Abstract
Obesity and hypertension are major risk factors for cardiovascular diseases, and their growing coexistence accounts for an increase in adverse cardiac events, but the mechanisms are yet to be determined. We hypothesized that obesity exacerbates mitochondrial dysregulation imposed by hypertension and augments left ventricular dysfunction. Obesity-prone Ossabaw pigs were randomized to lean (standard diet) and obese (high-fat diet), without (Lean-sham, Obese-sham) or with renovascular hypertension (Lean-Hypertension, Obese-Hypertension), induced after 12 weeks of diet (n=7 each). Cardiac function, myocardial perfusion and oxygenation, and microvascular remodeling were assessed 4 weeks later. Mitochondrial biogenesis signals and structural proteins, respiratory chain complex activities, and mitochondrial self-degradation were examined, as was fibrosis. Obesity alone exerted no apparent effect on mitochondrial dynamics, but aggravated in hypertensive hearts the reduction of mitochondrial proteins, deoxyribonucleic acid content, and respiratory chain complex IV subunits activity, and amplified mitochondrial self-degradation. Synergistic interaction of obesity with hypertension also exacerbated myocardial fibrosis and left ventricular diastolic dysfunction. Mitochondrial content, respiratory chain complex IV subunits activity, and mitophagy were correlated with myocardial fibrosis. These findings suggest that obesity aggravates in renovascular hypertension cardiac mitochondrial aberrations. Mitochondrial function may regulate the progression of cardiac injury and functional deterioration in hypertension concomitant with obesity.
Keywords: obesity, hypertension, renal artery stenosis, mitochondrial biogenesis, diastolic dysfunction
Introduction
Obesity remains prominent among public health concerns. According to recent national estimates, 16.9% of youth and 34.9% of adults are obese.1 Obesity has been shown to have adverse effects on the cardiovascular system, promote atherosclerotic plaques,2 and worsen outcomes in patients with coronary artery disease.3 Obesity can also induce cardiac remodeling,4 particularly left ventricular (LV) hypertrophy and diastolic dysfunction,5 which is directly related to mortality.6
The mechanisms by which obesity induces cardiac injury are yet to be fully determined. Inflammation and oxidative stress due to increased fatty acid substrates have been regarded as common pathogenic factors. Recent studies have also linked cardiac metabolism to obesity-related cardiac alterations. As a result of increases in circulating fatty acids and insulin resistance that commonly accompany obesity, the myocardium is exposed to excessive nutrient substrates. Nevertheless, energy production becomes less efficient, as overloaded mitochondria undergo stress and develop dysfunction.7, 8 High-fat diet has been shown to decrease mitochondrial biogenesis, reduce mitochondrial coupling efficiency, and impair ATP synthesis.9–11 These findings suggest that mitochondrial homeostasis can be modulated by energy supply and potentially impact cardiac health and function.
Hypertension (HT) is one of the most common causes of LV hypertrophy, and more prevalent in obese individuals than in the lean population.12 Particularly, renovascular hypertension (RVH), a common cause of secondary HT due to renal artery stenosis, accelerates LV remodeling,13 driven by the activated inflammation and renin-angiotensin system in response to decreased renal blood supply distal to the stenosis.14 We have recently shown that the renovascular hypertensive heart is characterized by attenuated myocardial mitochondrial biogenesis and by enhanced mitophagy.15 However, whether concurrent obesity affects mitochondria integrity in the hypertensive heart remains unclear. We hypothesized that co-existence of obesity would exacerbate myocardial mitochondrial dysregulation and fibrosis, and magnify LV diastolic dysfunction in renovascular hypertensive pigs.
Methods
Littermate Ossabaw pigs were randomized to lean (standard chow) and obese (high-fat diet), without (Lean-sham, Obese-sham) or with renovascular HT secondary to unilateral renal artery stenosis (Lean-HT, Obese-HT), induced after 12 weeks of diet (n=7 each). Cardiac structure, function and oxygenation were studied with multi-detector computed-tomography and blood-oxygenation-level-dependent-magnetic resonance imaging. The myocardium was examined ex-vivo for indices of mitochondrial biogenesis and function, and tissue damage (Detailed descriptions of all experimental methods are included in the Online-only Data Supplement http://hyper.ahajournals.org).
Results
Animal systemic characteristics
Diet increased body weight, total cholesterol, and low-density lipoprotein in Obese pigs compared to Lean (Table 1), as well as intra-abdominal and pericardial fat deposition (Figure S1A–D). Basal homeostasis model assessment-insulin resistance index was also increased in both obese groups, indicating insulin resistance (Table 1). Renal artery stenosis was similarly established in hypertensive Lean and Obese pigs (87.9±4.9% vs. 85.6±4.7%, p>0.10), increasing mean arterial pressure, which was further elevated in Obese-HT. HT tended to increase and obesity increased plasma renin activity (PRA), which resulted in elevation of PRA only in Obese-HT (Table 1), suggesting additive effects by coexistence of obesity and HT. HT elevated plasma tumor necrosis factor-α level in both groups, and interacted with diet to markedly increase sE-selectin levels only in Obese-HT (Table 1).
