Abstract
Plants containing resveratrol have been used effectively in traditional medicine for over 2000 years. It can be found in some plants, fruits, and derivatives, such as red wine. Therefore, it can be administered by either consuming these natural products or intaking nutraceutical pills. Resveratrol exhibits a wide range of beneficial properties, and this may be due to its molecular structure, which endow resveratrol with the ability to bind to many biomolecules. Among these properties its activity as an anticancer agent, a platelet antiaggregation agent, and an antioxidant, as well as its antiaging, antifrailty, anti-inflammatory, antiallergenic, and so forth activities, is worth highlighting. These beneficial biological properties have been extensively studied in humans and animal models, both in vitro and in vivo. The issue of bioavailability of resveratrol is of paramount importance and is determined by its rapid elimination and the fact that its absorption is highly effective, but the first hepatic step leaves little free resveratrol. Clarifying aspects like stability and pharmacokinetics of resveratrol metabolites would be fundamental to understand and apply the therapeutic properties of resveratrol.
1. Background
Resveratrol (3,5,4′-Trihydroxystilbene) is a natural polyphenol with a stilbene structure. Its chemical structure was characterized in 1940 by Takaoka, who isolated it from the root of Veratrum grandiflorum [1]. However, it has been present in medicinal preparations, such as darakchasava or manakka [2], for more than 2000 years. Its basic structure consists of two phenolic rings bonded together by a double styrene bond, which forms the 3,5,4′-Trihydroxystilbene (molecular weight 228.25 g/mol). This double bond is responsible for the isometric cis- and trans-forms of resveratrol (Figure 1). It is worth mentioning that the trans-isomer is the most stable from the steric point of view [3].
Figure 1.
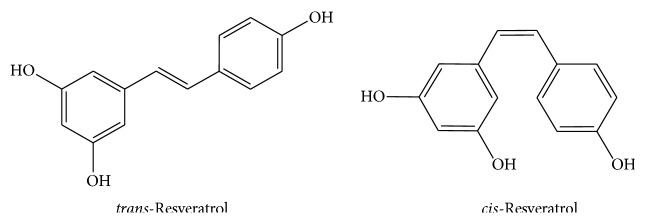
Chemical structures of trans-resveratrol and cis-resveratrol.
There are many synthetic and natural analogues of resveratrol, as well as adducts, derivatives, and conjugates, including glucosides [4].
The synthesis of resveratrol decreases regularly during the grape ripening process, which explains the increasing susceptibility of mature fruits to infection by Botrytis cinerea [5].
Resveratrol is a phytoalexin. These chemicals are characterized by their low molecular weight and their ability to inhibit the progress of certain infections. The accumulation of these substances in plants is produced by a mechanism of resistance to parasites and other adverse conditions, like fungal infection, UV radiation, chemical substances and, in general, stressful factors for the plant [5–7]. In fact, resveratrol is produced by more than 70 species of plants in response to such stressful situations [8].
The concentration of resveratrol in plants depends on various factors. For example, in vines, the two most important factors are the weather and presence of fungus [9–12].
Resveratrol can be found in some fruits, which are part of the human diet, such as blueberries (Vaccinium spp.), blackberries (Morus spp.), and peanuts (Arachis hypogaea) [13, 14]. However, red wine is the main source of resveratrol in the Mediterranean diet.
Resveratrol content in red wine comes from grapes (Vitaceae). In particular, the richest sources are the skin, seeds, petioles, and woody parts [15]. For that reason, red wine is richer in resveratrol than white wine, because during the production of red wine, parts of the grape where resveratrol is concentrated are macerated. This does not happen in white wine [16, 17]. Alcohol formation during grape fermentation facilitates its solubility and thus its extraction.
Cis- and trans-isomers coexist in plants and in wine. However, cis-resveratrol has never been found in grape extract [18, 19]. The trans-isomer appears to be the more predominant and stable natural form. Cis-isomerisation can occur when the trans-isoform is exposed to solar [20] or artificial light or ultraviolet radiation [21] at a wavelength of 254 [22] or 366 nm [23].
Although less important in our culture, the richest source of resveratrol is the Polygonum cuspidatum herb, whose root extract has played a very important role in Japanese and Chinese traditional medicine. In fact, it is the main active ingredient in ko-jo-kon, which is used in the treatment of several cardiovascular diseases [24].
Veratrum grandiflorum has a high content of resveratrol in leaves, when the plant is damaged by any chemical treatment [25]. Furthermore, the roots and rhizomes of Veratrum formosanum are also rich in resveratrol and, in fact, a preparation of this plant has been traditionally used in east Asia to treat hypertension [26].
2. Absorption, Metabolism, and Bioavailability
2.1. Absorption
The chemical structure of resveratrol leads to low water solubility (<0.05 mg/mL), which affects its absorption. In order to increase its solubility, ethanol (50 mg/mL) or organic solvents may be used.
It is important to highlight the ability of resveratrol to form a wide range of organic molecular complexes. Sterification of hydroxyl groups with aliphatic molecules can also be employed as a tool to increase its intestinal absorption and cellular permeability. For example, resveratrol acetylation can increase its absorption and its cellular capture without loss of activity [27–29].
At the intestinal level, resveratrol is absorbed by passive diffusion or forming complexes with membrane transporters, such as integrins. Once in the bloodstream, resveratrol can be found essentially in three different forms: glucuronide, sulfate, or free. The free form can be bound to albumin and lipoproteins such as LDL (low-density lipoprotein). These complexes, in turn, can be dissociated at cellular membranes that have receptors for albumin and LDL, leaving the resveratrol free and allowing it to enter cells. Resveratrol's affinity for albumin suggests that it could be a natural polyphenolic reservoir, playing an important role in its distribution and bioavailability [30].
Due to its chemical characteristics, resveratrol can interact with fatty acids. Recent studies in vitro show that more than 90% of free trans-resveratrol binds to human plasma lipoproteins. This binding is also found in vivo, as shown by the presence of dietary polyphenolic compounds detected in isolated LDL in blood samples of healthy human volunteers [31, 32].
Fatty acids facilitate a lipophilic environment, which favors resveratrol binding [33]. Normally they are employed as vectors because of their high affinity for the liver and their efficient cellular uptake, resulting from specific interactions with transmembrane transporters.
2.2. Metabolism
Phase II metabolism of resveratrol or its metabolites occurs in liver. There is enterohepatic transport in bile, which may result in some returning cycles to the small intestine [34]. Furthermore, resveratrol is able to induce its own metabolism, increasing the activity of phase II hepatic detoxifying enzymes [35].
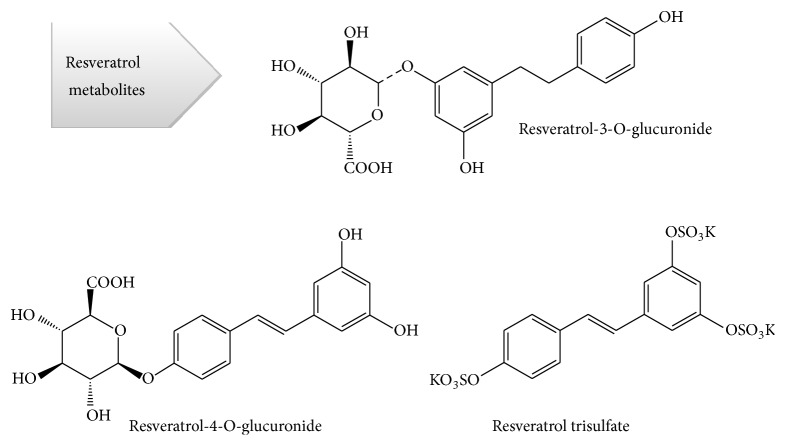
Resveratrol has a high metabolism, leading to the production of conjugated sulfates, glucuronides (Figure 2), which retain some biological activity [36], and up to five different metabolites present in the urine: resveratrol monosulfate, two isomeric forms of resveratrol monoglucuronide, monosulfate dihydroresveratrol, and monoglucuronide dihydroresveratrol. However, the nature and quantity of these metabolites can differ between subjects due to interindividual variability [37–39].
Figure 2.
Resveratrol metabolites.
Cis-metabolites have been identified in human urine samples, mainly as cis-resveratrol-4′-sulfate, cis-resveratrol-3-O-glucuronide, and cis-resveratrol-4′-O-glucuronide [32, 40]. Most research has been performed with trans-isomer due to the instability of cis-isomer [20]. However, data indicate that both of them can have different biological effects [41–43].
Other dietary flavonoids, such as quercetin, may inhibit resveratrol sulphation and glucuronidation in the liver and duodenal tissue, increasing its bioavailability [44].
2.3. Bioavailability
Resveratrol exhibits lipophilic characteristics, which lead to a high absorption. However, it should be noted that resveratrol absorption can vary depending on the way it is consumed and the kind of food ingested [45].
Low bioavailability of resveratrol is a factor that may reduce the efficacy of resveratrol. Although in vitro studies show a high efficacy in biologically beneficial effects of resveratrol in cells, it is known that its distribution in tissues is very low. Consequently, in vitro studies must be interpreted with caution when trying to extrapolate its effect in in vivo studies.
