Abstract
Background
We have limited knowledge of the geographic distribution of resistant EAC in the resected specimen and its clinical importance can be enormous.
Method
We selected patients with baseline stage III EAC who had chemoradiation followed by surgery, and had residual EAC (resistant cases only). Outcomes were correlated with various endpoints (% of resistant EAC, anatomic distribution).
Results
100 clinical stage III patients were studied. 90% had an R0 resection. 99% had either moderate or poorly differentiated EAC. 12% had >50% residual cancer, 31% had 11–50% residual cancer, 53% had 1–10% residual cancer, and 3% had positive nodes only. Each compartment was frequently involved: mucosa/submucosa=66%, muscularis propria=76%, serosa=62%, and all=35%. Lack of EAC (meaning response) was observed in mucosa/submucosa (34%), muscularis propria (24%), serosa (38%), and nodes (42%). Although the endoscopic biopsies prior to surgery had no EAC in 79% of patients, in the surgical specimen, however, resistant EAC was frequent (66%) in mucosa/submucosa.
Conclusion
Contrary to our belief that resistant EAC would be frequent in the nodes, our data show that its distribution is heterogeneous and unpredictable. Most importantly, the post-chemoradiation biopsies are misleading and a decision to delay/avoid surgery based on negative biopsies can be detrimental for the patients.
Keywords: Esophageal adenocarcinoma, chemoradiation, surgery, treatment decisions
Introduction
Esophageal adenocarcinoma (EAC) accounts for 1.1% of all new cancers in the United States. It is estimated that 18,170 new cases and 15,450 deaths from esophageal cancer (EC) will occur in 2014.1 EAC comprises of >67% of all cases of EC in the United States.1 The treatment of EAC depends on the clinical stage but the post-treatment surgical stage is a better determinant of prognosis.2–5 There is an inter-patient variability in the degree of response to chemoradiation and ~25% of patients achieve a complete response6 but the remaining have resistant EAC in the surgical specimen. Assessment of histopathologic response after treatment was proposed first in osteosarcoma using the percentage of residual tumor along with regressive changes but was further refined in ovarian cancer.7 Since then, it has been applied to many solid tumors. The Tumor Regression Grading system (TRG) for EC cancer was first described by Mandard et al.8 Chirieac et al.3 modified the TRG system and this modification has been validated in a multi-institutional setting.2
The geographical patterns of resistant EAC can be important in developing novel therapeutic strategies and consolidating the current ones; however, this information remains limited. Shapiro et al. reported one noteworthy effort9 in which 102 consecutive patients with EC (squamous and adenocarcinoma both and they included cases that did not have any residual cancer in the specimen) were analyzed for geographic distribution of EC after chemoradiation. Only 74 patients had EAC (it is not clear how many of these had resistant EAC). 70% of all EC patients had residual EC and among these only 57 patients had baseline T3 (may be most with baseline stage III but it is unclear how many had EAC) EC. Our analysis differs from the prior report. We entirely focused on EACs and on baseline stage III patients with resistant EACs. We selected baseline stage III EAC (because this is the most prevalent localized EAC population in the clinics) and we compared pre-surgery parameters (pre-surgery biopsies) with the findings in the surgical specimens. We also focused on the clinical importance of the distribution of resistant EAC in the surgical specimen. Our results support some of the current trends in the management of localized EAC.
Material and Methods
Patient Population
The purpose of this study was to assess the geographic distribution of resistant EAC in the surgical specimen after chemoradiation. We selected patients with baseline stage III EAC by 6th Edition of AJCC.10 Between years 2000 and 2013, 100 EAC patients who had chemoradiation followed by elective surgery at the University of Texas MD Anderson Cancer Center (UTMDACC) were identified. Patients with pathologic complete response were excluded. No other selection criteria were implemented. The UTMDACC IRB approved this analysis.
Staging and Grading
Pretreatment clinical stage was established by endoscopy and biopsies, endoscopic ultrasonography (EUS) with fine needle aspiration of suspected lymph nodes, if needed/feasible, computed tomography (CT) of the chest and abdomen, and positron emission tomography (PET). All staging data on each patient were reviewed in our multidisciplinary conference.
Chemoradiation and Surgery
All patients received concurrent chemotherapy (fluoropyrimidine [i.v. or oral] and the second agent was either a platinum compound or taxane) with a total radiation dose of 45–50.4 Gy, delivered in daily fractions of 1.8 Gy. ~6 weeks after chemoradiation,
All patients underwent pre-surgery evaluation including endoscopic biopsies and repeat PET within 7 week from the end of chemoradiation. Patients then proceeded to surgery and the primary surgeon selected the type of surgery.
