ABSTRACT
Background: Sub-Saharan African countries utilize whole blood (WB) to treat severe anemia secondary to severe blood loss or malaria on an emergency basis. In many areas with high prevalence of transfusion-transmissible agents, blood safety measures are insufficient. Pathogen reduction technology applied to WB might considerably improve blood safety. Methods: Whole blood from 40 different donors were treated with riboflavin and UV light (pathogen reduction technology) in order to inactivate malaria parasite replication. The extent of parasite inactivation was determined using quantitative polymerase chain reaction methods and was correlated to studies evaluating the replication of malaria parasites in culture. Products were also stored for 21 days at +4°C and monitored for cell quality throughout storage. Results: Plasmodium amplicon was present in 21 samples (>100 copies/mL), doubtful in four (10–100 genome equivalents [gEq]/mL), and negative in 15 U. The majority of asymptomatic parasitemic donors carried low parasite levels, with only six donors above 5,000 copies/mL (15%). After treatment with riboflavin and UV light, these six samples demonstrated a 0.5 to 1.2 log reduction in quantitative polymerase chain reaction amplification. This correlated to equal to or greater than 6.4 log reductions in infectivity. In treated WB units, cell quality parameters remained stable; however, plasma hemoglobin increased to 0.15 g/dL. All markers behaved similarly to published data for stored, untreated WB. Conclusions: Pathogen reduction technology treatment can inactivate malaria parasites in WB while maintaining adequate blood quality during posttreatment cold storage for 21 days.
KEYWORDS: Whole blood, pathogen reduction, cold storage, Sub-Saharan Africa
INTRODUCTION
Blood transfusion in Sub-Saharan Africa (SSA) is plagued by chronic blood shortages, scarce resources, and a high prevalence of infectious agents transmissible by transfusion. Although routine use of serological testing can limit the transmission risk for human immunodeficiency virus, hepatitis B virus, hepatitis C virus, and syphilis, the same cannot be said for malaria. Screening of blood units for malaria is afflicted with either the poor sensitivity of microscopy and antigen testing or the high costs associated with genomic detection (1,2) Thus, in SSA, transfusion-transmitted malaria is frequent and clinically consequential, especially in young children and pregnant women.
Recent data demonstrated that, when tested with a sensitive polymerase chain reaction (PCR) assay, greater than 50% of donated blood from healthy donors contained parasites (1) compared with 3% by traditional microscopic methods. It was also shown that nonparasitemic recipients of parasitemic whole blood (WB) were infected with the donor parasite in approximately 40% of cases regardless of parasite load or level of antibody to Plasmodium species in the patient (1). Parasite-naive children, semi-immune young children, and pregnant women are at the greatest risk of morbidity from malarial infection transmitted by transfusion (3).
A pathogen reduction technology (PRT) using riboflavin as a photosensitizer in combination with a UV light illumination device (Mirasol System for Whole Blood; Terumo BCT, Lakewood, Colo) has focused on reducing the infectivity of blood-borne pathogens from donated WB products. This PRT product is nontoxic and nonmutagenic, and riboflavin and UV light-treated components have been shown to be safe for transfusion recipients as well as for those handling blood products (4). When added to a WB unit, riboflavin molecules can associate with the nucleic acids (both RNA and DNA) of viruses, bacteria, white blood cells (WBCs), and parasites (5). Exposure to UV light then activates riboflavin, inducing a chemical alteration to the functional groups of the nucleic acids (primarily guanine bases), reducing the ability of a pathogen to successfully replicate (5).
Previous work done using this PRT technology has shown the ability of the process to inactivate WBCs (6,7) and reduce parasites (8–10) and viruses (6,11,12) in WB units. Preliminary studies examining the efficacy of the riboflavin and UV light method in WB spiked with Plasmodium falciparum clearly showed genomic damage and inhibition of parasite replication, suggesting a distinct potential clinical benefit of this PRT method (10).
The objective of this study was 3-fold: first to confirm the ability of the riboflavin and UV light process to inactivate malaria parasites in WB collected in a hyperendemic area, as measured by quantitative PCR (qPCR), second to measure the reduction in infectivity of a laboratory-adapted strain of P. falciparum using an in vitro culture model after treatment, and third to determine the characteristics of the treated WB after up to 21 days of storage at 4°C.
MATERIALS AND METHODS
All treatments with riboflavin and UV light were conducted at energy levels of 80 J/mLRBC following addition of 35 mL of 500 μM riboflavin (6.2 mg) solution in 0.9% saline to units of WB. Equipment and training required for the riboflavin and UV light treatment were provided by Terumo BCT. Details of treatment are provided in El Chaar et al. (10).
