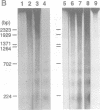
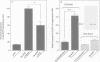
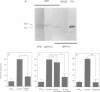
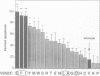
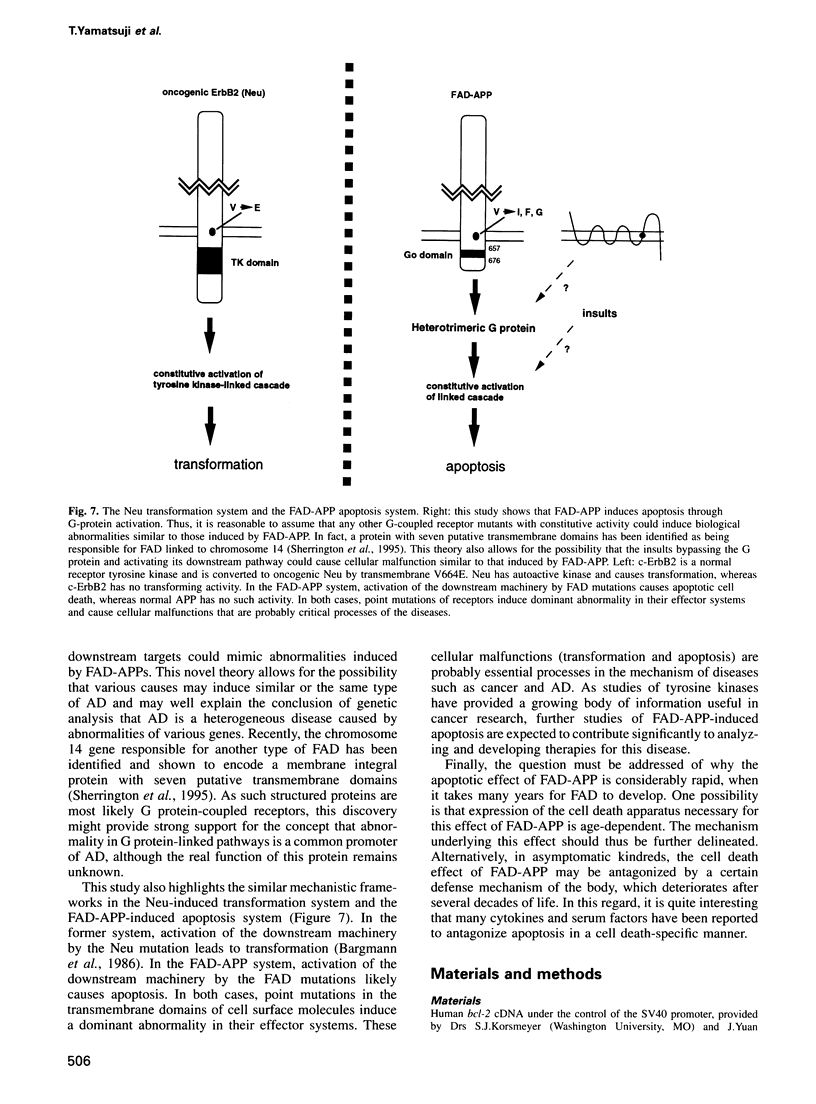
Abstract
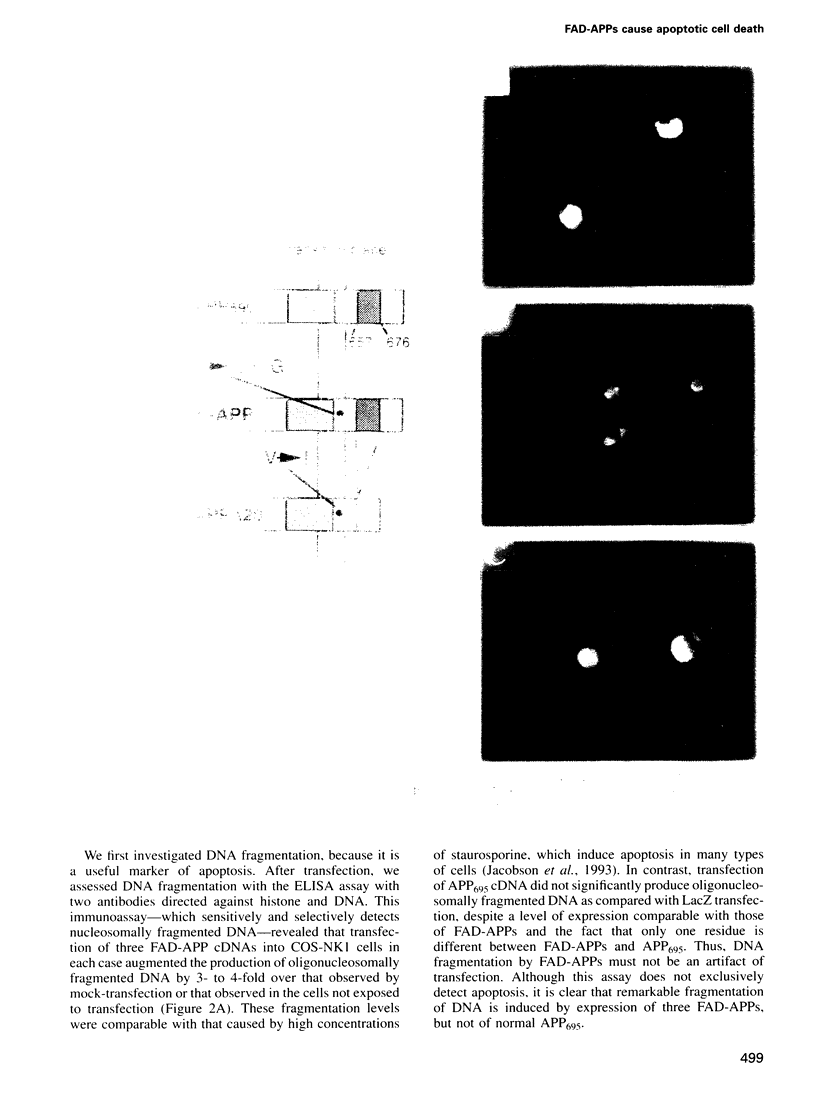
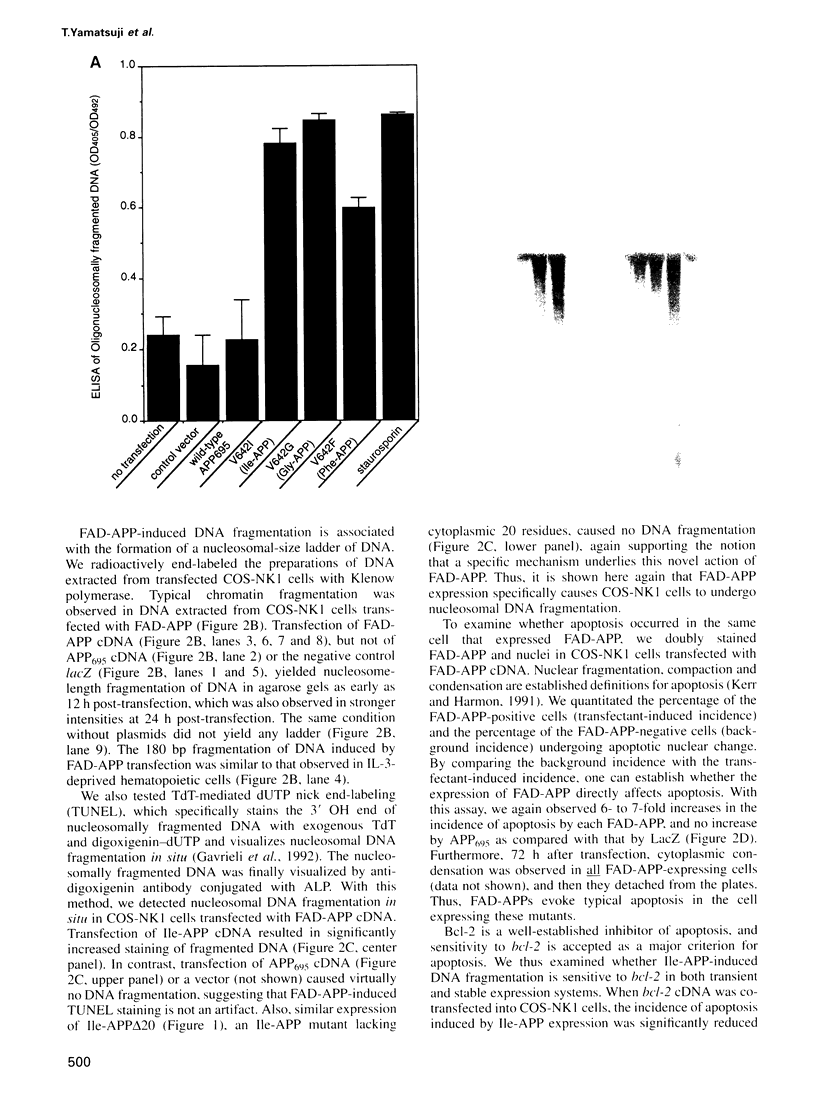
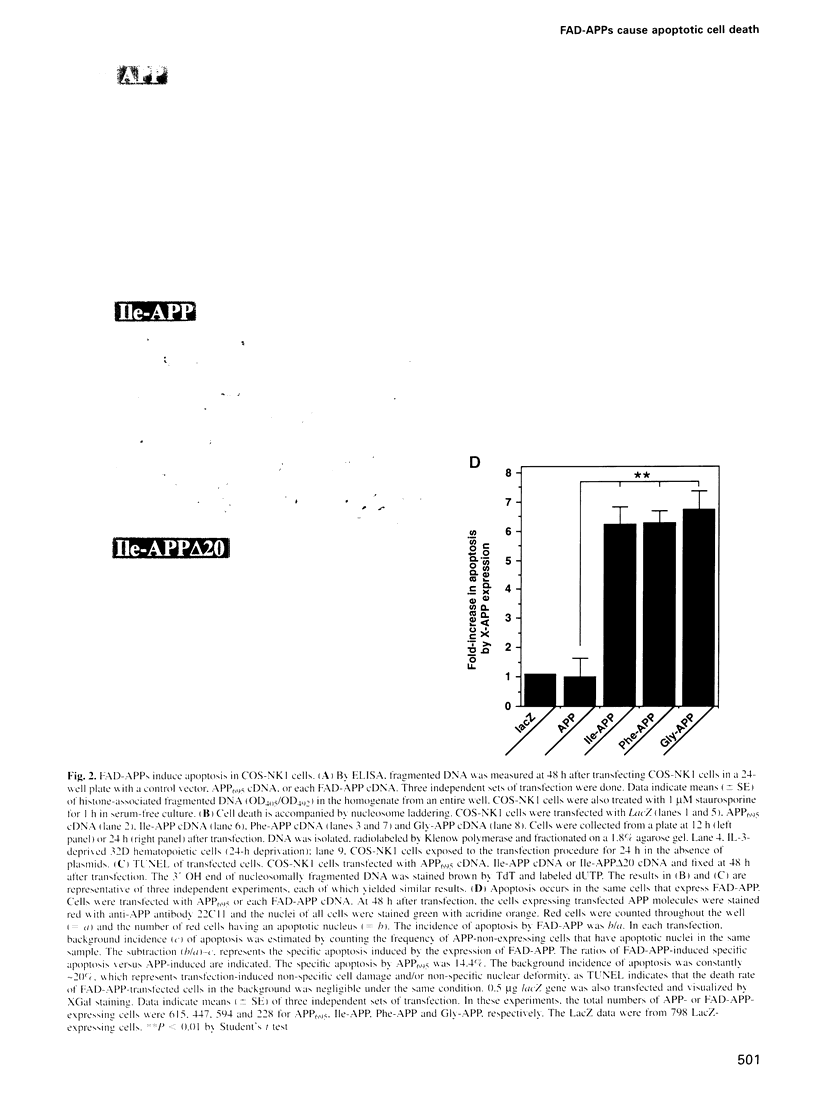
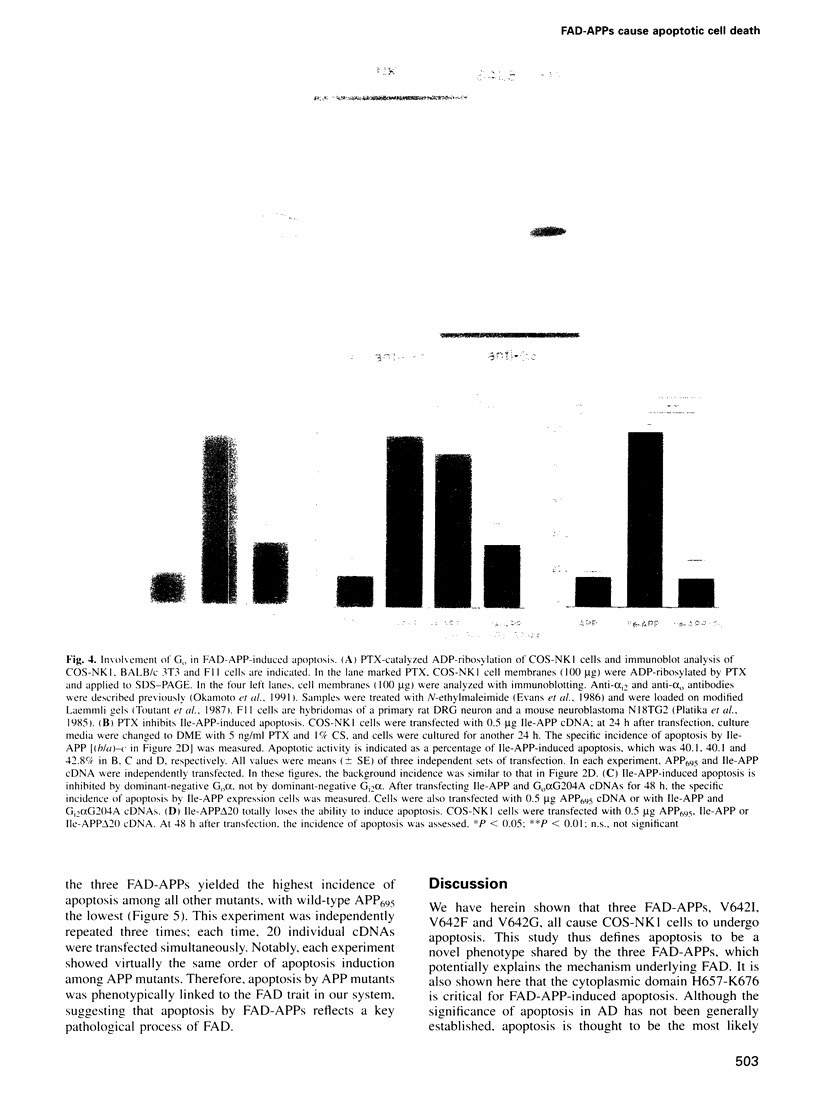
APP is a transmembrane precursor of beta-amyloid. In dominantly inherited familial Alzheimer's disease (FAD), point mutations V6421, V642F and V642G have been discovered in APP695. Here we show that expression of these mutants (FAD-APPs) causes a clone of COS cells to undergo apoptosis associated with DNA fragmentation. Apoptosis by the three FAD-APPs was the highest among all possible V642 mutants; normal APP695 had no effect on apoptosis, suggesting that apoptosis by APP mutants in this system is phenotypically linked to the FAD trait. FAD-APP-induced apoptosis was sensitive to bcl-2 and most probably mediated by heteromeric G proteins. This study presents a model system allowing analysis of the mechanism for FAD-APP-induced cytotoxicity.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baffy G., Yang L., Raj S., Manning D. R., Williamson J. R. G protein coupling to the thrombin receptor in Chinese hamster lung fibroblasts. J Biol Chem. 1994 Mar 18;269(11):8483–8487. [PubMed] [Google Scholar]
- Bargmann C. I., Hung M. C., Weinberg R. A. Multiple independent activations of the neu oncogene by a point mutation altering the transmembrane domain of p185. Cell. 1986 Jun 6;45(5):649–657. doi: 10.1016/0092-8674(86)90779-8. [DOI] [PubMed] [Google Scholar]
- Blass J. P., Baker A. C., Ko L., Sheu R. K., Black R. S. Expression of 'Alzheimer antigens' in cultured skin fibroblasts. Arch Neurol. 1991 Jul;48(7):709–717. doi: 10.1001/archneur.1991.00530190055016. [DOI] [PubMed] [Google Scholar]
