group, predicted to be at highest risk of developing substance use problems, was found to have significantly lower PDE in right parietal cortex when compared to the low-risk control subjects. Furthermore, PDE in this brain region correlated positively with the Information scale score of the Wechsler Intelligence Scale for Children, 3rd edition (WISC-III) in only the highest risk group. It was speculated that the difference in PDE concentrations could reflect heightened synaptic pruning or decreased activity-dependent synaptogenesis in right parietal cortex, consequently influencing information retrieval abilities. The group possessing only a single risk factor of family history of substance abuse did not differ significantly from either of the other two groups for any metabolite measure and no effects of age were observed. A significant main effect of sex for PCr levels did emerge, with females exhibiting lower levels than males across brain regions and risk groups, a result that may reflect differences in white and grey matter proportions within the regions studied (Moss, et al., 1997). Females also had lower frontal β-NTP levels than males, but higher β-NTP concentrations overall, mirroring findings from a separate study by the same group in older subjects (Moss, et al., 1997).
While there have been thirty-two MRS studies published investigating alcohol-related neurochemical consequences in alcohol abusing and dependent adult populations (see also, Meyerhoff, et al. 2011), no studies have been published to date that have examined neurochemistry using MRS in adolescents either with significant alcohol use histories or alcohol use disorders. Notably, the metabolites detected as abnormal in adult patients with alcohol abuse disorders overlap with metabolites that demonstrate maturational changes during healthy adolescence (e.g., NAA, Cho, Cr, and GABA). Furthermore, metabolite alterations observed in alcohol abuse cohorts occur widely in the frontal lobe, which is in line with other neuroimaging results demonstrating that this late-maturing brain region is particularly susceptible to alcohol effects. Given the impact of alcohol use and abuse observed across multiple domains of cognitive functioning in adults, the paucity of MRS studies investigating neurochemical correlates of adolescent alcohol use further highlights the critical need for research in this area.
Conclusions and Future Directions
Structural, functional, and neurochemical maturation in the healthy adolescent brain occurs in regions and in cognitive domains that overlap with those observed to be most vulnerable to alcohol abuse and dependence. While a substantial body of neuroimaging research exploring the impact of alcohol on the structure and functional activation of the adolescent brain exists, less is known about neurochemical changes occurring in the human adolescent brain that are associated with healthy development, or that are associated with adolescent alcohol use. Recent advances have made MRS technology more amenable to the study of pediatric populations, offering promise for understanding typical brain neurochemical development and maladaptive developmental trajectories associated with the manifestation of alcohol disorders or other adolescent psychopathologies.
Developmental characterization of neurochemistry via MRS during adolescence could yield mechanistic hypotheses regarding the neurobiological consequences of alcohol use, as well as the propensity for future, continued and heavy alcohol use. For example, animal models suggest reduced GABA in prefrontal control regions in adolescents versus adults may reduce the sedative effects of alcohol, allowing for longer and heavier bouts of drinking (Silveri & Spear, 2004), which is consistent with developmental changes in GABA concentrations measured in frontal regions of human adolescents (Silveri, et al., 2013). In addition, lower prefrontal concentrations of NAA have been reported in children and adolescents compared to adults (Costa, et al., 2002; Horska, et al., 2002; Kadota, et al., 2001), but also in recently detoxified alcoholics relative to healthy controls (Meyerhoff, et al., 2011), suggesting that alcohol use during adolescence may present a double-threat to neuronal integrity and volume in prefrontal regions critical to self-regulation. Given that PCr concentrations have been reported to increase with age (Goldstein, et al., 2009), the developing adolescent brain may be more susceptible to alcohol effects on this important high-energy resource pool, leading to deleterious alterations in brain function. Moreover, MRS can be integrated with other imaging techniques and behavioral assessments to provide an additional level of analysis and construct a more comprehensive profile of brain development. This profile would provide a foundation for quantifying the effects of alcohol use on the adolescent brain and for the development of biomarkers that could help identify adolescents at risk for initiating and escalating alcohol consumption, even before they start drinking.
For the majority of the currently published developmental studies using MRS, the sparse distribution of sampling from different age groups makes results difficult to interpret. Larger samples and longitudinal studies are needed to map the time course of age-related metabolite changes associated with structural and functional brain development (Figures 3, 4), as well as with cognitive and behavioral changes during adolescence. Furthermore, the majority of existing pediatric MRS studies were conducted at a relatively low field strength (1.5 Tesla), which limits the number of proton- and phosphorous-containing metabolites that can be detected and reliably quantified. Significant future advances in understanding pediatric neurochemical development will depend on a number of factors: 1) increased uniformity in protocols across studies and sites (improving feasibility and reliability in multi-site studies); 2) the inclusion of large sample sizes to achieve statistical power to detect significant group differences; and 3) collection of longitudinal data sets. It also will be important to consider the influence of sex differences and brain laterality on metabolite levels. The measurement of additional nuclei (e.g., 13C, 19F) and the integration of metabolite data with data from other MR imaging modalities, such as brain tissue volumes (MRI), indices of white matter microstructure integrity (DTI) and neuronal activation (fMRI), will provide a more comprehensive understanding of neurochemical brain development.
Taken together, MRS studies of adolescent populations hold promise for advancing developmental neuroscience by identifying the biochemical signatures associated with healthy brain development, as well as neurobiological predictors and consequences of alcohol use and abuse. Given the paucity of extant developmental MRS data, a better characterization of healthy neurochemical development is needed to identify potential risk factors for the manifestation of alcohol and substance abuse disorders, which can also be applied to better understand neurobiological alterations associated with a number co-occurring psychopathological conditions that often emerge during adolescence. Thus, the continued evolution of MRS is expected to contribute to our understanding of pathophysiology, mechanisms of treatment response and ultimately to contribute to advances in treatment development in both low and high-risk adolescent populations.
Acknowledgements
This work was supported by NIAAA grants K01 AA014651 and R01 AA018153 (MMS). The authors wish to thank Dr. Jennifer T. Sneider for her assistance in the preparation of this review.