Table 1.
Characteristics (mean±SEM) of lean or obese pigs with or without hypertension (HT, n=7 each group)
| Parameters | Sham
|
HT
|
P value for two-way ANOVA
|
||||
|---|---|---|---|---|---|---|---|
| Lean | Obese | Lean | Obese | Diet | HT | DietXHT | |
| Body weight (kg) | 31.4±4.3 | 45.0±5.7* | 35.7±2.1 | 45.3±1.1*† | 0.001 | 0.30 | 0.52 |
| MAP (mmHg) | 108.0±7.1 | 113.9±5.8 | 122.67±4.4* | 137.2±5.5*†‡ | 0.09 | 0.004 | 0.004 |
| Heart rate (bpm) | 61.3±2.6 | 67.0±7.0 | 76.6±5.4* | 84.5±6.4*‡ | 0.54 | 0.018 | 0.87 |
| RPP (mmHgxbpm) | 83.4±7.5 | 91.9±10.6 | 115.7±8.3* | 137.7±9.7*† | 0.24 | 0.002 | 0.017 |
| Total cholesterol (mg/dl) | 97.0±2.9 | 388.0±48.1* | 85.4±5.0 | 346.7±40.6*† | <0.001 | 0.81 | 0.58 |
| LDL (mg/dl) | 38.3±2.9 | 243.0±21.9* | 32.4±3.0 | 187.3±27.3*† | <0.001 | 0.66 | 0.09 |
| Triglycerides (mg/dl) | 26.0±5.6 | 37.6±13.9 | 17.4±2.9 | 34.6±5.6 | 0.16 | 0.77 | 0.10 |
| HOMA-IR (μU/ml×mg/dl) | 3.6±0.3 | 6.7±2.4* | 2.6±0.5 | 10.5±2.9*† | 0.005 | 0.63 | 0.006 |
| TNF-α (pg/ml) | 38.1±15.4 | 55.0±11.8 | 138.9±49.7* | 101.4±23.4* | 0.77 | 0.029 | 0.52 |
| sE-selectin (pg/ml) | 5.6±3.9 | 5.7±5.7 | 15.4±7.1 | 36.8±10.8*†‡ | 0.17 | 0.017 | 0.005 |
| 8-epi-Isoprostane (pg/ml) | 126.9±24.4 | 142.2±27.4 | 97.9±8.0 | 139.5±12.8 | 0.27 | 0.49 | 0.73 |
| PRA (ng/ml/hr) | 0.02±0.01 | 0.10±0.03 | 0.07±0.02 | 0.16±0.06* | 0.005 | 0.07 | 0.94 |
| LVMM (g) | 39.8±2.6 | 44.7±3.7 | 49.0±4.7 | 52.1±4.3* | 0.25 | 0.034 | 0.10 |
| E/A | 1.6±0.1 | 1.5±0.2 | 1.5±0.2 | 1.1±0.2*‡ | 0.28 | 0.021 | 0.023 |
| Stroke volume (ml) | 36.5±3.6 | 35.6±4.6 | 34.0±2.5 | 35.8±2.2 | 0.74 | 0.74 | 0.67 |
| Ejection fraction (%) | 59.5±4.2 | 62.0±3.3 | 63.8±2.0 | 62.4±3.7 | 0.96 | 0.54 | 0.60 |
| Cardiac output (mlxbpm) | 2.5±0.2 | 2.4±0.2 | 2.5±0.3 | 2.9±0.2‡ | 0.40 | 0.15 | 0.023 |
MAP: mean arterial pressure; RPP: rate pressure product; LDL: low-density lipoprotein; HOMA-IR: homeostasis model assessment insulin resistance; TNF-α: tumor necrosis factor-α; PRA: plasma renin activity; LVMM: left ventricular muscle mass.
p<0.05 vs. Lean-sham,
p<0.05 vs. Lean-HT,
p<0.05 vs. Obese-sham.
