Many of the recent eye-catching advances in zincate executed metallation chemistry have centered on the secondary monoamide 2,2,6,6-tetramethylpiperidide (TMP). Developed by Kondo, Uchiyama, and co-workers, the original TMP zincate reagent [LiZn(TMP)tBu2] is an effective base for both aromatic and heteroaromatic substrates.[1] Knochel and co-workers have since compiled a library of new TMP-based metalating agents, including the zinc pivalate [(TMP)Zn(OPiv)⋅LiCl] (OPiv=pivalate), which like [LiZn(TMP)tBu2], displays strong deprotonating power and exceptional functional-group tolerance but has the added advantage that its arylzincated derivatives boast prolonged air stability.[2] The area of zincate metalation chemistry that is best structurally defined is that involving the sodium TMP reagent [(TMEDA)Na(TMP)(tBu)Zn(tBu)] (1; TMEDA=N,N,N′,N′-tetramethylethylenediamine). This reagent, which exhibits enhanced reactivity over the aforementioned lithium zincates, and many of its arylzincated derivatives adopt similar structures, which are designed on an architectural template of a Na–anion–Zn–anion ring carrying terminal ligands, namely a neutral donor and an anion on Na and Zn, respectively (Figure 1). To break this template and develop new structural motifs that could stimulate new reactivity we have investigated replacing monofunctional TMP with multifunctional 2,2′-dipyridylamide [dpa, (2-NC5H4)2N]. Offering three potential ligating N sites, this N-bridged bis(N heterocycle) finds utility in a diverse range of applications including medicine,[3] catalysis,[4] photoluminescence,[5] and supramolecular chemistry.[6] Herein we report a remarkable set of novel structures incorporating dpa into neutral zinc, sodium zincate, and potassium zincate modifications containing tert-butyl ligands. Preliminary reactivity studies reveal that the mixed sodium-zinc dpa complexes can tert-butylate the ketone benzophenone at the para (1,6-addition) position, which is challenging, and hint that the reaction can be made substoichiometric in the sodium component.
Figure 1.
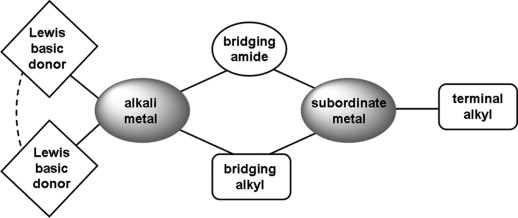
General structural motif observed for heteroleptic ate bases. Here the subordinate metal is zinc.
The neutral, heteroleptic zinc complex [[(dpa)Zn(tBu)]2] (2) was synthesized straightforwardly through the 1:1 stoichiometric combination of tBu2Zn and the amine dpa(H) in a hexane solution. NMR studies of the filtrate revealed the absolute yield was higher than the 70 % yield of the isolated crystals. Possessing an attractive hour-glass-shaped core, the centrosymmetric molecular structure of 2 (Scheme 1) is dimeric with the distorted tetrahedral zinc atom[7] bonded to the deprotonated N of one amido unit, which occupies the di-pyridyl pocket of the other and completes its coordination with a terminal tBu ligand. In the hour-glass description (see the Supporting Information), each bulb comprises a puckered, six-atom (NCNCNZn) ring with Zn situated 1.0991(3) Å from the nearest NCNCN plane and 1.7143(3) Å from the second NCNCN plane. This motif bears a strong resemblance to that of the isoelectronic neutral zinc dimer [[MeZnC(H)Py2]2](Py=2-pyridyl).[8] To our knowledge, 2 represents the first crystallographically characterized Zn/dpa complex showing a Zn=(bridgehead)N bond, although it has been noted in alkylated dimeric derivatives wherein the mode of dimerization is distinct from that in 2.[9] The anti/anti conformation[10] (i.e., both pyridyl N atoms directed away from the bridgehead N) within 2 also contrasts with the syn/anti conformations found in polymorphs of dpa(H)[11] and the aforementioned alkylated dpa derivatives.
Scheme 1.