- Cai X. D., Golde T. E., Younkin S. G. Release of excess amyloid beta protein from a mutant amyloid beta protein precursor. Science. 1993 Jan 22;259(5094):514–516. doi: 10.1126/science.8424174. [DOI] [PubMed] [Google Scholar]
- Doherty P., Ashton S. V., Moore S. E., Walsh F. S. Morphoregulatory activities of NCAM and N-cadherin can be accounted for by G protein-dependent activation of L- and N-type neuronal Ca2+ channels. Cell. 1991 Oct 4;67(1):21–33. doi: 10.1016/0092-8674(91)90569-k. [DOI] [PubMed] [Google Scholar]
- Evans T., Brown M. L., Fraser E. D., Northup J. K. Purification of the major GTP-binding proteins from human placental membranes. J Biol Chem. 1986 May 25;261(15):7052–7059. [PubMed] [Google Scholar]
- Games D., Adams D., Alessandrini R., Barbour R., Berthelette P., Blackwell C., Carr T., Clemens J., Donaldson T., Gillespie F. Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature. 1995 Feb 9;373(6514):523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson G. E., Toral-Barza L. Cytosolic free calcium in lymphoblasts from young, aged and Alzheimer subjects. Mech Ageing Dev. 1992 Mar 15;63(1):1–9. doi: 10.1016/0047-6374(92)90012-3. [DOI] [PubMed] [Google Scholar]
- Goate A., Chartier-Harlin M. C., Mullan M., Brown J., Crawford F., Fidani L., Giuffra L., Haynes A., Irving N., James L. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature. 1991 Feb 21;349(6311):704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- Goh J. W., Pennefather P. S. A pertussis toxin-sensitive G protein in hippocampal long-term potentiation. Science. 1989 May 26;244(4907):980–983. doi: 10.1126/science.2543072. [DOI] [PubMed] [Google Scholar]
- Granneman J. G., Kapatos G. Developmental expression of Go in neuronal cultures from rat mesencephalon and hypothalamus. J Neurochem. 1990 Jun;54(6):1995–2001. doi: 10.1111/j.1471-4159.1990.tb04903.x. [DOI] [PubMed] [Google Scholar]
