Abstract
The high mobility group proteins 1 and 2 (HMG1/2) and histone B4 are major components of chromatin within the nuclei assembled during the incubation of Xenopus sperm chromatin in Xenopus egg extract. To investigate their potential structural and functional roles, we have cloned and expressed Xenopus HMG1 and histone B4. Purified histone B4 and HMG1 form stable complexes with nucleosomes including Xenopus 5S DNA. Both proteins associate with linker DNA and stabilize it against digestion with micrococcal nuclease, in a similar manner to histone H1. However, neither histone B4 nor HMG1 influence the DNase I or hydroxyl radical digestion of DNA within the nucleosome core. We suggest that HMG1/2 and histone B4 have a shared structural role in organizing linker DNA in the nucleosome.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi Y., Mizuno S., Yoshida M. Efficient large-scale purification of non-histone chromosomal proteins HMG1 and HMG2 by using Polybuffer-exchanger PBE94. J Chromatogr. 1990 Aug 24;530(1):39–46. doi: 10.1016/s0378-4347(00)82300-2. [DOI] [PubMed] [Google Scholar]
- Allan J., Hartman P. G., Crane-Robinson C., Aviles F. X. The structure of histone H1 and its location in chromatin. Nature. 1980 Dec 25;288(5792):675–679. doi: 10.1038/288675a0. [DOI] [PubMed] [Google Scholar]
- Baxevanis A. D., Landsman D. The HMG-1 box protein family: classification and functional relationships. Nucleic Acids Res. 1995 May 11;23(9):1604–1613. doi: 10.1093/nar/23.9.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernués J., Querol E., Martinez P., Barris A., Espel E., Lloberas J. Detection by chemical cross-linking of interaction between high mobility group protein 1 and histone oligomers in free solution. J Biol Chem. 1983 Sep 25;258(18):11020–11024. [PubMed] [Google Scholar]
- Bianchi M. E., Beltrame M., Paonessa G. Specific recognition of cruciform DNA by nuclear protein HMG1. Science. 1989 Feb 24;243(4894 Pt 1):1056–1059. doi: 10.1126/science.2922595. [DOI] [PubMed] [Google Scholar]
- Bianchi M. E., Falciola L., Ferrari S., Lilley D. M. The DNA binding site of HMG1 protein is composed of two similar segments (HMG boxes), both of which have counterparts in other eukaryotic regulatory proteins. EMBO J. 1992 Mar;11(3):1055–1063. doi: 10.1002/j.1460-2075.1992.tb05144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi M. E. Prokaryotic HU and eukaryotic HMG1: a kinked relationship. Mol Microbiol. 1994 Oct;14(1):1–5. doi: 10.1111/j.1365-2958.1994.tb01261.x. [DOI] [PubMed] [Google Scholar]
- Bonne-Andrea C., Harper F., Sobczak J., De Recondo A. M. Rat liver HMG1: a physiological nucleosome assembly factor. EMBO J. 1984 May;3(5):1193–1199. doi: 10.1002/j.1460-2075.1984.tb01950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulikas T., Wiseman J. M., Garrard W. T. Points of contact between histone H1 and the histone octamer. Proc Natl Acad Sci U S A. 1980 Jan;77(1):127–131. doi: 10.1073/pnas.77.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin M., Lehn D. A., Landsman D. Structural features of the HMG chromosomal proteins and their genes. Biochim Biophys Acta. 1990 Jul 30;1049(3):231–243. doi: 10.1016/0167-4781(90)90092-g. [DOI] [PubMed] [Google Scholar]
- Camerini-Otero R. D., Sollner-Webb B., Felsenfeld G. The organization of histones and DNA in chromatin: evidence for an arginine-rich histone kernel. Cell. 1976 Jul;8(3):333–347. doi: 10.1016/0092-8674(76)90145-8. [DOI] [PubMed] [Google Scholar]