References
- Abe C, Mon A, Durazzo TC, Pennington DL, Schmidt TP, Meyerhoff DJ. Polysubstance and alcohol dependence: Unique abnormalities of magnetic resonance-derived brain metabolite levels. Drug and Alcohol Dependence. 2012 doi: 10.1016/j.drugalcdep.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer DP, Gossl C, Schirmer T, Czisch M. Improved analysis of 1H-MR spectra in the presence of mobile lipids. Magnetic Resonance in Medicine. 2001;46(3):615–618. doi: 10.1002/mrm.1235. [DOI] [PubMed] [Google Scholar]
- Barker PB, Breiter SN, Soher BJ, Chatham JC, Forder JR, Samphilipo MA, et al. Quantitative proton spectroscopy of canine brain: in vivo and in vitro correlations. Magnetic Resonance in Medicine. 1994;32(2):157–163. doi: 10.1002/mrm.1910320202. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, et al. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cerebral Cortex. 2005;15(12):1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- Bava S, Thayer R, Jacobus J, Ward M, Jernigan TL, Tapert SF. Longitudinal characterization of white matter maturation during adolescence. Brain Research. 2010;1327:38–46. doi: 10.1016/j.brainres.2010.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar KL, Rothman DL. In vivo nuclear magnetic resonance studies of glutamate-gamma-aminobutyric acid-glutamine cycling in rodent and human cortex: the central role of glutamine. Journal of Nutrition. 2001;131(9 Suppl):2498S–2504S. doi: 10.1093/jn/131.9.2498S. discussion 2523S–2494S. [DOI] [PubMed] [Google Scholar]
- Behar KL, Rothman DL, Petersen KF, Hooten M, Delaney R, Petroff OA, et al. Preliminary evidence of low cortical GABA levels in localized 1H-MR spectra of alcohol-dependent and hepatic encephalopathy patients. American Journal of Psychiatry. 1999;156(6):952–954. doi: 10.1176/ajp.156.6.952. [DOI] [PubMed] [Google Scholar]
- Behar KL, Rothman DL, Spencer DD, Petroff OA. Analysis of macromolecule resonances in 1H NMR spectra of human brain. Magnetic Resonance in Medicine. 1994;32(3):294–302. doi: 10.1002/mrm.1910320304. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Irvine RF. Inositol phosphates and cell signalling. Nature. 1989;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Bessman SP, Geiger PJ. Transport of energy in muscle: the phosphorylcreatine shuttle. Science. 1981;211(4481):448–452. doi: 10.1126/science.6450446. [DOI] [PubMed] [Google Scholar]
- Birken DL, Oldendorf WH. N-acetyl-L-aspartic acid: a literature review of a compound prominent in 1H-NMR spectroscopic studies of brain. Neuroscience Biobehavioral Review. 1989;13(1):23–31. doi: 10.1016/s0149-7634(89)80048-x. [DOI] [PubMed] [Google Scholar]
- Blakemore S-J, Choudhury S. Development of the adolescent brain: implications for executive function and social cognition. Journal of Child Psychology & Psychiatry. 2006;47(3/4):296–312. doi: 10.1111/j.1469-7610.2006.01611.x. [DOI] [PubMed] [Google Scholar]
- Boesch C, Gruetter R, Martin E, Duc G, Wuthrich K. Variations in the in vivo P-31 MR spectra of the developing human brain during postnatal life. Work in progress. Radiology. 1989;172(1):197–199. doi: 10.1148/radiology.172.1.2740503. [DOI] [PubMed] [Google Scholar]
- Bovey FA, Jelinski L, Mirau PA. Nuclear Magnetic Resonance Spectroscopy. San Diego: Academic Press; 1988. [Google Scholar]
- Boy F, Evans CJ, Edden RA, Singh KD, Husain M, Sumner P. Individual differences in subconscious motor control predicted by GABA concentration in SMA. Current Biology. 2010;20(19):1779–1785. doi: 10.1016/j.cub.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boy F, Evans CJ, Edden RAE, Lawrence AD, Singh KD, Husain M, et al. Dorsolateral prefrontal gamma-aminobutyric acid in men predicts individual differences in rash impulsivity. Biological Psychiatry. 2011;70(9):866–872. doi: 10.1016/j.biopsych.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A, Richter-Landsberg C, Leibfritz D. Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Developmental Neuroscience. 1993;15(3–5):289–298. doi: 10.1159/000111347. [DOI] [PubMed] [Google Scholar]
- Brown TR, Kincaid BM, Ugurbil K. NMR chemical shift imaging in three dimensions. Proceedings of the National Academy of Sciences of the United States of America. 1982;79(11):3523–3526. doi: 10.1073/pnas.79.11.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley PF, Moore C, Long H, Larkin C, Thompson P, Mulvany F, et al. 1H-magnetic resonance spectroscopy of the left temporal and frontal lobes in schizophrenia: Clinical, neurodevelopmental, and cognitive correlates. Biological Psychiatry. 1994;36(12):792–800. doi: 10.1016/0006-3223(94)90591-6. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Hare TA. The adolescent brain. Annals of the New York Academy of Sciences. 2008;1124(1):111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Trainor RJ, Orendi JL, Schubert AB, Nystrom LE, Giedd JN, et al. A developmental functional MRI study of prefrontal activation during performance of a Go-No-Go task. Journal of Cognitive Neuroscience. 1997;9(6):835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- Catani M, Cherubini A, Howard R, Tarducci R, Pelliccioli GP, Piccirilli M, et al. (1)H-MR spectroscopy differentiates mild cognitive impairment from normal brain aging. Neuroreport. 2001;12(11):2315–2317. doi: 10.1097/00001756-200108080-00007. [DOI] [PubMed] [Google Scholar]
- Charlton RA, McIntyre DJO, Howe FA, Morris RG, Markus HS. The relationship between white matter brain metabolites and cognition in normal aging: The GENIE study. Brain Research. 2007;1164:108–116. doi: 10.1016/j.brainres.2007.06.027. [DOI] [PubMed] [Google Scholar]