- Guillén A., Jallon J. M., Fehrentz J. A., Pantaloni C., Bockaert J., Homburger V. A Go-like protein in Drosophila melanogaster and its expression in memory mutants. EMBO J. 1990 May;9(5):1449–1455. doi: 10.1002/j.1460-2075.1990.tb08261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J. Framing beta-amyloid. Nat Genet. 1992 Jul;1(4):233–234. doi: 10.1038/ng0792-233. [DOI] [PubMed] [Google Scholar]
- Hayashi Y., Kashiwagi K., Yoshikawa K. Protease inhibitors generate cytotoxic fragments from Alzheimer amyloid protein precursor in cDNA-transfected glioma cells. Biochem Biophys Res Commun. 1992 Sep 30;187(3):1249–1255. doi: 10.1016/0006-291x(92)90437-p. [DOI] [PubMed] [Google Scholar]
- Hermouet S., Merendino J. J., Jr, Gutkind J. S., Spiegel A. M. Activating and inactivating mutations of the alpha subunit of Gi2 protein have opposite effects on proliferation of NIH 3T3 cells. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10455–10459. doi: 10.1073/pnas.88.23.10455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hescheler J., Rosenthal W., Trautwein W., Schultz G. The GTP-binding protein, Go, regulates neuronal calcium channels. 1987 Jan 29-Feb 4Nature. 325(6103):445–447. doi: 10.1038/325445a0. [DOI] [PubMed] [Google Scholar]
- Huff R. M., Axton J. M., Neer E. J. Physical and immunological characterization of a guanine nucleotide-binding protein purified from bovine cerebral cortex. J Biol Chem. 1985 Sep 5;260(19):10864–10871. [PubMed] [Google Scholar]
- Igarashi M., Strittmatter S. M., Vartanian T., Fishman M. C. Mediation by G proteins of signals that cause collapse of growth cones. Science. 1993 Jan 1;259(5091):77–79. doi: 10.1126/science.8418498. [DOI] [PubMed] [Google Scholar]
- Ito E., Oka K., Etcheberrigaray R., Nelson T. J., McPhie D. L., Tofel-Grehl B., Gibson G. E., Alkon D. L. Internal Ca2+ mobilization is altered in fibroblasts from patients with Alzheimer disease. Proc Natl Acad Sci U S A. 1994 Jan 18;91(2):534–538. doi: 10.1073/pnas.91.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito I., Okada D., Sugiyama H. Pertussis toxin suppresses long-term potentiation of hippocampal mossy fiber synapses. Neurosci Lett. 1988 Jul 19;90(1-2):181–185. doi: 10.1016/0304-3940(88)90808-7. [DOI] [PubMed] [Google Scholar]
