Abstract
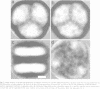
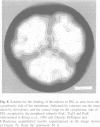
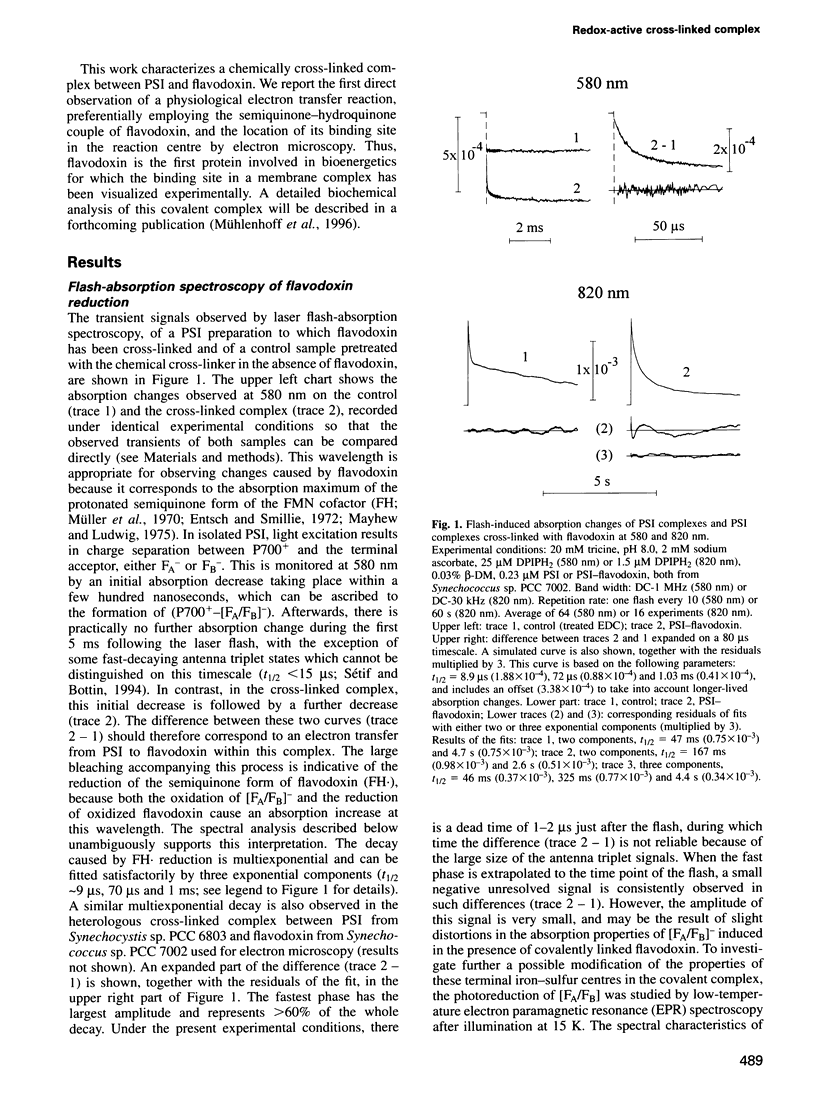
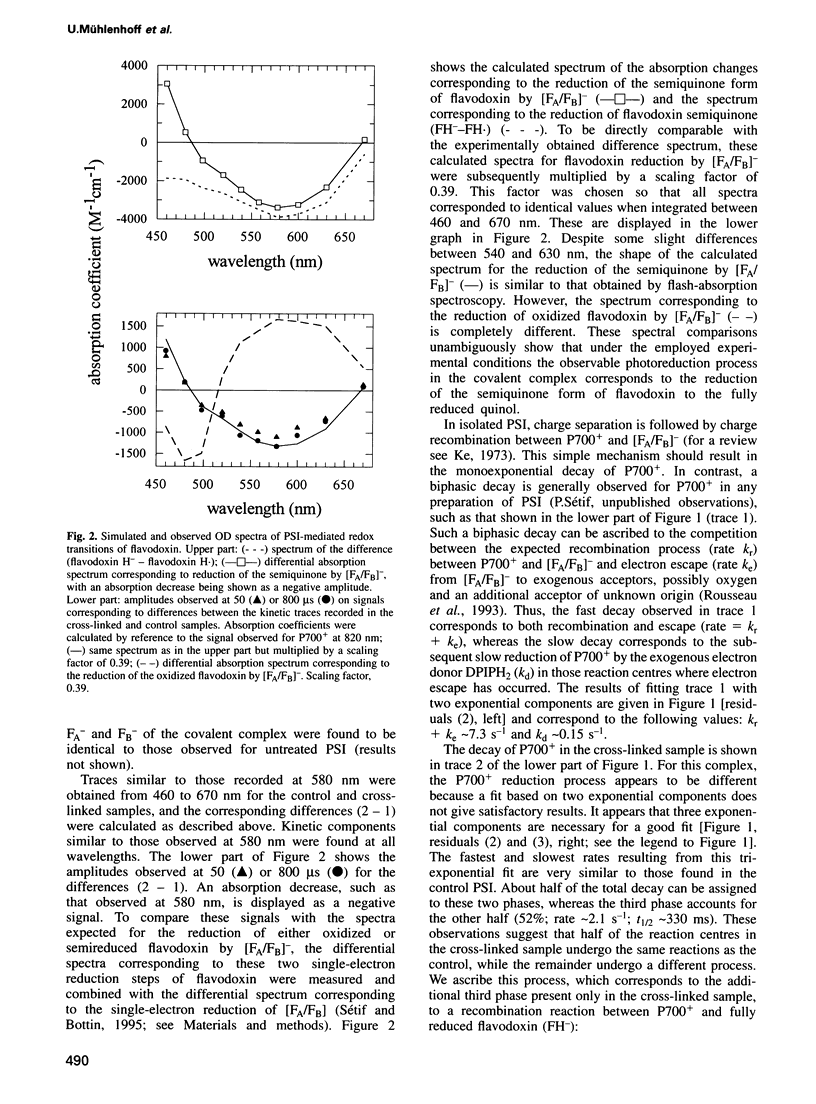
A covalent complex between photosystem I and flavodoxin from the cyanobacterium Synechococcus sp. PCC 7002 was generated by chemical cross-linking. Laser flash-absorption spectroscopy indicates that the bound flavodoxin of this complex is stabilized in the semiquinone state and is photoreduced to the quinol form upon light excitation. The kinetics of this photoreduction process, which takes place in approximately 50% of the reaction centres, displays three exponential components with half-lives of 9 microsec, 70 microsec and 1 ms. The fully reduced flavodoxin subsequently recombines with P700+ with a t1/2 of 330 ms. A corresponding flavodoxin semiquinone radical signal is readily observed in the dark by room temperature electron paramagnetic resonance, which reversibly disappears upon illumination. In contrast, the light-induced reduction of oxidized flavodoxin can be observed only by first-flash experiments following excessive dark adaptation. In addition, the docking site of flavodoxin on photosystem I was determined by electron microscopy in combination with image analysis. Flavodoxin binds to the cytoplasmic side of photosystem I at a distance of 7 nm from the centre of the trimer and in close contact to a ridge formed by the subunits PsaC, PsaD and PsaE.
Full text
PDF
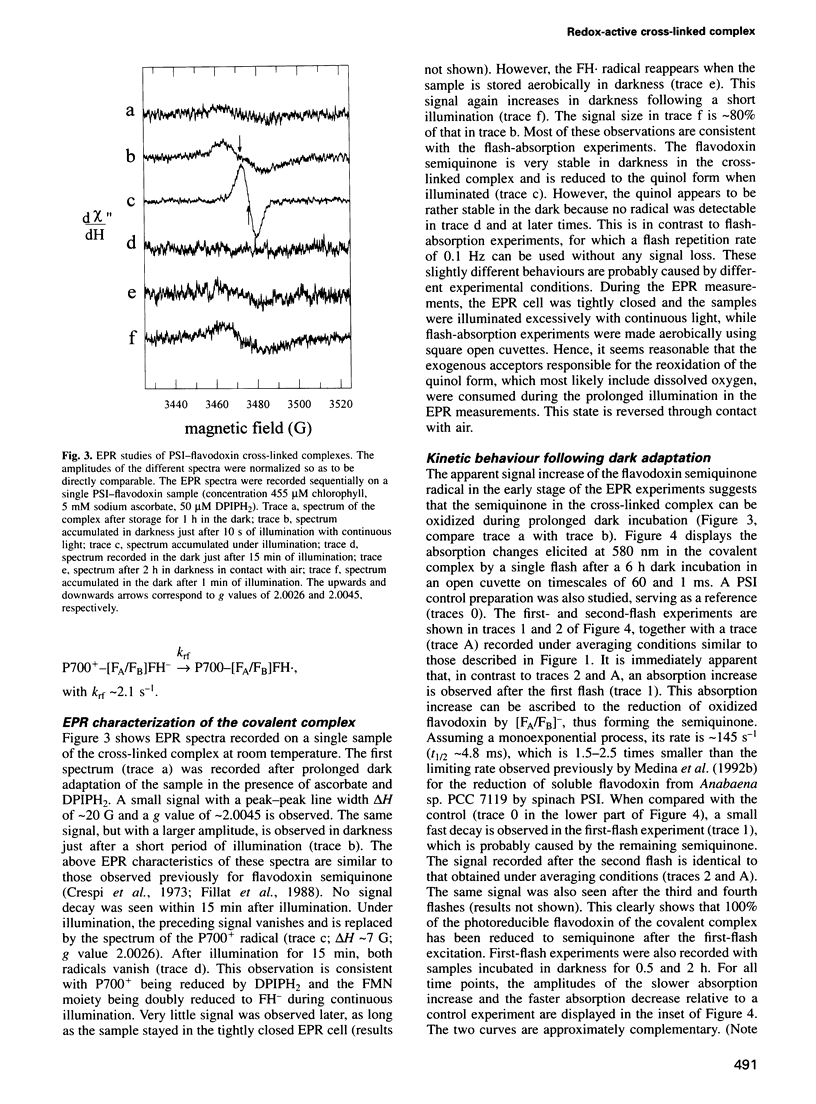
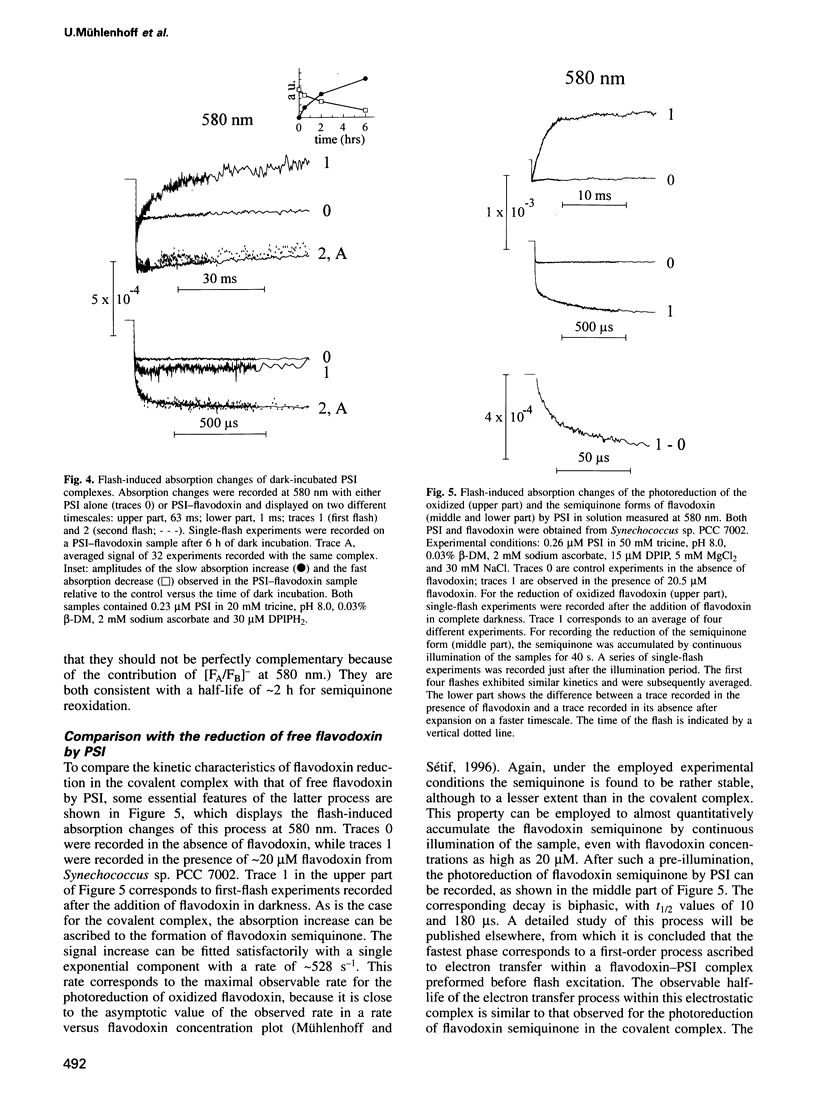




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boekema E. J., Boonstra A. F., Dekker J. P., Rögner M. Electron microscopic structural analysis of Photosystem I, Photosystem II, and the cytochrome b6/f complex from green plants and cyanobacteria. J Bioenerg Biomembr. 1994 Feb;26(1):17–29. doi: 10.1007/BF00763217. [DOI] [PubMed] [Google Scholar]
