Abstract
We have observed that stimulation of human natural killer cells with dibutyryl cAMP (Bt2cAMP) reproduced the effects of ADP ribosylation of the GTP binding protein RhoA by Clostridium botulinum C3 transferase: both agents induced similar morphological changes, inhibited cell motility and blocked the cytolytic function. We demonstrate here that cAMP-dependent protein kinase A (PKA) phosphorylates RhoA in its C-terminal region, on serine residue 188. This phosphorylation does not affect the ability of recombinant RhoA to bind guanine nucleotides, nor does it modify its intrinsic GTPase activity. However, treatment of cells with Bt2cAMP results in the translocation of membrane-associated RhoA towards the cytosol. Experiments using purified membrane preparations indicated that Rho-GDP dissociation inhibitor, which can complex phosphorylated RhoA in its GTP-bound state, was the effector of this translocation. Taken together, these data suggest that PKA phosphorylation of RhoA is a central event in mediating the cellular effects of cAMP, and support the existence of an alternative pathway for terminating RhoA signalling whereby GTP-bound RhoA, when phosphorylated, could be separated from its putative effector(s) independently of its GTP/GDP cycling.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo A., Webb M. R., Grogan A., Segal A. W. Activation of NADPH oxidase involves the dissociation of p21rac from its inhibitory GDP/GTP exchange protein (rhoGDI) followed by its translocation to the plasma membrane. Biochem J. 1994 Mar 15;298(Pt 3):585–591. doi: 10.1042/bj2980585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamson P., Marshall C. J., Hall A., Tilbrook P. A. Post-translational modifications of p21rho proteins. J Biol Chem. 1992 Oct 5;267(28):20033–20038. [PubMed] [Google Scholar]
- Aktories K., Rösener S., Blaschke U., Chhatwal G. S. Botulinum ADP-ribosyltransferase C3. Purification of the enzyme and characterization of the ADP-ribosylation reaction in platelet membranes. Eur J Biochem. 1988 Mar 1;172(2):445–450. doi: 10.1111/j.1432-1033.1988.tb13908.x. [DOI] [PubMed] [Google Scholar]
- Araki S., Kikuchi A., Hata Y., Isomura M., Takai Y. Regulation of reversible binding of smg p25A, a ras p21-like GTP-binding protein, to synaptic plasma membranes and vesicles by its specific regulatory protein, GDP dissociation inhibitor. J Biol Chem. 1990 Aug 5;265(22):13007–13015. [PubMed] [Google Scholar]
- Ashby B., Daniel J. L., Smith J. B. Mechanisms of platelet activation and inhibition. Hematol Oncol Clin North Am. 1990 Feb;4(1):1–26. [PubMed] [Google Scholar]
- Bailly E., McCaffrey M., Touchot N., Zahraoui A., Goud B., Bornens M. Phosphorylation of two small GTP-binding proteins of the Rab family by p34cdc2. Nature. 1991 Apr 25;350(6320):715–718. doi: 10.1038/350715a0. [DOI] [PubMed] [Google Scholar]
- Bokoch G. M., Knaus U. G. Ras-related GTP-binding proteins and leukocyte signal transduction. Curr Opin Hematol. 1994 Jan;1(1):53–60. [PubMed] [Google Scholar]