- Iwatsubo T., Odaka A., Suzuki N., Mizusawa H., Nukina N., Ihara Y. Visualization of A beta 42(43) and A beta 40 in senile plaques with end-specific A beta monoclonals: evidence that an initially deposited species is A beta 42(43). Neuron. 1994 Jul;13(1):45–53. doi: 10.1016/0896-6273(94)90458-8. [DOI] [PubMed] [Google Scholar]
- Jacobson M. D., Burne J. F., King M. P., Miyashita T., Reed J. C., Raff M. C. Bcl-2 blocks apoptosis in cells lacking mitochondrial DNA. Nature. 1993 Jan 28;361(6410):365–369. doi: 10.1038/361365a0. [DOI] [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987 Feb 19;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Karlinsky H., Vaula G., Haines J. L., Ridgley J., Bergeron C., Mortilla M., Tupler R. G., Percy M. E., Robitaille Y., Noldy N. E. Molecular and prospective phenotypic characterization of a pedigree with familial Alzheimer's disease and a missense mutation in codon 717 of the beta-amyloid precursor protein gene. Neurology. 1992 Aug;42(8):1445–1453. doi: 10.1212/wnl.42.8.1445. [DOI] [PubMed] [Google Scholar]
- Lassmann H., Bancher C., Breitschopf H., Wegiel J., Bobinski M., Jellinger K., Wisniewski H. M. Cell death in Alzheimer's disease evaluated by DNA fragmentation in situ. Acta Neuropathol. 1995;89(1):35–41. doi: 10.1007/BF00294257. [DOI] [PubMed] [Google Scholar]
- Mattson M. P., Barger S. W., Cheng B., Lieberburg I., Smith-Swintosky V. L., Rydel R. E. beta-Amyloid precursor protein metabolites and loss of neuronal Ca2+ homeostasis in Alzheimer's disease. Trends Neurosci. 1993 Oct;16(10):409–414. doi: 10.1016/0166-2236(93)90009-b. [DOI] [PubMed] [Google Scholar]
- Milward E. A., Papadopoulos R., Fuller S. J., Moir R. D., Small D., Beyreuther K., Masters C. L. The amyloid protein precursor of Alzheimer's disease is a mediator of the effects of nerve growth factor on neurite outgrowth. Neuron. 1992 Jul;9(1):129–137. doi: 10.1016/0896-6273(92)90228-6. [DOI] [PubMed] [Google Scholar]