Cell quality studies
Whole blood units were collected into CPD (Terumo CPD Collection Kit P/N: PB3AG456M8B) either at the Komfo Anokye Teaching Hospital (KATH) donor clinic in the hospital for family donors or from mobile collection (volunteer donors) sites in and around Kumasi. The time of blood collection, defined as the time the needle entered the vein of the donor, was noted for each unit. Blood units were labeled with “Not for Human Use” labels immediately after collection. Both the units collected at the hospital or via mobile collection were transported to the Transfusion Medicine Unit (TMU) at KATH laboratories in an isothermal container with cool packs, and upon arrival, the units were either treated immediately or stored overnight at 4°C until treatment. Processing with the Mirasol PRT System occurred within 24 h of collection.
Whole blood in the illumination bag was weighed and checked against the accepted guard band of 558 to 651 g. Riboflavin solution was added to the illumination bag through a sterile connection. Following transfer of the riboflavin solution, excess air in the bag was gently expelled. Whole blood and riboflavin were gently mixed until a homogenous solution was obtained. The target illumination volume was 493 to 603 mL (which includes 450 ±45 mL WB, 63 ±5 mL CPD, and 35 ± 5 mL riboflavin). A unit was excluded if it exceeded the manufacturer’s specifications for treatment volume. Prior to treatment, a sample pouch was connected to the inlet line to aseptically remove a 1-mL sample to determine the preillumination hematocrit. These samples were spun in a ZIPocrit (LW Scientific, Atlanta, Ga) hematocrit centrifuge for 5 min, and the value was recorded. The WB unit was weighed again to determine the final illumination weight.
Pretreatment and posttreatment (days 0, 7, 14, 21) WB samples were aseptically removed from the products and evaluated for blood gases (iSTAT System with CG4+ and EC4+ cartridges; Abbott Laboratories; Abbott Park, Ill), plasma hemoglobin (Plasma/Low Hb System; Hemocue, Ängelholm Sweden), and complete blood count (KX21-N Automated Hematology Analyzer; Sysmex America Inc., Lincolnshire, Ill). Cross-match compatibility was confirmed utilizing a Coombs test.
Analysis by PCR amplification
Analysis of PCR amplification inhibition and treatment of WB samples were conducted in the TMU facilities of KATH, a 1,200-bed tertiary hospital located in Kumasi, Ghana. This laboratory included a real-time PCR (qPCR) instrument (MX3000; Agilent, La Jolla, Calif) and a separate room with laminar flow hood where sample handling and nucleic acid extraction could be performed.
Training and supervision for the PCR methodology were provided by J.-P.A. and G.F. from University of Cambridge, UK.
Qualitative PCR and qPCR
The primers used in the study are described by Rougemont et al. (13). Two conserved regions of the P. falciparum genome equivalents (gEq) were amplified as previously reported (10,13,14). Species-specific primers (Sigma-Aldrich, St. Louis, Mo) were also designed for use in nested PCR as described by Freimanis et al. (1), one each against P. falciparum, Plasmodium vivax, Plasmodium malariae, and Plasmodium ovale.
All samples were tested in duplicate by qPCR first. Only samples that demonstrated a positive result with both replicates for the genus specific primers were quantified. Quantification of the 18S rRNA amplicon was performed as previously described (1) using the first WHO International Standard for P. falciparum DNA (NIBSC, code 04/176) appropriately diluted to construct a reference curve.
Genus- and species-specific nested PCR
Oligonucleotide primers for the nested PCR assay were obtained from Sigma-Aldrich and designed based on the Plasmodium small subunit ribosomal RNA genes (14). Nested PCR was performed as described by Freimanis et al. (1). Primers used for the assay are described by El Chaar et al. (10) and are specific to mtDNA.
In vitro culture of malaria parasites in treated blood samples
Plasmodium falciparum viability studies were carried out in the laboratory of Dr. Christine Olver at Colorado State University, Fort Collins, Colo. Treatment of these units used a scaled process in order to concentrate the P. falciparum. Each unit consisted of 210 mL of WB, 16 mL of riboflavin solution, and 10 mL of P. falciparum infected erythrocytes. Because of the lower volume, the units received an equivalent energy dose of 68 J/mLRBC. All other parameters remained the same. The P. falciparum was continuously cultured in human type A + red blood cells (RBCs) (initially from MR4 repository, www.mr4.org, 3D7 strain).
A 1-mL volume of packed RBCs with at least 1% parasitemia was cultured for 2 days in 50 mL (2% hematocrit) RPMI 1640 complete medium (CM) (RPMI; Gibco Life Technologies, Grand Island, New York) supplemented with HEPES, glutamine, gentamicin, sodium bicarbonate, and 10% human AB serum (Gibco Life Technologies, Grand Island, New York) in each of six 75-cm2 flasks. On day 3, the flasks were centrifuged, and spent medium was removed. Samples were resuspended in CM to a hematocrit of 50%. Parasitemia was determined using manual counting on thin smears. Inoculation of the P. falciparum into the WB unit yielded a theoretical final parasitemia of 0.5% to 0.6%. A 20-mL aliquot of blood was removed from the unit for pretreatment titer determination. Following treatment, a 20-mL aliquot of blood was removed from the unit for posttreatment titer determination.
