Abstract
Most studies examining associations between hypertension and brain white matter microstructure have focused on older adults or on cohorts with a large age range. Since hypertension effects on the brain may vary with age it is important to focus on middle age, when hypertension becomes more prevalent. We used linear mixed effect models to examine differences in white matter diffusion metrics as a function of hypertension in a well-characterized cohort of middle-aged men (N=316, mean 61.8 years; range 56.7–65.6). Diffusion metrics were examined in nine tracts reported to be sensitive to hypertension in older adults. Relative to normotensive individuals, individuals with longstanding hypertension (> 5.6 years) showed reduced fractional anisotropy or increased diffusivity in most tracts. Effects were stronger among carriers than non-carriers of the apolipoprotein E ε4 allele for two tracts connecting frontal regions with other brain areas. Significant differences were observed even after adjustment for potentially-related lifestyle and cardiovascular risk factors. Shorter duration of hypertension or better blood pressure control among hypertensive individuals did not lessen the adverse effects. These findings suggest that microstructural white matter alterations appear early in the course of hypertension and may persist despite adequate treatment. Although longitudinal studies are needed to confirm these findings, the results suggest that prevention—rather than management—of hypertension may be vital to preserving brain health in aging.
Keywords: diffusion-weighted imaging, APOE, fractional anisotropy, mean diffusivity, aging
Introduction
Hypertension is a well-known risk factor for cerebrovascular disease, stroke, and vascular dementia. It has been associated with silent brain injury, including grey matter atrophy, silent infarcts, microbleeds, and white matter damage1. Individuals with an APOE–ε4 allele may be particularly susceptible to adverse effects of hypertension2,3.
Middle age is an important period for emergence of hypertension, yet few studies have focused solely on this age range when examining hypertension effects on cerebral white matter microstructure. This is an important limitation because hypertension may have different effects in younger than older adults4. Additionally, because hypertension is associated with increased mortality, hypertension effects may be underestimated in studies of older adults due to survival bias, or results may be confounded by latent neurodegenerative pathology.
Diffusion-weighted neuroimaging enables quantification of the degree and direction of water molecule motion within tissue. It is a sensitive method for detecting differences in white matter microstructure as a function of injury or illness5,6 and has been used to investigate effects of hypertension in older adults7–10. For example, in a large study (N=4532) of adults aged 46–100 years (mean 63.8), severe, but not moderate, hypertension was associated with reduced fractional anisotropy (FA) or increased mean diffusivity (MD) in numerous white matter tracts11. No significant main effects of APOE-ε4 were observed, but this study did not examine whether hypertension effects differed by APOE-ε4 status. Although several studies have looked at main effects of the ε4 allele on diffusion metrics, with mixed results12,13, none have examined whether hypertension effects differ by APOE-ε4 status.
In one of the few studies focused on younger adults (ages 19–63, mean 39.2 years) increased systolic blood pressure (SBP) was associated with altered diffusion in several tracts, even though high BPs were mainly at pre-hypertensive or mild hypertensive levels14. This suggests that white matter microstructure may already be affected early in the course of the disease.
If white matter changes are an early occurrence in hypertension, it might be expected that severity of effects would be larger in those with longer duration hypertension. This has not been found consistently for macroscopic white matter lesions (WMLs), however15, and effects of duration of hypertension on white matter microstructure have not been assessed in middle-aged adults. Similarly, few studies have looked at whether adequate blood pressure control may mitigate effects of hypertension on white matter microstructure8, 9.
To address these gaps in the literature, we examined participants of the Vietnam Era Twin Study of Aging (VETSA), a longitudinal study of cognitive and brain aging beginning in midlife16,17. We hypothesized that, in middle-aged adults, long-duration hypertension is associated with altered white matter microstructure relative to normotensive individuals, with greater adverse effects in ε4 carriers than non-carriers. In secondary analyses, we explored whether hypertension of more recent onset is also associated with significant white matter alterations and whether white matter microstructure differs between hypertensive individuals with controlled and uncontrolled BP.
Methods
Participants
Participants in the parent VETSA cohort were recruited from the Vietnam-Era Twin Registry, a nationally distributed sample of male-male twin pairs who served in the United States military at some point between 1965 and 197518. Participants are similar in health and lifestyle characteristics to American men in their age range19. Although all VETSA participants are veterans, most (~80%) did not experience combat situations.