Despite its low bioavailability, resveratrol shows efficacy in vivo. This may be explained by the conversion of both sulfates and glucuronides again to resveratrol in target organs such as the liver [44, 46]. Another possible explanation could be the enterohepatic recirculation of resveratrol metabolites, followed by its deconjugation in the small intestine and its reabsorption [47]. Finally, in vivo effects could be explained by the activity of its metabolites.
Glucuronidation of the cis-form is faster (5–10 times) than that of the trans-form, thus leading to a lower bioavailability of the cis-form [44].
The presence of hydroxyl groups allows polyphenols to associate with proteins and other nutrients. The solubility of these compounds determines its physiological effects. Thus, complexes including these macronutrients and polyphenols, which maintain solubility, can be absorbed in the small intestine, while insoluble complexes are eliminated in feces, reducing their availability [48].
Two of the first human studies on the absorption and bioavailability of resveratrol used a single oral dose treatment of 25 mg [37, 49]. Despite the use of high sensitivity methods and a specific molecular analysis, it was difficult to detect the nonmetabolized resveratrol in circulating plasma. Approximate calculations showed maximal concentrations of <10 ng/mL (≈40 nM), 0.5–2 hours after the oral dose. Estimates of the plasmatic concentrations of resveratrol plus total metabolites were considerably higher, around 400–500 ng/mL (≈2 μM), indicating a very low oral bioavailability of free resveratrol, but a significant one of its metabolites [37, 49].
Urinary excretion of total metabolites after a radio-labeled dose was administered showed that about 75% of orally or intravenously administered resveratrol was absorbed [37]. This is an unusually high absorption for a dietary polyphenol, particularly in view of the poor aqueous solubility of this compound.
Several approaches have been used to increase the bioavailability of resveratrol in humans. The dose concentration curve seems to be a logical method, and it has been examined in two studies, with a dose range from 25 to 1000 mg [39, 50], covering the wide range used in chemoprevention studies. The absorption in these cases reached a maximum of between 0.3 and 2.4 μM, which does not reach the anticancer properties found at concentrations higher than 5 μM. Furthermore, in these studies an increase in the bioavailability of resveratrol during the treatment was found and a lack of metabolism saturation with the highest concentrations (500 mg/mL) [39]. Nevertheless, other studies in rats, which were administered resveratrol for 15–20 weeks, showed that a saturation of metabolites exists, and it leads to an increase of resveratrol in plasma and thus in tissues [51–53].
A pharmacokinetic study of repeated doses over two days concludes that tolerance is good, concentrations in plasma do not increase over time and even decrease, and the bioavailability is higher when administered in the morning [50].
Vitaglione et al. carried out an interesting study on the bioavailability of free trans-resveratrol present in red wine in humans [45]. Subjects were randomly divided into three experimental groups, consuming different types of food and red wine. The first group consumed 300 mL of red wine with a free trans-resveratrol content of 0.82 mg/L with a meal consisting of beef, egg, bread, corn oil, and French fries. The second group consumed 600 mL of red wine containing 3.2 mg/L free trans-resveratrol while fasting (before breakfast). Lastly, the third group consumed two different meals with different lipid content and 600 mL of red wine, which assured a free trans-resveratrol total ingestion of 0.48 mg. The authors concluded that the kind of food does not affect resveratrol bioavailability and found much variability between individuals. However, these results are inconsistent with those of other studies, in which a high-fat meal decreased its absorption [54]. Thus, we conclude that the different sample processing methods and the kind of analysis are the key to detect both free or conjugated resveratrol [45].
3. Biological Properties
The beneficial properties of the phenolic compounds present in grapes and wine have been studied after the discovery of the “French Paradox.” This term refers to the fact that in north France there is a high intake of saturated fat but low mortality from coronary heart disease compared to other countries where the same high saturated fat intake exists, being the Paradox attributable to high wine consumption [55]. In fact, there are more and more studies dealing with the ability of grape polyphenols and red wine to protect against different types of diseases [56, 57]. Resveratrol is one of the most studied red wine molecules and, in fact, there are more than 1000 references about its properties in the bibliography. Some of these studies in vivo and in vitro are described in Table 1.
Table 1.
Some of the resveratrol biological effects reported in vivo and in vitro.
(a) Clinical trials (humans)
| Article | Study type | Administered dose | Treatment time | Blood resveratrol | Dietary dose | Relevance |
|---|---|---|---|---|---|---|
| [37] | In vivo: human | Oral: 25 mg; intravenous: 1.5 mg | Once | <5 ng/mL | NO | Resveratrol is quickly metabolized |
|
| ||||||
| [49] | In vivo: human | Oral: 25 mg | Once | 10 to 40 nmol/L | NO | In vitro anticancer and anti-inflammatory effects of the free polyphenols are irrelevant in vivo |
|
| ||||||
| [45] | In vivo: human | Diet: 300 mL (0.82 mg/L) 600 mL (3.2 mg/L) 600 mL (0.48 mg/L) |
Once | Not detected | YES | The observed protective effect on cardiovascular diseases associated with a moderate consumption of wine may be due to the whole polyphenols contained in wine and not to resveratrol alone |
|
| ||||||
| [54] | In vivo: human | Oral: 2 times/day 2000 mg | 16 days | 1274 ± 790 ng/mL | NO | A high-fat meal decreases resveratrol absorption |
|
| ||||||
| [39] | In vivo: human | Oral: 1 g | Once | NO | A rapid, sensitive, and accurate method for the analysis of resveratrol and its metabolites in human plasma and urine | |
|
| ||||||
| [50] | In vivo: human | Oral: 6 times/day 25 mg 50 mg 100 mg 150 mg |
Two days | 3.89 ng/mL 7.39 ng/mL 23.1 ng/mL 63.8 ng/mL |
NO | Repeated administration was well-tolerated but produced relatively low plasma concentrations of trans-resveratrol, despite the different administrated doses; trans-resveratrol pharmacokinetics showed circadian variation; bioavailability was higher after morning administration |
|
| ||||||
| [55] | In vivo: human | Wine in diet | YES | A moderate wine consumption (alcohol) may be one explanation for protection from coronary heart disease | ||
|
| ||||||
| [116] | In vivo: human | Oral: 1 g/day 1.5 g/day 2 g/day |
28 days | NO | Resveratrol improves insulin sensitivity in subjects with impaired glucose tolerance | |
|
| ||||||
| [117] | In vivo: human | 150 mg/day | 30 days | 182.59 ± 30.33 ng/mL | NO | Resveratrol supplementation induces metabolic changes in obese humans, mimicking the effects of calorie restriction |
(b) In vivo (animals)
| Article | Study type | Administered dose | Treatment time | Relevance |
|---|---|---|---|---|
| [51] | In vivo: F344 rat | Oral: 200 μg/kg/day | 100 days | A protective role of resveratrol in colon carcinogenesis. |
|
| ||||
| [52] | In vivo: F344 rat | Orally or intraperitoneally: 1 mg/kg 2 mg/kg |
16 weeks 20 weeks |
Resveratrol may be a promising natural anticarcinogenesis agent for the prevention and treatment of human esophageal cancer |
|
| ||||
| [53] | In vivo: Sprague Dawley rat | Diet: 200 μg/rat/day | 120 days | Resveratrol suppresses 7,12-dimethylbenz(a)anthracene induced mammary carcinogenesis |
|
| ||||
| [60] |
In vivo: APfSD rat In vitro: Cos-1 cells hER-α Yeast |
Oral or subcutaneous: 0.03–120 mg/kg/day |
Weak estrogenicity of the red wine constituent resveratrol | |
|
| ||||
| [62] | In vivo: weanling rat | Oral: 1, 4, 10, 40, and 100 μg/day | Six days | Resveratrol has little or no estrogen agonism on reproductive and nonreproductive estrogen target tissues and may be an estrogen antagonist |
|
| ||||
| [103] |
In vivo: streptozotocin-induced diabetes mellitus Sprague-Dawley rats |
Oral: 0.75 mg/kg three times a day |
Eight weeks | Resveratrol improves energy metabolism and reduces protein wasting |
|
| ||||
| [99] | In vivo: rabbit | Oral: 4 mg/kg/day | 12 weeks | Resveratrol inhibits platelet aggregation |
|
| ||||
| [115] |
In vivo: Microcebus murinus |
Diet: 200 mg/kg/day | 21 months 33 months |
Resveratrol affects insulin sensitivity by improving glucose tolerance |
|
| ||||
| [105] |
In vivo: Caenorhabditis elegans Drosophila melanogaster |
Diet: 100 μM | Whole life | Resveratrol activates sirtuins in Caenorhabditis elegans and Drosophila melanogaster and extends their lifespan |
|
| ||||
| [108] |
In vivo: Drosophila melanogaster |
Diet: 50–500 μM | Whole life | Resveratrol extends lifespan |
|
| ||||
| [109] |
In vivo: Caenorhabditis elegans |