- Bourmeyster N., Stasia M. J., Garin J., Gagnon J., Boquet P., Vignais P. V. Copurification of rho protein and the rho-GDP dissociation inhibitor from bovine neutrophil cytosol. Effect of phosphoinositides on rho ADP-ribosylation by the C3 exoenzyme of Clostridium botulinum. Biochemistry. 1992 Dec 29;31(51):12863–12869. doi: 10.1021/bi00166a022. [DOI] [PubMed] [Google Scholar]
- Chardin P., Boquet P., Madaule P., Popoff M. R., Rubin E. J., Gill D. M. The mammalian G protein rhoC is ADP-ribosylated by Clostridium botulinum exoenzyme C3 and affects actin microfilaments in Vero cells. EMBO J. 1989 Apr;8(4):1087–1092. doi: 10.1002/j.1460-2075.1989.tb03477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavrier P., Parton R. G., Hauri H. P., Simons K., Zerial M. Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell. 1990 Jul 27;62(2):317–329. doi: 10.1016/0092-8674(90)90369-p. [DOI] [PubMed] [Google Scholar]
- Chijiwa T., Mishima A., Hagiwara M., Sano M., Hayashi K., Inoue T., Naito K., Toshioka T., Hidaka H. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J Biol Chem. 1990 Mar 25;265(9):5267–5272. [PubMed] [Google Scholar]
- Chuang T. H., Xu X., Knaus U. G., Hart M. J., Bokoch G. M. GDP dissociation inhibitor prevents intrinsic and GTPase activating protein-stimulated GTP hydrolysis by the Rac GTP-binding protein. J Biol Chem. 1993 Jan 15;268(2):775–778. [PubMed] [Google Scholar]
- Dickson B., Sprenger F., Morrison D., Hafen E. Raf functions downstream of Ras1 in the Sevenless signal transduction pathway. Nature. 1992 Dec 10;360(6404):600–603. doi: 10.1038/360600a0. [DOI] [PubMed] [Google Scholar]
- Downey G. P., Elson E. L., Schwab B., 3rd, Erzurum S. C., Young S. K., Worthen G. S. Biophysical properties and microfilament assembly in neutrophils: modulation by cyclic AMP. J Cell Biol. 1991 Sep;114(6):1179–1190. doi: 10.1083/jcb.114.6.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downward J. Signal transduction. Rac and Rho in tune. Nature. 1992 Sep 24;359(6393):273–274. doi: 10.1038/359273a0. [DOI] [PubMed] [Google Scholar]
- Fukumoto Y., Kaibuchi K., Hori Y., Fujioka H., Araki S., Ueda T., Kikuchi A., Takai Y. Molecular cloning and characterization of a novel type of regulatory protein (GDI) for the rho proteins, ras p21-like small GTP-binding proteins. Oncogene. 1990 Sep;5(9):1321–1328. [PubMed] [Google Scholar]
- Garrett M. D., Kabcenell A. K., Zahner J. E., Kaibuchi K., Sasaki T., Takai Y., Cheney C. M., Novick P. J. Interaction of Sec4 with GDI proteins from bovine brain, Drosophila melanogaster and Saccharomyces cerevisiae. Conservation of GDI membrane dissociation activity. FEBS Lett. 1993 Oct 4;331(3):233–238. doi: 10.1016/0014-5793(93)80343-s. [DOI] [PubMed] [Google Scholar]
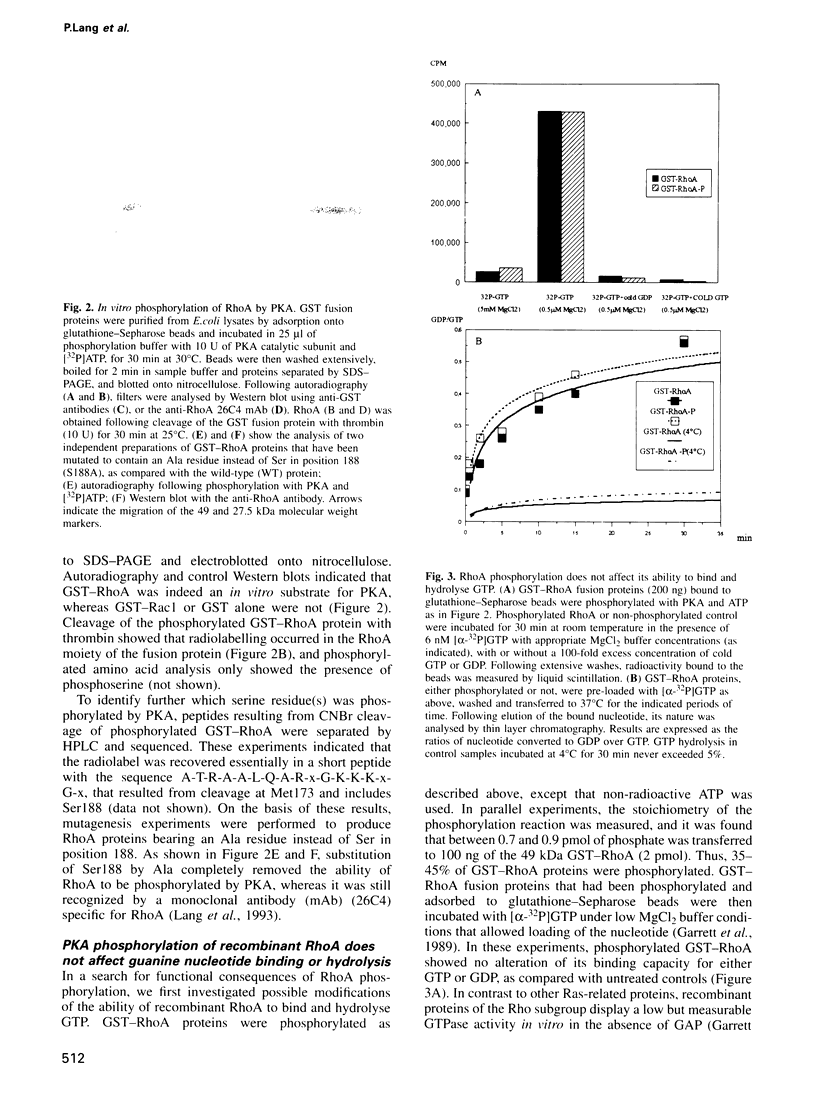
- Garrett M. D., Self A. J., van Oers C., Hall A. Identification of distinct cytoplasmic targets for ras/R-ras and rho regulatory proteins. J Biol Chem. 1989 Jan 5;264(1):10–13. [PubMed] [Google Scholar]