Inoculation of cultures with blood from untreated control (pretreatment) and treated units
For untreated samples, quadruplicate wells of a microtiter plate were cultured using 10-fold dilutions (in uninfected cells), starting with a 100 dilution (through 10−10). Wells were checked for the presence of parasite using thick smears every 2 days out to 24 days. A 50% media change (100 μL) was performed every other day. Fresh cells were added to any parasitemia-negative wells at day 12. One flask (75 cm2) containing 2.5 mL of WB from the untreated unit, adjusted in CM to a hematocrit of 2%, was cultured to ensure the parasite was growing and expanding.
For treated samples, four 75-cm2 flasks containing undiluted packed cells from the treated sample were cultured at a hematocrit of 2%. An additional four 75-cm2 flasks containing a 1:10 dilution (into packed, uninfected cells) of the packed cells from the treated sample were also cultured at a hematocrit of 2%. Parasitemia was checked using thin and thick smears every 2 days until day 24. Fresh medium was added every 2 days. A volume of fresh cells, equal to that of original culture, was added on day 12.
An ID50 was calculated for both the pretreatment and posttreatment samples using the Spearman-Karber method. The log reduction was determined with the equation for reduction factor (RF):
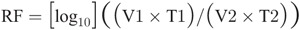
where V1 is volume of starting (input) material; T1, parasite concentration in starting material; V2, volume of material after delivery of UV light energy; T2, concentration of parasite after delivery of UV light energy.
Storage study of WB at +4°C for 21 days
Twenty-six WB units collected in CPD from either volunteer nonremunerated (15) or family (15) blood donors between November 10 and 21, 2013, were included in the study. Donor ages ranged between 17 and 45 years (median, 22 years). Eleven were females, and 15 were males. Two units were ultimately excluded from the analysis because they did not meet the inclusion criteria of the study. The 24 remaining units had a median collection weight of 572 ± 23 g and, following transfer of the units to illumination bags and the addition of 35 mL of riboflavin, a median pretreatment weight of 626 ± 19 g. During the interval between collection and initial testing, units were either treated upon arrival to KATH or were kept overnight at 4°C for median time of 2 h 20 min (range, 1–22 h). Because of the logistics of processing, ultimately only 2 U were stored overnight at 4°C prior to PRT, although all units collected outside the hospital were initially planned to be held overnight.
The riboflavin and UV treatment process was carried out at KATH with scientists trained to the process by Terumo BCT personnel (S.W. and S.D.K.).
Samples were exposed to UV light according to methods described previously at 80 J/mLRBC (10). After illumination, the treated WB unit was removed from the illuminator, and a 10-mL posttreatment sample was removed for in vitro testing. This unit was immediately stored at 4 ± 2°C with minimal exposure to ambient light and without agitation.
The riboflavin and UV-treated blood units were resampled for in vitro testing for complete blood count, pH, potassium, and plasma hemoglobin and cross-match testing on days 7, 14, and 21 of storage.
RESULTS
Plasmodium quantification in WB donation
In the 40 U WB tested at KATH by qPCR, the Plasmodium amplicon was clearly present in 21 samples (50%), doubtful in four (10%, where parasitemia <100 gEq/mL), and 15 (40%) were negative. This distribution (53% parasitemic) is fully compatible with what has been previously found in the same population of donors (1). Unsurprisingly, microscopy was positive in only 3 of 40 U (7.5%), including the 2 U with the highest parasitemia according to qPCR results. As previously described, P. falciparum was dominant (14/20); P. malariae (6/20) and P. ovale (2/20) were also observed, often associated with P. falciparum (5/6 P. malariae and one of two P. ovale) (1).