Of the 409 VETSA participants who underwent clinical assessment at wave 1 and brain magnetic resonance imaging (MRI) 5.6 years later, during wave 2, 9 were excluded due to missing APOE data, 63 due to missing or poor quality MRI data, and 21 due to use of antihypertensive medications for reasons other than blood pressure control. The final sample comprised 316 participants aged 61.8 (±2.58; range 56.7–65.6) at wave 2. Participants were predominantly Caucasian (88.9%), with an average education of 13.9 years (SD=2.0).
The study was conducted under local institutional review board supervision at the participating institutions and all participants provided signed informed consent.
Hypertensive Status
At both waves, SBP and diastolic blood pressure (DBP) were measured as the average of two A.M. and two P.M. seated BP readings by trained observers with an electronic sphygmomanometer (Omron Model HEM-757/Wave 1; Lifesource Model UA-789AC/Wave 2). Participants rested for 5 minutes prior to the first reading in each set, wearing a blood pressure cuff on their right arm with their arm resting on a table. Participants rested for one minute between the paired readings.
Participants were classified as having hypertension based on SBP≥140 mm Hg, DBP≥90 mm Hg, or self-report of a physician diagnosis. Normotensive individuals did not meet hypertension criteria at either wave 1 or wave 2 (N=101; 32.0%). Participants who met hypertensive criteria at wave 1 were classified as having longer duration hypertension (N=173; 54.7%), those who met hypertension criteria at wave 2 but not wave 1 were classified as having shorter duration hypertension (N=42; 13.3%).
To examine differences related to blood pressure control, individuals who met hypertensive criteria at wave 2 were categorized into controlled (SBP<140 mm Hg and DBP<90 mm Hg at Wave 2, N=142), and uncontrolled (N=73) hypertension groups.
Clinical and Lifestyle Covariates
During Wave 2, height and weight were measured, and body mass index (BMI) calculated. Diabetes status was ascertained from self-report of a doctor’s diagnosis or reported use of a diabetes-related medication. Following laboratory protocols, fasting morning blood samples were centrifuged after allowing 30–45 minutes for the sample to clot. Low- and high-density lipoprotein (LDL and HDL) cholesterol and triglycerides were assayed as part of a lipid panel via spectrophotometry. C-Reactive protein (CRP) levels were assessed with nephelometry. Assays were conducted by Quest Diagnostics Inc/Nichols Institute, San Juan Capistrano California. CRP values of 10 or higher were assumed to reflect acute infection and these cases (N=8) were excluded from analyses that included CRP. CRP, cholesterol and triglyceride levels were log-transformed prior to analyses.
Smoking, alcohol, and medication use were assessed at wave 2 as part of a structured medical history interview. Individuals were categorized into non-smokers, former smokers, or current smokers, and into non-drinkers, moderate drinkers (≤2 drinks per day) or heavy drinkers (>2 drinks per day) based on use over the prior two weeks.
APOE Genotype
Methods for APOE genotyping were previously described20. Participants were separated into APOE-ε4 carriers if they had at least 1 copy of the ε4 allele (25.3%) or non-carriers (74.7%).
Image Acquisition and Processing
T1-weighted and diffusion-weighted images were obtained using standardized protocols on 3T scanners at two sites. Image acquisition and processing methods are detailed in supplemental material (please see http://hyper.ahajournals.org). Diffusion metrics, including FA, a scalar value of the degree of anisotropic/directional diffusion within the voxel; MD, the average diffusion in all directions; longitudinal diffusivity (LD), the average diffusion along the primary axis of diffusion, and transverse diffusivity (TD), the average diffusion along the two non-primary axes, were derived from each fiber tract region of interest using a probabilistic diffusion tensor atlas of fiber tract locations and orientations (AtlasTrack21). We averaged diffusion metrics from homologous tracts in left and right hemispheres and examined nine major tracts previously shown to be affected by hypertension8,11,14: the uncinate fasciculus (UF), inferior fronto-occipital fasciculus (IFOF), inferior and superior lateral fasciculi (ILF and SLF), anterior thalamic radiations (ATR), cingulum portion of the cingulate bundle (CgC), the corticospinal tract, and forceps minor and forceps major (the anterior and posterior portions of the corpus callosum). Locations of these tracts are shown in Supplemental Figure S1.
Statistical analysis
Diffusion metrics were submitted to linear mixed effect models (Proc Mixed SAS, version 9.4). Base models included fixed effects of site (scanner) and age at wave 2. A “family ID” variable was included in all models as a random effect to control for non-independence of twin data. To test our primary hypothesis, we examined whether diffusion metrics differed between normotensive individuals and those with longer-duration hypertension as a function of APOE-ε4 status. Base models included fixed effects of hypertension and APOE-ε4 status, as well as an interaction term between hypertension and APOEε4. In separate models, we adjusted for covariates that differed between normotensive and hypertensive individuals at p<0.10: education level, BMI, LDL, CRP, diabetes, statin use, and alcohol use.