Diet: 100–1000 μM | Whole life | Lifespan extension in C. elegans is mediated by sir-2.1 |
|
| ||||
| [82] |
In vivo: Apis mellifera |
Diet: 30–130 μM | Whole life | Resveratrol significantly affects gustatory responsiveness and prolongs lifespan under normal oxygen conditions |
(c) In vitro
| Article | Study type | Administered dose | Relevance |
|---|---|---|---|
| [63] |
In vitro: MCF-7 cells T47D cells MDA-MB-231 cells |
3–10 μM | Resveratrol exhibits variable degrees of estrogen receptor agonism in different test systems |
|
| |||
| [89] |
In vitro: DU-145, PC-3, and JCA-1 human prostate cancer cells |
25 μM | Resveratrol negatively modulates prostate cancer cell growth |
|
| |||
| [90] |
In vitro: HL-60 cells Hepa LcLc7 cells |
11, 18, 21, 27 μM | Resveratrol is a potential cancer chemopreventive agent |
|
| |||
| [91] |
In vitro: MCF7 cells |
10 μM | Resveratrol blocks the aryl hydrocarbon receptor and has beneficial effects against some types of tumors |
|
| |||
| [92] |
In vitro: MCF7 cells |
10, 50, 100, 150 μM | The anticancer effect of resveratrol is via BCL-2 and NFκB |
|
| |||
| [93] |
In vitro: human lymphoblast cells |
2.5, 5, 10, 20, 40 μM | The anticancer effect of resveratrol is via p53 |
|
| |||
| [94] |
In vitro: L1210-R2 murine lymphoblastic leukemia cells K-562 human myelogenous leukemia cells P-815 murine mastocytoma cells |
0.1–1000 μM | The anticancer effect of resveratrol is via inhibiting ribonuclease reductase |
|
| |||
| [95] |
In vitro: murine 3T6 fibroblast |
0.3–30 μM | Reactive oxygen species and arachidonic acid might be involved in the control of 3T6 fibroblast growth by resveratrol |
|
| |||
| [98] |
In vitro: human platelets |
0.1, 1.0 and 10.0 μM | trans-Resveratrol is an inhibitor of store-operated Ca2+ channels in human platelets. This accounts for the ability of trans-resveratrol to inhibit platelet aggregation induced by thrombin |
|
| |||
| [104] |
In vitro: Saccharomyces cerevisiae |
0–500 μM | Resveratrol stimulates Sir2, thus increasing DNA stability and extending lifespan |
Because of its chemical and physical features, resveratrol can either cross passively cell membranes or interact with membrane receptors. Therefore, it may interact with extracellular and intracellular molecules. For this reason, its mechanism of action at the cellular level may be triggered by either activating signaling pathways when binding to cell membrane receptors, activating intracellular mechanisms, or even developing its effects inside the nucleus.
3.1. Phytoestrogenic Properties
In fact, resveratrol is able to bind to estrogen receptors alpha and beta (ER-α and ER-β) with similar affinities, but this interaction is 7000 times less powerful than that of estradiol [58]. Molecular studies have shown that the union of resveratrol to ER-α is stereoselective, that is, that the trans-isomer shows more affinity for this receptor than the cis-isomer [59].
The chemical structure of resveratrol is similar to that of 17-β-estradiol (Figure 3) or synthetic estrogens like diethylstilbestrol. Thus, several studies have been carried out in order to test its ability to act as a phytoestrogen [60–63].
Figure 3.
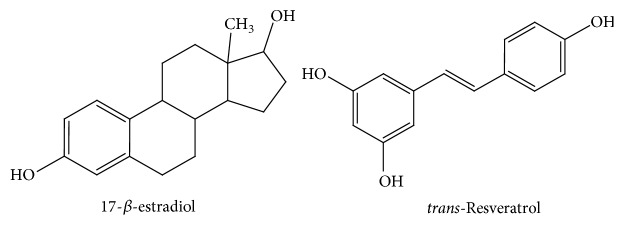
Comparison of the chemical structures of trans-resveratrol and 17-β-estradiol.
Estrogens and phytoestrogens exert almost all of their effects through binding to estrogen receptors. When estrogen binds to its receptor, it activates the transcription of target genes. We found that antioxidant genes were upregulated by this mechanism [64, 65]. Resveratrol can bind to estrogen receptors and activate the transcription of such genes with similar concentrations to those required for its other biological effects. In this regard, Gehm et al. demonstrated in 1997 that resveratrol behaves as an estradiol analog [63]. They used MCF-7 cells, which are rich in estrogenic receptors. Maximal efficiency binding was at 10 μM. This subsequently activated genes with estrogen responsive elements (ERE). Furthermore, to confirm that resveratrol starts ERE activation, they used estrogenic antagonists and the effect was inhibited. By contrast, it has also been shown that resveratrol, in its capacity of an estrogen receptor modulator, can also antagonize the effect of estradiol on increasing proliferation of MCF-7 cells, at higher doses [62].
Regarding its estrogenic activity, it has been shown that it does not have any effect on the growth and differentiation in the uterus of growing rats. In the same article, the authors did not find any effect of resveratrol on either radial bone growth, serum cholesterol levels, or animal body weight. This study concludes that resveratrol does not act as an agonist in rats at doses from 1 to 100 μg/day. Even with higher doses (1000 μg/day) the effect is insignificant and could also act as an estrogen antagonist [62].
3.2. Antioxidant Properties
Oxidative damage is involved in the pathogenesis of many important diseases, such as diabetes [66], cardiovascular diseases [67], neurodegenerative diseases [68], and cancer [69]. It also plays an important role in the aging process [70, 71]. Therefore, a great deal of attention has been focused on finding natural antioxidants, which could help in the treatment of all these diseases and, consequently, potential antioxidant effects of resveratrol have been studied in depth.
Its antioxidant activity has been determined in isolated rat brain mitochondria, which shows an inhibition of the mitochondrial respiration state when they are incubated with resveratrol. Furthermore, it inhibits the activity of complex III by competing with coenzyme Q. This fact is interesting because it determines its antioxidant activity in mitochondria, not only its activity in uptake capacity of unpaired electrons, but also by inhibiting a complex that generates free radicals [72].
Most published in vitro studies report using concentrations of resveratrol too high to be reached in the organism after red wine consumption. Therefore, it is very important to make sure that low plasma concentrations of free resveratrol are sufficient enough to be active as an antioxidant. In this regard, it has been shown that nutritionally relevant concentrations of resveratrol are able to decrease H2O2 levels in MCF-7 cells by inducing the expression of antioxidant genes, such as catalase [12] and manganese superoxide dismutase (MnSOD), through a mechanism that involves phosphatase and tensin homolog (PTEN) and proteinkinase-B (PKB or Akt) signaling pathway [73].
In the cardiovascular system it has been reported how this polyphenol, at a concentration of 20 μM, can reduce the malondialdehyde content in blood mononuclear cells isolated ex vivo from healthy individuals [74]. Thus, resveratrol preincubation of bovine aortic smooth muscle cells was able to attenuate oxidized low-density lipoprotein- (oxLDL-) induced increases in reactive oxygen species (ROS) and H2O2 levels [75]. In another study performed in human blood platelets treated with peroxynitrite, resveratrol inhibited protein carbonylation and nitration, as well as lipid peroxidation [76].
Regarding other physiological systems and tissues, resveratrol has also been shown to protect primary hepatocytes in culture against oxidative stress damage by increasing the activities of catalase, superoxide dismutase, glutathione peroxidase, NADPH quinone oxidoreductase, and glutathione-S-transferase. Furthermore, it increases the level of nuclear factor (erythroid-derived 2)-like 2 (Nrf2) and induces its translocation to the nucleus [77]. This factor can activate genes with antioxidant responsive elements (ARE).
In rat spinal cord, resveratrol was shown to protect it from secondary spinal cord injuries via improving the energy metabolism system and inhibiting the lipid peroxidation, at a dose between 50 and 100 mg/kg, reaching the maximal effect after 48 h of the spinal cord injury [78]. In a related article, resveratrol protected rabbit spinal cord from ischemia-reperfusion injury by decreasing lipid peroxidation (at a dose of 10 mg/kg) and increasing nitric oxide (NO) release (at doses of 1 mg/kg and 10 mg/kg) [76, 79].
Regarding the musculoskeletal system, it has been described how, in young and old rats submitted to a 14-day muscle disuse by hindlimb suspension, resveratrol (at a dose of 12.5 mg/Kg) was able to diminish oxidative stress by increasing gastrocnemius catalase activity, MnSOD activity, and MnSOD protein content. Interestingly, resveratrol was also able to regain the muscle isometric force, but apoptotic markers were not modified [80]. Another similar article also deals with the ability of resveratrol to protect against muscle and bone alterations after disuse and suggests resveratrol as a physical exercise mimetic [81].
The ability of resveratrol to act as an antioxidant has also been found in a model of senescence-accelerated mice, where resveratrol at different dosages (25, 50, and 100 mg/Kg/day) for 8 weeks increased the activity of superoxide dismutase (SOD) and glutathione peroxidase (GPx), as well as diminishing malondialdehyde levels [82, 83].