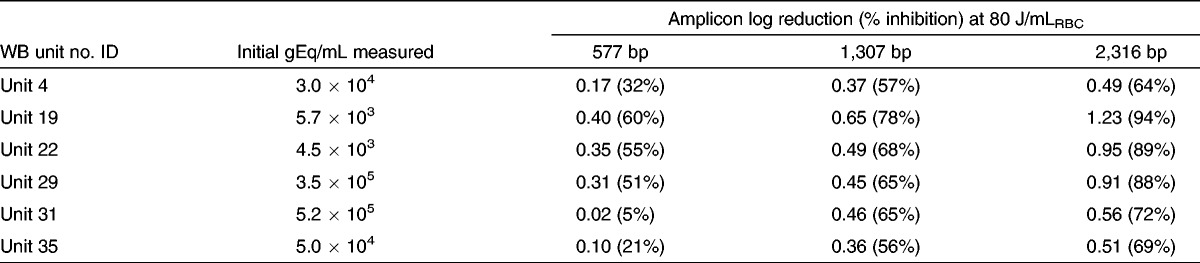
The distribution of parasitemia is shown in Figure 1. The majority of parasitemic donors carried levels between 10 and 1,000 gEq/mL, and only six were more than 5,000 gEq/mL. The limit of quantification for the qPCR assay is such that it can be applied only to samples containing more than 5,000 gEq/mL. As a result, only samples from unit 4: 3 × 104; unit 19: 5.7 × 103; unit 22: 4.5 × 103; unit 29: 3.5 × 105; unit 31: 5.2 × 105; and unit 35: 5.0 × 104 could be evaluated with the assay. Results shown in Table 1 were generally reproducible across the 6 U treated with 80 J/mLRBC energy. Log inhibition of the 2,316–base pair (bp) amplicon, the longest amplicon tested, ranged between 0.49 and 1.23 (mean, 0.78). Log inhibition of the amplification increased as the size of the amplicon increased, as expected and as has been reported previously (16–19). In 3 U, further treated to 120 J/mLRBC, the level of log inhibition did not increase notably (data not shown).
Fig. 1.
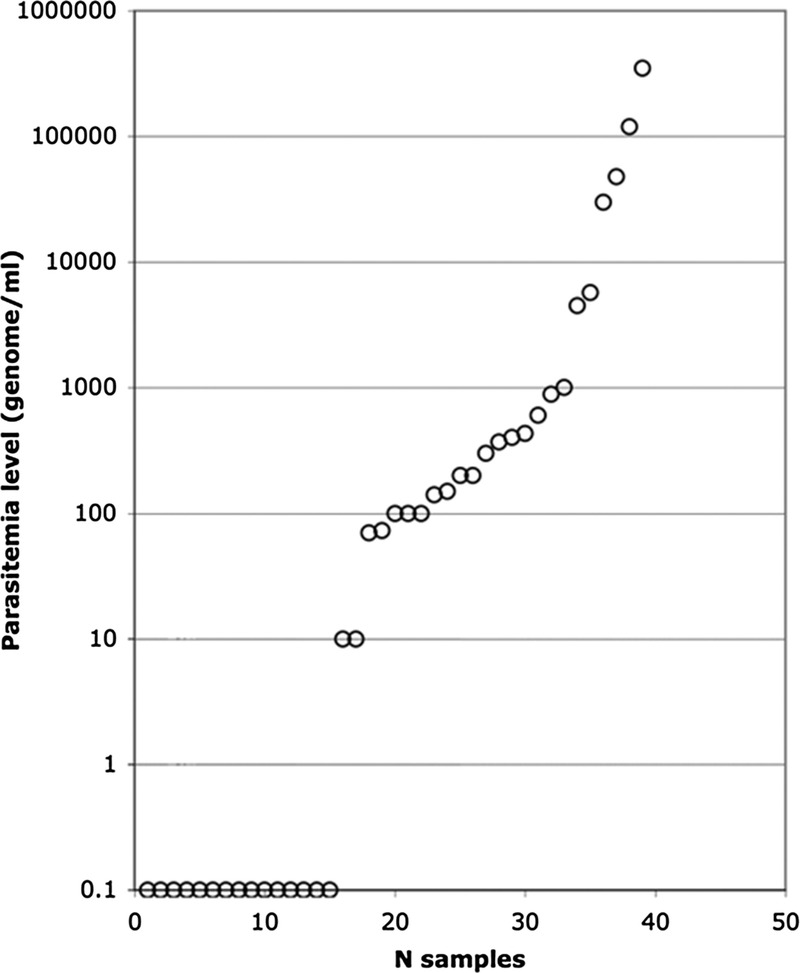
Distribution of Plasmodium gEq levels in KATH blood donors.
Table 1.
Amplicon inhibition in six high parasitemia WB units
P. falciparum culture
The results for four replicate experiments are shown in Table 2. Each sample set demonstrated that the Mirasol treatment markedly reduced the growth of P. falciparum parasites compared with untreated controls. The mean log reduction for the Mirasol treatment was 6.4 log or greater.
Table 2.