Evaluation of Surgical Specimen
Our validated method of surgical specimen examination has been in implementation.2, 3 In brief, each case was assessed for the percentage of resistant EAC, yp staging by the 7th Edition of AJCC11, tumor differentiation, and tumor distribution. Geographic distribution was designated in 4 compartments: (1) mucosa/submucosa, (2) muscularis propria, (3) serosa, and (4) nodes. Certain specimens were re-reviewed if the original pathology report was not comprehensive. We did not purposely re-review each specimen so that we could present the information in accordance with the normal flow of medical information (real time) in our system that leads to current patterns of treatment decisions. Re-review not being the standard of care could have introduced biases thus going forward re-review would not have been practical.
Statistical Analysis
Univariate Cox proportional hazards regression was used to identify any association between each factor and the each survival outcome. For each factor, medians, hazard ratios (HR), their 95% confidence intervals (CI), and proportional hazards regression p-values were established. Statistical analysis was performed using STATA/SE version 13.1 statistical software (Stata Corp. LP, College Station, TX).
Results
Baseline Patients Characteristics
Patient Characteristics are shown in Table 1
Table 1.
Summary Statistics of the Clinical and Demographic Characteristics { new }
| N | % | |
|---|---|---|
| Age | ||
| <60 years | 48 | 48 |
| ≥60 years | 52 | 52 |
| Gender | ||
| Female | 3 | 3 |
| Male | 97 | 97 |
| Baseline Stage | ||
| IIIA | 86 | 86 |
| IIIB | 11 | 11 |
| IIIC | 3 | 3 |
| Tumor grade | ||
| G1/G2 | 46 | 46 |
| G3 | 54 | 54 |
| Tumor grade | ||
| G1 | 1 | 1 |
| G2 | 45 | 45 |
| G3 | 54 | 54 |
| Surgical Stage | ||
| I | 13 | 13.5 |
| II | 41 | 42.7 |
| III | 42 | 43.8 |
Surgical Pathology
ypT0 was noted in 3 patients (these 3 had positive nodes), ypT1 in 22, ypT2 in 13 and ypT3 in 62, and ypN0 in 42. Surgical pathology stages were: I in 14 and II in 41 patients. Stage III EAC was most frequent and observed in 45 cases. Resection was R0 in 90 patients and R1 in 10. Tumor grade was: undetermined (n=11), well differentiated (n=1), moderately differentiated (n=18), and poorly differentiated (n=70). The degree of residual EAC was as follows: 1–10% (n=53), 11–50% (n=31), and >50% (n=12). The median number of examined lymph nodes was 23 (range: 3 to 52). The median number of positive lymph nodes was 1 (range: 0 to 20).
Geographic Distribution of Resistant EAC
Residual EAC was frequently found in more than one anatomic compartment. Mucosa and submucosa involvement was in 66 EACs (with 20 EACs not overlapping other compartments), muscularis propria was involved in 76 EACs (with 4 EACs not overlapping), serosa was involved in 62 patients, and nodes were involved in 58 EACs (with 3 not overlapping). In 35 EACs all anatomic compartments were involved.
The relative response rate was calculated by the absence of residual EAC in a given compartment and it was as follows: mucosa/submucosa in 34%, muscularis in 24%, serosa in 38%, and nodes in 42%.
Pre-surgery Biopsy and Residual EAC
79% of patients did not have EAC in the endoscopic biopsy specimens prior to surgery; however, 66% of resected specimen had EAC in mucosa/submucosa. Biopsy positive EACs had a median of 30% residual EAC, in contrast with biopsy negative EACs which had a median of 7% of residual (p=<0.001). Positive biopsy results did not correlate with the presence of positive nodes unlike we reported previously in a smaller number of patients.12
Survival and Recurrence
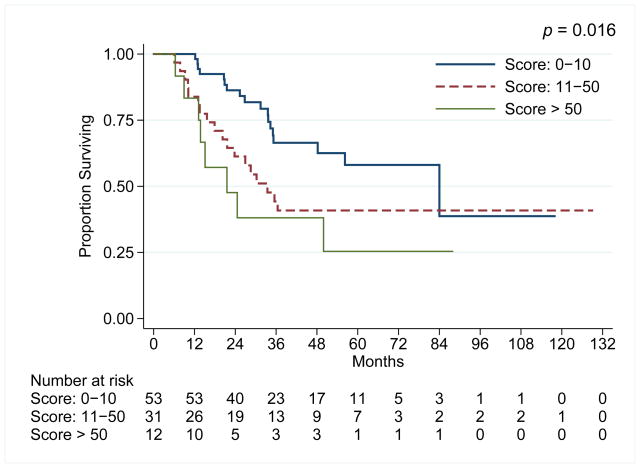
The median duration of follow-up was 33 months (range: 6 to 129 months). 47 patients have died. The median overall survival (OS) and relapse-free survival (RFS) were 50 months and 23 months, respectively. For the entire cohort, the 5-year OS rate was 47%. In the univariate analysis for OS, age, number of positive lymph nodes, residual tumor score, baseline TNM, tumor grade, and margins were significant. In the multivariate analysis, only tumor grade, baseline stage and margin status were independent for survival. Multivariate analysis is presented in Table 2. Additionally, % of residual EAC influenced survival (Figure 1) was as anticipated. Positive pre-surgery biopsy conferred poor survival (median: 24 months) compared to negative biopsy (56 months) but this was not significant (P=0.089) although it has been reported previously by others. 13, 14
Table 2.