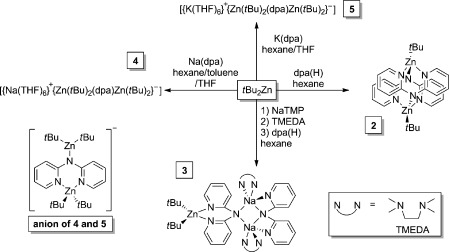
Synthesis of the neutral zinc dimer 2, disodium zincate 3, sodium zinczincate 4, and potassium zinczincate 5.
A simple transamination reaction[12] between TMP/zincate 1 and dpa(H) in a hexane solution was anticipated, but instead the isolated crystalline product was the disodium monozinc species [(TMEDA)2Na2(μ-dpa)2Zn(tBu)2] [3; 70 % yield based on the dpa(H) stoichiometry]. Formally transamination has occurred but the 2:1 Na/Zn stoichiometry of 3 is inconsistent with the 1:1 stoichiometry of the reaction. Its molecular structure (Figure 2) can be viewed as a cocomplex between a TMEDA-complexed sodium amide dimer and a bis(alkyl) zinc monomer with the connection being two dative Zn=N(pyr) bonds completing a distorted tetrahedral (C2N2) zinc environment. To bind to Zn, one dipyridylamide must adopt an anti/anti disposition with its pyridine N atoms oriented away from the [[Na(amido)N]2] ring, while the second has a syn/syn conformation with each Na bonded to the amido N and one or other of the pyridine N atoms. Resonance delocalization within the dipyridylamide scaffold is usually associated with shorter C-N(amido)-C bridges (e.g., about 1.34 Å each), longer C-N(pyr) bonds (e.g., about 1.38 Å), and a dihedral angle between the two pyridine ring planes approaching 0° as ascertained by data from several neutral dpa(H) and anionic dpa complexes[11,13] (see the Supporting Information). The corresponding dimensions within 3 [e.g., C10=N1, 1.371(2) Å; C15=N1, 1.373(2) Å; C10=N2, 1.347(2) Å; C15=N3, 1.346(2) Å; dihedral angle of unit bonded to Zn, 22.9°] suggest the degree of delocalization is small. This prompts the thought that though formally a higher-order zincate (conforming to the empirical formula Na2ZnR2R′2), 3 is best interpreted as a neutral donor-acceptor bis(alkyl)zinc complex with a metalloligand as a donor. Supporting this view, the structure of 3 is novel as it bears little resemblance to the common Weiss motif of dialkali-metal zincate and related ate structures[14] (Figure 3) but shows more in common with homonuclear zinc complexes such as [tBu2Zn[(iPrN(H)CH2CH2N(H)iPr]][15] or [(tBu2Zn)3(C4H4N2)4].[16]
Figure 2.
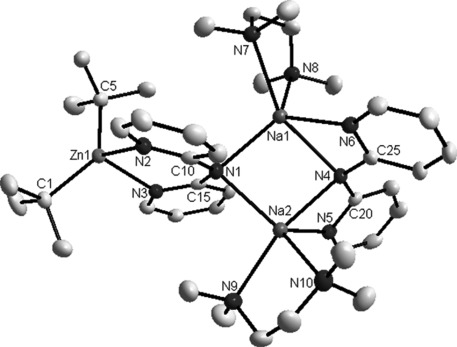
Molecular structure of 3[26] with thermal ellipsoids at 50 % probability level. Hydrogen atoms omitted for clarity. Selected bond lengths (Å) and angles (°): Zn1=C1 2.048(2), Zn1=C5 2.044(2), Zn1=N2 2.185(2), Zn1=N3 2.157(2), C10=N1 1.371(2), C10=N2 1.347(2), C15=N1 1.373(2), C15=N3 1.346(2); C1-Zn1-C5 130.31(7), C1-Zn1-N2 111.19(6), C1-Zn1-N3 110.41(6), N2-Zn1-N3 82.42(6), N2-Zn1-C5 108.19(6), N3-Zn1-C5 103.65(7), C10-N1-C15 125.1(2).
Figure 3.
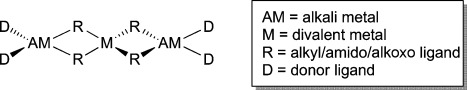
Graphical representation of a Weiss motif structure.