- Brosius J. Superpolylinkers in cloning and expression vectors. DNA. 1989 Dec;8(10):759–777. doi: 10.1089/dna.1989.8.759. [DOI] [PubMed] [Google Scholar]
- Cammack R., Rao K. K., Hall D. O. Metalloproteins in the evolution of photosynthesis. Biosystems. 1981;14(1):57–80. doi: 10.1016/0303-2647(81)90022-8. [DOI] [PubMed] [Google Scholar]
- Entsch B., Smillie R. M. Oxidation--reduction properties of phytoflavin, a flavoprotein from blue-green algae. Arch Biochem Biophys. 1972 Aug;151(2):378–386. doi: 10.1016/0003-9861(72)90512-7. [DOI] [PubMed] [Google Scholar]
- Fillat M. F., Borrias W. E., Weisbeek P. J. Isolation and overexpression in Escherichia coli of the flavodoxin gene from Anabaena PCC 7119. Biochem J. 1991 Nov 15;280(Pt 1):187–191. doi: 10.1042/bj2800187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M. P., Husain A., Rogers L. J. A constitutive flavodoxin from a eukaryotic alga. Biochem Biophys Res Commun. 1978 Mar 30;81(2):630–635. doi: 10.1016/0006-291x(78)91582-6. [DOI] [PubMed] [Google Scholar]
- Fukuyama K., Matsubara H., Rogers L. J. Crystal structure of oxidized flavodoxin from a red alga Chondrus crispus refined at 1.8 A resolution. Description of the flavin mononucleotide binding site. J Mol Biol. 1992 Jun 5;225(3):775–789. doi: 10.1016/0022-2836(92)90400-e. [DOI] [PubMed] [Google Scholar]
- Harauz G., Boekema E., van Heel M. Statistical image analysis of electron micrographs of ribosomal subunits. Methods Enzymol. 1988;164:35–49. doi: 10.1016/s0076-6879(88)64033-x. [DOI] [PubMed] [Google Scholar]
- Ke B. The primary electron acceptor of photosystem. I. Biochim Biophys Acta. 1973 Feb 12;301(1):1–33. doi: 10.1016/0304-4173(73)90010-4. [DOI] [PubMed] [Google Scholar]
