Abstract
Cyclophilins are peptidyl-prolyl cis-trans isomerases (PPIases) which have been implicated in intracellular protein folding, transport and assembly. Cyclophilins are also known as the intracellular receptors for the immunosuppressive drug cyclosporin A (CsA). The most common type of cyclophilins are the 18 kDa cytosolic proteins containing only the highly conserved core domain for PPIase and CsA binding activities. The wis2+ gene of the fission yeast Schizosaccharomyces pombe was isolated as a multicopy suppressor of wee1-50 cdc25-22 win1-1, a triple mutant strain which exhibits a cell cycle defect phenotype. Sequence analysis of wis2+ reveals that it encodes a 40 kDa cyclophilin-like protein, homologous to the mammalian cyclophilin 40. The 18 kDa cyclophilin domain (CyP-18) of wis2 is followed by a C-terminal region of 188 amino acids. The C-terminal region of wis2 is essential for suppression of the triple mutant defect. Furthermore this region of the protein is able to confer suppression activity on the 18 kDa S.pombe cyclophilin, cyp1, since a hybrid protein consisting of an 18 kDa S.pombe cyclophilin (cyp1) fused to the C-terminus of wis2 shows suppression activity. We also demonstrate that the level of wis2+ mRNA increases 10- to 20-fold upon heat shock of S.pombe cells suggesting a role for wis2+ in the heat-shock response.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aligue R., Akhavan-Niak H., Russell P. A role for Hsp90 in cell cycle control: Wee1 tyrosine kinase activity requires interaction with Hsp90. EMBO J. 1994 Dec 15;13(24):6099–6106. doi: 10.1002/j.1460-2075.1994.tb06956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S. K., Gallinger S., Roder J., Frey J., Young H. A., Ortaldo J. R. A cyclophilin-related protein involved in the function of natural killer cells. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):542–546. doi: 10.1073/pnas.90.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker E. K., Colley N. J., Zuker C. S. The cyclophilin homolog NinaA functions as a chaperone, forming a stable complex in vivo with its protein target rhodopsin. EMBO J. 1994 Oct 17;13(20):4886–4895. doi: 10.1002/j.1460-2075.1994.tb06816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach D., Durkacz B., Nurse P. Functionally homologous cell cycle control genes in budding and fission yeast. Nature. 1982 Dec 23;300(5894):706–709. doi: 10.1038/300706a0. [DOI] [PubMed] [Google Scholar]
- Booher R., Beach D. Site-specific mutagenesis of cdc2+, a cell cycle control gene of the fission yeast Schizosaccharomyces pombe. Mol Cell Biol. 1986 Oct;6(10):3523–3530. doi: 10.1128/mcb.6.10.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callebaut I., Renoir J. M., Lebeau M. C., Massol N., Burny A., Baulieu E. E., Mornon J. P. An immunophilin that binds M(r) 90,000 heat shock protein: main structural features of a mammalian p59 protein. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6270–6274. doi: 10.1073/pnas.89.14.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas M. E., Hemenway C., Muir R. S., Ye R., Fiorentino D., Heitman J. Immunophilins interact with calcineurin in the absence of exogenous immunosuppressive ligands. EMBO J. 1994 Dec 15;13(24):5944–5957. doi: 10.1002/j.1460-2075.1994.tb06940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H. C., Lindquist S. Conservation of Hsp90 macromolecular complexes in Saccharomyces cerevisiae. J Biol Chem. 1994 Oct 7;269(40):24983–24988. [PubMed] [Google Scholar]