Surprisingly when the same metal components in 3 were mixed together in the presence of THF and in the absence of TMEDA, the structural outcome was remarkably different (Scheme 1). Substituting TMEDA by THF can be trivial in organoalkalimetal chemistry with one bidentate TMEDA being replaced by two monodentate THF molecules without altering the basic structure of the complex, but the reactivity may be changed.[17] However, here the structural effect is more profound. Unlike the contact ion-pair 3 with its 2:1 Na/Zn stoichiometry, the isolated product is the solvent-separated monosodium dizinc complex [[Na(THF)6]+[Zn(tBu)2(dpa)Zn(tBu)2]−] (4). The octahedrally coordinated cation of 4 is known[18] but its anionic moiety (Figure 4) is unprecedented. Two tBu2Zn monomers connect through one dpa anion which, lying flat in an anti/anti conformation, presents a chelating pyridyl pocket to one Zn atom, thus making it four coordinate, and the bridgehead amido N to the other Zn, thus making it three coordinate. Unfortunately, the anion lies on a crystallographic center of symmetry, and as this symmetry can only be approximately satisfied by the dpa ligand, the structure is disordered over two sites. This prevents any discussion of dimensions, though the atomic connectivity is definite. We therefore turned to a DFT study using the B3LYP[19] method and the 6-311G (d,p)[20] basis set in which the anionic moiety of 4 was modeled. Its dimensions (see the Supporting Information for full details) revealed slightly more delocalization than that implied in 3. Key indicators are the C=N(amido) bond lengths [1.363 Å; 1.372 Å (mean) in 3] and the dihedral angle between the N(pyr)=C(H) bonds [C-N⋅⋅⋅N-C is 3.9° in 4; 26.0° in 3]. Also within the pyridyl rings there are long C=N bonds and two relatively short C=C bonds akin to a N-CC-C=C-C-N pattern (bond lengths 1.345/1.345, 1.380/1.380, 1.401/1.401, 1.374/1.374, 1.429/1.428, and 1.360/1.359 Å) similar to that found in a series of [Me2MPy2N]n complexes, where M is Al, Ga, In, or Tl.[13b] Formally 4 could be regarded as a sodium zinczincate[21] by combining a neutral tBu2Zn unit with a [Zn(dpa)tBu2]− ate, though the true electronic distribution, as reflected by the theoretical calculations, lies between these two extremes. We also successfully prepared the potassium congener [[K(THF)6]+[Zn(tBu)2(dpa)Zn(tBu)2]−] (5; see the Supporting Information for details).
Figure 4.
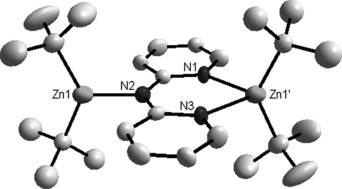
Anion of the solvent-separated ion pair structure of 4[26] with thermal ellipsoids at 50 % probability level. Hydrogen atoms and disordered components omitted for clarity. The anionic moiety of 5 is effectively identical.
Alkylselective addition to ketones by Grignard reagents has been much studied with benzophenone, which is often used as a benchmark as its reactions can have several outcomes. Excellent progress has been made in carbonyl (1,2) additions most recently by Ishihara and co-workers who showed that catalytic amounts of ZnCl2 (thus generating R3ZnMgCl intermediates) can greatly enhance such reactions.[22] Hevia and co-workers also reported that adding just 10 mol % of the magnesium-zinc hybrid [(THF)6Mg2Cl3]+[Zn2Et5]− to the reaction of EtMgCl and benzophenone gave the 1, 2-addition product in 90 % yield.[23] These bimetallic zinc-mediated successes inspired us to test the tert-butylation performance of 3 and 4 in a hexane solution, noting that 1 had previously delivered a tBu nucleophile to the para (1,6) position of benzophenone albeit on the sole evidence of an isolated crystalline enolato intermediate.[24] Table 1 reveals that the three sodium zincates 3, 4, and 1 give competitive yields of the challenging para-addition product 4-tbutylbenzophenone in contrast to the near 0 % yields witnessed for the homonuclear zinc species. A striking comparison is that the metalloligand solvated tBu2Zn produces 40 times as much para product as unsolvated tBu2Zn or its pyridine and TMEDA solvates. Olah and co-workers reported that reacting tBuLi with benzophenone in THF solvent gave a 52 % yield of ring alkylation products (1:9, ortho/para ratio), but only at −100 °C using toxic thionyl chloride as oxidant as an aqueous work-up dropped the combined yield to 28 °C and running the reaction in hexane at ambient temperature switched the major product to that of 1,2 addition.[23] Clearly the zincate systems are advantageous since they give comparable yields at ambient temperature using a mild aqueous work-up. The most intriguing result came when a 1:1 mixture of tBu2Zn and benzophenone was treated with only 10 mol % of the sodium amide [[(TMEDA)Na(dpa)]2], presumably generating 10 mol % of 3 in situ in a hexane solution. At ambient temperature, 4-tert-butylbenzophenone was obtained in a low yield of 11 % but this remarkably rose to 52 % under reflux conditions, thus implying that the substoichiometric sodium amide is recycling in some way.