Results from culture studies on parasitemia in treated and untreated units of WB

WB storage at +4°C after treatment with riboflavin and UV light
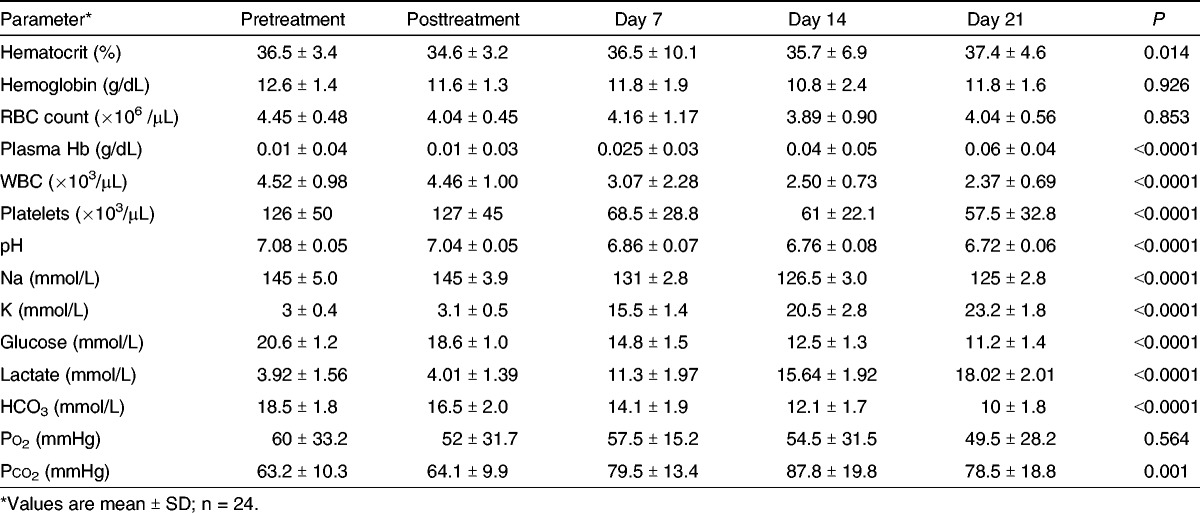
All 24 evaluable units were tested for multiple parameters prior to and after addition of riboflavin followed by UV illumination at 80 J/mLRBC per unit and stored at 4 ± 2°C for 21 days. Control WB units were not stored and tested in parallel in order to avoid depleting the TMU blood stock for patient treatment. Table 3 shows a summary of the data collected. First, the impact of the addition of riboflavin, transfer to the illumination bag, and illumination at 80 J/mLRBC was examined by comparing T-pre (before illumination) and T-post (after illumination) results. The addition of 35 mL of riboflavin caused a slight dilution of the WB units, which is reflected as a statistically significant drop in hematocrit, hemoglobin level, RBC count, glucose concentration, and pH (Table 3). The procedure did not cause immediate hemolysis because the plasma hemoglobin level was identical pre- and post-PRT.
Table 3.
Impact of riboflavin and UV illumination and 21-d storage time on 24 U WB
The impact of storage on treated WB for 21 days at 4°C ± 2°C was examined by comparing data between WB units immediately after PRT at T-post and at 21 days of storage. Detailed cell quality data can be found in Table 3. In order to maintain full availability of WB and blood components for clinical use during the 12-day entry period of the WB storage study, no control units were included, and comparisons were made with published data (Table 4).
Table 4.
Comparison of biological markers in WB and RBC concentrates during storage at +4°C

A low but significant amount of hemolysis took place as evidenced by the progressive increase in plasma hemoglobin over time reaching an average of 0.06 +/− 0.04 g/dL at day 21 of storage (Table 3). The dilution factor did not significantly affect the WBCs or platelet counts, but at greater than 21 days of storage, both cell type counts declined significantly (P < 0.001, Table 3). Ultimately, all units met the Council of Europe Guidelines posttreatment and after day 21 (pH >6.2, hemolysis <0.8%, total hemoglobin >45 g/U, and cross-match compatible with allogeneic plasma).
Storage significantly affected pH and electrolyte levels: moderate acidification and decrease in Na+ levels but considerable increase in K+ levels. Glucose levels significantly declined over time of storage, whereas lactate increased in parallel with potassium levels (Table 3). For both potassium and lactate, most of the increase was observed after 7 days of storage.
Bicarbonate levels steadily decreased after WB PRT. Po2 remained essentially unchanged (P value nonsignificant; Table 3), although large variations were observed between WB units. Pco2 significantly increased during storage also showing wide variations between individual units.
DISCUSSION
The objective of this study was to confirm that the PRT can inactivate malaria parasites in WB collected in a hyperendemic area, as measured by qPCR, and to correlate this to a laboratory-adapted strain of P. falciparum using an in vitro culture model after treatment. Last, the study was able to define the RBC quality characteristics of the treated WB after up to 21 days of storage at 4°C.
While the qPCR results showed a 1.2 log reduction, and the culture results gave an equal to or greater than 6.4 log reduction, it is important to remember that these two techniques measure different end points. The qPCR results only give an indication of the DNA damage to a small 2,316-bp target region out of the total 23 Mb gEq (23), whereas the culture result measures the infectivity of the organism. Thus, the 1.2 log reduction in amplification of the 2,316-bp amplicon corresponds to an equal to or greater than 6.4 log reduction in P. falciparum infectivity.