- Goto T., Herberman R. B., Maluish A., Strong D. M. Cyclic AMP as a mediator of prostaglandin E-induced suppression of human natural killer cell activity. J Immunol. 1983 Mar;130(3):1350–1355. [PubMed] [Google Scholar]
- Hall A. The cellular functions of small GTP-binding proteins. Science. 1990 Aug 10;249(4969):635–640. doi: 10.1126/science.2116664. [DOI] [PubMed] [Google Scholar]
- Hancock J. F., Cadwallader K., Marshall C. J. Methylation and proteolysis are essential for efficient membrane binding of prenylated p21K-ras(B). EMBO J. 1991 Mar;10(3):641–646. doi: 10.1002/j.1460-2075.1991.tb07992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock J. F., Hall A. A novel role for RhoGDI as an inhibitor of GAP proteins. EMBO J. 1993 May;12(5):1915–1921. doi: 10.1002/j.1460-2075.1993.tb05840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori Y., Kikuchi A., Isomura M., Katayama M., Miura Y., Fujioka H., Kaibuchi K., Takai Y. Post-translational modifications of the C-terminal region of the rho protein are important for its interaction with membranes and the stimulatory and inhibitory GDP/GTP exchange proteins. Oncogene. 1991 Apr;6(4):515–522. [PubMed] [Google Scholar]
- Isomura M., Kikuchi A., Ohga N., Takai Y. Regulation of binding of rhoB p20 to membranes by its specific regulatory protein, GDP dissociation inhibitor. Oncogene. 1991 Jan;6(1):119–124. [PubMed] [Google Scholar]
- Jalink K., van Corven E. J., Hengeveld T., Morii N., Narumiya S., Moolenaar W. H. Inhibition of lysophosphatidate- and thrombin-induced neurite retraction and neuronal cell rounding by ADP ribosylation of the small GTP-binding protein Rho. J Cell Biol. 1994 Aug;126(3):801–810. doi: 10.1083/jcb.126.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karniguian A., Zahraoui A., Tavitian A. Identification of small GTP-binding rab proteins in human platelets: thrombin-induced phosphorylation of rab3B, rab6, and rab8 proteins. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7647–7651. doi: 10.1073/pnas.90.16.7647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawata M., Kikuchi A., Hoshijima M., Yamamoto K., Hashimoto E., Yamamura H., Takai Y. Phosphorylation of smg p21, a ras p21-like GTP-binding protein, by cyclic AMP-dependent protein kinase in a cell-free system and in response to prostaglandin E1 in intact human platelets. J Biol Chem. 1989 Sep 15;264(26):15688–15695. [PubMed] [Google Scholar]
- Knaus U. G., Heyworth P. G., Kinsella B. T., Curnutte J. T., Bokoch G. M. Purification and characterization of Rac 2. A cytosolic GTP-binding protein that regulates human neutrophil NADPH oxidase. J Biol Chem. 1992 Nov 25;267(33):23575–23582. [PubMed] [Google Scholar]