Despite this antioxidant function, however, it can also suffer an autooxidation process, leading to the production of O2 ∙−, H2O2, and a complex mixture of semiquinones and quinones, which can become cytotoxic [84, 85]. The oxidized resveratrol molecule can generate complexes with copper that can fragment DNA [86].
3.3. Antitumor Effects
Resveratrol can interact with the αVβ3 integrin receptor in MCF-7 cells (a breast-cancer cell line) inducing apoptosis [87]. Besides, it shows antagonist actions when binding to the aryl hydrocarbon receptor, which has immunosuppressive and carcinogenic activity in cells [88].
Nevertheless, these studies conclude that resveratrol inhibits cellular proliferation at concentrations within 10–30 μM. In particular, the effect is locked in phase G/S2 of the cellular cycle, suggesting an inhibition in the enzymatic activities responsible for DNA duplication. These effects have been observed in a cell line of prostate cancer with a concentration of 25 μM but not with 2.5 μM [89].
Some studies show that it can exert its antitumor effects on the initiation, promotion, and progression of cancer in tumor cells [89]. In this regard, it has been shown how resveratrol at 15 μM is able to inhibit cyclooxigenase 1 (COX-1), a very active enzyme involved in tumor progression. In addition, at 11 μM, it induces phenotypic nonproliferative markers, like the reduction of the nitroblue tetrazolium activity. In the initiation of tumor cells, it acts to inhibit the formation of free radicals at 27 μM on leukemia cells (HL-60). In hepatoma cells (Hepa LcLc7), it inhibits hepatic reductase activity, an enzyme which produces hepatic toxicity, at concentrations of 21 μM. In addition, at 18 μM, the incorporation of thymidine is inhibited, indicating the end of differentiation and thus the transformation to a nonproliferative phenotype [90]. In MCF-7 cells, 10 μM resveratrol blocks the aryl hydrocarbon receptor obtaining beneficial effects against cancer, as it is reported that the activation of this receptor may be involved in some types of tumors [91]. The anticancer effect of resveratrol in MCF-7 has also been associated with BCL-2 and NF-kappaβ [92]. Between 10 and 40 μM, it induces apoptosis via p53 activation in human lymphoblast cell lines [93]. It can also inhibit ribonuclease reductase [94] or COX-2 activity [95]. For that reason, resveratrol has antitumor effects when administered in vitro.
In vivo studies show beneficial effects [96, 97]. For example, its preventive effect on the initiation of cancer has been determined in a skin cancer animal model, with a concentration between 1 and 25 μM of resveratrol, and administrated twice a week [90]. Thus, in vivo studies support the antitumor beneficial effects previously seen in in vitro studies.
3.4. Cardiovascular Effects
Platelet aggregation is inhibited by resveratrol both in vitro and in vivo. There are studies that suggest that resveratrol, at concentrations of 0.1, 1, and 10 μM binds to calcium channels producing 20, 30, and 50% inhibition of thrombin, respectively [98]. This is very beneficial for the cardiovascular system, due to its interference in the formation of blood clots.
Those effects on platelet aggregation showed in in vitro studies mentioned above have also been shown in an in vivo study in rabbits, when a dose of 4 mg/kg/day of resveratrol was administered [99].
Other cardiovascular effects attributed to resveratrol are the regulation of the accumulation of triglycerides and the regulation of the lipolysis in murine adipocytes. When human adipocyte cells (3T3-L1 and SGBS) are incubated with resveratrol at 100 μM, a decrease in triglycerides is observed by an induction of lipolysis, activating adipose triglyceride lipase, and by inducing lipid mobilization [100]. Thus, authors suggest a possible treatment for obesity.
3.5. Other Biological Effects
In a cellular model of leucocytes (RBL-2H3 cells), it has been observed that resveratrol at 15 μM has an antiallergenic effect by decreasing the β-hexosaminidase activity [101].
In human mesenchymal stem cells, resveratrol promotes a spontaneous osteogenesis, activating genes such as osteocalcin and RUNX2. It also prevents adipogenesis by repressing the expression of some genes such as PPARγ2 and leptin [102], suggesting beneficial effects of resveratrol on bone regeneration.
Resveratrol has been shown to have beneficial effects on experimental diabetes. It improves the health status of diabetic rats induced with streptozotocin, by enhancing the energy metabolism and reducing protein breakdown [103].
4. Resveratrol, Sirtuins, and Aging
4.1. Invertebrates
It has been reported that resveratrol can extend lifespan in some organisms, such as the budding yeast Saccharomyces cerevisiae, involving a similar mechanism to that of calorie restriction (CR) [104]. Further studies have confirmed these results in the Caenorhabditis elegans nematode and the Drosophila melanogaster fly [105]. This effect has been shown to be mediated by the activation of sirtuin 2 [105], an enzyme induced, for example, by calorie restriction, physical exercise, and ethanol consumption [106]. Sirtuins (Sirt or Sir, from “silent information regulator”) belong to a family of enzymes with deacetylase activity [107]. These enzymes have the ability to modify covalently the histones that cover the DNA by deacetylation, inhibiting the transcription of certain genes. They can also activate or inhibit important enzymes by deacetylation. These enzymes can be activated by resveratrol consumption. Further studies seem to verify this capacity of resveratrol to increase lifespan using Drosophila spp. [108] and C. elegans [109]. In this context, a CR-sirtuin-prolonging lifespan association was established, in which a low-calorie diet would activate sirtuins and regulate mechanisms which extend lifespan. It was suggested that resveratrol could activate the same mechanisms as CR so that the new association was resveratrol-sirtuin-prolonging lifespan [104, 110]. However, in the past years, the role of Sir2 and resveratrol in aging and the relationship with CR have brought about controversy [96, 111, 112]. In fact, some authors postulate that resveratrol cannot activate Sirt2 in vivo [111, 113] and that CR could increase fly lifespan regardless of Sirt2 activation [114]. Thus, a recent study performed on 30 and 130 μM resveratrol pretreated honey bees showed that resveratrol was able to extend lifespan under normal conditions, but not under hyperoxia [82].
4.2. Vertebrates
In a recent study, which was carried out on five-year-old male grey mouse lemurs (Microcebus murinus), resveratrol decreased glycemia after an oral glucose loading without decreasing fasting blood insulin, mimicking the effects of calorie restriction [115]. In humans, a recent study reported an improvement of insulin sensitivity in plasma glucose in subjects with impaired glucose tolerance, after four weeks of daily resveratrol administration (1 and 2 g/day), showing no differences between doses [116]. Regarding resveratrol's potential to mimic the beneficial effects of calorie restriction, in a recent human study on obese individuals who were administered 150 mg/day of resveratrol for 30 days, it induced similar metabolic changes as those achieved with CR, such as a reduction in sleeping and resting metabolic rate and an increase of AMPK, Sirt1, PGC-1α, and citrate synthase activity [117]. However, the above mentioned controversy still exists in humans [118].
Another effect attributed to resveratrol is the protection against the vulnerability associated with frailty [119]. It seems to act as a neuroprotective agent due to its anti-inflammatory activity and its ability to decrease the levels of tumor necrosis factor, cyclooxygenase 2, inducible nitric oxide synthase, and interleukins [120]. Such neuroprotective activity could also be mediated by increasing Sirt1 activity, because this enzyme is thought to increase neurotrophic factors, such as brain-derived neurotrophic factor (BDNF). Specifically, in mice with a phenotype of accelerated aging (SAMP8) an increase of Sir and BDNF is produced when rats are calorie-restricted [121].
5. Conclusions
According to the bibliography, we could state that resveratrol has many beneficial properties. Its small molecular structure and polyphenolic character endow resveratrol with antioxidant properties and the ability to bind to organic compounds present in many organisms, such as hormone receptors and enzymes. This ability to interact with biological molecules provides resveratrol with multiple biological activities that are evident and clear when studied in vitro. These include beneficial effects against tumor processes, cardiovascular parameters, and longevity. However, some discrepancies have been observed in in vivo studies.
The concentrations used in vitro are too high to be reached in the organism after red wine consumption. However, it is possible to achieve such high concentration of resveratrol in plasma by administering resveratrol supplements and that is how many of the in vitro results have been verified in animal tests. However, if nutrients containing resveratrol are used to test these effects in vitro, the results show little biological activity. This is due to the small amount of resveratrol present in natural products and its low bioavailability limits its activity in the target tissues. The issue of bioavailability is determined by its rapid elimination and the fact that its absorption is highly effective, but the first hepatic step leaves little free resveratrol. In fact, free resveratrol can even bind to plasma proteins that could serve as a reservoir.
Clarifying aspects like stability and pharmacokinetics of resveratrol metabolites would be fundamental to understand and apply the therapeutic properties of resveratrol.
Further research into resveratrol uptake, cellular destination, metabolism, and stability of the original molecule and that of its metabolites is needed to elucidate its biological activity and it would be crucial to take advantage of the efficiency of its properties [30].