In addition, the results from PCR analysis of donated blood from routine collections demonstrate that 50% of the collected products were infected with malaria parasites. These results are consistent with those reported previously (1). Likewise, the qPCR amplification inhibition observed for products collected locally agrees with data reported previously for samples spiked with the 3D7 laboratory-adapted strain of P. falciparum (10).
Given that the observed levels of parasitemia present in donated samples in this study did not exceed 105 gEq/mL, it would be expected that the transmissibility of parasitemia from infected donor units following treatment would be significantly curtailed.
In order for such a technique to be effective, treated WB unit quality and function should not be compromised. Studies of the toxicology (4) and effectiveness of transfused blood in animal models of hemorrhage (24,25) have shown the ability of treated blood to maintain functional characteristics without inducing enhanced toxicity or adverse events associated with transfusion. The present study also monitored 14 parameters for 24 U of WB prior to and after PRT and subsequent 21 days of refrigerated storage.
In order to maintain full availability of WB and blood components for clinical use during the 12-day entry period of the WB storage study, no control units were included, and comparisons were made with published data (Table 4). The closest comparison was with a group of eight WB units tested for hematocrit, RBC, WBC, and platelet counts; pH; and concentrations of lactate and glucose (26). Hematocrit and RBC counts remained stable in both groups, pH levels declined similarly to riboflavin- and UV-treated units. In published data, WBC counts remained stable but declined substantially in treated WB. In both published untreated WB studies and the present PRT WB results, platelet counts declined over storage but more markedly in treated than in untreated WB. This comparison suggests that although WBC and platelet counts did not significantly differ before and after PRT (Table 3), some damage took place on both cell types that became apparent during storage. After 21 days of storage, glucose levels declined 60% in untreated WB units and 40% in PRT WB. However, in the study by Pidcoke et al. (20), the decline in glucose levels during storage was identical between the eight control units and eight treated WB (21).
In the study by Latham et al. (21), 11 of the 14 markers reported in the present study were described for 10 U of WB collected in CPDA-1 (22). Similar to data in Table 3, hemoglobin level, hematocrit, RBC counts, and Na+ concentration remained stable (Table 4). Hemolysis reflected in plasma hemoglobin levels was 0.2% compared with 0.5% in the present study. White blood cell counts and potassium and lactate concentrations were similar; glucose declined by 26% versus 40% in the present study. Bicarbonate levels remained stable instead of significantly declining, and pH decreased by 2.4% instead of 4.6% (Table 3). In the absence of individual WB unit values, statistical comparison could not be performed, but except for glucose and bicarbonate levels, most biological markers of WB treated or untreated behaved comparably. Some markers monitored in treated WB were also compared with red cell concentrate (RCC) as reported by Burger et al. (22). At 21 days of storage of CPD RCC, hemolysis was 0.1%, but pH declined 6.5%, lactate increased 4.8 times, glucose level decreased 27.5%, and potassium level increased by 10%, suggesting that erythrocytes are slightly better conserved in WB than in RCC (Table 4).
Although WB is the most used blood product in SSA, blood components are systematically produced by major blood centers often supported by external funding. RCC are requested for treatment of severe anemia related to hemoglobinopathies or pediatric malaria; fresh frozen plasma (FFP) is frequently used in surgery and obstetrics-gynecology, and the relatively recent availability of chemotherapy for cancer treatment requires a limited supply of platelet concentrates. It would therefore be important to examine whether PRT WB can be further processed into components and meet the generally accepted quality guidelines. It was previously shown that 3 U of WB treated at an energy level of 44 J/mLRBC separated into RCC had a 24-h in vivo recovery of radiolabeled RBCs ranging between 60% and 75% after 42 days of storage (27). The UV illumination energy was lower (44 vs. 80 J/mLRBC), but the storage time was twice as long as in the present study (44 vs. 21 days), preventing direct comparison. Studies using samples treated at 80 J/mLRBC are currently ongoing in the United States under an Investigational Device Exemption (IMPROVE II study, NCT01907906). Preliminary results indicate that RBCs derived from these WB products meet Food and Drug Administration criteria for RBC recovery after 21 days of storage (personal communication, written and oral, August 2014, R.P.G.).
Another study compared clotting factors and platelet functions in PRT-treated and untreated WB immediately and over 21 days of 4°C storage (20). Prothrombin time, partial thromboplastin time, and thromboelastographic responses were not immediately affected by treatment, but at 24 h of storage, prothrombin time and partial thromboplastin times increased by 3 and 10 s, respectively. Levels of clotting factors (fibrinogen, factor V, factor VIII, von Willebrand factor, protein C, and antithrombin III) were not affected immediately by PRT, but after 24 h of storage, the level of the first three factors declined by 10% to 50%. These limited data suggest that FFP prepared immediately after PRT may preserve essential clotting ability.
