Abstract
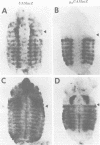
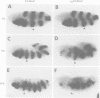
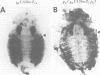
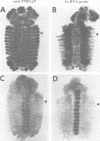
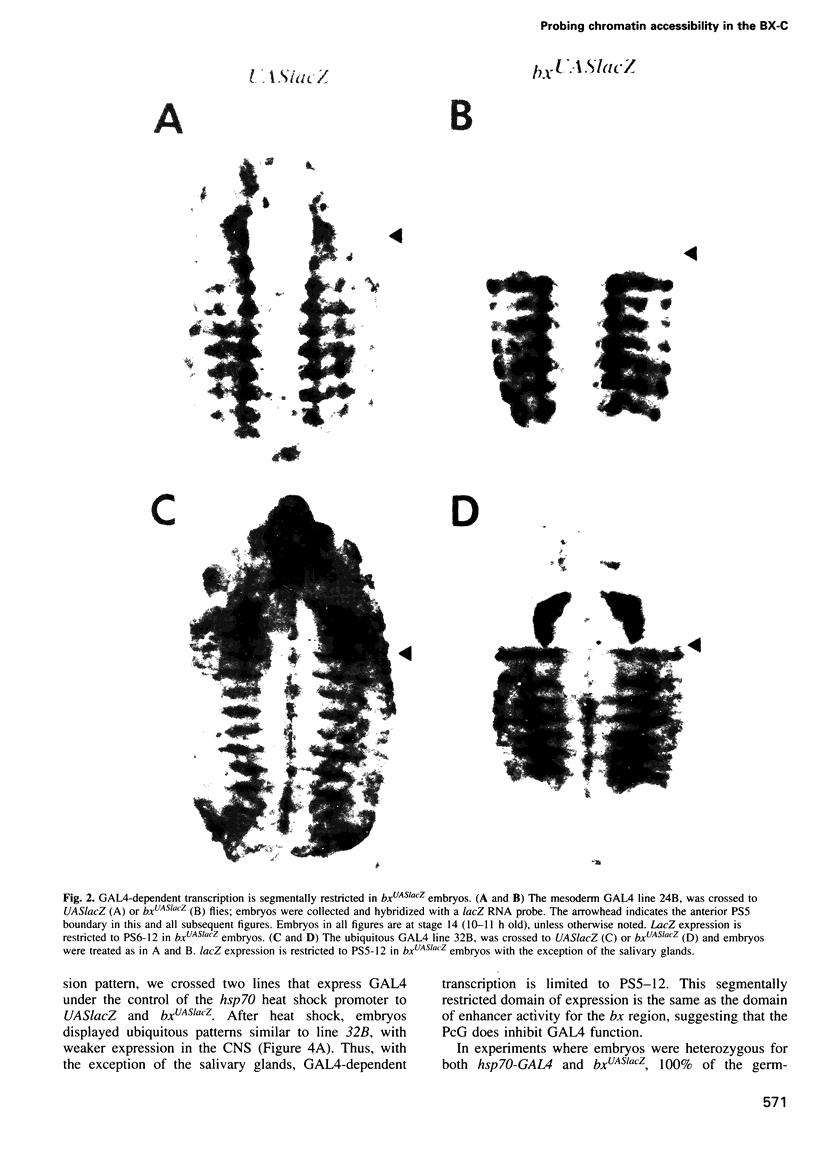
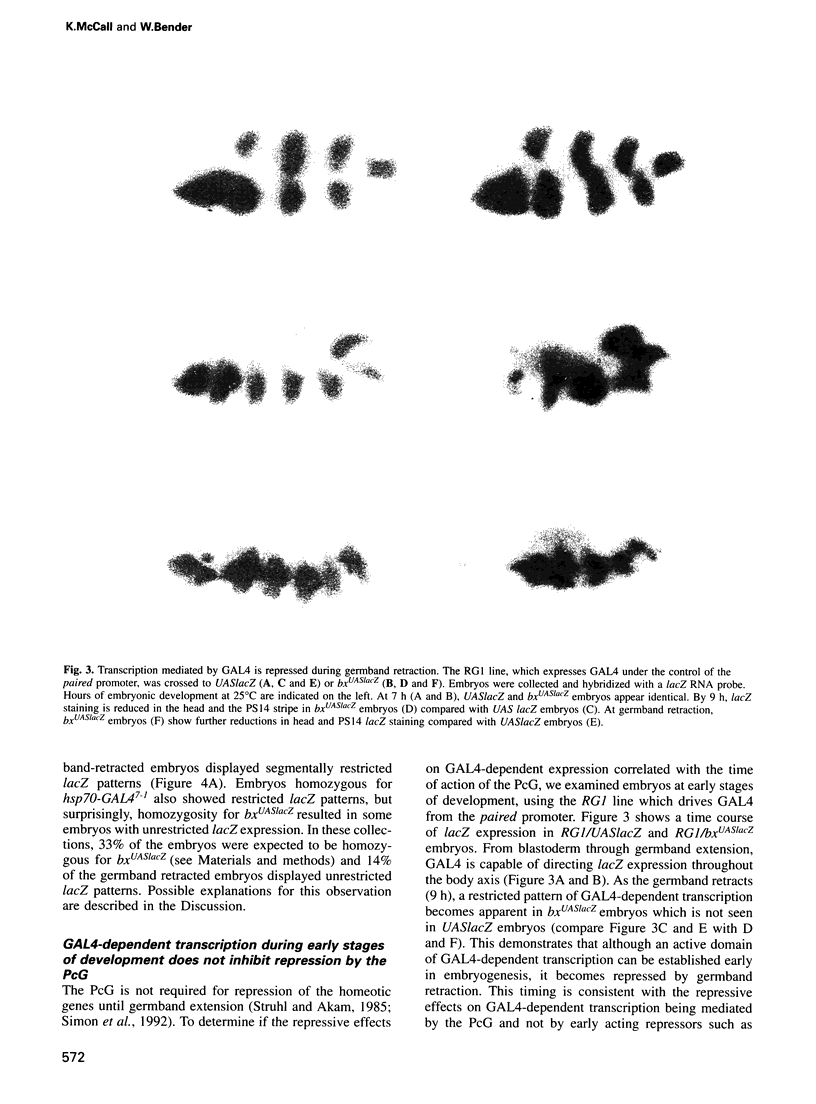
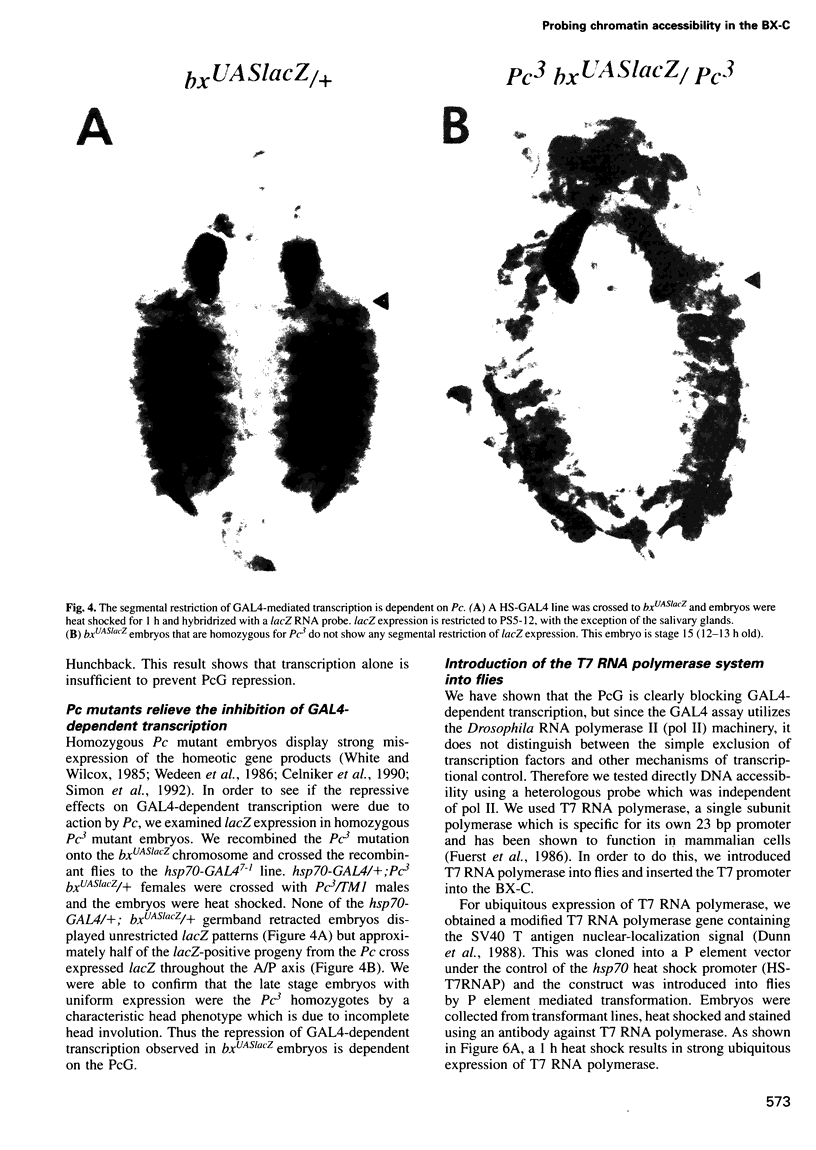
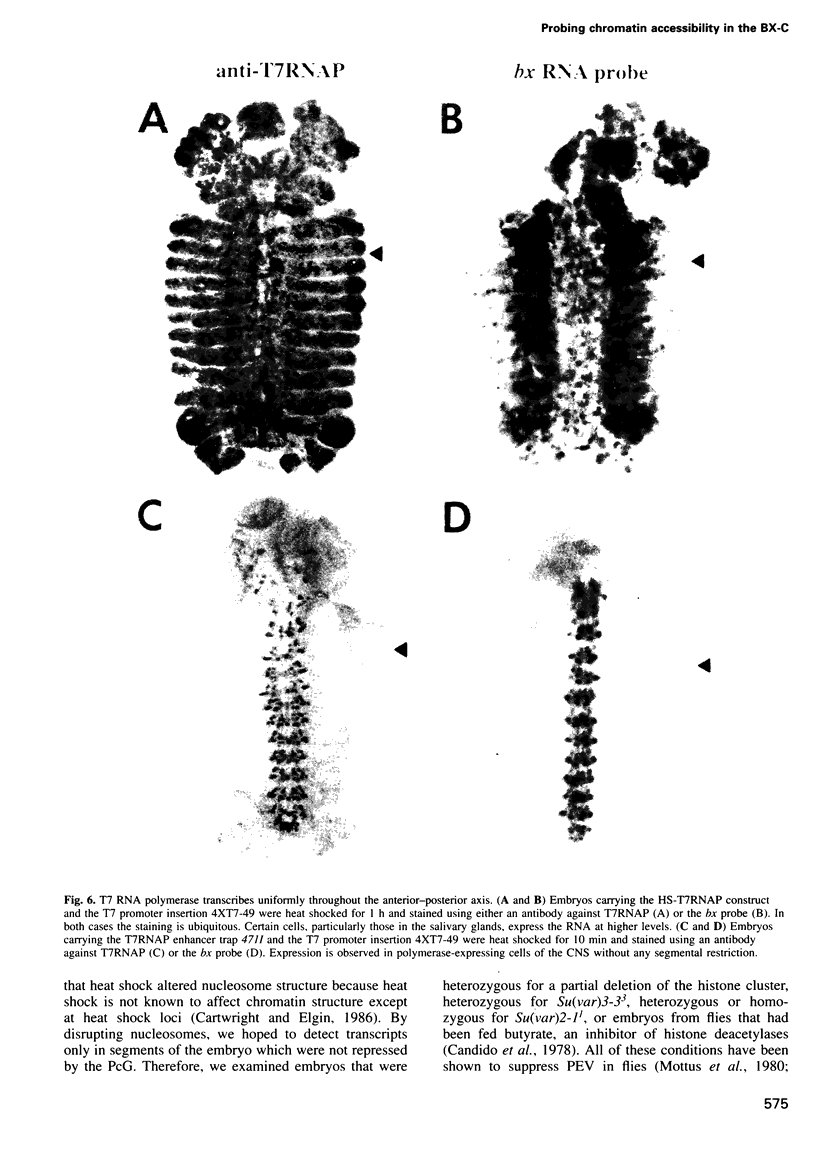
The Polycomb group (PcG) of genes are required for maintenance of the repressed state of the homeotic genes in Drosophila. There are similarities between the PcG repression and mating-type silencing in yeast or heterochromatic position effect in Drosophila, which suggest that PcG repression may involve a highly compacted chromatin structure. To test for such a structure, heterologous