Full and reduced multivariate analysis for OS
| Full Model | Reduced Model | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| HR | 95% CI for OS | p-value | HR | 95% CI for OS | p-value | |
|
| ||||||
| # positive LNs | 1.06 | (0.92, 1.23) | 0.410 | |||
| Age | ||||||
| <60 years | ||||||
| ≥60 years | 1.45 | (0.54, 3.89) | 0.463 | |||
| Tumor grade | ||||||
| G1/G2 | ||||||
| G3 | 7.50 | (2.03, 27.72) | 0.003 | 9.66 | (3.14, 29.73) | <0.001 |
| Residual Tumor Score | ||||||
| TRG2: Score:1–10 | ||||||
| TRG3:Score:11–50 | 1.30 | (0.37, 4.65) | 0.682 | |||
| TRG4:Score>50 | 3.32 | (0.63, 17.52) | 0.158 | |||
| Margin | ||||||
| Free | ||||||
| Involved Margins | 36.61 | (2.08, 645.913) | 0.014 | 81.66 | (6.58, 1012.637) | 0.001 |
Figure 1.
Kaplan-Meir overall survival plots according to the degree of residual EAC in the resected specimen.
The trends were similar for RFS. Higher tumor grade as reflected in primary diagnostic biopsy were related to lower RFS (p <0.001).
Discussion
The most important message from our results, which has not been previously reported, is that one should not make a decision to delay or avoid surgery based on post-chemoradiation negative endoscopic biopsies. Doing so can jeopardize trimodality-eligible patients who are likely to benefit from surgery. Our results also confirm previously reported finding that the degree of residual cancer correlates with OS of EAC patients. In this context, we made the observation of geographic distribution of resistant EAC in clinical stage III EAC patients treated with chemoradiation and surgery. Our results are complementary to other results and extend the observations more meaningfully and paint a more succinct picture of this phenomenon. Greater knowledge of where residual EAC resides should influence the clinical decision process and also help develop novel clinical strategies. At times, when a clinical complete response is achieved, there is the temptation on the part of the patient, and sometime physicians, to delay/avoid surgery. However, based on our results and those of others,9 it is clear that EAC is a highly resistant cancer and 70% of the time, residual cancer is found. Our data also contradict the conclusions made by Shapiro et al. that we can implement wait and see approach in some trimodality-eligible patients. We have previous reported in >300 patients who had a clinical complete response (meaning pre-surgery biopsies were negative and PET were physiological), 70% had resistant EAC in the surgical specimen.15 The fact that we have no reliable tools to recommend delay/avoidance of surgery in EAC patients is further strengthened by our current results suggesting that a negative post-chemoradiation biopsy result is frequently misleading.
Additionally, our results are contrary to what we anticipated: lymph node compartment turned out to be the most sensitive of all 4 compartments. Resistant EAC can be widely distributed in all 4 compartments and heterogeneous geographic pattern of resistant EAC is intriguing. Pre-surgery biopsy results highly correlate with the degree of residual EAC to be found in the surgical specimen. Our data also suggests that clinical stage III [IIIA,B,C] EAC patients should be encouraged to undergo surgery because there is considerable resistant EAC in the surgical specimen of these patients. Our previous report also suggested benefits from surgery for this particular group of patients.16
Our report is retrospective in nature, therefore, has its limits and does not provide guidance for individualizing therapy. It simply adds to our understanding of geographical distribution of resistant EAC. The shortcomings of our staging evaluations prior to surgery are evident and provide another glimpse into the complexity of treating EAC. The strength of report is that it is the largest report on clinical stage III [A,B,C] EAC. We have a novel observation of discrepancy between pre-surgery biopsy and what is found in the resected specimen and finally, the degree of residual EAC correlates with the results of pre-surgery biopsy.
In conclusion, resistant EAC after chemoradiation is unpredictably widely distributed in various anatomic compartments. Post-chemoradiation (i.e., pre-surgery) biopsy is misleading and should not be used to delay/avoidance of surgery recommendation.