- Knaff D. B., Hirasawa M. Ferredoxin-dependent chloroplast enzymes. Biochim Biophys Acta. 1991 Jan 22;1056(2):93–125. doi: 10.1016/s0005-2728(05)80277-4. [DOI] [PubMed] [Google Scholar]
- Kruip J., Boekema E. J., Bald D., Boonstra A. F., Rögner M. Isolation and structural characterization of monomeric and trimeric photosystem I complexes (P700.FA/FB and P700.FX) from the cyanobacterium Synechocystis PCC 6803. J Biol Chem. 1993 Nov 5;268(31):23353–23360. [PubMed] [Google Scholar]
- Laudenbach D. E., Reith M. E., Straus N. A. Isolation, sequence analysis, and transcriptional studies of the flavodoxin gene from Anacystis nidulans R2. J Bacteriol. 1988 Jan;170(1):258–265. doi: 10.1128/jb.170.1.258-265.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelong C., Sétif P., Lagoutte B., Bottin H. Identification of the amino acids involved in the functional interaction between photosystem I and ferredoxin from Synechocystis sp. PCC 6803 by chemical cross-linking. J Biol Chem. 1994 Apr 1;269(13):10034–10039. [PubMed] [Google Scholar]
- Leonhardt K., Straus N. A. An iron stress operon involved in photosynthetic electron transport in the marine cyanobacterium Synechococcus sp. PCC 7002. J Gen Microbiol. 1992 Aug;138(Pt 8):1613–1621. doi: 10.1099/00221287-138-8-1613. [DOI] [PubMed] [Google Scholar]
- Medina M., Hervás M., Navarro J. A., De la Rosa M. A., Gómez-Moreno C., Tollin G. A laser flash absorption spectroscopy study of Anabaena sp. PCC 7119 flavodoxin photoreduction by photosystem I particles from spinach. FEBS Lett. 1992 Nov 30;313(3):239–242. doi: 10.1016/0014-5793(92)81200-6. [DOI] [PubMed] [Google Scholar]