Platelet aggregation with ADP and thrombin receptor-activating peptide were moderately affected by riboflavin + UV and declined in parallel with the control when refrigerated. Aggregation with collagen, arachidonic acid antagonist, and high-dose ristocetin were not significantly affected at day 0. The functional data added to the platelet count decline suggest that PRT WB affects platelets and that cold storage rapidly reduces their hemostatic capacity. The Pidcoke et al. study investigated WB function via clot strength using thromboelastographic measurements and found that samples that were PRT treated, whether stored refrigerated or not, remained within normal subject limits throughout the duration of the study (21 days) (20). Studies need to be conducted to determine the properties of both FFP and platelet concentrate prepared immediately after WB PRT.
The results presented here represent the first report for inactivating malaria parasites in donated WB products using riboflavin and UV light while maintaining adequate cell storage characteristics for stored WB for up to 21 days after treatment. The ultimate test is to examine these results in vivo, and the efficacy of the treated product is currently being tested in a clinical trial in Ghana that investigates whether treatment can reduce transfusion-transmitted malaria (AIMS Study, NCT02118428). If proven effective in the clinical setting for preventing malaria transmission by transfusion, this technique may provide a practical method to greatly enhance current blood safety profiles for donated blood products in this region (28).
Footnotes
This work was supported by a grant (W81XWH-09-2-0100) from the US Department of Defense.
R.P.G., S.D.K., S.W., and J.M.M. are employees of Terumo BCT, an organization involved in the development of pathogen reduction technology. J.-P.A., S.O.-O., and C.O. have received research grant support from Terumo BCT.
REFERENCES
- 1. Freimanis G, Sedegah M, Owusu-Ofori S, Kumar S, Allain JP: Investigating the prevalence of transfusion transmission of Plasmodium within a hyperendemic blood donation system. Transfusion 53 (7): 1429– 41, 2013. [DOI] [PubMed] [Google Scholar]
- 2. Owusu-Ofori AK, Betson M, Parry CM, Stothard JR, Bates I: Transfusion-transmitted malaria in Ghana. Clin Infect Dis 56 (12): 1735– 1741, 2013. [DOI] [PubMed] [Google Scholar]
- 3. Owusu-Ofori AK, Parry C, Bates I: Transfusion-transmitted malaria in countries where malaria is endemic: a review of the literature from Sub-Saharan Africa. Clin Infect Dis 51 (10): 1192– 1198, 2010. [DOI] [PubMed] [Google Scholar]
- 4. Reddy HL, Dayan AD, Cavagnaro J, Gad S, Li J, Goodrich RP: Toxicity testing of a novel riboflavin-based technology for pathogen reduction and white blood cell inactivation. Transfus Med Rev 22 (2): 133– 153, 2008. [DOI] [PubMed] [Google Scholar]
- 5. Mundt JM, Rouse L, Van den Bossche J, Goodrich RP: Chemical and Biological Mechanisms of Pathogen Reduction Technologies. Photochem Photobiol 90 (5): 957– 964, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goodrich RP, Doane S, Reddy HL: Design and development of a method for the reduction of infectious pathogen load and inactivation of white blood cells in whole blood products. Biologicals 38 (1): 20– 30, 2010. [DOI] [PubMed] [Google Scholar]
- 7. Fast LD, Nevola M, Tavares J, Reddy HL, Goodrich RP, Marschner S: Treatment of whole blood with riboflavin plus ultraviolet light, an alternative to gamma irradiation in the prevention of transfusion-associated graft-versus-host disease? Transfusion 53 (2): 373– 81, 2013. [DOI] [PubMed] [Google Scholar]
- 8. Tonnetti L, Thorp AM, Reddy HL, Keil SD, Goodrich RP, Leiby DA: Evaluating pathogen reduction of Trypanosoma cruzi with riboflavin and ultraviolet light for whole blood. Transfusion 52 (2): 409– 416, 2012. [DOI] [PubMed] [Google Scholar]
- 9. Tonnetti L, Thorp AM, Reddy HL, Keil SD, Goodrich RP, Leiby DA: Riboflavin and ultraviolet light reduce the infectivity of Babesia microti in whole blood. Transfusion 53 (4): 860– 867, 2013. [DOI] [PubMed] [Google Scholar]
- 10. El Chaar M, Atwal S, Freimanis GL, Dinko B, Sutherland CJ, Allain JP: Inactivation of Plasmodium falciparum in whole blood by riboflavin plus irradiation. Transfusion 53 (12): 3174– 3183, 2013. [DOI] [PubMed] [Google Scholar]