- Lamb N. J., Fernandez A., Conti M. A., Adelstein R., Glass D. B., Welch W. J., Feramisco J. R. Regulation of actin microfilament integrity in living nonmuscle cells by the cAMP-dependent protein kinase and the myosin light chain kinase. J Cell Biol. 1988 Jun;106(6):1955–1971. doi: 10.1083/jcb.106.6.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang P., Gesbert F., Thiberge J. M., Troalen F., Dutartre H., Chavrier P., Bertoglio J. Characterization of a monoclonal antibody specific for the Ras-related GTP-binding protein Rho A. Biochem Biophys Res Commun. 1993 Nov 15;196(3):1522–1528. doi: 10.1006/bbrc.1993.2424. [DOI] [PubMed] [Google Scholar]
- Lang P., Guizani L., Vitté-Mony I., Stancou R., Dorseuil O., Gacon G., Bertoglio J. ADP-ribosylation of the ras-related, GTP-binding protein RhoA inhibits lymphocyte-mediated cytotoxicity. J Biol Chem. 1992 Jun 15;267(17):11677–11680. [PubMed] [Google Scholar]
- Lapetina E. G., Lacal J. C., Reep B. R., Molina y Vedia L. A ras-related protein is phosphorylated and translocated by agonists that increase cAMP levels in human platelets. Proc Natl Acad Sci U S A. 1989 May;86(9):3131–3134. doi: 10.1073/pnas.86.9.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerosey I., Pizon V., Tavitian A., de Gunzburg J. The cAMP-dependent protein kinase phosphorylates the rap1 protein in vitro as well as in intact fibroblasts, but not the closely related rap2 protein. Biochem Biophys Res Commun. 1991 Mar 15;175(2):430–436. doi: 10.1016/0006-291x(91)91582-w. [DOI] [PubMed] [Google Scholar]
- Lowy D. R., Willumsen B. M. Function and regulation of ras. Annu Rev Biochem. 1993;62:851–891. doi: 10.1146/annurev.bi.62.070193.004223. [DOI] [PubMed] [Google Scholar]
- Manser E., Leung T., Salihuddin H., Zhao Z. S., Lim L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature. 1994 Jan 6;367(6458):40–46. doi: 10.1038/367040a0. [DOI] [PubMed] [Google Scholar]
- Nelson W. J. Regulation of cell surface polarity from bacteria to mammals. Science. 1992 Nov 6;258(5084):948–955. doi: 10.1126/science.1439806. [DOI] [PubMed] [Google Scholar]
- Nemoto Y., Namba T., Teru-uchi T., Ushikubi F., Morii N., Narumiya S. A rho gene product in human blood platelets. I. Identification of the platelet substrate for botulinum C3 ADP-ribosyltransferase as rhoA protein. J Biol Chem. 1992 Oct 15;267(29):20916–20920. [PubMed] [Google Scholar]
- Nemoto Y., Namba T., Teru-uchi T., Ushikubi F., Morii N., Narumiya S. A rho gene product in human blood platelets. I. Identification of the platelet substrate for botulinum C3 ADP-ribosyltransferase as rhoA protein. J Biol Chem. 1992 Oct 15;267(29):20916–20920. [PubMed] [Google Scholar]
- Nishiki T., Narumiya S., Morii N., Yamamoto M., Fujiwara M., Kamata Y., Sakaguchi G., Kozaki S. ADP-ribosylation of the rho/rac proteins induces growth inhibition, neurite outgrowth and acetylcholine esterase in cultured PC-12 cells. Biochem Biophys Res Commun. 1990 Feb 28;167(1):265–272. doi: 10.1016/0006-291x(90)91760-p. [DOI] [PubMed] [Google Scholar]
- Novick P., Brennwald P. Friends and family: the role of the Rab GTPases in vesicular traffic. Cell. 1993 Nov 19;75(4):597–601. doi: 10.1016/0092-8674(93)90478-9. [DOI] [PubMed] [Google Scholar]