- Carlos S., Hunt D. F., Rocchini C., Arnott D. P., Ausio J. Post-translational cleavage of a histone H1-like protein in the sperm of Mytilus. J Biol Chem. 1993 Jan 5;268(1):195–199. [PubMed] [Google Scholar]
- Churchill M. E., Jones D. N., Glaser T., Hefner H., Searles M. A., Travers A. A. HMG-D is an architecture-specific protein that preferentially binds to DNA containing the dinucleotide TG. EMBO J. 1995 Mar 15;14(6):1264–1275. doi: 10.1002/j.1460-2075.1995.tb07110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D. J., Thomas J. O. Salt-dependent co-operative interaction of histone H1 with linear DNA. J Mol Biol. 1986 Feb 20;187(4):569–580. doi: 10.1016/0022-2836(86)90335-9. [DOI] [PubMed] [Google Scholar]
- Clark K. L., Halay E. D., Lai E., Burley S. K. Co-crystal structure of the HNF-3/fork head DNA-recognition motif resembles histone H5. Nature. 1993 Jul 29;364(6436):412–420. doi: 10.1038/364412a0. [DOI] [PubMed] [Google Scholar]
- Dasso M., Dimitrov S., Wolffe A. P. Nuclear assembly is independent of linker histones. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12477–12481. doi: 10.1073/pnas.91.26.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov S. I., Russanova V. R., Pashev I. G. The globular domain of histone H5 is internally located in the 30 nm chromatin fiber: an immunochemical study. EMBO J. 1987 Aug;6(8):2387–2392. doi: 10.1002/j.1460-2075.1987.tb02516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov S., Almouzni G., Dasso M., Wolffe A. P. Chromatin transitions during early Xenopus embryogenesis: changes in histone H4 acetylation and in linker histone type. Dev Biol. 1993 Nov;160(1):214–227. doi: 10.1006/dbio.1993.1299. [DOI] [PubMed] [Google Scholar]
- Dimitrov S., Dasso M. C., Wolffe A. P. Remodeling sperm chromatin in Xenopus laevis egg extracts: the role of core histone phosphorylation and linker histone B4 in chromatin assembly. J Cell Biol. 1994 Aug;126(3):591–601. doi: 10.1083/jcb.126.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong F., Hansen J. C., van Holde K. E. DNA and protein determinants of nucleosome positioning on sea urchin 5S rRNA gene sequences in vitro. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5724–5728. doi: 10.1073/pnas.87.15.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge H., Roeder R. G. The high mobility group protein HMG1 can reversibly inhibit class II gene transcription by interaction with the TATA-binding protein. J Biol Chem. 1994 Jun 24;269(25):17136–17140. [PubMed] [Google Scholar]
- Glotov B. O., Itkes A. V., Nikolaev L. G., Severin E. S. Evidence for the close proximity of histones H1 and H3 in chromatin of intact nuclei. FEBS Lett. 1978 Jul 1;91(1):149–152. doi: 10.1016/0014-5793(78)80037-4. [DOI] [PubMed] [Google Scholar]
- Goodwin G. H., Woodhead L., Johns E. W. The presence of high mobility group non-histone chromatin proteins in isolated nucleosomes. FEBS Lett. 1977 Jan 15;73(1):85–88. [PubMed] [Google Scholar]
- Grasser K. D., Feix G. Isolation and characterization of maize cDNAs encoding a high mobility group protein displaying a HMG-box. Nucleic Acids Res. 1991 May 25;19(10):2573–2577. doi: 10.1093/nar/19.10.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosschedl R., Giese K., Pagel J. HMG domain proteins: architectural elements in the assembly of nucleoprotein structures. Trends Genet. 1994 Mar;10(3):94–100. doi: 10.1016/0168-9525(94)90232-1. [DOI] [PubMed] [Google Scholar]