Acknowledgments
Supported by: We received generous support from the Caporella, Dallas, Sultan, Park, Smith, Frazier, Oaks, Vanstekelenberg, and Cantu Families. From the Schecter Private Foundation, Rivercreek Foundation, Kevin Fund, Myer Fund, Dio Fund, Milrod Fund, and Multidisciplinary Grant provided by the University of Texas M. D. Anderson Cancer Center, Houston, USA. Supported in part by RO1CA129906 and RO1CA 172741 from the National Cancer Institute (JAA)
Footnotes
The authors declare no conflict of interest.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Wu TT, Chirieac LR, Abraham SC, et al. Excellent interobserver agreement on grading the extent of residual carcinoma after preoperative chemoradiation in esophageal and esophagogastric junction carcinoma: a reliable predictor for patient outcome. Am J Surg Pathol. 2007;31:58–64. doi: 10.1097/01.pas.0000213312.36306.cc. [DOI] [PubMed] [Google Scholar]
- 3.Chirieac LR, Swisher SG, Ajani JA, et al. Posttherapy pathologic stage predicts survival in patients with esophageal carcinoma receiving preoperative chemoradiation. Cancer. 2005;103:1347–1355. doi: 10.1002/cncr.20916. [DOI] [PubMed] [Google Scholar]
- 4.Rizk NP, Venkatraman E, Bains MS, et al. American Joint Committee on Cancer staging system does not accurately predict survival in patients receiving multimodality therapy for esophageal adenocarcinoma. J Clin Oncol. 2007;25:507–512. doi: 10.1200/JCO.2006.08.0101. [DOI] [PubMed] [Google Scholar]
- 5.Berger AC, Farma J, Scott WJ, et al. Complete response to neoadjuvant chemoradiotherapy in esophageal carcinoma is associated with significantly improved survival. J Clin Oncol. 2005;23:4330–4337. doi: 10.1200/JCO.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 6.Ajani JA, Correa AM, Hofstetter WL, et al. Clinical parameters model for predicting pathologic complete response following preoperative chemoradiation in patients with esophageal cancer. Ann Oncol. 2012;23:2638–2642. doi: 10.1093/annonc/mds210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sassen S, Schmalfeldt B, Avril N, et al. Histopathologic assessment of tumor regression after neoadjuvant chemotherapy in advanced-stage ovarian cancer. Hum Pathol. 2007;38:926–934. doi: 10.1016/j.humpath.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Mandard AM, Dalibard F, Mandard JC, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73:2680–2686. doi: 10.1002/1097-0142(19940601)73:11<2680::aid-cncr2820731105>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 9.Shapiro J, ten Kate FJ, van Hagen P, Biermann K, Wijnhoven BP, van Lanschot JJ. Residual esophageal cancer after neoadjuvant chemoradiotherapy frequently involves the mucosa and submucosa. Ann Surg. 2013;258:678–688. doi: 10.1097/SLA.0b013e3182a6191d. discussion 688–679. [DOI] [PubMed] [Google Scholar]
- 10.Greene FLPD, Fleming ID, et al., editors. AJCC Cancer Staging Manual. 6. New York, NY: Springer-Verlag; 2002. [Google Scholar]
- 11.Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol. 2010;17:1721–1724. doi: 10.1245/s10434-010-1024-1. [DOI] [PubMed] [Google Scholar]
- 12.Yang Q, Cleary KR, Yao JC, et al. Significance of post-chemoradiation biopsy in predicting residual esophageal carcinoma in the surgical specimen. Dis Esophagus. 2004;17:38–43. doi: 10.1111/j.1442-2050.2004.00355.x. [DOI] [PubMed] [Google Scholar]
- 13.Sarkaria IS, Rizk NP, Bains MS, et al. Post-treatment endoscopic biopsy is a poor-predictor of pathologic response in patients undergoing chemoradiation therapy for esophageal cancer. Ann Surg. 2009;249:764–767. doi: 10.1097/SLA.0b013e3181a38e9e. [DOI] [PubMed] [Google Scholar]
- 14.Miyata H, Yamasaki M, Takiguchi S, et al. Prognostic value of endoscopic biopsy findings after induction chemoradiotherapy with and without surgery for esophageal cancer. Ann Surg. 2011;253:279–284. doi: 10.1097/SLA.0b013e318206824f. [DOI] [PubMed] [Google Scholar]
- 15.Cheedella NK, Suzuki A, Xiao L, et al. Association between clinical complete response and pathological complete response after preoperative chemoradiation in patients with gastroesophageal cancer: analysis in a large cohort. Ann Oncol. 2013;24:1262–1266. doi: 10.1093/annonc/mds617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy CC, Correa AM, Ajani JA, et al. Surgery is an essential component of multimodality therapy for patients with locally advanced esophageal adenocarcinoma. J Gastrointest Surg. 2013;17:1359–1369. doi: 10.1007/s11605-013-2223-4. [DOI] [PMC free article] [PubMed] [Google Scholar]