- Ojcius D. M., Zheng L. M., Sphicas E. C., Zychlinsky A., Young J. D. Subcellular localization of perforin and serine esterase in lymphokine-activated killer cells and cytotoxic T cells by immunogold labeling. J Immunol. 1991 Jun 15;146(12):4427–4432. [PubMed] [Google Scholar]
- Philips M. R., Pillinger M. H., Staud R., Volker C., Rosenfeld M. G., Weissmann G., Stock J. B. Carboxyl methylation of Ras-related proteins during signal transduction in neutrophils. Science. 1993 Feb 12;259(5097):977–980. doi: 10.1126/science.8438158. [DOI] [PubMed] [Google Scholar]
- Quilliam L. A., Mueller H., Bohl B. P., Prossnitz V., Sklar L. A., Der C. J., Bokoch G. M. Rap1A is a substrate for cyclic AMP-dependent protein kinase in human neutrophils. J Immunol. 1991 Sep 1;147(5):1628–1635. [PubMed] [Google Scholar]
- Quinn M. T., Evans T., Loetterle L. R., Jesaitis A. J., Bokoch G. M. Translocation of Rac correlates with NADPH oxidase activation. Evidence for equimolar translocation of oxidase components. J Biol Chem. 1993 Oct 5;268(28):20983–20987. [PubMed] [Google Scholar]
- ROSE G. G., POMERAT C. M., SHINDLER T. O., TRUNNELL J. B. A cellophane-strip technique for culturing tissue in multipurpose culture chambers. J Biophys Biochem Cytol. 1958 Nov 25;4(6):761–764. doi: 10.1083/jcb.4.6.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley A. J., Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992 Aug 7;70(3):389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley A. J., Paterson H. F., Johnston C. L., Diekmann D., Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992 Aug 7;70(3):401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Rubin E. J., Gill D. M., Boquet P., Popoff M. R. Functional modification of a 21-kilodalton G protein when ADP-ribosylated by exoenzyme C3 of Clostridium botulinum. Mol Cell Biol. 1988 Jan;8(1):418–426. doi: 10.1128/mcb.8.1.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T., Kato M., Takai Y. Consequences of weak interaction of rho GDI with the GTP-bound forms of rho p21 and rac p21. J Biol Chem. 1993 Nov 15;268(32):23959–23963. [PubMed] [Google Scholar]
- Sekine A., Fujiwara M., Narumiya S. Asparagine residue in the rho gene product is the modification site for botulinum ADP-ribosyltransferase. J Biol Chem. 1989 May 25;264(15):8602–8605. [PubMed] [Google Scholar]
- Self A. J., Paterson H. F., Hall A. Different structural organization of Ras and Rho effector domains. Oncogene. 1993 Mar;8(3):655–661. [PubMed] [Google Scholar]
- Smeal T., Binetruy B., Mercola D. A., Birrer M., Karin M. Oncogenic and transcriptional cooperation with Ha-Ras requires phosphorylation of c-Jun on serines 63 and 73. Nature. 1991 Dec 12;354(6353):494–496. doi: 10.1038/354494a0. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Stasia M. J., Jouan A., Bourmeyster N., Boquet P., Vignais P. V. ADP-ribosylation of a small size GTP-binding protein in bovine neutrophils by the C3 exoenzyme of Clostridium botulinum and effect on the cell motility. Biochem Biophys Res Commun. 1991 Oct 31;180(2):615–622. doi: 10.1016/s0006-291x(05)81110-6. [DOI] [PubMed] [Google Scholar]