- Guan K. L., Dixon J. E. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem. 1991 Feb 1;192(2):262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- Hankeln T., Schmidt E. R. New foldback transposable element TFB1 found in histone genes of the midge Chironomus thummi. J Mol Biol. 1990 Oct 20;215(4):477–482. doi: 10.1016/S0022-2836(05)80159-7. [DOI] [PubMed] [Google Scholar]
- Haqq C. M., King C. Y., Ukiyama E., Falsafi S., Haqq T. N., Donahoe P. K., Weiss M. A. Molecular basis of mammalian sexual determination: activation of Müllerian inhibiting substance gene expression by SRY. Science. 1994 Dec 2;266(5190):1494–1500. doi: 10.1126/science.7985018. [DOI] [PubMed] [Google Scholar]
- Hayashi T., Hayashi H., Iwai K. Tetrahymena HMG nonhistone chromosomal protein. Isolation and amino acid sequence lacking the N- and C-terminal domains of vertebrate HMG 1. J Biochem. 1989 Apr;105(4):577–581. doi: 10.1093/oxfordjournals.jbchem.a122707. [DOI] [PubMed] [Google Scholar]
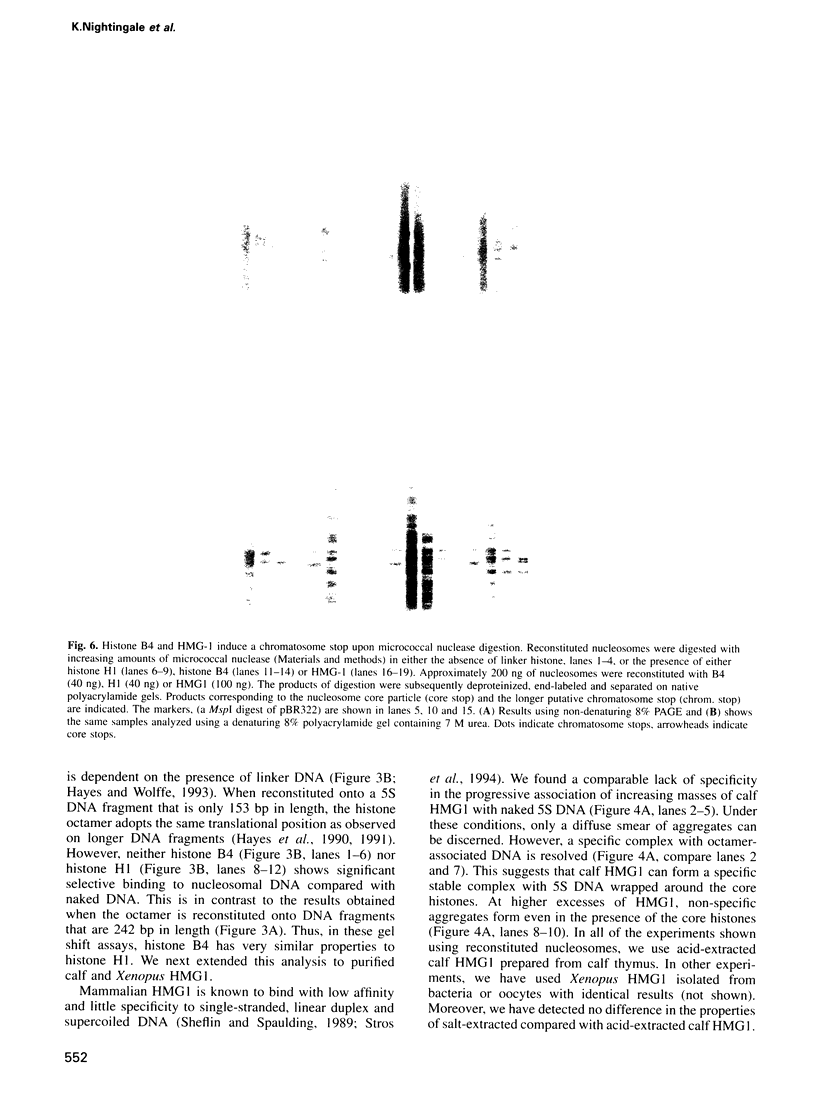
- Hayes J. J., Clark D. J., Wolffe A. P. Histone contributions to the structure of DNA in the nucleosome. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6829–6833. doi: 10.1073/pnas.88.15.6829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. J., Pruss D., Wolffe A. P. Contacts of the globular domain of histone H5 and core histones with DNA in a "chromatosome". Proc Natl Acad Sci U S A. 1994 Aug 2;91(16):7817–7821. doi: 10.1073/pnas.91.16.7817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. J., Tullius T. D., Wolffe A. P. The structure of DNA in a nucleosome. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7405–7409. doi: 10.1073/pnas.87.19.7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. J., Wolffe A. P. Histones H2A/H2B inhibit the interaction of transcription factor IIIA with the Xenopus borealis somatic 5S RNA gene in a nucleosome. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1229–1233. doi: 10.1073/pnas.89.4.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. J., Wolffe A. P. Preferential and asymmetric interaction of linker histones with 5S DNA in the nucleosome. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6415–6419. doi: 10.1073/pnas.90.14.6415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock R., Moorman A., Fischer D., Scheer U. Absence of somatic histone H1 in oocytes and preblastula embryos of Xenopus laevis. Dev Biol. 1993 Aug;158(2):510–522. doi: 10.1006/dbio.1993.1209. [DOI] [PubMed] [Google Scholar]
- Huang S. Y., Garrard W. T. Electrophoretic analyses of nucleosomes and other protein-DNA complexes. Methods Enzymol. 1989;170:116–142. doi: 10.1016/0076-6879(89)70044-6. [DOI] [PubMed] [Google Scholar]
- Isackson P. J., Debold W. A., Reeck G. R. Isolation and separation of chicken erythrocyte high mobility group non-histone chromatin proteins by chromatography on phosphocellulose. FEBS Lett. 1980 Oct 6;119(2):337–342. doi: 10.1016/0014-5793(80)80284-5. [DOI] [PubMed] [Google Scholar]
- Jackson J. B., Pollock J. M., Jr, Rill R. L. Chromatin fractionation procedure that yields nucleosomes containing near-stoichiometric amounts of high mobility group nonhistone chromosomal proteins. Biochemistry. 1979 Aug 21;18(17):3739–3748. doi: 10.1021/bi00584a015. [DOI] [PubMed] [Google Scholar]
- Jackson J. B., Rill R. L. Circular dichroism, thermal denaturation, and deoxyribonuclease I digestion studies of nucleosomes highly enriched in high mobility group proteins HMG 1 and HMG 2. Biochemistry. 1981 Feb 17;20(4):1042–1046. doi: 10.1021/bi00507a060. [DOI] [PubMed] [Google Scholar]