In exploratory analyses, we examined differences in diffusion metrics related to relative duration of hypertension and to control of hypertension. In these analyses, there were too few ε4 carriers in relevant subgroups to meaningfully assess differential effects of APOE. Base models included age, site, and family ID; secondary models adjusted for the covariates described above.
Results
Table 1 shows the demographic and clinical characteristics of normotensive individuals and those with longer-duration hypertension, by APOE-ε4 status. Normotensives had lower BMI, lower CRP levels, were less likely to have diabetes, less likely to take statin medication, and had higher LDL levels than those with hypertension. There were no significant effects of APOE or interactions between hypertension and APOE on any clinical or demographic variable.
Table 1.
Demographic and clinical characteristics of participants at wave 2. Values are mean (standard deviation) unless otherwise specified.
| Characteristic | Normotensive | Longer-Duration Hypertension | Hypertension | ||
|---|---|---|---|---|---|
| APOE ε4− N = 74 |
APOE ε4+ N=27 |
APOE ε4− N=134 |
APOE ε4+ N=39 |
Main Effect p value |
|
| Age, yrs | 62.0 (2.6) | 61.3 (2.7) | 62.0 (2.5) | 61.7 (2.6) | 0.548 |
| Education, yrs | 14.3 (2.2) | 14.2 (2.5) | 13.8 (1.7) | 13.5 (1.9) | 0.045 |
| BMI | 27.5 (4.5) | 26.6 (4.4) | 29.6 (4.0) | 29.2 (3.7) | <0.001 |
| Systolic BP | 118.5 (9.2) | 121.2 (9.0) | 133.0 (18.0) | 132.8 (16.8) | <0.001 |
| Diastolic BP | 73.9 (6.6) | 75.7 (7.2) | 81.6 (9.8) | 78.6 (9.8) | <0.001 |
| * Log Triglycerides | 4.63 (0.58) | 4.71 (0.59) | 4.77 (0.51) | 4.77 (0.47) | 0.197 |
| * Log HDL | 3.93 (0.27) | 3.89 (0.25) | 3.86 (0.30) | 3.83 (0.33) | 0.160 |
| * Log LDL | 4.72 (0.35) | 4.81 (0.32) | 4.60 (0.30) | 4.57 (0.38) | <0.001 |
| * Log CRP | 0.02 (0.96) | −0.10 (1.04) | 0.52 (0.93) | 0.11 (0.91) | 0.011 |
| HTN Meds (%) | 0 | 0 | 71.6 | 74.4 | <0.001 |
| Statins (%) | 25.7 | 18.5 | 41.0 | 46.2 | <.016 |
| Diabetes (%) | 5.4 | 3.7 | 17.9 | 12.8 | 0.03 |
| Smoking (% former/current) | 35.1 / 25.7 | 29.6 / 11.1 | 36.6/ 17.9 | 41.0 / 19.3 | 0.520 |
| Alcohol (% moderate / heavy) | 56.8 / 5.4 | 44.4 / 11.1 | 53.4 / 15.8 | 33.3 / 25.6 | 0.067 |
Yrs indicates years, BMI body mass index, BP blood pressure, HDL high density lipoprotein; LDL low density lipoprotein; CRP, C-reactive protein; HTN Meds antihypertensive medication.
Lipid and CRP data were missing for a small number of participants. N’s for LDL and trigylcerides were 70, 24, 122, 38. N’s for HLD were 70, 25,123, 38. N’s for CRP were 70, 26,123, 37.
Table 2 shows the main effects of hypertension and the interaction of hypertension with APOE for the four diffusion metrics within the nine tracts. In base models, hypertension was associated with significantly lower FA or increased diffusivity in all tracts except the CgC and forceps major. MD and TD were more often affected than LD.
Table 2.