- Miura M., Zhu H., Rotello R., Hartwieg E. A., Yuan J. Induction of apoptosis in fibroblasts by IL-1 beta-converting enzyme, a mammalian homolog of the C. elegans cell death gene ced-3. Cell. 1993 Nov 19;75(4):653–660. doi: 10.1016/0092-8674(93)90486-a. [DOI] [PubMed] [Google Scholar]
- Molchan S. E., Manji H., Chen G., Dou L., Little J., Potter W. Z., Sunderland T. Effects of chronic lithium treatment on platelet PKC isozymes in Alzheimer's and elderly control subjects. Neurosci Lett. 1993 Nov 12;162(1-2):187–191. doi: 10.1016/0304-3940(93)90592-9. [DOI] [PubMed] [Google Scholar]
- Mulheron J. G., Casañas S. J., Arthur J. M., Garnovskaya M. N., Gettys T. W., Raymond J. R. Human 5-HT1A receptor expressed in insect cells activates endogenous G(o)-like G protein(s). J Biol Chem. 1994 Apr 29;269(17):12954–12962. [PubMed] [Google Scholar]
- Mönning U., König G., Banati R. B., Mechler H., Czech C., Gehrmann J., Schreiter-Gasser U., Masters C. L., Beyreuther K. Alzheimer beta A4-amyloid protein precursor in immunocompetent cells. J Biol Chem. 1992 Nov 25;267(33):23950–23956. [PubMed] [Google Scholar]
- Müller U., Cristina N., Li Z. W., Wolfer D. P., Lipp H. P., Rülicke T., Brandner S., Aguzzi A., Weissmann C. Behavioral and anatomical deficits in mice homozygous for a modified beta-amyloid precursor protein gene. Cell. 1994 Dec 2;79(5):755–765. doi: 10.1016/0092-8674(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Nishimoto I., Okamoto T., Matsuura Y., Takahashi S., Okamoto T., Murayama Y., Ogata E. Alzheimer amyloid protein precursor complexes with brain GTP-binding protein G(o) Nature. 1993 Mar 4;362(6415):75–79. doi: 10.1038/362075a0. [DOI] [PubMed] [Google Scholar]
- Okamoto T., Takeda S., Murayama Y., Ogata E., Nishimoto I. Ligand-dependent G protein coupling function of amyloid transmembrane precursor. J Biol Chem. 1995 Mar 3;270(9):4205–4208. doi: 10.1074/jbc.270.9.4205. [DOI] [PubMed] [Google Scholar]