- Kaplan D. J., Duncan C. H. Full length cDNA sequence for bovine high mobility group 1 (HMG1) protein. Nucleic Acids Res. 1988 Nov 11;16(21):10375–10375. doi: 10.1093/nar/16.21.10375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita M., Hatada S., Asashima M., Noda M. HMG-X, a Xenopus gene encoding an HMG1 homolog, is abundantly expressed in the developing nervous system. FEBS Lett. 1994 Sep 26;352(2):191–196. doi: 10.1016/0014-5793(94)00909-0. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt J. A., Scheer U., Dabauvalle M. C., Bustin M., Franke W. W. High mobility group proteins of amphibian oocytes: a large storage pool of a soluble high mobility group-1-like protein and involvement in transcriptional events. J Cell Biol. 1983 Sep;97(3):838–848. doi: 10.1083/jcb.97.3.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodrubetz D., Burgum A. Duplicated NHP6 genes of Saccharomyces cerevisiae encode proteins homologous to bovine high mobility group protein 1. J Biol Chem. 1990 Feb 25;265(6):3234–3239. [PubMed] [Google Scholar]
- Kremer H., Hennig W. Isolation and characterization of a Drosophila hydei histone DNA repeat unit. Nucleic Acids Res. 1990 Mar 25;18(6):1573–1580. doi: 10.1093/nar/18.6.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger W., Herskowitz I. A negative regulator of HO transcription, SIN1 (SPT2), is a nonspecific DNA-binding protein related to HMG1. Mol Cell Biol. 1991 Aug;11(8):4135–4146. doi: 10.1128/mcb.11.8.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley D. M. DNA--protein interactions. HMG has DNA wrapped up. Nature. 1992 May 28;357(6376):282–283. doi: 10.1038/357282a0. [DOI] [PubMed] [Google Scholar]
- Lohka M. J., Masui Y. Formation in vitro of sperm pronuclei and mitotic chromosomes induced by amphibian ooplasmic components. Science. 1983 May 13;220(4598):719–721. doi: 10.1126/science.6601299. [DOI] [PubMed] [Google Scholar]
- Lohka M. J., Masui Y. Roles of cytosol and cytoplasmic particles in nuclear envelope assembly and sperm pronuclear formation in cell-free preparations from amphibian eggs. J Cell Biol. 1984 Apr;98(4):1222–1230. doi: 10.1083/jcb.98.4.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar A., Brown D., Kerby S., Rudzinski I., Polte T., Randhawa Z., Seidman M. M. Sequence of human HMG2 cDNA. Nucleic Acids Res. 1991 Dec 11;19(23):6643–6643. doi: 10.1093/nar/19.23.6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meersseman G., Pennings S., Bradbury E. M. Chromatosome positioning on assembled long chromatin. Linker histones affect nucleosome placement on 5 S rDNA. J Mol Biol. 1991 Jul 5;220(1):89–100. doi: 10.1016/0022-2836(91)90383-h. [DOI] [PubMed] [Google Scholar]
- Ner S. S., Churchill M. E., Searles M. A., Travers A. A. dHMG-Z, a second HMG-1-related protein in Drosophila melanogaster. Nucleic Acids Res. 1993 Sep 11;21(18):4369–4371. doi: 10.1093/nar/21.18.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ner S. S., Travers A. A. HMG-D, the Drosophila melanogaster homologue of HMG 1 protein, is associated with early embryonic chromatin in the absence of histone H1. EMBO J. 1994 Apr 15;13(8):1817–1822. doi: 10.1002/j.1460-2075.1994.tb06450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport J., Kirschner M. A major developmental transition in early Xenopus embryos: I. characterization and timing of cellular changes at the midblastula stage. Cell. 1982 Oct;30(3):675–686. doi: 10.1016/0092-8674(82)90272-0. [DOI] [PubMed] [Google Scholar]