- Chen X, Pavan M, Heinzer-Schweizer S, Boesiger P, Henning A. Optically transmitted and inductively coupled electric reference to access in vivo concentrations for quantitative proton-decoupled 13C magnetic resonance spectroscopy. Magnetic Resonance in Medicine. 2012;67(1):1–7. doi: 10.1002/mrm.23110. [DOI] [PubMed] [Google Scholar]
- Choi CG, Ko TS, Lee HK, Lee JH, Suh DC. Localized proton MR spectroscopy of the allocortex and isocortex in healthy children. American Journal of Neuroradiology. 2000;21(7):1354–1358. [PMC free article] [PubMed] [Google Scholar]
- Chugani HT. A critical period of brain development: studies of cerebral glucose utilization with PET. Preventive Medicine. 1998;27(2):184–188. doi: 10.1006/pmed.1998.0274. [DOI] [PubMed] [Google Scholar]
- Cohen-Gilbert JE, Thomas KM. Inhibitory control during emotional distraction across adolescence and early adulthood. Child Development. 2013 doi: 10.1111/cdev.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa MO, Lacerda MT, Garcia Otaduy MC, Cerri GG, Da Costa Leite C. Proton magnetic resonance spectroscopy: normal findings in the cerebellar hemisphere in childhood. Pediatric Radiology. 2002;32(11):787–792. doi: 10.1007/s00247-002-0777-5. [DOI] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, Donahue S, van Leijenhorst L, Bunge S. Neurocognitive development of the ability to manipulate information in working memory. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(24):9315–9320. doi: 10.1073/pnas.0510088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl RE. Affect regulation, brain development and behavioral/emotional health in adolescence. CNS Spectrums. 2001;6(1):60–72. doi: 10.1017/s1092852900022884. [DOI] [PubMed] [Google Scholar]
- Davanzo P, Yue K, Thomas MA, Belin T, Mintz J, Venkatraman TN, et al. Proton magnetic resonance spectroscopy of bipolar disorder versus intermittent explosive disorder in children and adolescents. American Journal of Psychiatry. 2003;160(8):1442–1452. doi: 10.1176/appi.ajp.160.8.1442. [DOI] [PubMed] [Google Scholar]
- de Graaf RA. In vivo NMR spectroscopy: principles and techniques. 2nd ed. Chichester, West Sussex, England; Hoboken, NJ: John Wiley & Sons; 2002. [Google Scholar]
- DeBellis MD, Clark DB, Beers SR, Soloff PH, Boring AM, Hall J, et al. Hippocampal volume in adolescent-onset alcohol use disorders. American Journal of Psychiatry. 2000;157(5):737–744. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- Demougeot C, Marie C, Giroud M, Beley A. N-acetylaspartate: a literature review of animal research on brain ischaemia. Journal of Neurochemistry. 2004;90(4):776–783. doi: 10.1111/j.1471-4159.2004.02583.x. [DOI] [PubMed] [Google Scholar]
- Downes CP, Macphee CH. myo-inositol metabolites as cellular signals. European Journal of Biochemistry. 1990;193(1):1–18. doi: 10.1111/j.1432-1033.1990.tb19297.x. [DOI] [PubMed] [Google Scholar]
- Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, et al. A shift from diffuse to focal cortical activity with development. Developmental Science. 2006;9(1):1–20. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- Eaton DK, Kann L, Kinchen S, Ross J, Hawkins J, Harris WA, et al. Youth risk behavior surveillance - United States, 2005. Journal of School Health. 2006;76(7):353–372. doi: 10.1111/j.1746-1561.2006.00127.x. [DOI] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, et al. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. NeuroImage. 2005;25(4):1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Fayed N, Modrego PJ. Comparative study of cerebral white matter in autism and attention-deficit/hyperactivity disorder by means of magnetic resonance spectroscopy. Academic Radiology. 2005;12(5):566–569. doi: 10.1016/j.acra.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Ferguson KJ, MacLullich AMJ, Marshall I, Deary IJ, Starr JM, Seckl JR, et al. Magnetic resonance spectroscopy and cognitive function in healthy elderly men. Brain. 2002;125(12):2743–2749. doi: 10.1093/brain/awf278. [DOI] [PubMed] [Google Scholar]
- Gabbay V, Mao X, Klein RG, Ely BA, Babb JS, Panzer AM, et al. Anterior cingulate cortex gamma-aminobutyric acid in depressed adolescents: relationship to anhedonia. Archives of General Psychiatry. 2012;69(2):139–149. doi: 10.1001/archgenpsychiatry.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, et al. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. The Journal of Neuroscience. 2006;26(25):6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gimenez M, Junque C, Narberhaus A, Caldu X, Segarra D, Vendrell P, et al. Medial temporal MR spectroscopy is related to memory performance in normal adolescent subjects. Neuroreport. 2004;15(4):703–707. doi: 10.1097/00001756-200403220-00026. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein G, Panchalingam K, McClure RJ, Stanley JA, Calhoun VD, Pearlson GD, et al. Molecular neurodevelopment: An in vivo 31P-1H MRSI study. Journal of the International Neuropsychological Society. 2009;15(05):671–683. doi: 10.1017/S1355617709990233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez R, Behar KL, Watzl J, Weinzimer SA, Gulanski B, Sanacora G, et al. Intravenous ethanol infusion decreases human cortical gamma-aminobutyric acid and N-acetylaspartate as measured with proton magnetic resonance spectroscopy at 4 Tesla. Biological Psychiatry. 2011;71(3):239–246. doi: 10.1016/j.biopsych.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grachev ID, Apkarian AV. Chemical heterogeneity of the living human brain: A proton MR spectroscopy study on the effects of sex, age, and brain region. NeuroImage. 2000;11(5):554–563. doi: 10.1006/nimg.2000.0557. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the national longitudinal alcohol epidemiologic survey. Journal of Substance Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Gyulai L, Roth Z, Leigh JS, Jr, Chance B. Bioenergetic studies of mitochondrial oxidative phosphorylation using 31phosphorus NMR. Journal of Biological Chemistry. 1985;260(7):3947–3954. [PubMed] [Google Scholar]