F and p values of the main effect of longer-duration hypertension and the hypertension by APOE ε4 interaction, for each diffusion metric for each tract.
| Fiber Tract | Effect | FA | MD | TD | LD |
|---|---|---|---|---|---|
| UF | htn main effect | 8.81; 0.004 | 18.16; <0.001 | 15.98; 0.002 | 10.11; 0.002 |
| APOE ε4 x htn | 4.54; 0.037 | 5.27; 0.025 | 5.43; 0.023 | 1.42; 0.237 | |
| IFOF | htn main effect | 5.13; 0.027 | 7.35; 0.008 | 7.57; 0.008 | 4.35; 0.040 |
| APOE ε4 x htn | 2.53; 0.116 | 4.38; 0.040 | 4.71; 0.033 | 2.27; 0.136 | |
| ILF | htn main effect | 4.57; 0.034 | 7.23; 0.009 | 8.59; 0.005 | 3.40; 0.069 |
| APOE ε4 x htn | 2.58; 0.112 | 3.28; 0.074 | 4.43; 0.039 | 1.12; 0.293 | |
| SLF | htn main effect | 10. 70; 0.002 | 11.97; <0.001 | 13.44; <0.001 | 3.93; 0.051 |
| APOE ε4 x htn | 1.81; 0.182 | 1.16; 0.286 | 1.61; 0.208 | 0.11; 0.738 | |
| CgC | htn main effect | 3.82; 0.055 | 1.77; 0.188 | 0.81; 0.372 | 0.10; 0.747 |
| APOE ε4 x htn | 0.63; 0.432 | 0.01; 0.938 | 0.15; 0.699 | 0.44; 0.507 | |
| ATR | htn main effect | 11.00; 0.001 | 8.48; 0.005 | 10.08; 0.002 | 4.21; 0.044 |
| APOE ε4 x htn | 2.04; 0.158 | 0.81; 0.370 | 1.12; 0.293 | 0.37; 0.547 | |
| F Min | htn main effect | 4.78; 0.032 | 4.83; 0.031 | 5.03; 0.028 | 2.24; 0.139 |
| APOE ε4 x htn | 1.04; 0.310 | 2.58; 0.112 | 1.95; .167 | 2.62; 0.110 | |
| F Maj | htn main effect | 0.74; 0.393 | 1.96; 0.166 | 1.35; 0.249 | 2.56; 0.114 |
| APOE ε4 x htn | 0.00; 0.950 | 0.92; 0.340 | 0.32; 0.575 | 2.95; 0.090 | |
| CST | htn main effect | 4.77; 0.032 | 3.45; 0.067 | 5.19; 0.026 | 0.11; 0.743 |
| APOE ε4 x htn | 3.69; 0.059 | 3.92; 0.052 | 4.84; 0.031 | 0.83; 0.364 |
Values are from base models which corrected for age, scanner site and non-independence of twin data; p values < .05 are shown in bold; underlined values remained significant after separate adjustment for education level, BMI, diabetes, LDL, CRP, statin use, and alcohol use.
FA indicates fractional anisotropy; MD, mean diffusivity; TD, transverse diffusivity; LD, longitudinal diffusivity; htn hypertension; UF, uncinate fasciculi; IFOF, inferior fronto-occipital fasciculi; ILF, the inferior lateral fasciculi; SLF, the superior lateral fasciculi; ATR, anterior thalamic radiations; CgC, cingulum portion of the cingulate bundle; Fmin, forceps minor; Fmax, forceps major, and CST, corticospinal tract.
Significant interactions between APOE-ε4 and hypertension were found for the UF, IFOF and ILF; hypertension was associated with significantly lower FA or higher MD or TD among ε4 carriers only.
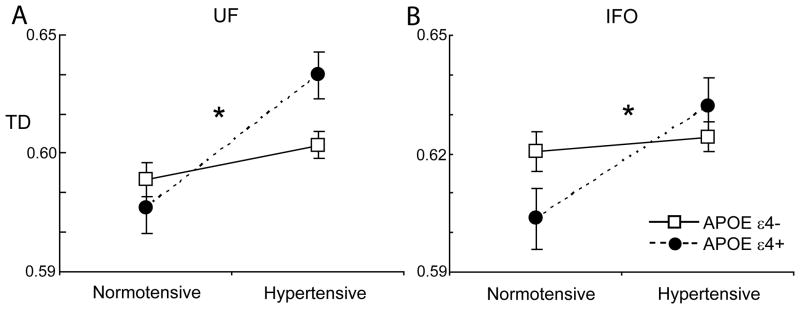
With adjustment for potentially confounding covariates, the APOE-hypertension interaction remained significant for FA, MD, TD in the UF, and TD in the IFOF (Figure 1). Main effects of hypertension on diffusion metrics in the UF, IFOF, ILF, SLF, ATR and forceps minor remained significant with adjustment for covariates (Table 2).
Figure 1.
Interactions between APOE-ε4 status and longer-duration hypertension for transverse diffusivity (TD) in the uncinate fasciculus (UF; A) and inferior fronto-occipital fasciculus (IFOF; B). Means are adjusted for age and scanner site. *p<.05 for interaction with adjustment for potential confounders.