Table 1.
Reaction of zinc reagents with benzophenone in a hexane solution for 18 h. 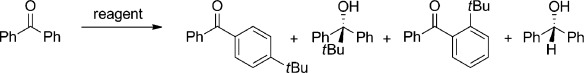
| Entry | Reagent | T | Yield [%][a] | |||||
|---|---|---|---|---|---|---|---|---|
| [°C] | para addi- tion (1,6) | Carbonyl addition (1,2) | ortho addi- tion (1,4) | benzhydrol (H− addition) | total | |||
| Stoichiometric | ||||||||
| 1 | tBu2Zn (1 equiv) | RT | 1 | 0 | 0 | 0 | 1 | |
| 2 | tBu2Zn⋅2 pyridine (1 equiv) | RT | 1 | 0 | 0 | 0 | 1 | |
| 3 | tBu2Zn⋅TMEDA (1 equiv) | RT | 1 | 1 | 0 | 0 | 2 | |
| 4 | [(TMEDA)Na(TMP)(tBu)Zn(tBu)] (1; 1 equiv) | RT | 58 | 14 | 3 | 0 | 75 | |
| 5 | [(TMEDA)2Na2(μ-dpa)2Zn(tBu)2] (3; 1 equiv) | RT | 40 | 6 | 0 | 11 | 57 | |
| 6 | [[Na(THF)6]+ [Zn(tBu)2(dpa)Zn(tBu)2]−] (4; 1 equiv) | RT | 42 | 13 | 0 | 3 | 58 | |
| Substoichiometric | ||||||||
| 7 | tBu2Zn (1 equiv) | 75 | 11 | 1 | 0 | 8 | 20 | |
| 8 | tBu2Zn (1 equiv)+[(TMEDA)Na(TMP)] (0.1 equiv) | 75 | 33 | 8 | 0 | 6 | 47 | |
| 9 | tBu2Zn (1 equiv)+[(TMEDA)Na(dpa)]2 (0.1 equiv) | 75 | 52 | 12 | 0 | 7 | 71 | |
| 10 | tBu2Zn (1 equiv)+[[Na(THF)6]+[Zn(tBu)2(dpa)Zn(tBu)2]−] (0.1 equiv) | 75 | 33 | 7 | 0 | 9 | 49 | |
Yields were determined by 1H NMR spectroscopy using hexamethylbenzene (10 mol %) as an internal standard.
To conclude, this study has uncovered a set of alkylzinc-based compounds with unprecedented structures by utilizing a secondary amide equipped with two pyridyl appendages as a structural template-changing co-ligand. Unlike conventional alkylzinc reagents these heteroleptic compounds can directly alkylate (tert-butylate) benzophenone in the challenging para position. Most intriguingly, substoichiometric amounts of sodium amide [[(TMEDA)Na(dpa)]2] can activate tBu2Zn towards this tert-butylation through the intermediacy of the zincate 3, the structure of which masquerades as a neutral donor-acceptor complex.
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.201302426.
