Abstract
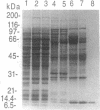
Previously we reported the isolation of two cytosolic fractions (A and B) from Xenopus ovary that are required sequentially to support protein import into the nuclei of digitonin-permeabilized cells. Fraction A is required for recognition of the nuclear localization sequence and targeting to the nuclear envelope, whereas fraction B is required for the subsequent translocation of the bound substrate into the nucleus. The first protein required for fraction B activity to be purified was the small GTPase Ran (ras-related nuclear protein). Here we report the purification of the second (and final) protein required for fraction B activity. By SDS/PAGE, the purified protein appeared as a single band with an apparent molecular mass of 10 kDa, but the native protein fractionated upon gel filtration chromatography with an apparent size of 30 kDa. Peptide sequence analysis indicated that the purified protein was highly homologous to a previously identified human protein of unknown function called placental protein 15 (pp15) and to the predicted protein product of a yeast open reading frame from Saccharomyces cerevisiae.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam E. J., Adam S. A. Identification of cytosolic factors required for nuclear location sequence-mediated binding to the nuclear envelope. J Cell Biol. 1994 May;125(3):547–555. doi: 10.1083/jcb.125.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff F. R., Klebe C., Kretschmer J., Wittinghofer A., Ponstingl H. RanGAP1 induces GTPase activity of nuclear Ras-related Ran. Proc Natl Acad Sci U S A. 1994 Mar 29;91(7):2587–2591. doi: 10.1073/pnas.91.7.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff F. R., Ponstingl H. Catalysis of guanine nucleotide exchange on Ran by the mitotic regulator RCC1. Nature. 1991 Nov 7;354(6348):80–82. doi: 10.1038/354080a0. [DOI] [PubMed] [Google Scholar]
- Bischoff F. R., Ponstingl H. Mitotic regulator protein RCC1 is complexed with a nuclear ras-related polypeptide. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10830–10834. doi: 10.1073/pnas.88.23.10830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn H., Johannsen R., Kraus W. Neues Plazentaprotein (PP15) mit immunsuppressiver Wirkung. Arch Gynecol. 1980;230(2):167–172. doi: 10.1007/BF02108272. [DOI] [PubMed] [Google Scholar]
- Bohn H., Kraus W., Winckler W. New soluble placental tissue proteins: their isolation, characterization, localization and quantification. Placenta Suppl. 1982;4:67–81. [PubMed] [Google Scholar]
- Coutavas E., Ren M., Oppenheim J. D., D'Eustachio P., Rush M. G. Characterization of proteins that interact with the cell-cycle regulatory protein Ran/TC4. Nature. 1993 Dec 9;366(6455):585–587. doi: 10.1038/366585a0. [DOI] [PubMed] [Google Scholar]
- Dalrymple M. A., Petersen-Bjorn S., Friesen J. D., Beggs J. D. The product of the PRP4 gene of S. cerevisiae shows homology to beta subunits of G proteins. Cell. 1989 Sep 8;58(5):811–812. doi: 10.1016/0092-8674(89)90930-6. [DOI] [PubMed] [Google Scholar]
- Dasso M. RCC1 in the cell cycle: the regulator of chromosome condensation takes on new roles. Trends Biochem Sci. 1993 Mar;18(3):96–101. doi: 10.1016/0968-0004(93)90161-f. [DOI] [PubMed] [Google Scholar]
- Duronio R. J., Gordon J. I., Boguski M. S. Comparative analysis of the beta transducin family with identification of several new members including PWP1, a nonessential gene of Saccharomyces cerevisiae that is divergently transcribed from NMT1. Proteins. 1992 May;13(1):41–56. doi: 10.1002/prot.340130105. [DOI] [PubMed] [Google Scholar]
- Grundmann U., Nerlich C., Rein T., Lottspeich F., Küpper H. A. Isolation of cDNA coding for the placental protein 15 (PP15). Nucleic Acids Res. 1988 May 25;16(10):4721–4721. doi: 10.1093/nar/16.10.4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounsbury K. M., Beddow A. L., Macara I. G. A family of proteins that stabilize the Ran/TC4 GTPase in its GTP-bound conformation. J Biol Chem. 1994 Apr 15;269(15):11285–11290. [PubMed] [Google Scholar]
- Melchior F., Paschal B., Evans J., Gerace L. Inhibition of nuclear protein import by nonhydrolyzable analogues of GTP and identification of the small GTPase Ran/TC4 as an essential transport factor. J Cell Biol. 1993 Dec;123(6 Pt 2):1649–1659. doi: 10.1083/jcb.123.6.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M. S., Blobel G. A G protein involved in nucleocytoplasmic transport: the role of Ran. Trends Biochem Sci. 1994 May;19(5):211–216. doi: 10.1016/0968-0004(94)90024-8. [DOI] [PubMed] [Google Scholar]
- Moore M. S., Blobel G. The GTP-binding protein Ran/TC4 is required for protein import into the nucleus. Nature. 1993 Oct 14;365(6447):661–663. doi: 10.1038/365661a0. [DOI] [PubMed] [Google Scholar]
- Moore M. S., Blobel G. The two steps of nuclear import, targeting to the nuclear envelope and translocation through the nuclear pore, require different cytosolic factors. Cell. 1992 Jun 12;69(6):939–950. doi: 10.1016/0092-8674(92)90613-h. [DOI] [PubMed] [Google Scholar]
- Newmeyer D. D., Forbes D. J. An N-ethylmaleimide-sensitive cytosolic factor necessary for nuclear protein import: requirement in signal-mediated binding to the nuclear pore. J Cell Biol. 1990 Mar;110(3):547–557. doi: 10.1083/jcb.110.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newmeyer D. D., Forbes D. J. Nuclear import can be separated into distinct steps in vitro: nuclear pore binding and translocation. Cell. 1988 Mar 11;52(5):641–653. doi: 10.1016/0092-8674(88)90402-3. [DOI] [PubMed] [Google Scholar]
- Nishimoto T., Eilen E., Basilico C. Premature of chromosome condensation in a ts DNA- mutant of BHK cells. Cell. 1978 Oct;15(2):475–483. doi: 10.1016/0092-8674(78)90017-x. [DOI] [PubMed] [Google Scholar]
- Novick P., Garrett M. D. Vesicular transport. No exchange without receipt. Nature. 1994 May 5;369(6475):18–19. doi: 10.1038/369018a0. [DOI] [PubMed] [Google Scholar]
- Richardson W. D., Mills A. D., Dilworth S. M., Laskey R. A., Dingwall C. Nuclear protein migration involves two steps: rapid binding at the nuclear envelope followed by slower translocation through nuclear pores. Cell. 1988 Mar 11;52(5):655–664. doi: 10.1016/0092-8674(88)90403-5. [DOI] [PubMed] [Google Scholar]