- Hanaoka S, Takashima S, Morooka K. Study of the maturation of the child's brain using 31P-MRS. Pediatric Neurology. 1998;18(4):305–310. doi: 10.1016/s0887-8994(97)00201-4. [DOI] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biological Psychiatry. 2008;63(10):927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GJ, Jaffin SK, Hodge SM, Kennedy D, Caviness VS, Marinkovic K, et al. Frontal White Matter and Cingulum Diffusion Tensor Imaging Deficits in Alcoholism. Alcoholism: Clinical and Experimental Research. 2008;32(6):1001–1013. doi: 10.1111/j.1530-0277.2008.00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzer-Schweizer S, De Zanche N, Pavan M, Mens G, Sturzenegger U, Henning A, et al. In-vivo assessment of tissue metabolite levels using 1H MRS and the Electric REference To access In vivo Concentrations (ERETIC) method. NMR in Biomedicine. 2010;23(4):406–413. doi: 10.1002/nbm.1476. [DOI] [PubMed] [Google Scholar]
- Hill SY, Wang S, Kostelnik B, Carter H, Holmes B, McDermott M, et al. Disruption of orbitofrontal cortex laterality in offspring from multiplex alcohol dependence families. Biological Psychiatry. 2009;65(2):129–136. doi: 10.1016/j.biopsych.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper CJ, Luciana M, Conklin HM, Yarger RS. Adolescents' performance on the Iowa Gambling Task: implications for the development of decision making and ventromedial prefrontal cortex. Developmental Psychology. 2004;40(6):1148–1158. doi: 10.1037/0012-1649.40.6.1148. [DOI] [PubMed] [Google Scholar]
- Horska A, Kaufmann WE, Brant LJ, Naidu S, Harris JC, Barker PB. In vivo quantitative proton MRSI study of brain development from childhood to adolescence. Journal of Magnetic Resonance Imaging. 2002;15(2):137–143. doi: 10.1002/jmri.10057. [DOI] [PubMed] [Google Scholar]
- Huizinga M, Dolan CV, van der Molen MW. Age-related change in executive function: Developmental trends and a latent variable analysis. Neuropsychologia. 2006;44(11):2017–2036. doi: 10.1016/j.neuropsychologia.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Hüppi PS, Posse S, Lazeyras F, Burri R, Bossi E, Herschkowitz Magnetic resonance in preterm and term newborns: 1H spectroscopy in developing human brain. Pediatric Research. 1991;30(6):574–578. doi: 10.1203/00006450-199112000-00017. [DOI] [PubMed] [Google Scholar]
- Inglese M, Rusinek H, George IC, Babb JS, Grossman RI, Gonen O. Global average gray and white matter N-acetylaspartate concentration in the human brain. Neuroimage. 2008;41(2):270–276. doi: 10.1016/j.neuroimage.2008.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen JE, Frederick BB, Renshaw PF. Grey and white matter GABA level differences in the human brain using two-dimensional, J-resolved spectroscopic imaging. NMR in Biomedicine. 2005;18(8):570–576. doi: 10.1002/nbm.994. [DOI] [PubMed] [Google Scholar]
- Jensen JE, Licata SC, Ongur D, Friedman SD, Prescot AP, Henry ME, et al. Quantification of J-resolved proton spectra in two-dimensions with LCModel using GAMMA-simulated basis sets at 4 Tesla. NMR in Biomedicine. 2009;22(7):762–769. doi: 10.1002/nbm.1390. [DOI] [PubMed] [Google Scholar]
- Johnstone SJ, Pleffer CB, Barry RJ, Clarke AR, Smith JL. Development of inhibitory processing during the Go/NoGo task: a behavioral and event-related potential study of children and adults. Journal of Psychophysiology. 2005;19(1):11–23. [Google Scholar]
- Jung RE, Brooks WM, Yeo RA, Chiulli SJ, Weers DC, Sibbitt WL. Biochemical Markers of Intelligence: A Proton MR Spectroscopy Study of Normal Human Brain. Proceedings: Biological Sciences. 1999;266(1426):1375–1379. doi: 10.1098/rspb.1999.0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung RE, Gasparovic C, Chavez RS, Caprihan A, Barrow R, Yeo RA. Imaging intelligence with proton magnetic resonance spectroscopy. Intelligence. 2009;37(2):192–198. doi: 10.1016/j.intell.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung RE, Haier RJ, Yeo RA, Rowland LM, Petropoulos H, Levine AS, et al. Sex differences in N-acetylaspartate correlates of general intelligence: An 1H-MRS study of normal human brain. NeuroImage. 2005;26(3):965–972. doi: 10.1016/j.neuroimage.2005.02.039. [DOI] [PubMed] [Google Scholar]
- Jung RE, Yeo RA, Chiulli SJ, Sibbitt WL, Jr, Brooks WM. Myths of Neuropsychology: intelligence, neurometabolism, and cognitive ability. Clinical Neuropsychologist. 2000;14(4):535. doi: 10.1076/clin.14.4.535.7198. [DOI] [PubMed] [Google Scholar]
- Kadota T, Horinouchi T, Kuroda C. Development and aging of the cerebrum: assessment with proton MR spectroscopy. American Journal of Neuroradiology. 2001;22(1):128–135. [PMC free article] [PubMed] [Google Scholar]
- Kantarci K, Smith GE, Ivnik RJ, Petersen RC, Boeve BF, Knopman DS, et al. 1H magnetic resonance spectroscopy, cognitive function, and apolipoprotein E genotype in normal aging, mild cognitive impairment and Alzheimer's disease. Journal of the International Neuropsychological Society. 2002;8(07):934–942. doi: 10.1017/s1355617702870084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keltner JR, Wald LL, Frederick BD, Renshaw PF. In vivo detection of GABA in human brain using a localized double-quantum filter technique. Magnetic Resonance in Medicine. 1997;37(3):366–371. doi: 10.1002/mrm.1910370312. [DOI] [PubMed] [Google Scholar]
- Kemp GJ. Non-invasive methods for studying brain energy metabolism: what they show and what it means. Developmental Neuroscience. 2000;22(5–6):418–428. doi: 10.1159/000017471. [DOI] [PubMed] [Google Scholar]