Acknowledgments
This work was supported by Grants SAF2013-44663-R from the Spanish Ministry of Education and Science (MEC), ISCIII2012-RED-43-029 from the Red Temática de Investigación Cooperativa en Envejecimiento y Fragilidad (RETICEF), PROMETEO2014/056 from “Conselleria d'Educació, Cultura i Esport de la Generalitat Valenciana”, RS2012-609 Intramural Grant from INCLIVA and EU Funded CM1001, and FRAILOMIC-HEALTH.2012.2.1.1-2. The study has been cofinanced by FEDER funds from the European Union.
Conflict of Interests
The authors declare that they have no financial/commercial conflict of interests regarding the publication of this paper.
References
- 1.Takaoka M. J. Of the phenolic substances of white hellebore (Veratrum grandiflorum Loes. fil.) Journal of the Faculty of Science, Hokkaido Imperial University. 1940;3:1–16. [Google Scholar]
- 2.Paul B., Masih I., Deopujari J., Charpentier C. Occurrence of resveratrol and pterostilbene in age-old darakchasava, an ayurvedic medicine from India. Journal of Ethnopharmacology. 1999;68(1–3):71–76. doi: 10.1016/s0378-8741(99)00044-6. [DOI] [PubMed] [Google Scholar]
- 3.Trela B. C., Waterhouse A. L. Resveratrol: isomeric molar absorptivities and stability. Journal of Agricultural and Food Chemistry. 1996;44(5):1253–1257. doi: 10.1021/jf9504576. [DOI] [Google Scholar]
- 4.Aggarwal B. B., Takada Y., Oommen O. V. From chemoprevention to chemotherapy: common targets and common goals. Expert Opinion on Investigational Drugs. 2004;13(10):1327–1338. doi: 10.1517/13543784.13.10.1327. [DOI] [PubMed] [Google Scholar]
- 5.Jeandet P., Douillet-Breuil A.-C., Bessis R., Debord S., Sbaghi M., Adrian M. Phytoalexins from the vitaceae: biosynthesis, phytoalexin gene expression in transgenic plants, antifungal activity, and metabolism. Journal of Agricultural and Food Chemistry. 2002;50(10):2731–2741. doi: 10.1021/jf011429s. [DOI] [PubMed] [Google Scholar]
- 6.Langcake P., Pryce R. J. A new class of phytoalexins from grapevines. Experientia. 1977;33(2):151–152. doi: 10.1007/BF02124034. [DOI] [PubMed] [Google Scholar]
- 7.Adrian M., Jeandet P., Veneau J., Weston L. A., Bessis R. Biological activity of resveratrol, a stilbenic compound from grapevines, against Botrytis cinerea, the causal agent for gray mold. Journal of Chemical Ecology. 1997;23(7):1689–1702. doi: 10.1023/b:joec.0000006444.79951.75. [DOI] [Google Scholar]
- 8.Maier-Salamon A., Böhmdrfer M., Thalhammer T., Szekeres T., Jaeger W. Hepatic glucuronidation of resveratrol: interspecies comparison of enzyme kinetic profiles in human, mouse, rat, and dog. Drug Metabolism and Pharmacokinetics. 2011;26(4):364–373. doi: 10.2133/dmpk.dmpk-11-rg-006. [DOI] [PubMed] [Google Scholar]
- 9.Hathway D. E., Seakins J. W. Hydroxystilbenes of Eucalyptus wandoo . The Biochemical Journal. 1959;72:369–374. doi: 10.1042/bj0720369b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pace-Asciak C. R., Hahn S., Diamandis E. P., Soleas G., Goldberg D. M. The red wine phenolics trans-resveratrol and quercetin block human platelet aggregation and eicosanoid synthesis: implications for protection against coronary heart disease. Clinica Chimica Acta. 1995;235(2):207–219. doi: 10.1016/0009-8981(95)06045-1. [DOI] [PubMed] [Google Scholar]
- 11.Ferrero M. E., Bertelli A. A. E., Pellegatta F., Fulgenzi A., Corsi M. M., Bertelli A. Phytoalexin resveratrol (3-4′-5-trihydroxystilbene) modulates granulocyte and monocyte endothelial adhesion. Transplantation Proceedings. 1998;30(8):4191–4193. doi: 10.1016/s0041-1345(98)01388-8. [DOI] [PubMed] [Google Scholar]
- 12.Ferrero M. E., Bertelli A. A. E., Fulgenzi A., et al. Activity in vitro of resveratrol on granulocyte and monocyte adhesion to endothelium. The American Journal of Clinical Nutrition. 1998;68(6):1208–1214. doi: 10.1093/ajcn/68.6.1208. [DOI] [PubMed] [Google Scholar]
- 13.Soleas G. J., Diamandis E. P., Goldberg D. M. Resveratrol: a molecule whose time has come? And gone? Clinical Biochemistry. 1997;30(2):91–113. doi: 10.1016/s0009-9120(96)00155-5. [DOI] [PubMed] [Google Scholar]
- 14.Sato M., Maulik G., Bagchi D., Das D. K. Myocardial protection by protykin, a novel extract of trans-resveratrol and emodin. Free Radical Research. 2000;32(2):135–144. doi: 10.1080/10715760000300141. [DOI] [PubMed] [Google Scholar]
- 15.Elíes-Gómez J. Efectos de los isómeros del resveratrol sobre la homeostasis del calcio y del óxido nítrico en células vasculares [Ph.D. thesis] Santiago de Compostela, Spain: Universidade de Santiago de Compostela; 2009. [Google Scholar]
- 16.Siemann E. H., Creasy L. L. Concentration of the phytoalexin resveratrol in wine. American Journal of Enology and Viticulture. 1992;43(1):49–52. [Google Scholar]
- 17.Soleas G. J., Diamandis E. P., Goldberg D. M. Wine as a biological fluid: history, production, and role in disease prevention. Journal of Clinical Laboratory Analysis. 1997;11(5):287–313. doi: 10.1002/(SICI)1098-2825(1997)11:5<287::AID-JCLA6>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palomino O., Gómez-Serranillos M. P., Slowing K., Carretero E., Villar A. Study of polyphenols in grape berries by reversed-phase high-performance liquid chromatography. Journal of Chromatography A. 2000;870(1-2):449–451. doi: 10.1016/S0021-9673(99)01225-X. [DOI] [PubMed] [Google Scholar]
- 19.Burns J., Yokota T., Ashihara H., Lean M. E. J., Crozier A. Plant foods and herbal sources of resveratrol. Journal of Agricultural and Food Chemistry. 2002;50(11):3337–3340. doi: 10.1021/jf0112973. [DOI] [PubMed] [Google Scholar]
- 20.Chen X., He H., Wang G., et al. Stereospecific determination of cis- and trans-resveratrol in rat plasma by HPLC: application to pharmacokinetic studies. Biomedical Chromatography. 2007;21(3):257–265. doi: 10.1002/bmc.747. [DOI] [PubMed] [Google Scholar]
- 21.Camont L., Cottart C. H., Rhayem Y., et al. Simple spectrophotometric assessment of the trans-/cis-resveratrol ratio in aqueous solutions. Analytica Chimica Acta. 2009;634(1):121–128. doi: 10.1016/j.aca.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Blache D., Rustan I., Durand P., Lesgards G., Loreau N. Gas chromatographic analysis of resveratrol in plasma, lipoproteins and cells after in vitro incubations. Journal of Chromatography B: Biomedical Applications. 1997;702(1-2):103–110. doi: 10.1016/S0378-4347(97)00383-6. [DOI] [PubMed] [Google Scholar]
- 23.Basly J.-P., Marre-Fournier F., Bail J.-C. L., Habrioux G., Chulia A. J. Estrogenic/antiestrogenic and scavenging properties of (E)- and (Z)-resveratrol. Life Sciences. 2000;66(9):769–777. doi: 10.1016/s0024-3205(99)00650-5. [DOI] [PubMed] [Google Scholar]
- 24.Nonomura S., Kanagawa H., Makimoto A. Chemical constituents of polygonaceous plants. I. Studies on the components of Ko-J O-Kon. (Polygonum Cuspidatum Sieb. Et Zucc.) Yakugaku Zasshi. 1963;83:988–990. [PubMed] [Google Scholar]
- 25.Hanawa F., Tahara S., Mizutani J. Antifungal stress compounds from Veratrum grandiflorum leaves treated with cupric chloride. Phytochemistry. 1992;31(9):3005–3007. doi: 10.1016/0031-9422(92)83436-3. [DOI] [Google Scholar]
- 26.Chung M.-I., Teng C.-M., Cheng K.-L., Ko F.-N., Lin C.-N. An antiplatelet principle of Veratrum formosanum . Planta Medica. 1992;58(3):274–276. doi: 10.1055/s-2006-961453. [DOI] [PubMed] [Google Scholar]
- 27.Laza-Knoerr A. L., Gref R., Couvreur P. Cyclodextrins for drug delivery. Journal of Drug Targeting. 2010;18(9):645–656. doi: 10.3109/10611861003622552. [DOI] [PubMed] [Google Scholar]
- 28.Colin D., Gimazane A., Lizard G., et al. Effects of resveratrol analogs on cell cycle progression, cell cycle associated proteins and 5fluoro-uracil sensitivity in human derived colon cancer cells. International Journal of Cancer. 2009;124(12):2780–2788. doi: 10.1002/ijc.24264. [DOI] [PubMed] [Google Scholar]
- 29.Marel A.-K., Lizard G., Izard J.-C., Latruffe N., Delmas D. Inhibitory effects of trans-resveratrol analogs molecules on the proliferation and the cell cycle progression of human colon tumoral cells. Molecular Nutrition & Food Research. 2008;52(5):538–548. doi: 10.1002/mnfr.200700185. [DOI] [PubMed] [Google Scholar]