- Clipstone N. A., Crabtree G. R. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature. 1992 Jun 25;357(6380):695–697. doi: 10.1038/357695a0. [DOI] [PubMed] [Google Scholar]
- Featherstone C., Russell P. Fission yeast p107wee1 mitotic inhibitor is a tyrosine/serine kinase. Nature. 1991 Feb 28;349(6312):808–811. doi: 10.1038/349808a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fischer G., Wittmann-Liebold B., Lang K., Kiefhaber T., Schmid F. X. Cyclophilin and peptidyl-prolyl cis-trans isomerase are probably identical proteins. Nature. 1989 Feb 2;337(6206):476–478. doi: 10.1038/337476a0. [DOI] [PubMed] [Google Scholar]
- Foor F., Parent S. A., Morin N., Dahl A. M., Ramadan N., Chrebet G., Bostian K. A., Nielsen J. B. Calcineurin mediates inhibition by FK506 and cyclosporin of recovery from alpha-factor arrest in yeast. Nature. 1992 Dec 17;360(6405):682–684. doi: 10.1038/360682a0. [DOI] [PubMed] [Google Scholar]
- Freskgård P. O., Bergenhem N., Jonsson B. H., Svensson M., Carlsson U. Isomerase and chaperone activity of prolyl isomerase in the folding of carbonic anhydrase. Science. 1992 Oct 16;258(5081):466–468. doi: 10.1126/science.1357751. [DOI] [PubMed] [Google Scholar]
- Friedman J., Weissman I. Two cytoplasmic candidates for immunophilin action are revealed by affinity for a new cyclophilin: one in the presence and one in the absence of CsA. Cell. 1991 Aug 23;66(4):799–806. doi: 10.1016/0092-8674(91)90123-g. [DOI] [PubMed] [Google Scholar]
- Galat A. Peptidylproline cis-trans-isomerases: immunophilins. Eur J Biochem. 1993 Sep 15;216(3):689–707. doi: 10.1111/j.1432-1033.1993.tb18189.x. [DOI] [PubMed] [Google Scholar]
- Goebl M., Yanagida M. The TPR snap helix: a novel protein repeat motif from mitosis to transcription. Trends Biochem Sci. 1991 May;16(5):173–177. doi: 10.1016/0968-0004(91)90070-c. [DOI] [PubMed] [Google Scholar]
- Gould K. L., Nurse P. Tyrosine phosphorylation of the fission yeast cdc2+ protein kinase regulates entry into mitosis. Nature. 1989 Nov 2;342(6245):39–45. doi: 10.1038/342039a0. [DOI] [PubMed] [Google Scholar]
- Handschumacher R. E., Harding M. W., Rice J., Drugge R. J., Speicher D. W. Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science. 1984 Nov 2;226(4674):544–547. doi: 10.1126/science.6238408. [DOI] [PubMed] [Google Scholar]
- Harding M. W., Handschumacher R. E., Speicher D. W. Isolation and amino acid sequence of cyclophilin. J Biol Chem. 1986 Jun 25;261(18):8547–8555. [PubMed] [Google Scholar]
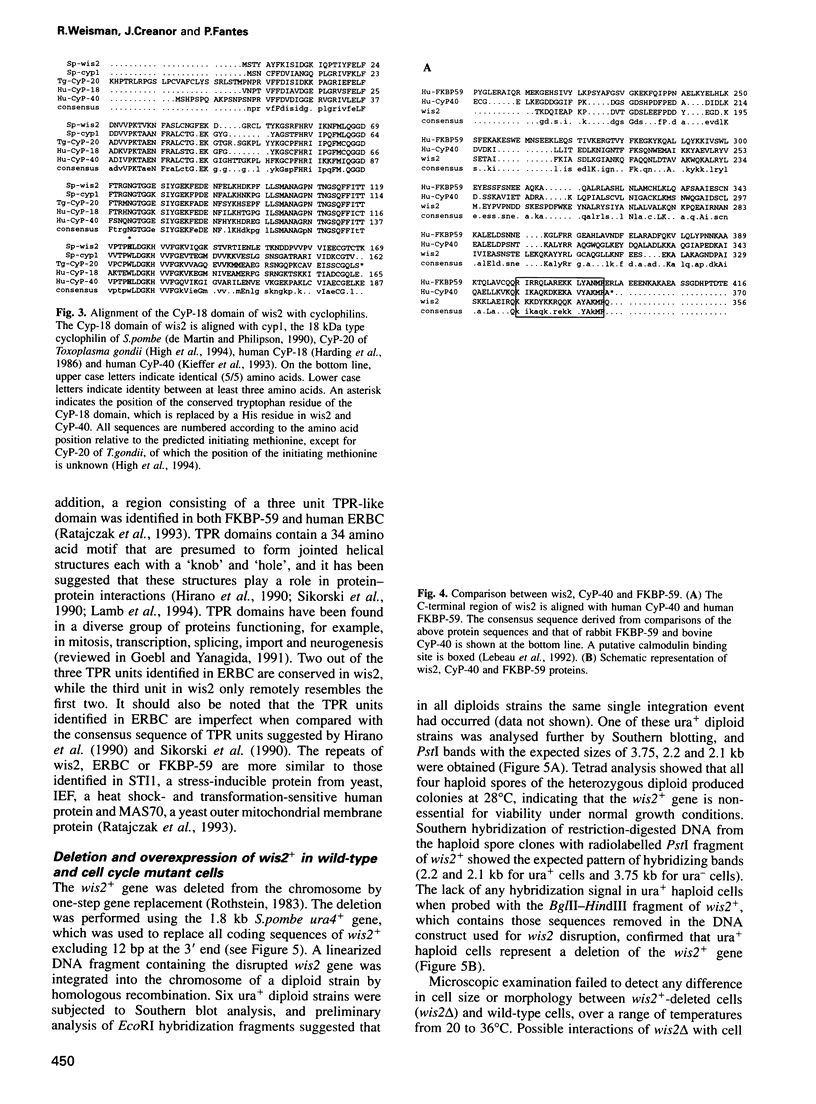
- High K. P., Joiner K. A., Handschumacher R. E. Isolation, cDNA sequences, and biochemical characterization of the major cyclosporin-binding proteins of Toxoplasma gondii. J Biol Chem. 1994 Mar 25;269(12):9105–9112. [PubMed] [Google Scholar]
- Hirano T., Kinoshita N., Morikawa K., Yanagida M. Snap helix with knob and hole: essential repeats in S. pombe nuclear protein nuc2+. Cell. 1990 Jan 26;60(2):319–328. doi: 10.1016/0092-8674(90)90746-2. [DOI] [PubMed] [Google Scholar]
- Hoffmann K., Handschumacher R. E. Cyclophilin-40: evidence for a dimeric complex with hsp90. Biochem J. 1995 Apr 1;307(Pt 1):5–8. doi: 10.1042/bj3070005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallen J., Spitzfaden C., Zurini M. G., Wider G., Widmer H., Wüthrich K., Walkinshaw M. D. Structure of human cyclophilin and its binding site for cyclosporin A determined by X-ray crystallography and NMR spectroscopy. Nature. 1991 Sep 19;353(6341):276–279. doi: 10.1038/353276a0. [DOI] [PubMed] [Google Scholar]