Hypertension Duration
Characteristics of normotensive individuals and those with shorter or longer duration hypertension are shown in supplemental Table S1. There were no significant differences between the two hypertensive groups on any demographic or clinical measure.
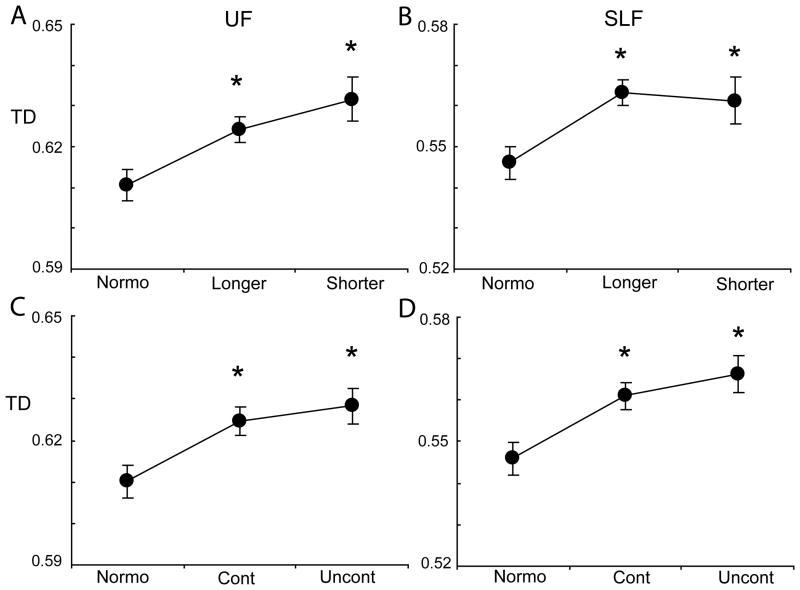
Mixed effects models showed significant effects of hypertension on FA, MD or TD in the UF, ILF, SLF, and ATR (supplemental Table S2). In pairwise comparisons, the hypertensive groups differed from the normotensive group, but there were no significant differences between the two hypertensive groups on any measure (Figure 2 and supplement Figure S2). Controlling for additional covariates did not materially affect the findings.
Figure 2.
Transverse diffusivity (TD) in the uncinate fasciculus (UF; A) and superior lateral fasciculus (SLF; B) by relative duration of hypertension and by control of hypertension (UF C, SLF D). Means are adjusted for age and scanner site. *p<.05, ** p < .01 for differences of the hypertensive subgroup relative to normotensives; the longer and shorter duration hypertension groups did not significantly differ from each other, nor did the controlled and uncontrolled hypertension groups.
Controlled Versus Uncontrolled Hypertension
Characteristics of hypertensive individuals who achieved good BP control and those who did not are shown in Supplemental Table S3. The two treatment groups did not differ in age, proportion of APOE-ε4 carriers, HDL, triglyceride levels, or smoking. The controlled hypertension group had a higher proportion of individuals with diabetes (22.5% vs 9.6 %), a higher proportion of individuals on statin medication, and lower LDL levels than the uncontrolled hypertension group. The uncontrolled hypertension group had higher BMI, SBP, and DBP, and consumed more alcohol than the controlled hypertension group. Less than half of those in the uncontrolled hypertensive group were taking antihypertensive medications (47.9%).
Mixed-effects models showed significant effects of hypertension on diffusion metrics in the UF, ILF, SLF, and ATR (Supplemental Table S4). Pairwise comparisons revealed significant differences between normotensives and those with hypertension, but no significant differences on any measure between the controlled and uncontrolled hypertension groups (Figure 2 and supplement Figure S3). Adjustment for additional covariates did not materially affect the findings.
Discussion
We examined the association of hypertension with tract-specific diffusion metrics in a relatively large cohort of middle-aged men. Several important findings emerged: 1) Consistent with prior studies involving older adults or comprising a wide age range, hypertension was associated with altered diffusion properties in several tracts. Alterations included lower FA and higher MD or TD, with few effects on LD. 2) The APOE-ε4 allele was associated with increased susceptibility to the effects of hypertension in the UF and IFOF. 3) Longer duration of hypertension was not associated with greater white matter microstructural differences than hypertension of more recent onset. 4) White matter microstructural alterations were observed in both controlled and uncontrolled hypertension groups. These findings suggest that hypertension-related alterations in white matter microstructure occur early in the course of the disease and may persist despite adequate BP control.