Cardiac function and hemodynamics
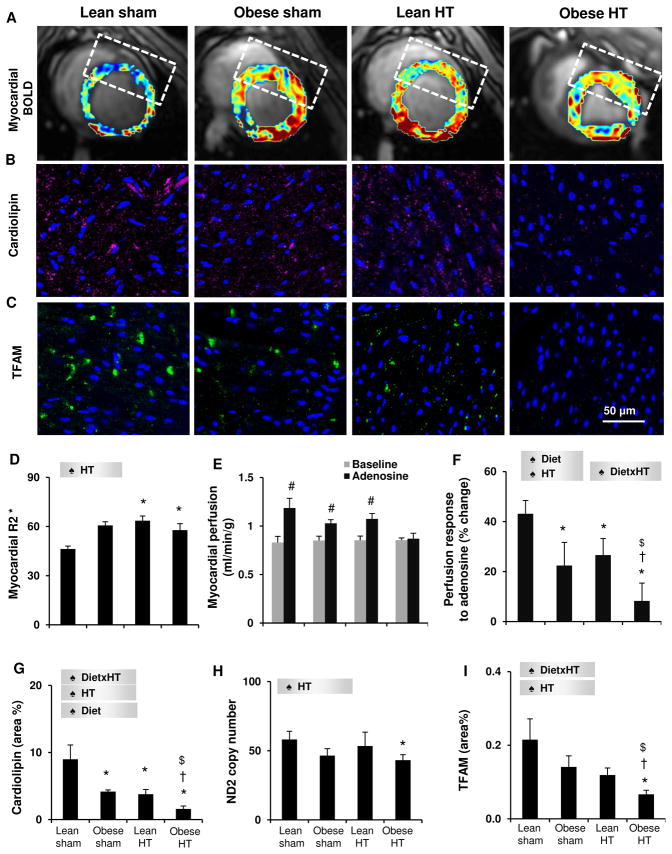
HT raised left ventricular muscle mass (LVMM), but the increase reached statistical significance only in Obese-HT (Table 1). Obese-HT also suppressed E/A ratio, indicating exacerbating impact of early obesity on HT to induce LV diastolic dysfunction. HT increased heart rate in both groups compared to their sham groups, and elevated cardiac output in Obese-HT compared to Obese-sham. HT also increased rate-pressure-product in both groups and to a greater extent in Obese-HT (Table 1), indicating increased LV workload, while myocardial oxygenation was similarly decreased in both HT groups (elevated R2*, Figure 1A,D). Stroke volume and ejection fraction remained unchanged. Although basal myocardial perfusion was unaltered, its response to adenosine was blunted by diet and HT, and synergistically further suppressed in Obese-HT (Figure 1E–F). Diet also interacted with HT to increase media/lumen ratio and downregulate endothelial nitric-oxide-synthase expression only in Obese-HT (Figure S2A,C,D,F). These results suggest that co-existence of obesity and HT precipitates diastolic LV dysfunction, accompanied by microvascular remodeling and dysfunction, which were subtle in HT or diet alone.
Figure 1.
A,D: Representative blood-oxygen level-dependent (BOLD) images from the left ventricle and derived R2* values. B–C, G, I: Myocardial expression of mitochondrial cardiolipin and mitochondrial transcription factor A (TFAM). E–F: Myocardial perfusion and its response to adenosine measured by multi-detector computed tomography. H: Copy number of mitochondrial DNA-encoded NADH dehydrogenase (ND)2. ♠ Diet: significant effect of diet. ♠ HT: significant effect of HT. ♠ DietxHT: significant interaction (Two-way ANOVA). *p<0.05 vs. Lean-sham, $p<0.05 vs. Obese-sham, †p<0.05 vs. Lean-HT, #p<0.05 vs. baseline.
Mitochondrial dynamics
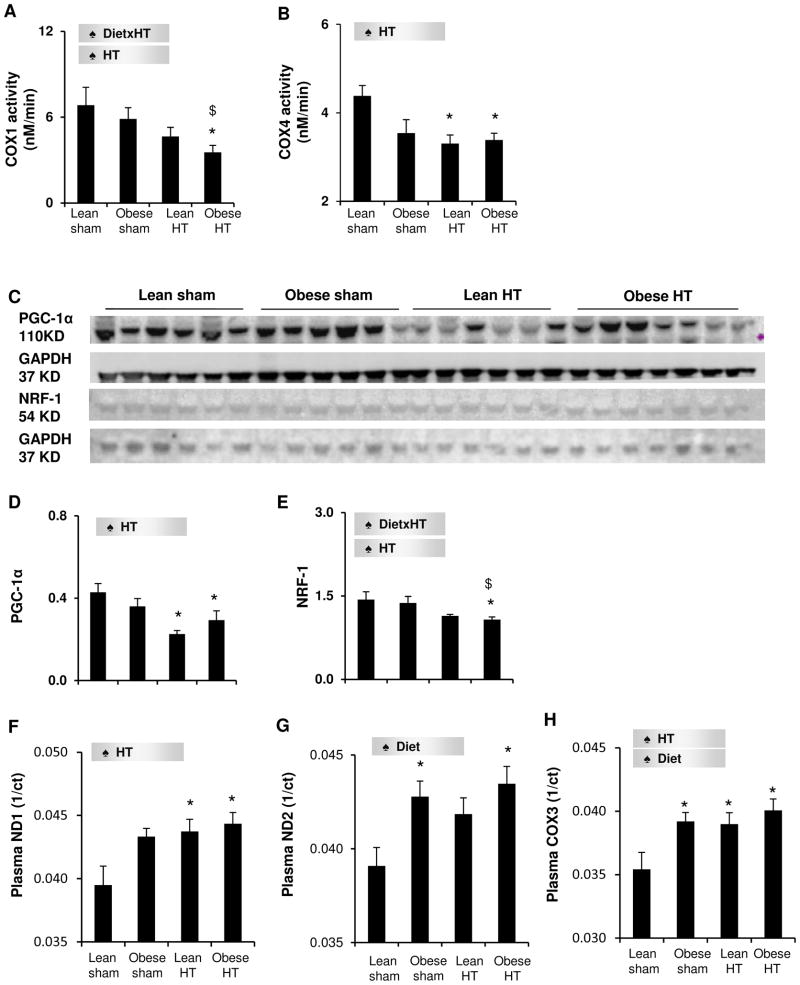
The mitochondrial phospholipid cardiolipin decreased in all experimental groups, particularly by the synergistic interaction of diet and HT (Figure 1B,G). Obese-HT alone also reduced mitochondrial DNA-encoded NADH dehydrogenase (ND)2 gene copy number in the myocardium (Figure 1H), and markedly blunted mitochondrial transcription factor-A (TFAM) immunoreactivity (Figure 1C,I), indicating decreased mitochondrial DNA content. In addition, Obese-HT inhibited the activities of mitochondrial respiratory chain complex IV (COX) subunits 1 and 4, suggesting compromised mitochondrial function, whereas Lean-HT only suppressed COX4 activity (Figure 2A–B). In concert, HT decreased the expression of the mitochondrial biogenesis regulator peroxisome proliferator-activated receptor-gamma coactivator (PGC)1-α in both groups, but its cofactor nuclear respiratory factor (NRF)-1 only in Obese-HT (Figure 2C–E). Further, HT increased circulating plasma levels of