- Olate J., Jorquera H., Purcell P., Codina J., Birnbaumer L., Allende J. E. Molecular cloning and sequence determination of a cDNA coding for the alpha-subunit of a Go-type protein of Xenopus laevis oocytes. FEBS Lett. 1989 Feb 13;244(1):188–192. doi: 10.1016/0014-5793(89)81190-1. [DOI] [PubMed] [Google Scholar]
- Peterson C., Goldman J. E. Alterations in calcium content and biochemical processes in cultured skin fibroblasts from aged and Alzheimer donors. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2758–2762. doi: 10.1073/pnas.83.8.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platika D., Boulos M. H., Baizer L., Fishman M. C. Neuronal traits of clonal cell lines derived by fusion of dorsal root ganglia neurons with neuroblastoma cells. Proc Natl Acad Sci U S A. 1985 May;82(10):3499–3503. doi: 10.1073/pnas.82.10.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rösl F. A simple and rapid method for detection of apoptosis in human cells. Nucleic Acids Res. 1992 Oct 11;20(19):5243–5243. doi: 10.1093/nar/20.19.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandbrink R., Masters C. L., Beyreuther K. Beta A4-amyloid protein precursor mRNA isoforms without exon 15 are ubiquitously expressed in rat tissues including brain, but not in neurons. J Biol Chem. 1994 Jan 14;269(2):1510–1517. [PubMed] [Google Scholar]
- Schubert W., Prior R., Weidemann A., Dircksen H., Multhaup G., Masters C. L., Beyreuther K. Localization of Alzheimer beta A4 amyloid precursor protein at central and peripheral synaptic sites. Brain Res. 1991 Nov 1;563(1-2):184–194. doi: 10.1016/0006-8993(91)91532-6. [DOI] [PubMed] [Google Scholar]
- Schuch U., Lohse M. J., Schachner M. Neural cell adhesion molecules influence second messenger systems. Neuron. 1989 Jul;3(1):13–20. doi: 10.1016/0896-6273(89)90111-6. [DOI] [PubMed] [Google Scholar]