Of the nine tracts examined, all but the forceps major and CgC showed associations with hypertension. Some studies have suggested that anterior white matter may be more susceptible to hypertension than posterior white matter22. Our finding of significant effects in anterior but not posterior corpus callosum is consistent with this, but we also observed significant effects in more centrally or posteriorly situated tracts, as have others7–9. This suggests that hypertension has widespread effects on brain white matter. Prior findings that alterations in white matter diffusion metrics are associated with decreased cognitive performance suggest that these changes are not benign7,23,24.
Negative effects of hypertension were greater in APOE-ε4 carriers than non-carriers in the UF, which connects orbitofrontal regions to anterior temporal and limbic regions, and the IFOF, which connects frontal regions to occipital regions. This is consistent with prior findings in healthy adults of greater susceptibility of frontal regions than other brain regions to APOE-ε420. It is also consistent with prior findings that APOE-ε4 confers greater vulnerability to effects of hypertension on cognition3,25 and WMLs2.
Hypertension was associated with decreased FA and increased MD, with TD more often affected than LD. Animal studies suggest that such a pattern is consistent with myelin damage26. However, mechanisms by which hypertension affects brain white matter are varied and complex1,27,28. Chronic hypertension is associated with vascular remodeling and reduced vascular reserve, which may lead to ischemia. Hypertension also interferes with perivascular lymphatic drainage and increases blood-brain permeability, resulting in fluid accumulation that may be toxic to cells28. Both tissue damage and fluid accumulation will alter diffusion. Thus, the complexity of fiber projections in the human brain and the multitude of factors that can affect diffusion preclude inference of the neurobiological basis of the observed differences29,30.
Longer duration of hypertension was not associated with greater white matter differences. Few studies have looked at effects of hypertension duration on white matter microstructure. In a 10-year follow-up of much older adults (mean 83 years at imaging) high and variable BP was associated with strongest detrimental effects on white matter measures10. Macroscopic WMLs did not differ between individuals with recent onset hypertension and those with 3- or 6- year hypertension in a study of adults aged 55–72 years at entry15. In a study of adults aged 60–90 (mean 72) years at entry, 20-year, but not 5-year duration of hypertension was associated with increased odds of WMLs, but only among individuals with onset of hypertension before middle age2. With longer follow-up, effects of longer duration of hypertension may become detectable in our sample. Additionally, our shorter-duration hypertensive group was relatively small, perhaps precluding detection of subtle differences related to hypertension duration.
The lack of significant differences in diffusion properties between those with controlled and uncontrolled hypertension is consistent with the interpretation that microstructural damage to white matter tracts occurs relatively early in the course of the disease, and suggests that this damage may not be easily reversible. A prior study (aged 50–85, mean 66 years), also found significantly lower FA in both adequately and inadequately treated hypertensive groups relative to normotensives8.
The current study has several limitations. The cohort is restricted to men, and largely Caucasian; thus, results may not generalize to women, other races or ethnicities. Image analyses were cross-sectional; longitudinal imaging studies are needed to confirm the time course of microstructural changes in relation to the onset of hypertension and impact of treatment over time. Finally, the low number of ε4 carriers in shorter-duration and uncontrolled hypertension subgroups limited our ability to determine whether hypertension duration or treatment interacts with this genetic risk factor.
Study strengths include the large number of men representative of the general population in health and lifestyle characteristics, and the narrow age-range centered at middle age. This minimizes differential survival effects and potential confounding due to latent neurodegenerative pathology. We were also able to control for numerous cardiovascular and behavioral risk factors. That significant differences remained between hypertensive and normotensive individuals after covariate adjustment indicates that the effects on white matter were not due to differences in education, age, BMI, diabetes, inflammation, lipid levels, statin therapy, or alcohol use.
Perspectives
This study demonstrates that hypertension is associated with significant microstructural differences in cerebral white matter in middle-aged men, and that APOE-ε4 carriers may show greater vulnerability to hypertension than non-carriers. Hypertension-related differences appear to occur early in the course of hypertension and are apparent even in those with adequately controlled hypertension. This suggests that prevention, rather than management, of hypertension may be vital to preserving brain health in aging.
Supplementary Material
Novelty and Significance.
1. What is New?
In middle-aged men, hypertension, whether controlled or uncontrolled, was associated with reduction of white matter microstructural organization in many major white matter tracts.
Similar effects were observed in individuals with recent onset (within 6 years) and those with longer duration hypertension.
Individuals with a copy of the APOE-ε4 allele, a genetic risk factor for Alzheimer’s disease, showed greater hypertension-related differences than those without the genetic risk factor.
2. What is Relevant?
Hypertension appears to have adverse effects on the brain early in the course of the disease; these effects may be difficult to reverse with BP management.