- 30.Delmas D., Aires V., Limagne E., et al. Transport, stability, and biological activity of resveratrol. Annals of the New York Academy of Sciences. 2011;1215(1):48–59. doi: 10.1111/j.1749-6632.2010.05871.x. [DOI] [PubMed] [Google Scholar]
- 31.Urpí-Sardà M., Jáuregui O., Lamuela-Raventós R. M., et al. Uptake of diet resveratrol into the human low-density lipoprotein. Identification and quantification of resveratrol metabolites by liquid chromatography coupled with tandem mass spectrometry. Analytical Chemistry. 2005;77(10):3149–3155. doi: 10.1021/ac0484272. [DOI] [PubMed] [Google Scholar]
- 32.Urpi-Sarda M., Zamora-Ros R., Lamuela-Raventos R., et al. HPLC-tandem mass spectrometric method to characterize resveratrol metabolism in humans. Clinical Chemistry. 2007;53(2):292–299. doi: 10.1373/clinchem.2006.071936. [DOI] [PubMed] [Google Scholar]
- 33.Jannin B., Menzel M., Berlot J.-P., Delmas D., Lançon A., Latruffe N. Transport of resveratrol, a cancer chemopreventive agent, to cellular targets: plasmatic protein binding and cell uptake. Biochemical Pharmacology. 2004;68(6):1113–1118. doi: 10.1016/j.bcp.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 34.Crozier A., Jaganath I. B., Clifford M. N. Dietary phenolics: chemistry, bioavailability and effects on health. Natural Product Reports. 2009;26(8):1001–1043. doi: 10.1039/b802662a. [DOI] [PubMed] [Google Scholar]
- 35.Lançon A., Delma D., Osman H., Thénot J.-P., Jannin B., Latruffe N. Human hepatic cell uptake of resveratrol: involvement of both passive diffusion and carrier-mediated process. Biochemical and Biophysical Research Communications. 2004;316(4):1132–1137. doi: 10.1016/j.bbrc.2004.02.164. [DOI] [PubMed] [Google Scholar]
- 36.Wang L.-X., Heredia A., Song H., et al. Resveratrol glucuronides as the metabolites of resveratrol in humans: characterization, synthesis, and anti-HIV activity. Journal of Pharmaceutical Sciences. 2004;93(10):2448–2457. doi: 10.1002/jps.20156. [DOI] [PubMed] [Google Scholar]
- 37.Walle T., Hsieh F., DeLegge M. H., Oatis J. E., Jr., Walle U. K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metabolism and Disposition. 2004;32(12):1377–1382. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- 38.Cottart C.-H., Nivet-Antoine V., Laguillier-Morizot C., Beaudeux J.-L. Resveratrol bioavailability and toxicity in humans. Molecular Nutrition & Food Research. 2010;54(1):7–16. doi: 10.1002/mnfr.200900437. [DOI] [PubMed] [Google Scholar]
- 39.Boocock D. J., Patel K. R., Faust G. E. S., et al. Quantitation of trans-resveratrol and detection of its metabolites in human plasma and urine by high performance liquid chromatography. Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences. 2007;848(2):182–187. doi: 10.1016/j.jchromb.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zamora-Ros R., Urpí-Sardà M., Lamuela-Raventós R. M., et al. Diagnostic performance of urinary resveratrol metabolites as a biomarker of moderate wine consumption. Clinical Chemistry. 2006;52(7):1373–1380. doi: 10.1373/clinchem.2005.065870. [DOI] [PubMed] [Google Scholar]
- 41.Orallo F. Comparative studies of the antioxidant effects of Cis- and trans-resveratrol. Current Medicinal Chemistry. 2006;13(1):87–98. doi: 10.2174/092986706775197953. [DOI] [PubMed] [Google Scholar]
- 42.Campos-Toimil M., Elíes J., Álvarez E., Verde I., Orallo F. Effects of trans- and cis-resveratrol on Ca2+ handling in A7r5 vascular myocytes. European Journal of Pharmacology. 2007;577(1–3):91–99. doi: 10.1016/j.ejphar.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 43.Yáñez M., Fraiz N., Cano E., Orallo F. Inhibitory effects of cis- and trans-resveratrol on noradrenaline and 5-hydroxytryptamine uptake and on monoamine oxidase activity. Biochemical and Biophysical Research Communications. 2006;344(2):688–695. doi: 10.1016/j.bbrc.2006.03.190. [DOI] [PubMed] [Google Scholar]
- 44.Wenzel E., Somoza V. Metabolism and bioavailability of trans-resveratrol. Molecular Nutrition and Food Research. 2005;49(5):472–481. doi: 10.1002/mnfr.200500010. [DOI] [PubMed] [Google Scholar]
- 45.Vitaglione P., Sforza S., Galaverna G., et al. Bioavailability of trans-resveratrol from red wine in humans. Molecular Nutrition and Food Research. 2005;49(5):495–504. doi: 10.1002/mnfr.200500002. [DOI] [PubMed] [Google Scholar]
- 46.Vitrac X., Desmoulière A., Brouillaud B., et al. Distribution of [14C]-trans-resveratrol, a cancer chemopreventive polyphenol, in mouse tissues after oral administration. Life Sciences. 2003;72(20):2219–2233. doi: 10.1016/s0024-3205(03)00096-1. [DOI] [PubMed] [Google Scholar]
- 47.Marier J.-F., Vachon P., Gritsas A., Zhang J., Moreau J.-P., Ducharme M. P. Metabolism and disposition of resveratrol in rats: extent of absorption, glucuronidation, and enterohepatic recirculation evidenced by a linked-rat model. The Journal of Pharmacology and Experimental Therapeutics. 2002;302(1):369–373. doi: 10.1124/jpet.102.033340. [DOI] [PubMed] [Google Scholar]
- 48.Bravo L., Abia R., Saura-Calixto F. Polyphenols as dietary fiber associated compounds. Comparative study on in vivo and in vitro properties. Journal of Agricultural and Food Chemistry. 1994;42(7):1481–1487. doi: 10.1021/jf00043a017. [DOI] [Google Scholar]
- 49.Goldberg D. M., Yan J., Soleas G. J. Absorption of three wine-related polyphenols in three different matrices by healthy subjects. Clinical Biochemistry. 2003;36(1):79–87. doi: 10.1016/S0009-9120(02)00397-1. [DOI] [PubMed] [Google Scholar]
- 50.Almeida L., Vaz-da-Silva M., Falcão A., et al. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Molecular Nutrition and Food Research. 2009;53(supplement 1):S7–S15. doi: 10.1002/mnfr.200800177. [DOI] [PubMed] [Google Scholar]
- 51.Tessitore L., Davit A., Sarotto I., Caderni G. Resveratrol depresses the growth of colorectal aberrant crypt foci by affecting bax and p21(CIP) expression. Carcinogenesis. 2000;21(8):1619–1622. doi: 10.1093/carcin/21.8.1619. [DOI] [PubMed] [Google Scholar]
- 52.Li Z. G., Hong T., Shimada Y., et al. Suppression of N-nitrosomethylbenzylamine (NMBA)-induced esophageal tumorigenesis in F344 rats by resveratrol. Carcinogenesis. 2002;23(9):1531–1536. doi: 10.1093/carcin/23.9.1531. [DOI] [PubMed] [Google Scholar]
- 53.Banerjee S., Bueso-Ramos C., Aggarwal B. B. Suppression of 7,12-dimethylbenz(a)anthracene-induced mammary carcinogenesis in rats by resveratrol: role of nuclear factor-kappaB, cyclooxygenase 2, and matrix metalloprotease 9. Cancer Research. 2002;62(17):4945–4954. [PubMed] [Google Scholar]
- 54.La Porte C., Voduc N., Zhang G., et al. Steady-state pharmacokinetics and tolerability of trans-resveratrol 2000 mg twice daily with food, quercetin and alcohol (ethanol) in healthy human subjects. Clinical Pharmacokinetics. 2010;49(7):449–454. doi: 10.2165/11531820-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 55.Renaud S., de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. The Lancet. 1992;339(8808):1523–1526. doi: 10.1016/0140-6736(92)91277-f. [DOI] [PubMed] [Google Scholar]
- 56.Yilmaz Y., Toledo R. T. Health aspects of functional grape seed constituents. Trends in Food Science & Technology. 2004;15(9):422–433. doi: 10.1016/j.tifs.2004.04.006. [DOI] [Google Scholar]
- 57.Sun A. Y., Simonyi A., Wang Q., Sun G. Beyond the French paradox: protection of grape polyphenols against neurodegenerative processes. Alcoholism: Clinical & Experimental Research. 2004;28 [Google Scholar]
- 58.Bowers J. L., Tyulmenkov V. V., Jernigan S. C., Klinge C. M. Resveratrol acts as a mixed agonist/antagonist for estrogen receptors α and β . Endocrinology. 2000;141(10):3657–3667. doi: 10.1210/endo.141.10.7721. [DOI] [PubMed] [Google Scholar]
- 59.Abou-Zeid L. A., El-Mowafy A. M. Differential recognition of resveratrol isomers by the human estrogen receptor-α: molecular dynamics evidence for stereoselective ligand binding. Chirality. 2004;16(3):190–195. doi: 10.1002/chir.20007. [DOI] [PubMed] [Google Scholar]