the mitochondrial DNA-encoded ND1 and COX3, and diet increased COX3 and ND2, consistent with release of mitochondrial components into the circulation due to cellular mitochondrial injury. Notably, Obese-HT elevated all three markers (Figure 2F–H), suggesting more severe mitochondrial damage than obesity or hypertension alone, possibly mediated by their additive effects. Furthermore, Obese-HT stimulated myocardial mitophagy, reflected by translocation of parkin to the mitochondrial outer membrane indicated by its marker Tom20, and upregulated expression of dynamin related protein-1 (Figure S2B,C,E,G). Collectively, Obese-HT amplified dysregulation of mitochondrial turnover and function compared to HT alone.
Figure 2.
A–B: Mitochondrial respiratory chain complex IV (COX) subunits 1 and 4 activities, respectively. C–E: Myocardial expression of peroxisome proliferator-activated receptor-gamma coactivator (PGC)-1α and nuclear respiratory factor (NRF)-1. F–H: Circulating plasma levels of mitochondrial DNA-encoded NADH dehydrogenase (ND)1, ND2, and COX3. ct: threshold cycle. ♠ Diet: significant effect of diet. ♠ HT: significant effect of HT. ♠ DietxHT: significant interaction (Two-way ANOVA). *p<0.05 vs. Lean-sham, $p<0.05 vs. Obese-sham, †p<0.05 vs. Lean-HT.
Oxidative stress and fibrosis
Systemic level of the oxidative stress marker 8-epi-isoprostane did not differ among the groups (Table 1), whereas myocardial oxidative stress (dihydroethidium staining) was enhanced only in Obese-HT (Figure S3A). The interaction of diet and HT also aggravated myocardial fibrosis in Obese-HT compared to either alone (Figure S3B). Notably, myocardial fibrosis was inversely associated with the content of mitochondrial cardiolipin and COX activity, and directly with the extent of mitophagy (Figure S3C–F).
Discussion
The present study demonstrates that despite subtle cardiac alterations in obesity and HT alone, their coexistence markedly dysregulated mitochondrial turnover and function, including suppressed mitochondrial biogenesis and respiratory chain complex activities, and enhanced mitochondrial self-degradation. This might subserve myocardial fibrosis, and is associated with LV diastolic dysfunction. Therefore, co-existing obesity may facilitate the progression of myocardial injury in early RVH by enhancing mitochondrial dysregulation. We have previously observed mitochondrial damage in the post-stenotic kidney in RVH.16 The present study extends our previous observations and shows that in the heart, superimposition of obesity on RVH leads to decreased levels of mitochondrial mtDNA, mitochondrial biogenesis regulators, and respiratory chain complex activities, and bolsters mitochondrial degradation and spillover. These novel findings significantly suggest a wide-range of mitochondrial damage involving their quantity, quality and dynamics in the RVH heart with co-existing obesity.