- Sebok K., Woodside D., al-Aoukaty A., Ho A. D., Gluck S., Maghazachi A. A. IL-8 induces the locomotion of human IL-2-activated natural killer cells. Involvement of a guanine nucleotide binding (Go) protein. J Immunol. 1993 Feb 15;150(4):1524–1534. [PubMed] [Google Scholar]
- Sherrington R., Rogaev E. I., Liang Y., Rogaeva E. A., Levesque G., Ikeda M., Chi H., Lin C., Li G., Holman K. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer's disease. Nature. 1995 Jun 29;375(6534):754–760. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- Slepak V. Z., Wilkie T. M., Simon M. I. Mutational analysis of G protein alpha subunit G(o) alpha expressed in Escherichia coli. J Biol Chem. 1993 Jan 15;268(2):1414–1423. [PubMed] [Google Scholar]
- Small D. H., Nurcombe V., Reed G., Clarris H., Moir R., Beyreuther K., Masters C. L. A heparin-binding domain in the amyloid protein precursor of Alzheimer's disease is involved in the regulation of neurite outgrowth. J Neurosci. 1994 Apr;14(4):2117–2127. doi: 10.1523/JNEUROSCI.14-04-02117.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spicher K., Klinz F. J., Rudolph U., Codina J., Birnbaumer L., Schultz G., Rosenthal W. Identification of the G-protein alpha-subunit encoded by alpha o2 cDNA as a 39 kDa pertussis toxin substrate. Biochem Biophys Res Commun. 1991 Mar 15;175(2):473–479. doi: 10.1016/0006-291x(91)91588-4. [DOI] [PubMed] [Google Scholar]
- Strittmatter S. M., Fishman M. C., Zhu X. P. Activated mutants of the alpha subunit of G(o) promote an increased number of neurites per cell. J Neurosci. 1994 Apr;14(4):2327–2338. doi: 10.1523/JNEUROSCI.14-04-02327.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter S. M., Valenzuela D., Kennedy T. E., Neer E. J., Fishman M. C. G0 is a major growth cone protein subject to regulation by GAP-43. Nature. 1990 Apr 26;344(6269):836–841. doi: 10.1038/344836a0. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Cheung T. T., Cai X. D., Odaka A., Otvos L., Jr, Eckman C., Golde T. E., Younkin S. G. An increased percentage of long amyloid beta protein secreted by familial amyloid beta protein precursor (beta APP717) mutants. Science. 1994 May 27;264(5163):1336–1340. doi: 10.1126/science.8191290. [DOI] [PubMed] [Google Scholar]