- Newport J., Kirschner M. A major developmental transition in early Xenopus embryos: II. Control of the onset of transcription. Cell. 1982 Oct;30(3):687–696. doi: 10.1016/0092-8674(82)90273-2. [DOI] [PubMed] [Google Scholar]
- Nightingale K., Wolffe A. P. Methylation at CpG sequences does not influence histone H1 binding to a nucleosome including a Xenopus borealis 5 S rRNA gene. J Biol Chem. 1995 Mar 3;270(9):4197–4200. doi: 10.1074/jbc.270.9.4197. [DOI] [PubMed] [Google Scholar]
- Ohsumi K., Katagiri C., Kishimoto T. Chromosome condensation in Xenopus mitotic extracts without histone H1. Science. 1993 Dec 24;262(5142):2033–2035. doi: 10.1126/science.8266099. [DOI] [PubMed] [Google Scholar]
- Paonessa G., Frank R., Cortese R. Nucleotide sequence of rat liver HMG1 cDNA. Nucleic Acids Res. 1987 Nov 11;15(21):9077–9077. doi: 10.1093/nar/15.21.9077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paull T. T., Haykinson M. J., Johnson R. C. The nonspecific DNA-binding and -bending proteins HMG1 and HMG2 promote the assembly of complex nucleoprotein structures. Genes Dev. 1993 Aug;7(8):1521–1534. doi: 10.1101/gad.7.8.1521. [DOI] [PubMed] [Google Scholar]
- Pentecost B. T., Wright J. M., Dixon G. H. Isolation and sequence of cDNA clones coding for a member of the family of high mobility group proteins (HMG-T) in trout and analysis of HMG-T-mRNA's in trout tissues. Nucleic Acids Res. 1985 Jul 11;13(13):4871–4888. doi: 10.1093/nar/13.13.4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpott A., Leno G. H. Nucleoplasmin remodels sperm chromatin in Xenopus egg extracts. Cell. 1992 May 29;69(5):759–767. doi: 10.1016/0092-8674(92)90288-n. [DOI] [PubMed] [Google Scholar]
- Pierrou S., Hellqvist M., Samuelsson L., Enerbäck S., Carlsson P. Cloning and characterization of seven human forkhead proteins: binding site specificity and DNA bending. EMBO J. 1994 Oct 17;13(20):5002–5012. doi: 10.1002/j.1460-2075.1994.tb06827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pil P. M., Lippard S. J. Specific binding of chromosomal protein HMG1 to DNA damaged by the anticancer drug cisplatin. Science. 1992 Apr 10;256(5054):234–237. doi: 10.1126/science.1566071. [DOI] [PubMed] [Google Scholar]
- Pruss D., Hayes J. J., Wolffe A. P. Nucleosomal anatomy--where are the histones? Bioessays. 1995 Feb;17(2):161–170. doi: 10.1002/bies.950170211. [DOI] [PubMed] [Google Scholar]
- Read C. M., Cary P. D., Preston N. S., Lnenicek-Allen M., Crane-Robinson C. The DNA sequence specificity of HMG boxes lies in the minor wing of the structure. EMBO J. 1994 Dec 1;13(23):5639–5646. doi: 10.1002/j.1460-2075.1994.tb06902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeck G. R., Isackson P. J., Teller D. C. Domain structure in high molecular weight high mobility group nonhistone chromatin proteins. Nature. 1982 Nov 4;300(5887):76–78. doi: 10.1038/300076a0. [DOI] [PubMed] [Google Scholar]
- Ruberti I., Worcel A. Mechanism of chromatin assembly in Xenopus oocytes. J Mol Biol. 1986 Jun 5;189(3):457–476. doi: 10.1016/0022-2836(86)90317-7. [DOI] [PubMed] [Google Scholar]
- Russanova V., Venkov C., Tsanev R. A comparison of histone variants in different rat tissues. Cell Differ. 1980 Dec;9(6):339–350. doi: 10.1016/0045-6039(80)90033-0. [DOI] [PubMed] [Google Scholar]
- Schröter H., Bode J. The binding sites for large and small high-mobility-group (HMG) proteins. Studies on HMG-nucleosome interactions in vitro. Eur J Biochem. 1982 Oct;127(2):429–436. doi: 10.1111/j.1432-1033.1982.tb06890.x. [DOI] [PubMed] [Google Scholar]