Studies have suggested impaired mitochondrial biogenesis in obesity.7, 9 In the present study, although preexisting obesity alone had a minor impact on mitochondrial biogenesis, it was predominant in Obese-HT, where HT suppressed mitochondrial function most prominently, underscoring the detrimental interaction of obesity with HT. While systemic oxidative stress remained unchanged, local oxidative stress (myocardial dihydroethidium) evoked by the co-existence of diet and HT can increase nitric oxide degradation17 or decrease its production,18 and in turn reduce mitochondrial biogenesis.19 PGC-1α downregulated by HT also decreased mitochondrial turn-over by its effector NRF-1.20 By binding to and coactivating the transcriptional function of its downstream NRF-1 on the promoter for TFAM, PGC-1α increases the activity of TFAM, a direct regulator of replication/transcription of mitochondrial DNA,21 which encodes subunits of NADH dehydrogenase and complex IV, such as ND2 and COX1,22 respectively. NRF-1 also regulates nucleus-encoded COX4.23 As a result, a decrease in these mitochondrial biogenic regulators impaired mitochondrial function. Importantly, spillover of circulating mtDNA markers and mitochondrial proteins due to cellular injury,24 as we observed, may also contribute to depletion of functional tissue mitochondria. Further, in addition to the excessive nutrient abundance that stresses the mitochondria by increasing their work-load,7, 8 activated renin-angiotensin system involved in adiposity and pressure overload in Obese-HT might suppress mitochondrial biogenesis and magnify mitophagy.15, 25 Increased mitochondrial degradation in Obese-HT could in turn magnify depletion of mitochondria. Consequently, dysregulated mitochondrial turnover and function ensue in Obese-HT, where the nutrient overload, cell injury, and pressure overload in concert magnify a fall in mitochondrial quantity and quality compared to either factor alone.
Interestingly, despite mitochondrial dysfunction and associated myocardial fibrosis (Figure S3), Obese-HT exhibited preserved cardiac systolic function (cardiac output, stroke volume). Tissue injury like fibrosis often precedes and accompanies development of LVH.26 Similarly, we have observed that their co-existence amplified kidney injury and fibrosis, whereas renal function was relatively preserved.27 Possibly, increased insulin levels and extracellular volume may sustain or enhance renal and cardiac function in the early stages of obesity. Furthermore, mitochondrial dysfunction contributes to early stages of hypertensive heart remodeling, preceding systolic dysfunction.28, 29 Decreased ATP production contributes to isolated LV diastolic dysfunction,30 and a fall in TFAM impairs myocyte relaxation.31 Notably, the severity of mitochondrial dysfunction is important in the transition from hypertrophy to systolic dysfunction,28, 32 as does its duration,33 and interventions that confer mitochondrial protection may ameliorate or reverse cardiac hypertrophy.29, 34 Indeed, we have shown previously that stabilization of mitochondrial cardiolipin normalized LV E/A ratio in RVH.29 Collectively, these findings signify the adverse impact of mitochondrial dysfunction on diastolic function at the early stages of hypertensive heart disease. Nevertheless, more severe and prolonged mitochondrial damage might eventually lead to cardiac systolic dysfunction.
In the present study, obesity alone did not increase blood pressure, but magnified its increment in HT (Table 1), underscoring their significant interaction. It has been shown that obese subjects have higher circulating angiotensinogen, renin and aldosterone. Body weight control by 5% leads to a reduction of renin-angiotensin-aldosterone not only in the adipose tissue, but also in the plasma, which correlates with the waist circumference decline35. These observations link obesity with increased circulating renin-angiotensin levels, which possibly contributes to greater MAP in Obese-HT compared to Lean-HT. Yet, while PRA was increased in Obese-HT compared to Lean-sham, it was not higher than Lean-HT, implicating additional factors in the higher MAP in Obese-HT compared to Lean-HT. For example, increased renal sodium reabsorption due to renal artery stenosis can be enhanced in obesity as proximal tubules cells may undergo hypertrophy,36 leading to volume expansion. Adipokines released from adipose tissue in obesity are related to increased sympathetic nerve activity,37 and insulin resistance (reflected by homeostasis model assessment-insulin resistance index) may also promote hypertension.38 These factors working in concert might increase MAP in Obese-HT compared to Lean-HT. Due to increased pressure load, cardiac oxygen demand (rate-pressure-product) rises. Alas, proliferating microvascular smooth muscle cells and decreased endothelial nitric oxide bioavailability in Obese-HT, resulting in diminished perfusion reserve, may restrict oxygen supply and lead to myocardial hypoxia. Interestingly, however, BOLD R2* in Obese-HT was comparable to that in Lean-HT and Obese-sham, suggesting relatively preserved oxygenation. Possibly, blunted mitochondrial COX1 and 4 activities and loss of mitochondrial genome may decrease oxygen utilization capacity and reduce oxygen consumption, leading to a paradoxical hypoxic-to-normoxic shift in the tissue.39
This study is limited by our relatively young animals, which may show greater resistance to insults exerted by obesity and HT, yet this relatively short-term exposure provided with an opportunity to capture the initial pathophysiological changes that they induce. Our findings underlie the adverse effects of renovascular disease with coexisting obesity on myocardial mitochondrial quantity, quality and dynamics. Further studies need to examine the potential alterations in myocardial ATP levels in obesity and pressure-overload, and the causal link between mitochondrial dynamics and cardiac outcomes. The potential protective effects of obesity management integrated with antihypertensive measures on mitochondrial homeostasis in individuals with obesity and HT also need to be investigated.