- Kieffer L. J., Seng T. W., Li W., Osterman D. G., Handschumacher R. E., Bayney R. M. Cyclophilin-40, a protein with homology to the P59 component of the steroid receptor complex. Cloning of the cDNA and further characterization. J Biol Chem. 1993 Jun 15;268(17):12303–12310. [PubMed] [Google Scholar]
- Kieffer L. J., Thalhammer T., Handschumacher R. E. Isolation and characterization of a 40-kDa cyclophilin-related protein. J Biol Chem. 1992 Mar 15;267(8):5503–5507. [PubMed] [Google Scholar]
- Koser P. L., Livi G. P., Levy M. A., Rosenberg M., Bergsma D. J. A Candida albicans homolog of a human cyclophilin gene encodes a peptidyl-prolyl cis-trans isomerase. Gene. 1990 Dec 15;96(2):189–195. doi: 10.1016/0378-1119(90)90252-m. [DOI] [PubMed] [Google Scholar]
- Käufer N. F., Simanis V., Nurse P. Fission yeast Schizosaccharomyces pombe correctly excises a mammalian RNA transcript intervening sequence. Nature. 1985 Nov 7;318(6041):78–80. doi: 10.1038/318078a0. [DOI] [PubMed] [Google Scholar]
- Lamb J. R., Michaud W. A., Sikorski R. S., Hieter P. A. Cdc16p, Cdc23p and Cdc27p form a complex essential for mitosis. EMBO J. 1994 Sep 15;13(18):4321–4328. doi: 10.1002/j.1460-2075.1994.tb06752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang K., Schmid F. X., Fischer G. Catalysis of protein folding by prolyl isomerase. Nature. 1987 Sep 17;329(6136):268–270. doi: 10.1038/329268a0. [DOI] [PubMed] [Google Scholar]
- Lebeau M. C., Massol N., Herrick J., Faber L. E., Renoir J. M., Radanyi C., Baulieu E. E. P59, an hsp 90-binding protein. Cloning and sequencing of its cDNA and preparation of a peptide-directed polyclonal antibody. J Biol Chem. 1992 Mar 5;267(7):4281–4284. [PubMed] [Google Scholar]
- Liu J., Chen C. M., Walsh C. T. Human and Escherichia coli cyclophilins: sensitivity to inhibition by the immunosuppressant cyclosporin A correlates with a specific tryptophan residue. Biochemistry. 1991 Mar 5;30(9):2306–2310. doi: 10.1021/bi00223a003. [DOI] [PubMed] [Google Scholar]
- Liu J., Farmer J. D., Jr, Lane W. S., Friedman J., Weissman I., Schreiber S. L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991 Aug 23;66(4):807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- Luan S., Lane W. S., Schreiber S. L. pCyP B: a chloroplast-localized, heat shock-responsive cyclophilin from fava bean. Plant Cell. 1994 Jun;6(6):885–892. doi: 10.1105/tpc.6.6.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan S., Li W., Rusnak F., Assmann S. M., Schreiber S. L. Immunosuppressants implicate protein phosphatase regulation of K+ channels in guard cells. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2202–2206. doi: 10.1073/pnas.90.6.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren K., Walworth N., Booher R., Dembski M., Kirschner M., Beach D. mik1 and wee1 cooperate in the inhibitory tyrosine phosphorylation of cdc2. Cell. 1991 Mar 22;64(6):1111–1122. doi: 10.1016/0092-8674(91)90266-2. [DOI] [PubMed] [Google Scholar]
- Maundrell K. nmt1 of fission yeast. A highly transcribed gene completely repressed by thiamine. J Biol Chem. 1990 Jul 5;265(19):10857–10864. [PubMed] [Google Scholar]
- McLaughlin M. M., Bossard M. J., Koser P. L., Cafferkey R., Morris R. A., Miles L. M., Strickler J., Bergsma D. J., Levy M. A., Livi G. P. The yeast cyclophilin multigene family: purification, cloning and characterization of a new isoform. Gene. 1992 Feb 1;111(1):85–92. doi: 10.1016/0378-1119(92)90606-p. [DOI] [PubMed] [Google Scholar]