- Sheflin L. G., Spaulding S. W. High mobility group protein 1 preferentially conserves torsion in negatively supercoiled DNA. Biochemistry. 1989 Jun 27;28(13):5658–5664. doi: 10.1021/bi00439a048. [DOI] [PubMed] [Google Scholar]
- Shirakawa H., Tsuda K., Yoshida M. Primary structure of non-histone chromosomal protein HMG2 revealed by the nucleotide sequence. Biochemistry. 1990 May 8;29(18):4419–4423. doi: 10.1021/bi00470a022. [DOI] [PubMed] [Google Scholar]
- Shykind B. M., Kim J., Sharp P. A. Activation of the TFIID-TFIIA complex with HMG-2. Genes Dev. 1995 Jun 1;9(11):1354–1365. doi: 10.1101/gad.9.11.1354. [DOI] [PubMed] [Google Scholar]
- Simpson R. T. Structure of the chromatosome, a chromatin particle containing 160 base pairs of DNA and all the histones. Biochemistry. 1978 Dec 12;17(25):5524–5531. doi: 10.1021/bi00618a030. [DOI] [PubMed] [Google Scholar]
- Sparrow D. B., Wells J. R. Sequence of a cDNA encoding chicken high-mobility-group protein-2. Gene. 1992 May 15;114(2):289–290. doi: 10.1016/0378-1119(92)90590-l. [DOI] [PubMed] [Google Scholar]
- Stolzenburg F., Dinkl E., Grummt F. Nucleotide sequence of a mouse cDNA encoding the non-histone chromosomal high mobility group protein-2 (HMG-2) Nucleic Acids Res. 1992 Sep 25;20(18):4927–4927. doi: 10.1093/nar/20.18.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stros M., Stokrová J., Thomas J. O. DNA looping by the HMG-box domains of HMG1 and modulation of DNA binding by the acidic C-terminal domain. Nucleic Acids Res. 1994 Mar 25;22(6):1044–1051. doi: 10.1093/nar/22.6.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugarman B. J., Dodgson J. B., Engel J. D. Genomic organization, DNA sequence, and expression of chicken embryonic histone genes. J Biol Chem. 1983 Jul 25;258(14):9005–9016. [PubMed] [Google Scholar]
- Tafuri S. R., Wolffe A. P. Xenopus Y-box transcription factors: molecular cloning, functional analysis and developmental regulation. Proc Natl Acad Sci U S A. 1990 Nov;87(22):9028–9032. doi: 10.1073/pnas.87.22.9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatchell K., Van Holde K. E. Reconstitution of chromatin core particles. Biochemistry. 1977 Nov 29;16(24):5295–5303. doi: 10.1021/bi00643a021. [DOI] [PubMed] [Google Scholar]
- Teo S. H., Grasser K. D., Hardman C. H., Broadhurst R. W., Laue E. D., Thomas J. O. Two mutations in the HMG-box with very different structural consequences provide insights into the nature of binding to four-way junction DNA. EMBO J. 1995 Aug 1;14(15):3844–3853. doi: 10.1002/j.1460-2075.1995.tb00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. O., Rees C., Finch J. T. Cooperative binding of the globular domains of histones H1 and H5 to DNA. Nucleic Acids Res. 1992 Jan 25;20(2):187–194. doi: 10.1093/nar/20.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd R. D., Garrard W. T. Two-dimensional electrophoretic analysis of polynucleosomes. J Biol Chem. 1977 Jul 10;252(13):4729–4738. [PubMed] [Google Scholar]
- Travers A. A., Ner S. S., Churchill M. E. DNA chaperones: a solution to a persistence problem? Cell. 1994 Apr 22;77(2):167–169. doi: 10.1016/0092-8674(94)90306-9. [DOI] [PubMed] [Google Scholar]
- Tremethick D. J., Molloy P. L. High mobility group proteins 1 and 2 stimulate transcription in vitro by RNA polymerases II and III. J Biol Chem. 1986 May 25;261(15):6986–6992. [PubMed] [Google Scholar]
- Tsuda K., Kikuchi M., Mori K., Waga S., Yoshida M. Primary structure of non-histone protein HMG1 revealed by the nucleotide sequence. Biochemistry. 1988 Aug 9;27(16):6159–6163. doi: 10.1021/bi00416a050. [DOI] [PubMed] [Google Scholar]