- Medina M., Peleato M. L., Mendez E., Gomez-Moreno C. Identification of specific carboxyl groups on Anabaena PCC 7119 flavodoxin which are involved in the interaction with ferredoxin-NADP+ reductase. Eur J Biochem. 1992 Feb 1;203(3):373–379. doi: 10.1111/j.1432-1033.1992.tb16560.x. [DOI] [PubMed] [Google Scholar]
- Müller F., Hemmerich P., Ehrenberg A., Palmer G., Massey V. The chemical and electronic structure of the neutral flavin radical as revealed by electron spin resonance spectroscopy of chemically and isotopically substituted derivatives. Eur J Biochem. 1970 May 1;14(1):185–196. doi: 10.1111/j.1432-1033.1970.tb00277.x. [DOI] [PubMed] [Google Scholar]
- Palma P. N., Moura I., LeGall J., Van Beeumen J., Wampler J. E., Moura J. J. Evidence for a ternary complex formed between flavodoxin and cytochrome c3: 1H-NMR and molecular modeling studies. Biochemistry. 1994 May 31;33(21):6394–6407. doi: 10.1021/bi00187a003. [DOI] [PubMed] [Google Scholar]
- Proceedings of the 8th European Bioenergetics Conference. Valencia, Spain, 12-17 September 1994. Biochim Biophys Acta. 1994 Aug 30;1187(2):99–276. [PubMed] [Google Scholar]
- Rousseau F., Sétif P., Lagoutte B. Evidence for the involvement of PSI-E subunit in the reduction of ferredoxin by photosystem I. EMBO J. 1993 May;12(5):1755–1765. doi: 10.1002/j.1460-2075.1993.tb05823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rögner M., Nixon P. J., Diner B. A. Purification and characterization of photosystem I and photosystem II core complexes from wild-type and phycocyanin-deficient strains of the cyanobacterium Synechocystis PCC 6803. J Biol Chem. 1990 Apr 15;265(11):6189–6196. [PubMed] [Google Scholar]
- Saxton W. O., Baumeister W. The correlation averaging of a regularly arranged bacterial cell envelope protein. J Microsc. 1982 Aug;127(Pt 2):127–138. doi: 10.1111/j.1365-2818.1982.tb00405.x. [DOI] [PubMed] [Google Scholar]