- Millar J. B., Lenaers G., Russell P. Pyp3 PTPase acts as a mitotic inducer in fission yeast. EMBO J. 1992 Dec;11(13):4933–4941. doi: 10.1002/j.1460-2075.1992.tb05600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar J. B., McGowan C. H., Lenaers G., Jones R., Russell P. p80cdc25 mitotic inducer is the tyrosine phosphatase that activates p34cdc2 kinase in fission yeast. EMBO J. 1991 Dec;10(13):4301–4309. doi: 10.1002/j.1460-2075.1991.tb05008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molz L., Booher R., Young P., Beach D. cdc2 and the regulation of mitosis: six interacting mcs genes. Genetics. 1989 Aug;122(4):773–782. doi: 10.1093/genetics/122.4.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S., Hayles J., Nurse P. Regulation of p34cdc2 protein kinase during mitosis. Cell. 1989 Jul 28;58(2):361–372. doi: 10.1016/0092-8674(89)90850-7. [DOI] [PubMed] [Google Scholar]
- Moreno S., Klar A., Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990 Apr 5;344(6266):503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- Ogden J. E., Fantes P. A. Isolation of a novel type of mutation in the mitotic control of Schizosaccharomyces pombe whose phenotypic expression is dependent on the genetic background and nutritional environment. Curr Genet. 1986;10(7):509–514. doi: 10.1007/BF00447384. [DOI] [PubMed] [Google Scholar]
- Ondek B., Hardy R. W., Baker E. K., Stamnes M. A., Shieh B. H., Zuker C. S. Genetic dissection of cyclophilin function. Saturation mutagenesis of the Drosophila cyclophilin homolog ninaA. J Biol Chem. 1992 Aug 15;267(23):16460–16466. [PubMed] [Google Scholar]
- Parker L. L., Atherton-Fessler S., Lee M. S., Ogg S., Falk J. L., Swenson K. I., Piwnica-Worms H. Cyclin promotes the tyrosine phosphorylation of p34cdc2 in a wee1+ dependent manner. EMBO J. 1991 May;10(5):1255–1263. doi: 10.1002/j.1460-2075.1991.tb08067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perisic O., Xiao H., Lis J. T. Stable binding of Drosophila heat shock factor to head-to-head and tail-to-tail repeats of a conserved 5 bp recognition unit. Cell. 1989 Dec 1;59(5):797–806. doi: 10.1016/0092-8674(89)90603-x. [DOI] [PubMed] [Google Scholar]
- Polanshek M. M. Effects of heat shock and cycloheximide on growth and division of the fission yeast, Schizosaccharomyces pombe. With an Appendix. Estimation of division delay for S. pombe from cell plate index curves. J Cell Sci. 1977 Feb;23:1–23. doi: 10.1242/jcs.23.1.1. [DOI] [PubMed] [Google Scholar]
- Pratt W. B. The role of heat shock proteins in regulating the function, folding, and trafficking of the glucocorticoid receptor. J Biol Chem. 1993 Oct 15;268(29):21455–21458. [PubMed] [Google Scholar]
- Radanyi C., Chambraud B., Baulieu E. E. The ability of the immunophilin FKBP59-HBI to interact with the 90-kDa heat shock protein is encoded by its tetratricopeptide repeat domain. Proc Natl Acad Sci U S A. 1994 Nov 8;91(23):11197–11201. doi: 10.1073/pnas.91.23.11197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak T., Carrello A., Mark P. J., Warner B. J., Simpson R. J., Moritz R. L., House A. K. The cyclophilin component of the unactivated estrogen receptor contains a tetratricopeptide repeat domain and shares identity with p59 (FKBP59). J Biol Chem. 1993 Jun 25;268(18):13187–13192. [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Sanchez E. R. Hsp56: a novel heat shock protein associated with untransformed steroid receptor complexes. J Biol Chem. 1990 Dec 25;265(36):22067–22070. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozaki K., Akhavan-Niaki H., McGowan C. H., Russell P. Protein phosphatase 2C, encoded by ptc1+, is important in the heat shock response of Schizosaccharomyces pombe. Mol Cell Biol. 1994 Jun;14(6):3742–3751. doi: 10.1128/mcb.14.6.3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski R. S., Boguski M. S., Goebl M., Hieter P. A repeating amino acid motif in CDC23 defines a family of proteins and a new relationship among genes required for mitosis and RNA synthesis. Cell. 1990 Jan 26;60(2):307–317. doi: 10.1016/0092-8674(90)90745-z. [DOI] [PubMed] [Google Scholar]