- Ura K., Hayes J. J., Wolffe A. P. A positive role for nucleosome mobility in the transcriptional activity of chromatin templates: restriction by linker histones. EMBO J. 1995 Aug 1;14(15):3752–3765. doi: 10.1002/j.1460-2075.1995.tb00045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ura K., Wolffe A. P., Hayes J. J. Core histone acetylation does not block linker histone binding to a nucleosome including a Xenopus borealis 5 S rRNA gene. J Biol Chem. 1994 Nov 4;269(44):27171–27174. [PubMed] [Google Scholar]
- Varga-Weisz P., Zlatanova J., Leuba S. H., Schroth G. P., van Holde K. Binding of histones H1 and H5 and their globular domains to four-way junction DNA. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3525–3529. doi: 10.1073/pnas.91.9.3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshavsky A. J., Bakayev V. V., Georgiev G. P. Heterogeneity of chromatin subunits in vitro and location of histone H1. Nucleic Acids Res. 1976 Feb;3(2):477–492. doi: 10.1093/nar/3.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waga S., Mizuno S., Yoshida M. Nonhistone proteins HMG1 and HMG2 suppress the nucleosome assembly at physiological ionic strength. Biochim Biophys Acta. 1989 Mar 1;1007(2):209–214. doi: 10.1016/0167-4781(89)90041-9. [DOI] [PubMed] [Google Scholar]
- Wagner C. R., Hamana K., Elgin S. C. A high-mobility-group protein and its cDNAs from Drosophila melanogaster. Mol Cell Biol. 1992 May;12(5):1915–1923. doi: 10.1128/mcb.12.5.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir H. M., Kraulis P. J., Hill C. S., Raine A. R., Laue E. D., Thomas J. O. Structure of the HMG box motif in the B-domain of HMG1. EMBO J. 1993 Apr;12(4):1311–1319. doi: 10.1002/j.1460-2075.1993.tb05776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisbrod S., Wickens M. P., Whytock S., Gurdon J. B. Active chromatin of oocytes injected with somatic cell nuclei or cloned DNA. Dev Biol. 1982 Nov;94(1):216–229. doi: 10.1016/0012-1606(82)90085-9. [DOI] [PubMed] [Google Scholar]
- Wen L., Huang J. K., Johnson B. H., Reeck G. R. A human placental cDNA clone that encodes nonhistone chromosomal protein HMG-1. Nucleic Acids Res. 1989 Feb 11;17(3):1197–1214. doi: 10.1093/nar/17.3.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner M. H., Huth J. R., Gronenborn A. M., Clore G. M. Molecular basis of human 46X,Y sex reversal revealed from the three-dimensional solution structure of the human SRY-DNA complex. Cell. 1995 Jun 2;81(5):705–714. doi: 10.1016/0092-8674(95)90532-4. [DOI] [PubMed] [Google Scholar]
- Wiśniewski J. R., Schulze E. Insect proteins homologous to mammalian high mobility group protein 1. Characterization and DNA-binding properties. J Biol Chem. 1992 Aug 25;267(24):17170–17177. [PubMed] [Google Scholar]
- Wolffe A. P. Dominant and specific repression of Xenopus oocyte 5S RNA genes and satellite I DNA by histone H1. EMBO J. 1989 Feb;8(2):527–537. doi: 10.1002/j.1460-2075.1989.tb03407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolffe A. P. Transcriptional activation of Xenopus class III genes in chromatin isolated from sperm and somatic nuclei. Nucleic Acids Res. 1989 Jan 25;17(2):767–780. doi: 10.1093/nar/17.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yotov W. V., St-Arnaud R. Nucleotide sequence of a mouse cDNA encoding the nonhistone chromosomal high mobility group protein-1 (HMG1). Nucleic Acids Res. 1992 Jul 11;20(13):3516–3516. doi: 10.1093/nar/20.13.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]