- 60.Ashby J., Tinwell H., Pennie W., et al. Partial and weak oestrogenicity of the red wine constituent resveratrol: consideration of its superagonist activity in MCF-7 cells and its suggested cardiovascular protective effects. Journal of Applied Toxicology. 1999;19(1):39–45. doi: 10.1002/(sici)1099-1263(199901/02)19:1<39::aid-jat534>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 61.Kopp P. Resveratrol, a phytoestrogen found in red wine. A possible explanation for the conundrum of the ‘French paradox’? European Journal of Endocrinology. 1998;138(6):619–620. doi: 10.1530/eje.0.1380619. [DOI] [PubMed] [Google Scholar]
- 62.Turner R. T., Evans G. L., Zhang M., Maran A., Sibonga J. D. Is resveratrol an estrogen agonist in growing rats? Endocrinology. 1999;140(1):50–54. doi: 10.1210/en.140.1.50. [DOI] [PubMed] [Google Scholar]
- 63.Gehm B. D., McAndrews J. M., Chien P.-Y., Jameson J. L. Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(25):14138–14143. doi: 10.1073/pnas.94.25.14138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Borrás C., Gambini J., Gómez-Cabrera M. C., et al. 17β-oestradiol up-regulates longevity-related, antioxidant enzyme expression via the ERK1 and ERK2[MAPK]/NFκB cascade. Aging Cell. 2005;4(3):113–118. doi: 10.1111/j.1474-9726.2005.00151.x. [DOI] [PubMed] [Google Scholar]
- 65.Borrás C., Gambini J., Gómez-Cabrera M. C., et al. Genistein, a soy isoflavone, up-regulates expression of antioxidant genes: involvement of estrogen receptors, ERK1/2, and NFkappaB. The FASEB Journal. 2006;20(12):2136–2138. doi: 10.1096/fj.05-5522fje. [DOI] [PubMed] [Google Scholar]
- 66.Bashan N., Kovsan J., Kachko I., Ovadia H., Rudich A. Positive and negative regulation of insulin signaling by reactive oxygen and nitrogen species. Physiological Reviews. 2009;89(1):27–71. doi: 10.1152/physrev.00014.2008. [DOI] [PubMed] [Google Scholar]
- 67.Madamanchi N. R., Vendrov A., Runge M. S. Oxidative stress and vascular disease. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25(1):29–38. doi: 10.1161/01.ATV.0000150649.39934.13. [DOI] [PubMed] [Google Scholar]
- 68.Viña J., Lloret A., Vallés S. L., et al. Mitochondrial oxidant signalling in Alzheimer's disease. Journal of Alzheimer's Disease. 2007;11(2):175–181. doi: 10.3233/jad-2007-11205. [DOI] [PubMed] [Google Scholar]
- 69.Reuter S., Gupta S. C., Chaturvedi M. M., Aggarwal B. B. Oxidative stress, inflammation, and cancer: how are they linked? Free Radical Biology and Medicine. 2010;49(11):1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pacifici R. E., Davies K. J. A. Protein, lipid and DNA repair systems in oxidative stress: the free-radical theory of aging revisited. Gerontology. 1991;37(1–3):166–180. doi: 10.1159/000213257. [DOI] [PubMed] [Google Scholar]
- 71.Viña J., Borrás C., Miquel J. Theories of ageing. IUBMB Life. 2007;59(4-5):249–254. doi: 10.1080/15216540601178067. [DOI] [PubMed] [Google Scholar]
- 72.Zini R., Morin C., Bertelli A., Bertelli A. A. E., Tillement J.-P. Effects of resveratrol on the rat brain respiratory chain. Drugs under Experimental and Clinical Research. 1999;25(2-3):87–97. [PubMed] [Google Scholar]
- 73.Inglés M., Gambini J., Miguel M. G., et al. PTEN mediates the antioxidant effect of resveratrol at nutritionally relevant concentrations. BioMed Research International. 2014;2014:6. doi: 10.1155/2014/580852.580852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Losa G. A. Resveratrol modulates apoptosis and oxidation in human blood mononuclear cells. European Journal of Clinical Investigation. 2003;33(9):818–823. doi: 10.1046/j.1365-2362.2003.01219.x. [DOI] [PubMed] [Google Scholar]
- 75.Liu Y., Liu G. Isorhapontigenin and resveratrol suppress oxLDL-induced proliferation and activation of ERK1/2 mitogen-activated protein kinases of bovine aortic smooth muscle cells. Biochemical Pharmacology. 2004;67(4):777–785. doi: 10.1016/j.bcp.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 76.Olas B., Wachowicz B., Nowak P., et al. Comparative studies of the antioxidant effects of a naturally occurring resveratrol analogue—trans-3,3′,5,5′-tetrahydroxy-4′- methoxystilbene and resveratrol—against oxidation and nitration of biomolecules in blood platelets. Cell Biology and Toxicology. 2008;24(4):331–340. doi: 10.1007/s10565-007-9045-7. [DOI] [PubMed] [Google Scholar]
- 77.Rubiolo J. A., Mithieux G., Vega F. V. Resveratrol protects primary rat hepatocytes against oxidative stress damage: activation of the Nrf2 transcription factor and augmented activities of antioxidant enzymes. European Journal of Pharmacology. 2008;591(1–3):66–72. doi: 10.1016/j.ejphar.2008.06.067. [DOI] [PubMed] [Google Scholar]
- 78.Yang Y.-B., Piao Y.-J. Effects of resveratrol on secondary damages after acute spinal cord injury in rats. Acta Pharmacologica Sinica. 2003;24(7):703–710. [PubMed] [Google Scholar]
- 79.Kiziltepe U., Turan N. N. D., Han U., Ulus A. T., Akar F. Resveratrol, a red wine polyphenol, protects spinal cord from ischemia-reperfusion injury. Journal of Vascular Surgery. 2004;40(1):138–145. doi: 10.1016/j.jvs.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 80.Jackson J. R., Ryan M. J., Hao Y., Alway S. E. Mediation of endogenous antioxidant enzymes and apoptotic signaling by resveratrol following muscle disuse in the gastrocnemius muscles of young and old rats. The American Journal of Physiology—Regulatory Integrative and Comparative Physiology. 2010;299(6):R1572–R1581. doi: 10.1152/ajpregu.00489.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Momken I., Stevens L., Bergouignan A., et al. Resveratrol prevents the wasting disorders of mechanical unloading by acting as a physical exercise mimetic in the rat. The FASEB Journal. 2011;25(10):3646–3660. doi: 10.1096/fj.10-177295. [DOI] [PubMed] [Google Scholar]
- 82.Rascón B., Hubbard B. P., Sinclair D. A., Amdam G. V. The lifespan extension effects of resveratrol are conserved in the honey bee and may be driven by a mechanism related to caloric restriction. Aging. 2012;4(7):499–508. doi: 10.18632/aging.100474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu G.-S., Zhang Z.-S., Yang B., He W. Resveratrol attenuates oxidative damage and ameliorates cognitive impairment in the brain of senescence-accelerated mice. Life Sciences. 2012;91(17-18):872–877. doi: 10.1016/j.lfs.2012.08.033. [DOI] [PubMed] [Google Scholar]
- 84.Sang S., Yang I., Buckley B., Ho C.-T., Yang C. S. Autoxidative quinone formation in vitro and metabolite formation in vivo from tea polyphenol (-)-epigallocatechin-3-gallate: studied by real-time mass spectrometry combined with tandem mass ion mapping. Free Radical Biology and Medicine. 2007;43(3):362–371. doi: 10.1016/j.freeradbiomed.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Klaus V., Hartmann T., Gambini J., et al. 1,4-Naphthoquinones as inducers of oxidative damage and stress signaling in HaCaT human keratinocytes. Archives of Biochemistry and Biophysics. 2010;496(2):93–100. doi: 10.1016/j.abb.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 86.Hadi S. M., Ullah M. F., Azmi A. S., et al. Resveratrol mobilizes endogenous copper in human peripheral lymphocytes leading to oxidative DNA breakage: a putative mechanism for chemoprevention of cancer. Pharmaceutical Research. 2010;27(6):979–988. doi: 10.1007/s11095-010-0055-4. [DOI] [PubMed] [Google Scholar]
- 87.Lin H.-Y., Lansing L., Merillon J.-M., et al. Integrin alphaVbeta3 contains a receptor site for resveratrol. The FASEB Journal. 2006;20(10):1742–1744. doi: 10.1096/fj.06-5743fje. [DOI] [PubMed] [Google Scholar]
- 88.Casper R. F., Quesne M., Rogers I. M., et al. Resveratrol has antagonist activity on the aryl hydrocarbon receptor: implications for prevention of dioxin toxicity. Molecular Pharmacology. 1999;56(4):784–790. [PubMed] [Google Scholar]