- Tamaoka A., Odaka A., Ishibashi Y., Usami M., Sahara N., Suzuki N., Nukina N., Mizusawa H., Shoji S., Kanazawa I. APP717 missense mutation affects the ratio of amyloid beta protein species (A beta 1-42/43 and a beta 1-40) in familial Alzheimer's disease brain. J Biol Chem. 1994 Dec 30;269(52):32721–32724. [PubMed] [Google Scholar]
- Toutant M., Aunis D., Bockaert J., Homburger V., Rouot B. Presence of three pertussis toxin substrates and Go alpha immunoreactivity in both plasma and granule membranes of chromaffin cells. FEBS Lett. 1987 May 11;215(2):339–344. doi: 10.1016/0014-5793(87)80174-6. [DOI] [PubMed] [Google Scholar]
- Uéda K., Cole G., Sundsmo M., Katzman R., Saitoh T. Decreased adhesiveness of Alzheimer's disease fibroblasts: is amyloid beta-protein precursor involved? Ann Neurol. 1989 Mar;25(3):246–251. doi: 10.1002/ana.410250307. [DOI] [PubMed] [Google Scholar]
- Van Huynh T., Cole G., Katzman R., Huang K. P., Saitoh T. Reduced protein kinase C immunoreactivity and altered protein phosphorylation in Alzheimer's disease fibroblasts. Arch Neurol. 1989 Nov;46(11):1195–1199. doi: 10.1001/archneur.1989.00520470049026. [DOI] [PubMed] [Google Scholar]
- Yoshikawa K., Aizawa T., Hayashi Y. Degeneration in vitro of post-mitotic neurons overexpressing the Alzheimer amyloid protein precursor. Nature. 1992 Sep 3;359(6390):64–67. doi: 10.1038/359064a0. [DOI] [PubMed] [Google Scholar]
- Zheng H., Jiang M., Trumbauer M. E., Sirinathsinghji D. J., Hopkins R., Smith D. W., Heavens R. P., Dawson G. R., Boyce S., Conner M. W. beta-Amyloid precursor protein-deficient mice show reactive gliosis and decreased locomotor activity. Cell. 1995 May 19;81(4):525–531. doi: 10.1016/0092-8674(95)90073-x. [DOI] [PubMed] [Google Scholar]