References
- [1].Kondo Y, Shilai M, Uchiyama M, Sakamoto T. J. Am. Chem. Soc. 1999;121:3539–3540. [Google Scholar]
- [2a].Bernhardt S, Manolikakes G, Kunz T, Knochel P. Angew. Chem. 2011;123:9372–9376. doi: 10.1002/anie.201104291. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2011;50 [Google Scholar]
- [2b].Stathakis CI, Bernhardt S, Quint V, Knochel P. Angew. Chem. 2012;124:9563–9567. doi: 10.1002/anie.201204526. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2012;51 [Google Scholar]
- [3].Lalia-Kantouri M, Gdaniec M, Choli-Papadopoulou T, Badounas A, Papadopoulos CD, Czapik A, Geromichalos GD, Sahpazidou D, Tsitouroudi F. J. Inorg. Biochem. 2012;117:25–34. doi: 10.1016/j.jinorgbio.2012.08.022. [DOI] [PubMed] [Google Scholar]
- [4].Hong SJ, Kim C, Kim HJ, Kim S-J, Kim Y, Kwak H, Lee SH, Lee YM. Inorg. Chem. Commun. 2007;10:287–291. [Google Scholar]
- [5].Ho K-Y, Yu W-Y, Cheung K-K, Che C-M. Dalton Trans. 1999:1581–1586. [Google Scholar]
- [6].Dey B, Mukhopadhyay S, Seth SK, Kar TJ. J. Mol. Struct. 2010;973:81–88. [Google Scholar]
- [7].According to the method published by Houser, the Zn centre of 2ττ. Yang L, Powell DR, Houser RP. Dalton Trans. 2007:955–964. doi: 10.1039/b617136b. has a distorted tetrahedral geometry, with a 4 value of 0.78. The value of 4 indicates a sliding scale where 1.00 represents an ideal tetrahedral geometry and 0.00 indicates an ideal square-planar geometry. [DOI] [PubMed] [Google Scholar]
- [8].Gornitzka H, Hemmert C, Bertrand G, Pfeiffer M, Stalke D. Organometallics. 2000;19:112–114. [Google Scholar]
- [9].Zheng Z, Elmkaddem MK, Fischmeister C, Roisnel T, Thomas CM, Carpentier J-F, Renaud J-L. New J. Chem. 2008;32:2150–2158. [Google Scholar]
- [10].Pfeiffer M, Baier F, Stey T, Leusser D, Stalke D, Engels B, Moigno D, Kiefer W. J. Mol. Model. 2000;6:299–311. [Google Scholar]
- [11a].Jacobsen RA, Johnson JE. Acta Crystallogr. Sect. B. 1973;29:1669–1674. [Google Scholar]
- [11b].Pyrka GJ, Pinkerton AA. Acta Crystallogr. Sect. C. 1992;48:91–94. [Google Scholar]
- [11c].Schödel H, Näther C, Bock H, Butenschön F. Acta Crystallogr. Sect. B. 1996;52:842–853. [Google Scholar]
- [12].Armstrong DR, Clegg W, Dale SH, García-Álvarez J, Harrington R, Hevia E, Honeyman GW, Kennedy AR, Mulvey RE, O’Hara CT. Chem. Commun. 2008:187–189. doi: 10.1039/b713987j. [DOI] [PubMed] [Google Scholar]
- [13a].Cotton FA, Daniels LM, Jordan GT, IV, Murillo CA. Polyhedron. 1998;17:589–597. [Google Scholar]
- [13b].Gornitzka H, Stalke D. Eur. J. Inorg. Chem. 1998:311–317. [Google Scholar]
- [13c].Pfeiffer M, Murso A, Mahalakshmi L, Moigno D, Kiefer W, Stalke D. Eur. J. Inorg. Chem. 2002:3222–3234. [Google Scholar]
- [14a].Weiss E. Angew. Chem. 1993;105:1565–1587. [Google Scholar]; Angew. Chem. Int. Ed. Engl. 1993;32 [Google Scholar]
- [14b].Mulvey RE. Acc. Chem. Res. 2009;42:743–755. doi: 10.1021/ar800254y. [DOI] [PubMed] [Google Scholar]
- [14c].Garden JA, Kennedy AR, Mulvey RE, Robertson SD. Dalton Trans. 2011;40:11945–11954. doi: 10.1039/c1dt11430a. [DOI] [PubMed] [Google Scholar]
- [15].Campbell R, García-Álvarez P, Kennedy AR, Mulvey RE. Chem. Eur. J. 2010;16:9964–9968. doi: 10.1002/chem.201001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Baillie SE, Blair VL, Blakemore DC, Hay D, Kennedy AR, Pryde DC, Hevia E. Chem. Commun. 2012;48:1985–1987. doi: 10.1039/c2cc16959b. [DOI] [PubMed] [Google Scholar]