- 89.Hsieh T.-C., Wu J. M. Differential effects on growth, cell cycle arrest, and induction of apoptosis by resveratrol in human prostate cancer cell lines. Experimental Cell Research. 1999;249(1):109–115. doi: 10.1006/excr.1999.4471. [DOI] [PubMed] [Google Scholar]
- 90.Jang M., Cai L., Udeani G. O., et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275(5297):218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 91.Nebert D. W., Dalton T. P., Okey A. B., Gonzalez F. J. Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. The Journal of Biological Chemistry. 2004;279(23):23847–23850. doi: 10.1074/jbc.r400004200. [DOI] [PubMed] [Google Scholar]
- 92.Pozo-Guisado E., Merino J. M., Mulero-Navarro S., et al. Resveratrol-induced apoptosis in MCF-7 human breast cancer cells involves a caspase-independent mechanism with downregulation of Bcl-2 and NF-κB. International Journal of Cancer. 2005;115(1):74–84. doi: 10.1002/ijc.20856. [DOI] [PubMed] [Google Scholar]
- 93.Huang C., Ma W.-Y., Goranson A., Dong Z. Resveratrol suppresses cell transformation and induces apoptosis through a p53-dependent pathway. Carcinogenesis. 1999;20(2):237–242. doi: 10.1093/carcin/20.2.237. [DOI] [PubMed] [Google Scholar]
- 94.Fontecave M., Lepoivre M., Elleingand E., Gerez C., Guittet O. Resveratrol, a remarkable inhibitor of ribonucleotide reductase. FEBS Letters. 1998;421(3):277–279. doi: 10.1016/s0014-5793(97)01572-x. [DOI] [PubMed] [Google Scholar]
- 95.Moreno J. J. Resveratrol modulates arachidonic acid release, prostaglandin synthesis, and 3T6 fibroblast growth. Journal of Pharmacology and Experimental Therapeutics. 2000;294(1):333–338. [PubMed] [Google Scholar]
- 96.Baur J. A., Sinclair D. A. Therapeutic potential of resveratrol: the in vivo evidence. Nature Reviews Drug Discovery. 2006;5(6):493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 97.Gescher A. J., Steward W. P. Relationship between mechanisms, bioavailibility, and preclinical chemopreventive efficacy of resveratrol: a conundrum. Cancer Epidemiology Biomarkers and Prevention. 2003;12(10):953–957. [PubMed] [Google Scholar]
- 98.Dobrydneva Y., Williams R. L., Blackmore P. F. trans-Resveratrol inhibits calcium influx in thrombin-stimulated human platelets. British Journal of Pharmacology. 1999;128(1):149–157. doi: 10.1038/sj.bjp.0702749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhu Y., Lin J., Li D., et al. Effect of resveratrol on platelet aggregation in vivo and in vitro. Chinese Medical Journal. 2002;115(3):378–380. [PubMed] [Google Scholar]
- 100.Lasa A., Schweiger M., Kotzbeck P., et al. Resveratrol regulates lipolysis via adipose triglyceride lipase. Journal of Nutritional Biochemistry. 2012;23(4):379–384. doi: 10.1016/j.jnutbio.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 101.Cheong H., Ryu S.-Y., Kim K.-M. Anti-allergic action of resveratrol and related hydroxystilbenes. Planta Medica. 1999;65(3):266–268. doi: 10.1055/s-2006-960773. [DOI] [PubMed] [Google Scholar]
- 102.Tseng P.-C., Hou S.-M., Chen R.-J., et al. Resveratrol promotes osteogenesis of human mesenchymal stem cells by upregulating RUNX2 gene expression via the SIRT1/FOXO3A axis. Journal of Bone and Mineral Research. 2011;26(10):2552–2563. doi: 10.1002/jbmr.460. [DOI] [PubMed] [Google Scholar]
- 103.Chen K. H., Cheng M. L., Jing Y. H., Chiu D. T., Shiao M. S., Chen J. K. Resveratrol ameliorates metabolic disorders and muscle wasting in streptozotocin-induced diabetic rats. The American Journal of Physiology: Endocrinology and metabolism. 2011;301(5):E853–E863. doi: 10.1152/ajpendo.00048.2011. [DOI] [PubMed] [Google Scholar]
- 104.Howitz K. T., Bitterman K. J., Cohen H. Y., et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425(6954):191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 105.Wood J. G., Regina B., Lavu S., et al. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430(7000):686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 106.Gambini J., Gomez-Cabrera M. C., Borras C., et al. Free [NADH]/[NAD+] regulates sirtuin expression. Archives of Biochemistry and Biophysics. 2011;512(1):24–29. doi: 10.1016/j.abb.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 107.Imai S.-I., Armstrong C. M., Kaeberlein M., Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403(6771):795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- 108.Bauer J. H., Goupil S., Garber G. B., Helfand S. L. An accelerated assay for the identification of lifespan-extending interventions in Drosophila melanogaster . Proceedings of the National Academy of Sciences of the United States of America. 2004;101(35):12980–12985. doi: 10.1073/pnas.0403493101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Viswanathan M., Kim S. K., Berdichevsky A., Guarente L. A role for SIR-2.1 regulation of ER stress response genes in determining C. elegans life span. Developmental Cell. 2005;9(5):605–615. doi: 10.1016/j.devcel.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 110.Bass T. M., Weinkove D., Houthoofd K., Gems D., Partridge L. Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans . Mechanisms of Ageing and Development. 2007;128(10):546–552. doi: 10.1016/j.mad.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 111.Kaeberlein M., McDonagh T., Heltweg B., et al. Substrate-specific activation of sirtuins by resveratrol. The Journal of Biological Chemistry. 2005;280(17):17038–17045. doi: 10.1074/jbc.m500655200. [DOI] [PubMed] [Google Scholar]
- 112.Pacholec M., Bleasdale J. E., Chrunyk B., et al. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. The Journal of Biological Chemistry. 2010;285(11):8340–8351. doi: 10.1074/jbc.m109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Borra M. T., Smith B. C., Denu J. M. Mechanism of human SIRT1 activation by resveratrol. The Journal of Biological Chemistry. 2005;280(17):17187–17195. doi: 10.1074/jbc.m501250200. [DOI] [PubMed] [Google Scholar]
- 114.Burnett C., Valentini S., Cabreiro F., et al. Absence of effects of Sir2 overexpression on lifespan in C. elegans and Drosophila . Nature. 2011;477(7365):482–485. doi: 10.1038/nature10296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Marchal J., Blanc S., Epelbaum J., Aujard F., Pifferi F. Effects of chronic calorie restriction or dietary resveratrol supplementation on insulin sensitivity markers in a primate, Microcebus murinus . PLoS ONE. 2012;7(3) doi: 10.1371/journal.pone.0034289.e34289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Crandall J. P., Oram V., Trafirescu G., et al. Pilot study of resveratrol in older adults with impaired glucose tolerance. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2012;67(12):1307–1312. doi: 10.1093/gerona/glr235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Timmers S., Konings E., Bilet L., et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metabolism. 2011;14(5):612–622. doi: 10.1016/j.cmet.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hu Y., Liu J., Wang J., Liu Q. The controversial links among calorie restriction, SIRT1, and resveratrol. Free Radical Biology and Medicine. 2011;51(2):250–256. doi: 10.1016/j.freeradbiomed.2011.04.034. [DOI] [PubMed] [Google Scholar]
- 119.Cristòfol R., Porquet D., Corpas R., et al. Neurons from senescence-accelerated SAMP8 mice are protected against frailty by the sirtuin 1 promoting agents melatonin and resveratrol. Journal of Pineal Research. 2012;52(3):271–281. doi: 10.1111/j.1600-079X.2011.00939.x. [DOI] [PubMed] [Google Scholar]
- 120.Cuzzola V. F., Ciurleo R., Giacoppo S., Marino S., Bramanti P. Role of resveratrol and its analogues in the treatment of neurodegenerative diseases: focus on recent discoveries. CNS and Neurological Disorders—Drug Targets. 2011;10(7):849–862. doi: 10.2174/187152711798072310. [DOI] [PubMed] [Google Scholar]
- 121.Tajes M., Gutierrez-Cuesta J., Folch J., et al. Neuroprotective role of intermittent fasting in senescence-accelerated mice P8 (SAMP8) Experimental Gerontology. 2010;45(9):702–710. doi: 10.1016/j.exger.2010.04.010. [DOI] [PubMed] [Google Scholar]