- Smith W. W., Burnett R. M., Darling G. D., Ludwig M. L. Structure of the semiquinone form of flavodoxin from Clostridum MP. Extension of 1.8 A resolution and some comparisons with the oxidized state. J Mol Biol. 1977 Nov 25;117(1):195–225. doi: 10.1016/0022-2836(77)90031-6. [DOI] [PubMed] [Google Scholar]
- Smith W. W., Pattridge K. A., Ludwig M. L., Petsko G. A., Tsernoglou D., Tanaka M., Yasunobu K. T. Structure of oxidized flavodoxin from Anacystis nidulans. J Mol Biol. 1983 Apr 25;165(4):737–753. doi: 10.1016/s0022-2836(83)80277-0. [DOI] [PubMed] [Google Scholar]
- Sétif P. Q., Bottin H. Laser flash absorption spectroscopy study of ferredoxin reduction by photosystem I in Synechocystis sp. PCC 6803: evidence for submicrosecond and microsecond kinetics. Biochemistry. 1994 Jul 19;33(28):8495–8504. doi: 10.1021/bi00194a014. [DOI] [PubMed] [Google Scholar]
- Sétif P. Q., Bottin H. Laser flash absorption spectroscopy study of ferredoxin reduction by photosystem I: spectral and kinetic evidence for the existence of several photosystem I-ferredoxin complexes. Biochemistry. 1995 Jul 18;34(28):9059–9070. doi: 10.1021/bi00028a015. [DOI] [PubMed] [Google Scholar]
- Watt W., Tulinsky A., Swenson R. P., Watenpaugh K. D. Comparison of the crystal structures of a flavodoxin in its three oxidation states at cryogenic temperatures. J Mol Biol. 1991 Mar 5;218(1):195–208. doi: 10.1016/0022-2836(91)90884-9. [DOI] [PubMed] [Google Scholar]
- Xu Q., Jung Y. S., Chitnis V. P., Guikema J. A., Golbeck J. H., Chitnis P. R. Mutational analysis of photosystem I polypeptides in Synechocystis sp. PCC 6803. Subunit requirements for reduction of NADP+ mediated by ferredoxin and flavodoxin. J Biol Chem. 1994 Aug 26;269(34):21512–21518. [PubMed] [Google Scholar]