- Kim H, McGrath BM, Silverstone PH. A review of the possible relevance of inositol and the phosphatidylinositol second messenger system (PI-cycle) to psychiatric disorders--focus on magnetic resonance spectroscopy (MRS) studies. Human Psychopharmacology. 2005;20(5):309–326. doi: 10.1002/hup.693. [DOI] [PubMed] [Google Scholar]
- Kreis R, Ernst T, Ross BD. Development of the human brain: in vivo quantification of metabolite and water content with proton magnetic resonance spectroscopy. Magnetic Resonance in Medicine. 1993;30(4):424–437. doi: 10.1002/mrm.1910300405. [DOI] [PubMed] [Google Scholar]
- Krupitsky EM, Rudenko AA, Burakov AM, Slavina TY, Grinenko AA, Pittman B, et al. Antiglutamatergic strategies for ethanol detoxification: comparison with placebo and diazepam. Alcoholism: Clinical and Experimental Research. 2007;31(4):604–611. doi: 10.1111/j.1530-0277.2007.00344.x. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Limoncelli D, Webb E, Gueorgueva R, D'Souza DC, et al. Altered NMDA glutamate receptor antagonist response in recovering ethanol-dependent patients. Neuropsychopharmacology. 2003;28(11):2020–2028. doi: 10.1038/sj.npp.1300252. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Mason G, Trevisan L, D'Souza DC. N-methyl-D-aspartate glutamate receptors and alcoholism: reward, dependence, treatment, and vulnerability. Pharmacology & Therapeutics. 2003;99(1):79–94. doi: 10.1016/s0163-7258(03)00054-8. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Staley J, Mason G, Petrakis IL, Kaufman J, Harris RA, et al. Gamma-aminobutyric acid type A receptors and alcoholism: intoxication, dependence, vulnerability, and treatment. Archives of General Psychiatry. 2006;63(9):957–968. doi: 10.1001/archpsyc.63.9.957. [DOI] [PubMed] [Google Scholar]
- Licata SC, Jensen J, Penetar D, Prescot A, Lukas S, Renshaw P. A therapeutic dose of zolpidem reduces thalamic GABA in healthy volunteers: a proton MRS study at 4 T. Psychopharmacology. 2009;203(4):819–829. doi: 10.1007/s00213-008-1431-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Watts R, Tottenham N, Davidson MC, Niogi S, Ulug AM, et al. Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cerebral Cortex. 2006;16(4):553–560. doi: 10.1093/cercor/bhj003. [DOI] [PubMed] [Google Scholar]
- Luna B, Padmanabhan A, O'Hearn K. What has fMRI told us about the Development of Cognitive Control through Adolescence? Brain and Cognition. 2010;72(1):101–113. doi: 10.1016/j.bandc.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyoo IK, Yoon SJ, Musen G, Simonson DC, Weinger K, Bolo N, et al. Altered Prefrontal Glutamate-Glutamine-{gamma}-Aminobutyric Acid Levels and Relation to Low Cognitive Performance and Depressive Symptoms in Type 1 Diabetes Mellitus. Archives of General Psychiatry. 2009;66(8):878. doi: 10.1001/archgenpsychiatry.2009.86. [DOI] [PubMed] [Google Scholar]
- Mangia S, Tkac I, Gruetter R, De Moortele V, Giove F, Maraviglia B, et al. Sensitivity of single-voxel 1H-MRS in investigating the metabolism of the activated human visual cortex at 7 T. Magnetic Resonance Imaging. 2006;24(4):343–348. doi: 10.1016/j.mri.2005.12.023. [DOI] [PubMed] [Google Scholar]
- Mason GF, Petrakis IL, de Graaf RA, Gueorguieva R, Guidone E, Coric V, et al. Cortical gamma-aminobutyric acid levels and the recovery from ethanol dependence: preliminary evidence of modification by cigarette smoking. Biological Psychiatry. 2006;59(1):85–93. doi: 10.1016/j.biopsych.2005.06.009. [DOI] [PubMed] [Google Scholar]
- McCormick DA. GABA as an inhibitory neurotransmitter in human cerebral cortex. Journal of Neurophysiology. 1989;62(5):1018–1027. doi: 10.1152/jn.1989.62.5.1018. [DOI] [PubMed] [Google Scholar]
- McQueeny T, Schweinsburg BC, Schweinsburg AD, Jacobus J, Bava S, Frank LR, et al. Altered White Matter Integrity in Adolescent Binge Drinkers. Alcoholism: Clinical and Experimental Research. 2009;33(7):1278–1285. doi: 10.1111/j.1530-0277.2009.00953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekle R, Mlynárik V, Gambarota G, Hergt M, Krueger G, Gruetter R. MR spectroscopy of the human brain with enhanced signal intensity at ultrashort echo times on a clinical platform at 3T and 7T. Magnetic Resonance in Medicine. 2009;61(6):1279–1285. doi: 10.1002/mrm.21961. [DOI] [PubMed] [Google Scholar]
- Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR in Biomedicine. 1998;11(6):266–272. doi: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Meyerhoff DJ, Durazzo TC, Ende G. Chronic alcohol consumption, abstinence and relapse: Brain proton magnetic resonance spectroscopy studies in animals and humans. Behavioral Neurobiology of Alcohol Addiction. 2011;13:511–540. doi: 10.1007/7854_2011_131. [DOI] [PubMed] [Google Scholar]
- Miller BL. A review of chemical issues in 1H NMR spectroscopy: N-acetyl-L-aspartate, creatine and choline. NMR in Biomedicine. 1991;4(2):47–52. doi: 10.1002/nbm.1940040203. [DOI] [PubMed] [Google Scholar]
- Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AM. N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Progress in Neurobiology. 2007;81(2):89–131. doi: 10.1016/j.pneurobio.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mon A, Durazzo TC, Meyerhoff DJ. Glutamate, GABA, and other cortical metabolite concentrations during early abstinence from alcohol and their associations with neurocognitive changes. Drug and Alcohol Dependence. 2012;125(1–2):27–36. doi: 10.1016/j.drugalcdep.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CM, Breeze JL, Kukes TJ, Rose SL, Dager SR, Cohen BM, et al. Effects of myo-inositol ingestion on human brain myo-inositol levels: a proton magnetic resonance spectroscopic imaging study. Biological Psychiatry. 1999;45(9):1197–1202. doi: 10.1016/s0006-3223(98)00249-2. [DOI] [PubMed] [Google Scholar]