- 11. Marschner S, Goodrich R: Pathogen reduction technology treatment of platelets, plasma and whole blood using riboflavin and UV light. Transfus Med Hemother 38: 8– 18, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Keil S, Rapaport R, Doane S, Young R, Marschner S, Campbell T: Viral reduction of intracellular HIV using the Mirasol system for whole blood [poster abstract]. Vox Sang 103: 68– 271, 2012. [Google Scholar]
- 13. Rougemont M, Van SM, Sahli R, Hinrikson HP, Bille J, Jaton K: Detection of four Plasmodium species in blood from humans by 18S rRNA gene subunit-based and species-specific real-time PCR assays. J Clin Microbiol 42 (12): 5636– 5643, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Singh B, Bobogare A, Cox-Singh J, Snounou G, Abdullah MS, Rahman HA: A genus- and species-specific nested polymerase chain reaction malaria detection assay for epidemiologic studies. Am J Trop Med Hyg 60 (4): 687– 692, 1999. [DOI] [PubMed] [Google Scholar]
- 15. Ala F, Allain JP, Bates I, Boukef K, Boulton F, Brandful J, Dax EM, El EM, Farrugia A, Gorlin J, et al. : External financial aid to blood transfusion services in Sub-Saharan Africa: a need for reflection. PLoS Med 9 (9): e1001309, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bakkour S, Chafets DM, Wen L, van der Meer PF, Mundt JM, Marschner S, Goodrich RP, Busch MP, Lee TH: Development of a mitochondrial DNA real-time polymerase chain reaction assay for quality control of pathogen reduction with riboflavin and ultraviolet light. Vox Sang 107 (4): 351– 359, 2014. [DOI] [PubMed] [Google Scholar]
- 17. Aytay S, Ohagen A, Busch MR, Alford B, Chapman JR, Lazo A: Development of a sensitive PCR inhibition method to demonstrate HBV nucleic acid inactivation. Transfusion 44 (4): 476– 484, 2004. [DOI] [PubMed] [Google Scholar]
- 18. Allain JP, Hsu J, Pranmeth M, Hanson D, Stassinopoulos A, Fischetti L, Corash L, Lin L: Quantification of viral inactivation by photochemical treatment with amotosalen and UV A light, using a novel polymerase chain reaction inhibition method with preamplification. J Infect Dis 194 (12): 1737– 1744, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sawyer L, Hanson D, Castro G, Luckett W, Dubensky TW, Jr, Stassinopoulos A: Inactivation of parvovirus B19 in human platelet concentrates by treatment with amotosalen and ultraviolet A illumination. Transfusion 47 (6): 1062– 1070, 2007. [DOI] [PubMed] [Google Scholar]
- 20. Pidcoke H, McFaul S, Ramasubramanian A, Parida B, Mora A, Fedyk C, Valdez-Delgado K, Montgomery R, Reddoch K, Rodriguez A, et al. : Primary hemostatic capacity of whole blood: a comprehensive analysis of pathogen reduction and refrigeration effects over time. Transfusion 53 Suppl 1: 137S– 49S, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Latham JT, Jr, Bove JR, Weirich FL: Chemical and hematologic changes in stored CPDA-1 blood. Transfusion 22 (2): 158– 159, 1982. [DOI] [PubMed] [Google Scholar]
- 22. Burger P, Korsten H, Verhoeven AJ, de Korte D, van BR: Collection and storage of red blood cells with anticoagulant and additive solution with a physiologic pH. Transfusion 52 (6): 1245– 1252, 2012. [DOI] [PubMed] [Google Scholar]
- 23. Gardner MJ, Hall N, Fung E, White O, Berriman M, Hyman RW, Carlton JM, Pain A, Nelson KE, Bowman S, et al. : Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 419 (6906): 498– 511, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Okoye O, Reddy H, Wong M, Doane S, Resnick S, Karamanos E: Large animal evaluation of the safety and efficacy of whole blood treated with the Mirasol System in a diffuse, non-surgical bleeding model. Transfusion 53 (Suppl 1): 82A, 2013. [Google Scholar]
- 25. Reddy H, Doane S, Keil S, Marschner S, Goodrich R: Development of a riboflavin and ultraviolet light–based device to treat whole blood. Transfusion 53 (Supp 1): 131S– 6S, 2013. [DOI] [PubMed] [Google Scholar]
- 26. Parpart AK, Gregg JR, Lorenz PB, Parpart ER, Chase AM: Whole blood preservation; a problem in general physiology. An in vitro analysis of the problem of blood storage. J Clin Invest 26 (4): 641– 654, 1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cancelas JA, Rugg N, Fletcher D, Pratt PG, Worsham DN, Dunn SK, Marschner S, Reddy HL, Goodrich RP: In vivo viability of stored red blood cells derived from riboflavin plus ultraviolet light–treated whole blood. Transfusion 51 (7): 1460– 1468, 2011. [DOI] [PubMed] [Google Scholar]
- 28. Allain J, Owusu-Ofori S, Bates I: Blood transfusion in Sub-Saharan Africa. TATM 6: 16– 23, 2004. [Google Scholar]