Perspectives
Our study demonstrates that coexistence of obesity and renovascular HT impairs mitochondrial biogenesis and function, and enhances mitochondrial self-degradation. This is associated with magnified tissue remodeling and LV diastolic dysfunction. As the concurrence of HT and obesity is becoming increasingly prevalent, greater understanding of cardiac mitochondrial metabolic dynamics and their potential roles as therapeutic targets in cardiac remodeling and dysfunction are in growing need.
Supplementary Material
Novelty and Significance.
1) What Is New
Our study implicates synergistic dysregulation of mitochondrial homeostasis as an important contributor in mediating myocardial injury in renovascular hypertension coexisting with obesity.
2) What Is Relevant
Renovascular hypertension and obesity often coexist and may therefore worsen cardiac outcomes, but the mechanisms are incompletely understood. Out study implicates mitochondrial dynamics in mediating cardiac alteration under renal vascular hypertension with concurrent obesity.
3) Summary
Synergistic mitochondrial loss and respiratory chain complex dysfunction may play important roles in mediating cardiac damage during coexisting renovascular hypertension and obesity.
Acknowledgments
Zi-Lun Li was supported by the China Scholarship Council under the authority of the Ministry of Education of the People’s Republic of China.
Source(s) of Funding
This study was partly supported by NIH grants numbers DK73608, HL12160, HL121561, DK104273, DK 102325 and C06-RR018898, and the American Heart Association.
Footnotes
Disclosures
NONE
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the united states, 2011–2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wee CC, Girotra S, Weinstein AR, Mittleman MA, Mukamal KJ. The relationship between obesity and atherosclerotic progression and prognosis among patients with coronary artery bypass grafts the effect of aggressive statin therapy. J Am Coll Cardiol. 2008;52:620–625. doi: 10.1016/j.jacc.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 3.Coutinho T, Goel K, Correa de Sa D, Carter RE, Hodge DO, Kragelund C, Kanaya AM, Zeller M, Park JS, Kober L, Torp-Pedersen C, Cottin Y, Lorgis L, Lee SH, Kim YJ, Thomas R, Roger VL, Somers VK, Lopez-Jimenez F. Combining body mass index with measures of central obesity in the assessment of mortality in subjects with coronary disease: Role of “normal weight central obesity”. J Am Coll Cardiol. 2013;61:553–560. doi: 10.1016/j.jacc.2012.10.035. [DOI] [PubMed] [Google Scholar]
- 4.Chang Y, Kim BK, Yun KE, Cho J, Zhang Y, Rampal S, Zhao D, Jung HS, Choi Y, Ahn J, Lima JA, Shin H, Guallar E, Ryu S. Metabolically-healthy obesity and coronary artery calcification. J Am Coll Cardiol. 2014;63:2679–2686. doi: 10.1016/j.jacc.2014.03.042. [DOI] [PubMed] [Google Scholar]
- 5.Olivotto I, Maron BJ, Tomberli B, Appelbaum E, Salton C, Haas TS, Gibson CM, Nistri S, Servettini E, Chan RH, Udelson JE, Lesser JR, Cecchi F, Manning WJ, Maron MS. Obesity and its association to phenotype and clinical course in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2013;62:449–457. doi: 10.1016/j.jacc.2013.03.062. [DOI] [PubMed] [Google Scholar]
- 6.Tobias DK, Pan A, Jackson CL, O’Reilly EJ, Ding EL, Willett WC, Manson JE, Hu FB. Body-mass index and mortality among adults with incident type 2 diabetes. N Engl J Med. 2014;370:233–244. doi: 10.1056/NEJMoa1304501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lipina C, Macrae K, Suhm T, Weigert C, Blachnio-Zabielska A, Baranowski M, Gorski J, Burgess K, Hundal HS. Mitochondrial substrate availability and its role in lipid-induced insulin resistance and proinflammatory signaling in skeletal muscle. Diabetes. 2013;62:3426–3436. doi: 10.2337/db13-0264. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Abel ED. Obesity stresses cardiac mitochondria even when you are young. J Am Coll Cardiol. 2011;57:586–589. doi: 10.1016/j.jacc.2010.09.039. [DOI] [PubMed] [Google Scholar]
- 9.Sparks LM, Xie H, Koza RA, Mynatt R, Hulver MW, Bray GA, Smith SR. A high-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes. 2005;54:1926–1933. doi: 10.2337/diabetes.54.7.1926. [DOI] [PubMed] [Google Scholar]