- Moss HB, Talagala SL. 31P magnetic resonance spectroscopy of normal peripubertal children: Effects of sex and fronto-occipital location. Proceedings of the 5th Annual Meeting of the International Society for Magnetic Resonance in Medicine. 1997;2:1219. [Google Scholar]
- Moss HB, Talagala SL, Kirisci L. Phosphorus-31 magnetic resonance brain spectroscopy of children at risk for a substance use disorder: preliminary results. Psychiatry Research: Neuroimaging. 1997;76(2–3):101–112. doi: 10.1016/s0925-4927(97)00067-x. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Wong MM, Martel MM, Jester JM, Puttler LI, Glass JM, et al. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. Journal of the American Academy of Child & Adolescent Psychiatry. 2006;45(4):468–475. doi: 10.1097/01.chi.0000199028.76452.a9. [DOI] [PubMed] [Google Scholar]
- Nioka S, Smith DS, Chance B, Subramanian HV, Butler S, Katzenberg M. Oxidative phosphorylation system during steady-state hypoxia in the dog brain. Journal of Applied Physiology. 1990;68(6):2527–2535. doi: 10.1152/jappl.1990.68.6.2527. [DOI] [PubMed] [Google Scholar]
- Olvera RL, Caetano SC, Stanley JA, Chen HH, Nicoletti M, Hatch JP, et al. Reduced medial prefrontal N-acetyl-aspartate levels in pediatric major depressive disorder: a multi-voxel in vivo(1)H spectroscopy study. Psychiatry Research. 2010;184(2):71–76. doi: 10.1016/j.pscychresns.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M. Learning and memory deficits in detoxified alcoholics. NIDA Research Monographs. 1990;101:136–155. [PubMed] [Google Scholar]
- Oscar-Berman M. Neuropsychological vulnerabilities in chronic alcoholism. In: Noronha A, Eckardt M, Warren K, editors. Review of NIAAA’s Neuroscience and Behavioral Research Portfolio. Bethesda, MD: National Institutes of Health; 2000. pp. 437–472. Vol. NIAAA Research Monograph No. 34. [Google Scholar]
- Oscar-Berman M, Marinkovic K. Alcoholism and the brain: an overview. Alcohol Research and Health. 2003;27(2):125–133. [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, Marinkovic K. Alcohol: Effects on Neurobehavioral Functions and the Brain. Neuropsychology Review. 2007;17(3):239–257. doi: 10.1007/s11065-007-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozturk A, Degaonkar M, Matson MA, Wells CT, Mahone EM, Horska A. Proton MR Spectroscopy Correlates of Frontal Lobe Function in Healthy Children. American Journal of Neuroradiology. 2009;30(7):1308–1314. doi: 10.3174/ajnr.A1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons OA, Nixon SJ. Cognitive functioning in sober social drinkers: a review of the research since. Journal of Studies on Alcohol 1986. 1998;59(2):180–190. doi: 10.15288/jsa.1998.59.180. [DOI] [PubMed] [Google Scholar]
- Patel AB, de Graaf RA, Mason GF, Rothman DL, Shulman RG, Behar KL. The contribution of GABA to glutamate/glutamine cycling and energy metabolism in the rat cortex in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(15):5588–5593. doi: 10.1073/pnas.0501703102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Pulido C, Schuckit MA. Alcohol attenuates load-related activation during a working memory task: relation to level of response to alcohol. Alcoholism: Clinical and Experimental Research. 2006;30(8):1363–1371. doi: 10.1111/j.1530-0277.2006.00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nature Reviews Neuroscience. 2008;9(12):947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroff OA, Mattson RH, Rothman DL. Proton MRS: GABA and glutamate. Advances in Neurology. 2000;83:261–271. [PubMed] [Google Scholar]
- Pettegrew JW, Keshavan MS, Panchalingam K, Strychor S, Kaplan DB, Tretta MG, et al. Alterations in brain high-energy phosphate and membrane phospholipid metabolism in first-episode, drug-naive schizophrenics. A pilot study of the dorsal prefrontal cortex by in vivo phosphorus 31 nuclear magnetic resonance spectroscopy. Archives of General Psychiatry. 1991;48(6):563–568. doi: 10.1001/archpsyc.1991.01810300075011. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Spielman D, Sullivan EV, Lim KO. In Vivo Brain Concentrations of N-Acetyl Compounds, Creatine, and Choline in Alzheimer Disease. Archives of General Psychiatry. 1999;56(2):185–192. doi: 10.1001/archpsyc.56.2.185. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcoholism: Clinical and Experimental Research. 1997;21(3):521–529. doi: 10.1111/j.1530-0277.1997.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Pfleiderer B, Ohrmann P, Suslow T, Wolgast M, Gerlach AL, Heindel W, et al. N-acetylaspartate levels of left frontal cortex are associated with verbal intelligence in women but not in men: a proton magnetic resonance spectroscopy study. Neuroscience. 2004;123(4):1053–1058. doi: 10.1016/j.neuroscience.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Pouwels PJ, Brockmann K, Kruse B, Wilken B, Wick M, Hanefeld F, et al. Regional age dependence of human brain metabolites from infancy to adulthood as detected by quantitative localized proton MRS. Pediatric Research. 1999;46(4):474–485. doi: 10.1203/00006450-199910000-00019. [DOI] [PubMed] [Google Scholar]
- Pouwels PJ, Frahm J. Differential distribution of NAA and NAAG in human brain as determined by quantitative localized proton MRS. NMR in Biomedicine. 1997;10(2):73–78. doi: 10.1002/(sici)1099-1492(199704)10:2<73::aid-nbm448>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Pouwels PJ, Frahm J. Regional metabolite concentrations in human brain as determined by quantitative localized proton MRS. Magnetic Resonance in Medicine. 1998;39(1):53–60. doi: 10.1002/mrm.1910390110. [DOI] [PubMed] [Google Scholar]