- [17].Slocum DW, Reinscheld TK, White CB, Timmons MD, Shelton PA, Slocum MG, Sandlin RD, Holland EG, Kusmic D, Jennings JA, Tekin KC, Nguyen Q, Bush SJ, Keller JM, Whitley PE. Organometallics. 2013;32:1674–1686. [Google Scholar]
- [18].Schumann H, Genthe W, Hahn E, Hossain MB, van der Helm D. J. Organomet. Chem. 1986;299:67–84. [Google Scholar]
- [19].Kohn W, Becke AD, Parr RG. J. Phys. Chem. 1996;100:12974. [Google Scholar]
- [20].McLean AD, Chandler GS. J. Chem. Phys. 1980;72:5639–5648. [Google Scholar]
- [21].Clegg W, Dale SH, Hevia E, Hogg LM, Honeyman GW, Mulvey RE, O’Hara CT, Russo L. Angew. Chem. 2008;120:743–746. doi: 10.1002/anie.200704341. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2008;47 [Google Scholar]
- [22].Hatano M, Suzuki S, Ishihara K. J. Am. Chem. Soc. 2006;128:9998–9999. doi: 10.1021/ja0628405. [DOI] [PubMed] [Google Scholar]
- [23].Armstrong DR, Clegg W, García-Álvarez P, McCall MD, Nuttall L, Kennedy AR, Russo L, Hevia E. Chem. Eur. J. 2011;17:4470–4479. doi: 10.1002/chem.201002544. [DOI] [PubMed] [Google Scholar]
- [24].Hevia E, Honeyman GW, Kennedy AR, Mulvey RE. J. Am. Chem. Soc. 2005;127:13106–13107. doi: 10.1021/ja053756c. [DOI] [PubMed] [Google Scholar]
- [25].Olah GA, Wu A, Farooq O. Synthesis. 1991:1179–1182. [Google Scholar]
- [26].Single-crystal data were measured at 123(2) K on Oxford Diffraction instruments using graphite-monochromated MoKαλF2[27]4523452283462P1cabcβV3ZRcalcd−3RIσIωRS34066102P1nabcβV3ZRcalcd−3RIσIωRS45092362PabcαβγV3ZRcalcd−3RIσIωRS. radiation ( =0.71073 Å). The structures were refined to convergence on and against all independent reflections by full-matrix least-squares using the SHELXL-97 program. Compounds and are structurally closely related. Both contain a large number of disordered groups (butyl, THF, and dipyridylamide) which required restraints and constraints to be applied to both bond lengths and displacement ellipsoids. Of particular relevance to the structural discussion is that the dipyridylamide ligands are disordered about the crystallographic center of symmetry. Selected parameters are given in the Supporting Information and full details are given in the deposited cif files. CCDC reference numbers 930517 ( ), 930518 ( ), 930519 ( ), and 930520 ( ) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif. Crystal data for : C H N Zn ; a colorless block gave monoclinic space group 2 =9.4833(3), =10.4377(4), =14.0888(5) Å, =105.844(4)°, =1341.58(8) Å =2, =1.449 Mg m 1=0.0413 [for 2840 reflections with > 2 )], 2=0.1039 and =1.065 for 166 parameters and 3451 independent reflections. Crystal data for : C H N Na Zn; an orange block gave monoclinic space group 2 =11.5137(3), =21.6403(4), =18.3837(3) Å; =93.636(2)°, =4571.26(16) Å =4, =1.160 Mg m 1=0.0388 [for 8121 reflections with >2 )], 2=0.0864 and =1.032 for 492 parameters and 10 516 independent reflections. Crystal data for : C H N NaO Zn ; an orange block gave triclinic space group −1, =10.0075(11), =11.1283(9), =13.3084(15) Å; =69.353(9), =85.491(9), =84.723(8)°; =1379.3(2) Å =1, =1.186 Mg m 1=0.0606 [for 5614 reflections with >2 )], 2=0.1679 and =1.057 for 321 parameters and 6820 independent reflections.
- [27].Sheldrick GM. Acta Crystallogr. Sect. A. 2008;64:112–122. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