3. Summary
Hypertension is associated with adverse effects on multiple major white matter tracts. Since these effects appear early in the course of the disease and are apparent even those who achieve good BP control, prevention—rather than management—of hypertension may be critical for preserved brain health in aging.
Acknowledgments
Sources of Funding
VETSA was supported by NIA grants [R01s AG018386, AG022381, AG022982 to W.S.K. and R01 AG018384 to M.J.L.]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIA/NIH, or the VA. L.K.M. was supported by NIAAA AA021187.
Footnotes
Conflicts of Interest/Disclosures
McEvoy has stock options in CorTechs Labs, Inc
Dale is a Founder of and holds equity in CorTechs Labs, Inc, and serves on its Scientific Advisory Board. He is a member of the Scientific Advisory Board of Human Longevity, Inc. and receives funding through research agreements with General Electric Healthcare and Medtronic, Inc. The terms of these arrangements have been reviewed and approved by UCSD in accordance with its conflict of interest policies.
Other authors declare no conflict of interest.
References
- 1.Gasecki D, Kwarciany M, Nyka W, Narkiewicz K. Hypertension, brain damage and cognitive decline. Curr Hypertens Rep. 2013;15:547–558. doi: 10.1007/s11906-013-0398-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Leeuw FE, Richard F, de Groot JC, van Duijn CM, Hofman A, Van Gijn J, Breteler MM. Interaction between hypertension, APOE, and cerebral white matter lesions. Stroke. 2004;35:1057–1060. doi: 10.1161/01.STR.0000125859.71051.83. [DOI] [PubMed] [Google Scholar]
- 3.de Frias CM, Schaie KW, Willis SL. Hypertension moderates the effect of APOE on 21-year cognitive trajectories. Psychol Aging. 2014;29:431–439. doi: 10.1037/a0036828. [DOI] [PubMed] [Google Scholar]
- 4.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4:487–499. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- 5.Le Bihan D. Molecular diffusion, tissue microdynamics and microstructure. NMR Biomed. 1995;8:375–386. doi: 10.1002/nbm.1940080711. [DOI] [PubMed] [Google Scholar]
- 6.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy KM, Raz N. Pattern of normal age-related regional differences in white matter microstructure is modified by vascular risk. Brain Res. 2009;1297:41–56. doi: 10.1016/j.brainres.2009.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gons RA, van Oudheusden LJ, de Laat KF, van Norden AG, van Uden IW, Norris DG, Zwiers MP, van Dijk E, de Leeuw FE. Hypertension is related to the microstructure of the corpus callosum: The RUN DMC Study. J Alzheimers Dis. 2012;32:623–631. doi: 10.3233/JAD-2012-121006. [DOI] [PubMed] [Google Scholar]
- 9.Salat DH, Williams VJ, Leritz EC, Schnyer DM, Rudolph JL, Lipsitz LA, McGlinchey RE, Milberg WP. Inter-individual variation in blood pressure is associated with regional white matter integrity in generally healthy older adults. Neuroimage. 2012;59:181–192. doi: 10.1016/j.neuroimage.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosano C, Abebe KZ, Aizenstein HJ, Boudreau R, Jennings JR, Venkatraman V, Harris TB, Yaffe K, Satterfield S, Newman AB, Health ABCS. Longitudinal systolic blood pressure characteristics and integrity of white matter tracts in a cohort of very old black and white adults. Am J Hypertens. 2015;28:326–334. doi: 10.1093/ajh/hpu134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Groot M, Ikram MA, Akoudad S, Krestin GP, Hofman A, van der Lugt A, Niessen WJ, Vernooij MW. Tract-specific white matter degeneration in aging: The Rotterdam Study. Alzheimers Dement. 2015;11:321–330. doi: 10.1016/j.jalz.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 12.Westlye LT, Reinvang I, Rootwelt H, Espeseth T. Effects of APOE on brain white matter microstructure in healthy adults. Neurology. 2012;79:1961–1969. doi: 10.1212/WNL.0b013e3182735c9c. [DOI] [PubMed] [Google Scholar]