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magnetic Resonance in Medicine. 1993;30(6):672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR in Biomedicine. 2001;14(4):260–264. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- Purdon SE, Valiakalayil A, Hanstock CC, Seres P, Tibbo P. Elevated 3T proton MRS glutamate levels associated with poor Continuous Performance Test (CPT-0X) scores and genetic risk for schizophrenia. Schizophrenia Research. 2008;99(1):218–224. doi: 10.1016/j.schres.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Raininko R, Mattsson P. Metabolite concentrations in supraventricular white matter from teenage to early old age: A short echo time 1H magnetic resonance spectroscopy (MRS) study. Acta Radiologica. 2010;51(3):309–315. doi: 10.3109/02841850903476564. [DOI] [PubMed] [Google Scholar]
- Ross AJ, Sachdev PS, Wen W, Valenzuela MJ, Brodaty H. Cognitive correlates of 1H MRS measures in the healthy elderly brain. Brain Research Bulletin. 2005;66(1):9–16. doi: 10.1016/j.brainresbull.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Taylor E, Brammer M. Linear age-correlated functional development of right inferior fronto-striatal-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Human Brain Mapping. 2007;28(11):1163–1177. doi: 10.1002/hbm.20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, et al. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Human Brain Mapping. 2006;27(12):973–993. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA. Results from the 2003 National Survey on Drug Use and Health: National Findings (Office of Applied Studies, NSDUH Series H-25, DHHS Publication No. SMA 04-3964) 2004 [Google Scholar]
- Scherf KS, Sweeney JA, Luna B. Brain basis of developmental change in visuospatial working memory. Journal of Cognitive Neuroscience. 2006;18(7):1045–1058. doi: 10.1162/jocn.2006.18.7.1045. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Correlation of white matter diffusivity and anisotropy with age during childhood and adolescence: A cross-sectional diffusion-tensor MR imaging study. Radiology. 2002;222(1):212–218. doi: 10.1148/radiol.2221010626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, McQueeny T, Nagel BJ, Eyler LT, Tapert SF. A preliminary study of functional magnetic resonance imaging response during verbal encoding among adolescent binge drinkers. Alcohol. 2010;44(1):111–117. doi: 10.1016/j.alcohol.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman RG, Rothman DL, Behar KL, Hyder F. Energetic basis of brain activity: implications for neuroimaging. Trends in Neurosciences. 2004;27(8):489–495. doi: 10.1016/j.tins.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Sikoglu EM, Jensen JE, Vitaliano G, Liso Navarro AA, Renshaw PF, Frazier JA, et al. Bioenergetic measurements in children with bipolar disorder: a pilot 31P magnetic resonance spectroscopy study. PLoS One. 2013;8(1):e54536. doi: 10.1371/journal.pone.0054536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri MM, Crowley DJ, Covell MJ, Acharya D, Sneider JT, Rosso IM, et al. Frontal lobe GABA levels during adolescence: Associations with impulsivity and response inhibition. Biological Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri MM, Rogowska J, McCaffrey A, Yurgelun-Todd DA. Adolescents at risk for alcohol abuse demonstrate altered frontal lobe activation during stroop performance. Alcoholism: Clinical and Experimental Research. 2011;35(2):1–11. doi: 10.1111/j.1530-0277.2010.01337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. The effects of NMDA and GABAA pharmacological manipulations on acute and rapid tolerance to ethanol during ontogeny. Alcoholism: Clinical and Experimental Research. 2004;28(6):884–894. doi: 10.1097/01.alc.0000128221.68382.ba. [DOI] [PubMed] [Google Scholar]
- Soliva JC, Moreno A, Fauquet J, Bielsa A, Carmona S, Gispert JD, et al. Cerebellar neurometabolite abnormalities in pediatric attention/deficit hyperactivity disorder: a proton MR spectroscopic study. Neuroscience Letters. 2010;470(1):60–64. doi: 10.1016/j.neulet.2009.12.056. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Jones RM, Casey BJ. A time of change: Behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain and Cognition. 2010;72(1):124–133. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nature Neuroscience. 1999;2(10):859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Developmental Medicine and Child Neurology. 2002;44:4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- Spadoni AD, Norman AL, Schweinsburg AD, Tapert SF. Effects of family history of alcohol use disorders on spatial working memory BOLD response in adolescents. Alcoholism: Clinical and Experimental Research. 2008;32(7):1135–1145. doi: 10.1111/j.1530-0277.2008.00694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Squeglia LM, Schweinsburg AD, Pulido C, Tapert SF. Adolescent binge drinking linked to abnormal spatial working memory brain activation: Differential gender effects. Alcoholism: Clinical and Experimental Research. 2011;35(10):1831–1841. doi: 10.1111/j.1530-0277.2011.01527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. Cognitive and affective development in adolescence. Trends in Cognitive Sciences. 2005;9(2):69–74. doi: 10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Steinberg L. A behavioral scientist looks at the science of adolescent brain development. Brain and Cognition. 2010;72(1):160–164. doi: 10.1016/j.bandc.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn JR, Chu WJ, Whitsel RM, Weber WA, Norris MM, Adler CM, et al. A Pilot Study of Anterior Cingulate Cortex Neurochemistry in Adolescents with Generalized Anxiety Disorder. Neuropsychobiology. 2013;67(4):224–229. doi: 10.1159/000347090. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Adalsteinsson E, Hedehus M, Ju C, Moseley M, Lim KO, et al. Equivalent disruption of regional white matter microstructure in ageing healthy men and women. Neuroreport. 2001;12(1):99–104. doi: 10.1097/00001756-200101220-00027. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology (Berl) 2005;180(4):583–594. doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- Sumner P, Edden RAE, Bompas A, Evans CJ, Singh KD. More GABA, less distraction: a neurochemical predictor of motor decision speed. Nature Neuroscience. 2010;13(7):825–827. doi: 10.1038/nn.2559. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Brown GG, Kindermann SS, Cheung EH, Frank LR, Brown SA. fMRI measurement of brain dysfunction in alcohol-dependent young women. Alcoholism: Clinical and Experimental Research. 2001;25(2):236–245. [PubMed] [Google Scholar]