- Taylor S. S., Buechler J. A., Yonemoto W. cAMP-dependent protein kinase: framework for a diverse family of regulatory enzymes. Annu Rev Biochem. 1990;59:971–1005. doi: 10.1146/annurev.bi.59.070190.004543. [DOI] [PubMed] [Google Scholar]
- Tominaga T., Sugie K., Hirata M., Morii N., Fukata J., Uchida A., Imura H., Narumiya S. Inhibition of PMA-induced, LFA-1-dependent lymphocyte aggregation by ADP ribosylation of the small molecular weight GTP binding protein, rho. J Cell Biol. 1993 Mar;120(6):1529–1537. doi: 10.1083/jcb.120.6.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda T., Kikuchi A., Ohga N., Yamamoto J., Takai Y. Purification and characterization from bovine brain cytosol of a novel regulatory protein inhibiting the dissociation of GDP from and the subsequent binding of GTP to rhoB p20, a ras p21-like GTP-binding protein. J Biol Chem. 1990 Jun 5;265(16):9373–9380. [PubMed] [Google Scholar]
- Ullrich O., Horiuchi H., Bucci C., Zerial M. Membrane association of Rab5 mediated by GDP-dissociation inhibitor and accompanied by GDP/GTP exchange. Nature. 1994 Mar 10;368(6467):157–160. doi: 10.1038/368157a0. [DOI] [PubMed] [Google Scholar]
- Ullrich O., Stenmark H., Alexandrov K., Huber L. A., Kaibuchi K., Sasaki T., Takai Y., Zerial M. Rab GDP dissociation inhibitor as a general regulator for the membrane association of rab proteins. J Biol Chem. 1993 Aug 25;268(24):18143–18150. [PubMed] [Google Scholar]
- Vitté-Mony I., Stancou R., Bertoglio J. H. Functional consequences of cAMP accumulation in human natural killer cells. Implications for IL-2 and IL-4 signal transduction. J Immunol. 1990 Dec 15;145(12):4272–4278. [PubMed] [Google Scholar]
- Vojtek A. B., Hollenberg S. M., Cooper J. A. Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell. 1993 Jul 16;74(1):205–214. doi: 10.1016/0092-8674(93)90307-c. [DOI] [PubMed] [Google Scholar]
- Yodoi J., Teshigawara K., Nikaido T., Fukui K., Noma T., Honjo T., Takigawa M., Sasaki M., Minato N., Tsudo M. TCGF (IL 2)-receptor inducing factor(s). I. Regulation of IL 2 receptor on a natural killer-like cell line (YT cells). J Immunol. 1985 Mar;134(3):1623–1630. [PubMed] [Google Scholar]
- Zhu J., Reynet C., Caldwell J. S., Kahn C. R. Characterization of Rad, a new member of Ras/GTPase superfamily, and its regulation by a unique GTPase-activating protein (GAP)-like activity. J Biol Chem. 1995 Mar 3;270(9):4805–4812. doi: 10.1074/jbc.270.9.4805. [DOI] [PubMed] [Google Scholar]
- van der Sluijs P., Hull M., Huber L. A., Mâle P., Goud B., Mellman I. Reversible phosphorylation--dephosphorylation determines the localization of rab4 during the cell cycle. EMBO J. 1992 Dec;11(12):4379–4389. doi: 10.1002/j.1460-2075.1992.tb05538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]