- Smith B. J., Yaffe M. P. A mutation in the yeast heat-shock factor gene causes temperature-sensitive defects in both mitochondrial protein import and the cell cycle. Mol Cell Biol. 1991 May;11(5):2647–2655. doi: 10.1128/mcb.11.5.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamnes M. A., Rutherford S. L., Zuker C. S. Cyclophilins: a new family of proteins involved in intracellular folding. Trends Cell Biol. 1992 Sep;2(9):272–276. doi: 10.1016/0962-8924(92)90200-7. [DOI] [PubMed] [Google Scholar]
- Sykes K., Gething M. J., Sambrook J. Proline isomerases function during heat shock. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5853–5857. doi: 10.1073/pnas.90.12.5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai P. K., Albers M. W., Chang H., Faber L. E., Schreiber S. L. Association of a 59-kilodalton immunophilin with the glucocorticoid receptor complex. Science. 1992 May 29;256(5061):1315–1318. doi: 10.1126/science.1376003. [DOI] [PubMed] [Google Scholar]
- Tai P. K., Maeda Y., Nakao K., Wakim N. G., Duhring J. L., Faber L. E. A 59-kilodalton protein associated with progestin, estrogen, androgen, and glucocorticoid receptors. Biochemistry. 1986 Sep 9;25(18):5269–5275. doi: 10.1021/bi00366a043. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Hayano T., Suzuki M. Peptidyl-prolyl cis-trans isomerase is the cyclosporin A-binding protein cyclophilin. Nature. 1989 Feb 2;337(6206):473–475. doi: 10.1038/337473a0. [DOI] [PubMed] [Google Scholar]
- Thériault Y., Logan T. M., Meadows R., Yu L., Olejniczak E. T., Holzman T. F., Simmer R. L., Fesik S. W. Solution structure of the cyclosporin A/cyclophilin complex by NMR. Nature. 1993 Jan 7;361(6407):88–91. doi: 10.1038/361088a0. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Warbrick E., Fantes P. A. Five novel elements involved in the regulation of mitosis in fission yeast. Mol Gen Genet. 1992 Apr;232(3):440–446. doi: 10.1007/BF00266249. [DOI] [PubMed] [Google Scholar]
- Warbrick E., Fantes P. A. The wis1 protein kinase is a dosage-dependent regulator of mitosis in Schizosaccharomyces pombe. EMBO J. 1991 Dec;10(13):4291–4299. doi: 10.1002/j.1460-2075.1991.tb05007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yem A. W., Tomasselli A. G., Heinrikson R. L., Zurcher-Neely H., Ruff V. A., Johnson R. A., Deibel M. R., Jr The Hsp56 component of steroid receptor complexes binds to immobilized FK506 and shows homology to FKBP-12 and FKBP-13. J Biol Chem. 1992 Feb 15;267(5):2868–2871. [PubMed] [Google Scholar]
- Yoshida T., Toda T., Yanagida M. A calcineurin-like gene ppb1+ in fission yeast: mutant defects in cytokinesis, cell polarity, mating and spindle pole body positioning. J Cell Sci. 1994 Jul;107(Pt 7):1725–1735. doi: 10.1242/jcs.107.7.1725. [DOI] [PubMed] [Google Scholar]
- de Martin R., Philipson L. The gene for cyclophilin (peptidyl-prolyl cis-trans isomerase) from Schizosaccharomyces pombe. Nucleic Acids Res. 1990 Aug 25;18(16):4917–4917. doi: 10.1093/nar/18.16.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]