- Tapert SF, Pulido C, Paulus MP, Schuckit MA, Burke C. Level of response to alcohol and brain response during visual working memory. Journal of Studies on Alcohol and Drugs. 2004;65(6):692–700. doi: 10.15288/jsa.2004.65.692. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Barlett VC, Brown SA, Frank LR, Brown GG, et al. Blood oxygen level dependent response and spatial working memory in adolescents with alcohol use disorders. Alcoholism: Clinical and Experimental Research. 2004;28(10):1577–1586. doi: 10.1097/01.alc.0000141812.81234.a6. [DOI] [PubMed] [Google Scholar]
- Tkac I, Andersen P, Adriany G, Merkle H, Ugurbil K, Gruetter R. In vivo 1H NMR spectroscopy of the human brain at 7 T. Magnetic Resonance in Medicine. 2001;46(3):451–456. doi: 10.1002/mrm.1213. [DOI] [PubMed] [Google Scholar]
- Tkac I, Oz G, Adriany G, Ugurbil K, Gruetter R. In vivo 1H NMR spectroscopy of the human brain at high magnetic fields: metabolite quantification at 4T vs. 7T. Magnetic Resonance in Medicine. 2009;62(4):868–879. doi: 10.1002/mrm.22086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toft PB, Christiansen P, Pryds O, Lou HC, Henriksen O. T1, T2, and concentrations of brain metabolites in neonates and adolescents estimated with H-1 MR spectroscopy. Journal of Magnetic Resonance Imaging. 1994;4(1):1–5. doi: 10.1002/jmri.1880040102. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM, Sowell ER. Mapping brain maturation. Trends in Neurosciences. 2006;29(3):148–159. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai G, Coyle JT. The role of glutamatergic neurotransmission in the pathophysiology of alcoholism. Annual Review of Medicine. 1998;49:173–184. doi: 10.1146/annurev.med.49.1.173. [DOI] [PubMed] [Google Scholar]
- Valenzuela MJ, Sachdev PS, Wen W, Shnier R, Brodaty H, Gillies D. Dual voxel proton magnetic resonance spectroscopy in the healthy elderly: subcortical-frontal axonal N-acetylaspartate levels are correlated with fluid cognitive abilities independent of structural brain changes. Neuroimage. 2000;12(6):747–756. doi: 10.1006/nimg.2000.0629. [DOI] [PubMed] [Google Scholar]
- van der Knaap MS, van der Grond J, van Rijen PC, Faber JA, Valk J, Willemse K. Age-dependent changes in localized proton and phosphorus MR spectroscopy of the brain. Radiology. 1990;176(2):509–515. doi: 10.1148/radiology.176.2.2164237. [DOI] [PubMed] [Google Scholar]
- Van Leijenhorst L, Zanolie K, Van Meel CS, Westenberg PM, Rombouts SARB, Crone EA. What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cerebral Cortex. 2011;20(1):61–69. doi: 10.1093/cercor/bhp078. [DOI] [PubMed] [Google Scholar]
- Volz HP, Hübner G, Rzanny R, Rößger G, Preußler B, Eichhorn M, et al. High-energy phosphates in the frontal lobe correlate with Wisconsin Card Sort Test performance in controls, not in schizophrenics: a 31phosphorus magnetic resonance spectroscopic and neuropsychological investigation. Schizophrenia Research. 1998;31(1):37–47. doi: 10.1016/s0920-9964(97)00157-6. [DOI] [PubMed] [Google Scholar]
- Wallimann T, Wyss M, Brdiczka D, Nicolay K, Eppenberger HM. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the 'phosphocreatine circuit' for cellular energy homeostasis. Biochemical Journal. 1992;281(Pt 1):21–40. doi: 10.1042/bj2810021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber OM, Trabesinger AH, Duc CO, Meier D, Boesiger P. Detection of hidden metabolites by localized proton magnetic resonance spectroscopy in vivo. Technology and Health Care. 1997;5(6):471–491. [PubMed] [Google Scholar]
- Whiteside SP, Abramowitz JS, Port JD. Decreased caudate N-acetyl-l-aspartic acid in pediatric obsessive-compulsive disorder and the effects of behavior therapy. Psychiatry Research. 2012;202(1):53–59. doi: 10.1016/j.pscychresns.2011.11.010. [DOI] [PubMed] [Google Scholar]
- Wiedermann D, Schuff N, Matson GB, Soher BJ, Du AT, Maudsley AA, et al. Short echo time multislice proton magnetic resonance spectroscopic imaging in human brain: metabolite distributions and reliability. Magnetic Resonance Imaging. 2001;19(8):1073–1080. doi: 10.1016/s0730-725x(01)00441-6. [DOI] [PubMed] [Google Scholar]
- Wolfson M, Bersudsky Y, Hertz E, Berkin V, Zinger E, Hertz L. A model of inositol compartmentation in astrocytes based upon efflux kinetics and slow inositol depletion after uptake inhibition. Neurochemical Research. 2000;25(7):977–982. doi: 10.1023/a:1007556509371. [DOI] [PubMed] [Google Scholar]
- Yakovlev PI, Lecours AR. Regional Development of the Brain in Early Life. Oxford: Blackwell Scientific; 1967. The myelogenetic cycles of regional maturation of the brain; pp. 3–70. [Google Scholar]
- Yeo RA, Hill D, Campbell R, Vigil J, Brooks WM. Developmental instability and working memory ability in children: A magnetic resonance spectroscopy investigation. Developmental Neuropsychology. 2000;17(2):143–143. doi: 10.1207/S15326942DN1702_01. [DOI] [PubMed] [Google Scholar]