- 13.Adluru N, Destiche DJ, Lu SY, Doran ST, Birdsill AC, Melah KE, Okonkwo OC, Alexander AL, Dowling NM, Johnson SC, Sager MA, Bendlin BB. White matter microstructure in late middle-age: Effects of apolipoprotein ε4 and parental family history of Alzheimer’s disease. Neuroimage Clin. 2014;4:730–742. doi: 10.1016/j.nicl.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maillard P, Seshadri S, Beiser A, Himali JJ, Au R, Fletcher E, Carmichael O, Wolf PA, DeCarli C. Effects of systolic blood pressure on white-matter integrity in young adults in the Framingham Heart Study. Lancet Neurol. 2012;11:1039–1047. doi: 10.1016/S1474-4422(12)70241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liao D, Cooper L, Cai J, Toole JF, Bryan NR, Hutchinson RG, Tyroler HA. Presence and severity of cerebral white matter lesions and hypertension, its treatment, and its control. The ARIC Study. Stroke. 1996;27:2262–2270. doi: 10.1161/01.str.27.12.2262. [DOI] [PubMed] [Google Scholar]
- 16.Kremen WS, Thompson-Brenner H, Leung YM, Grant MD, Franz CE, Eisen SA, Jacobson KC, Boake C, Lyons MJ. Genes, environment, and time: The Vietnam Era Twin Study of Aging. Twin Res Hum Genet. 2006;9:1009–1022. doi: 10.1375/183242706779462750. [DOI] [PubMed] [Google Scholar]
- 17.Kremen WS, Franz CE, Lyons MJ. Vetsa: The Vietnam Era Twin Study of Aging. Twin Res Hum Genet. 2013;16:399–402. doi: 10.1017/thg.2012.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldberg J, Curran B, Vitek ME, Henderson WG, Boyko EJ. The Vietnam Era Twin Registry. Twin Res. 2002;5:476–481. doi: 10.1375/136905202320906318. [DOI] [PubMed] [Google Scholar]
- 19.Schoenborn CA, Heyman KM. Health characteristics of adults aged 55 years and over: United States, 2004–2007. Natl Health Stat Report. 2009:1–31. [PubMed] [Google Scholar]
- 20.Fennema-Notestine C, Panizzon MS, Thompson WR, Chen CH, Eyler LT, Fischl B, Franz CE, Grant MD, Jak AJ, Jernigan TL, Lyons MJ, Neale MC, Seidman LJ, Tsuang MT, Xian H, Dale AM, Kremen WS. Presence of APOE epsilon4 allele associated with thinner frontal cortex in middle age. J Alzheimers Dis. 2011;26 (Suppl 3):49–60. doi: 10.3233/JAD-2011-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hagler DJ, Jr, Ahmadi ME, Kuperman J, Holland D, McDonald CR, Halgren E, Dale AM. Automated white-matter tractography using a probabilistic diffusion tensor atlas: Application to temporal lobe epilepsy. Hum Brain Mapp. 2009;30:1535–1547. doi: 10.1002/hbm.20619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raz N, Rodrigue KM, Acker JD. Hypertension and the brain: Vulnerability of the prefrontal regions and executive functions. Behav Neurosci. 2003;117:1169–1180. doi: 10.1037/0735-7044.117.6.1169. [DOI] [PubMed] [Google Scholar]
- 23.Ryan L, Walther K, Bendlin BB, Lue LF, Walker DG, Glisky EL. Age-related differences in white matter integrity and cognitive function are related to APOE status. Neuroimage. 2011;54:1565–1577. doi: 10.1016/j.neuroimage.2010.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobs HI, Leritz EC, Williams VJ, Van Boxtel MP, van der Elst W, Jolles J, Verhey FR, McGlinchey RE, Milberg WP, Salat DH. Association between white matter microstructure, executive functions, and processing speed in older adults: The impact of vascular health. Hum Brain Mapp. 2013;34:77–95. doi: 10.1002/hbm.21412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peila R, White LR, Petrovich H, Masaki K, Ross GW, Havlik RJ, Launer LJ. Joint effect of the APOE gene and midlife systolic blood pressure on late-life cognitive impairment: The Honolulu-Asia aging study. Stroke. 2001;32:2882–2889. doi: 10.1161/hs1201.100392. [DOI] [PubMed] [Google Scholar]
- 26.Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, Armstrong RC. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 27.Manolio TA, Olson J, Longstreth WT. Hypertension and cognitive function: Pathophysiologic effects of hypertension on the brain. Curr Hypertens Rep. 2003;5:255–261. doi: 10.1007/s11906-003-0029-6. [DOI] [PubMed] [Google Scholar]
- 28.Mohammadi MT, Dehghani GA. Acute hypertension induces brain injury and blood-brain barrier disruption through reduction of claudins MRNA expression in rat. Pathol Res Pract. 2014;210:985–990. doi: 10.1016/j.prp.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 30.Wheeler-Kingshott CA, Cercignani M. About “axial” and “radial” diffusivities. Magn Reson Med. 2009;61:1255–1260. doi: 10.1002/mrm.21965. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.