- 10.Hirabara SM, Curi R, Maechler P. Saturated fatty acid-induced insulin resistance is associated with mitochondrial dysfunction in skeletal muscle cells. J Cell Physiol. 2010;222:187–194. doi: 10.1002/jcp.21936. [DOI] [PubMed] [Google Scholar]
- 11.Boudina S, Sena S, O’Neill BT, Tathireddy P, Young ME, Abel ED. Reduced mitochondrial oxidative capacity and increased mitochondrial uncoupling impair myocardial energetics in obesity. Circulation. 2005;112:2686–2695. doi: 10.1161/CIRCULATIONAHA.105.554360. [DOI] [PubMed] [Google Scholar]
- 12.Julius S, Valentini M, Palatini P. Overweight and hypertension: A 2-way street? Hypertension. 2000;35:807–813. doi: 10.1161/01.hyp.35.3.807. [DOI] [PubMed] [Google Scholar]
- 13.Losito A, Fagugli RM, Zampi I, Parente B, de Rango P, Giordano G, Cao P. Comparison of target organ damage in renovascular and essential hypertension. Am J Hypertens. 1996;9:1062–1067. doi: 10.1016/0895-7061(96)00199-9. [DOI] [PubMed] [Google Scholar]
- 14.Mehta PK, Griendling KK. Angiotensin ii cell signaling: Physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Li ZL, Crane JA, Jordan KL, Pawar AS, Textor SC, Lerman A, Lerman LO. Valsartan regulates myocardial autophagy and mitochondrial turnover in experimental hypertension. Hypertension. 2014;64:87–93. doi: 10.1161/HYPERTENSIONAHA.113.02151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eirin A, Ebrahimi B, Zhang X, Zhu XY, Woollard JR, He Q, Textor SC, Lerman A, Lerman LO. Mitochondrial protection restores renal function in swine atherosclerotic renovascular disease. Cardiovasc Res. 2014;103:461–472. doi: 10.1093/cvr/cvu157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lavi S, Yang EH, Prasad A, Mathew V, Barsness GW, Rihal CS, Lerman LO, Lerman A. The interaction between coronary endothelial dysfunction, local oxidative stress, and endogenous nitric oxide in humans. Hypertension. 2008;51:127–133. doi: 10.1161/HYPERTENSIONAHA.107.099986. [DOI] [PubMed] [Google Scholar]
- 18.Chen C, Jiang J, Lu JM, Chai H, Wang X, Lin PH, Yao Q. Resistin decreases expression of endothelial nitric oxide synthase through oxidative stress in human coronary artery endothelial cells. Am J Physiol Heart Circ Physiol. 2010;299:H193–201. doi: 10.1152/ajpheart.00431.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nisoli E, Carruba MO. Nitric oxide and mitochondrial biogenesis. J Cell Sci. 2006;119:2855–2862. doi: 10.1242/jcs.03062. [DOI] [PubMed] [Google Scholar]
- 20.Scarpulla RC. Metabolic control of mitochondrial biogenesis through the pgc-1 family regulatory network. Biochim Biophys Acta. 2011;1813:1269–1278. doi: 10.1016/j.bbamcr.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator pgc-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 22.Schon EA, DiMauro S, Hirano M. Human mitochondrial DNA: Roles of inherited and somatic mutations. Nat Rev Genet. 2012;13:878–890. doi: 10.1038/nrg3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dhar SS, Ongwijitwat S, Wong-Riley MT. Nuclear respiratory factor 1 regulates all ten nuclear-encoded subunits of cytochrome c oxidase in neurons. J Biol Chem. 2008;283:3120–3129. doi: 10.1074/jbc.M707587200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial damps cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abadir PM, Foster DB, Crow M, Cooke CA, Rucker JJ, Jain A, Smith BJ, Burks TN, Cohn RD, Fedarko NS, Carey RM, O’Rourke B, Walston JD. Identification and characterization of a functional mitochondrial angiotensin system. Proc Natl Acad Sci U S A. 2011;108:14849–14854. doi: 10.1073/pnas.1101507108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diez J, Querejeta R, Lopez B, Gonzalez A, Larman M, Martinez Ubago JL. Losartan-dependent regression of myocardial fibrosis is associated with reduction of left ventricular chamber stiffness in hypertensive patients. Circulation. 2002;105:2512–2517. doi: 10.1161/01.cir.0000017264.66561.3d. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X, Li ZL, Woollard JR, Eirin A, Ebrahimi B, Crane JA, Zhu XY, Pawar AS, Krier JD, Jordan KL, Tang H, Textor SC, Lerman A, Lerman LO. Obesity-metabolic derangement preserves hemodynamics but promotes intrarenal adiposity and macrophage infiltration in swine renovascular disease. Am J Physiol Renal Physiol. 2013;305:F265–276. doi: 10.1152/ajprenal.00043.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dai DF, Johnson SC, Villarin JJ, Chin MT, Nieves-Cintron M, Chen T, Marcinek DJ, Dorn GW, 2nd, Kang YJ, Prolla TA, Santana LF, Rabinovitch PS. Mitochondrial oxidative stress mediates angiotensin ii-induced cardiac hypertrophy and galphaq overexpression-induced heart failure. Circ Res. 2011;108:837–846. doi: 10.1161/CIRCRESAHA.110.232306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eirin A, Williams BJ, Ebrahimi B, Zhang X, Crane JA, Lerman A, Textor SC, Lerman LO. Mitochondrial targeted peptides attenuate residual myocardial damage after reversal of experimental renovascular hypertension. J Hypertens. 2014;32:154–165. doi: 10.1097/HJH.0b013e3283658a53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diamant M, Lamb HJ, Groeneveld Y, Endert EL, Smit JW, Bax JJ, Romijn JA, de Roos A, Radder JK. Diastolic dysfunction is associated with altered myocardial metabolism in asymptomatic normotensive patients with well-controlled type 2 diabetes mellitus. J Am Coll Cardiol. 2003;42:328–335. doi: 10.1016/s0735-1097(03)00625-9. [DOI] [PubMed] [Google Scholar]
- 31.Watanabe A, Arai M, Koitabashi N, Niwano K, Ohyama Y, Yamada Y, Kato N, Kurabayashi M. Mitochondrial transcription factors tfam and tfb2m regulate serca2 gene transcription. Cardiovasc Res. 2011;90:57–67. doi: 10.1093/cvr/cvq374. [DOI] [PubMed] [Google Scholar]
- 32.Riehle C, Wende AR, Zaha VG, Pires KM, Wayment B, Olsen C, Bugger H, Buchanan J, Wang X, Moreira AB, Doenst T, Medina-Gomez G, Litwin SE, Lelliott CJ, Vidal-Puig A, Abel ED. Pgc-1beta deficiency accelerates the transition to heart failure in pressure overload hypertrophy. Circ Res. 2011;109:783–793. doi: 10.1161/CIRCRESAHA.111.243964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arany Z, Novikov M, Chin S, Ma Y, Rosenzweig A, Spiegelman BM. Transverse aortic constriction leads to accelerated heart failure in mice lacking ppar-gamma coactivator 1alpha. Proc Natl Acad Sci U S A. 2006;103:10086–10091. doi: 10.1073/pnas.0603615103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walther T, Tschope C, Sterner-Kock A, Westermann D, Heringer-Walther S, Riad A, Nikolic A, Wang Y, Ebermann L, Siems WE, Bader M, Shakibaei M, Schultheiss HP, Dorner A. Accelerated mitochondrial adenosine diphosphate/adenosine triphosphate transport improves hypertension-induced heart disease. Circulation. 2007;115:333–344. doi: 10.1161/CIRCULATIONAHA.106.643296. [DOI] [PubMed] [Google Scholar]
- 35.Engeli S, Bohnke J, Gorzelniak K, Janke J, Schling P, Bader M, Luft FC, Sharma AM. Weight loss and the renin-angiotensin-aldosterone system. Hypertension. 2005;45:356–362. doi: 10.1161/01.HYP.0000154361.47683.d3. [DOI] [PubMed] [Google Scholar]
- 36.Tobar A, Ori Y, Benchetrit S, Milo G, Herman-Edelstein M, Zingerman B, Lev N, Gafter U, Chagnac A. Proximal tubular hypertrophy and enlarged glomerular and proximal tubular urinary space in obese subjects with proteinuria. PLoS One. 2013;8:e75547. doi: 10.1371/journal.pone.0075547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith MM, Minson CT. Obesity and adipokines: Effects on sympathetic overactivity. J Physiol. 2012;590:1787–1801. doi: 10.1113/jphysiol.2011.221036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakamura M, Yamazaki O, Shirai A, Horita S, Satoh N, Suzuki M, Hamasaki Y, Noiri E, Kume H, Enomoto Y, Homma Y, Seki G. Preserved na/hco3 cotransporter sensitivity to insulin may promote hypertension in metabolic syndrome. Kidney Int. 2015;87:535–542. doi: 10.1038/ki.2014.351. [DOI] [PubMed] [Google Scholar]
- 39.Cook CC, Kim A, Terao S, Gotoh A, Higuchi M. Consumption of oxygen: A mitochondrial-generated progression signal of advanced cancer. Cell Death Dis. 2012;3:e258. doi: 10.1038/cddis.2011.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.