DNA- binding proteins were used as probes for DNA accessibility in Drosophila embryos. Binding sites for the yeast transcriptional activator GAL4 and for bacteriophage T7 RNA polymerase were inserted into the bithorax (bx) regulatory region of the endogenous Ultrabithorax (Ubx) gene, which is regulated by the PcG. Ubiquitously expressed GAL4 protein directs transcription through its binding sites only in the posterior segments where the bx region is active. The block to GAL4 activation in the more anterior segments is dependent on Polycomb (Pc) function. In contrast, T7 RNA polymerase can transcribe from its target promoter in all segments of the embryo. Thus, Pc-mediated repression blocks activated polymerase II transcription, but does not simply exclude all proteins.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts B., Sternglanz R. Gene expression. Chromatin contract to silence. Nature. 1990 Mar 15;344(6263):193–194. doi: 10.1038/344193a0. [DOI] [PubMed] [Google Scholar]
- Aparicio O. M., Gottschling D. E. Overcoming telomeric silencing: a trans-activator competes to establish gene expression in a cell cycle-dependent way. Genes Dev. 1994 May 15;8(10):1133–1146. doi: 10.1101/gad.8.10.1133. [DOI] [PubMed] [Google Scholar]
- Brand A. H., Manoukian A. S., Perrimon N. Ectopic expression in Drosophila. Methods Cell Biol. 1994;44:635–654. doi: 10.1016/s0091-679x(08)60936-x. [DOI] [PubMed] [Google Scholar]
- Chan C. S., Rastelli L., Pirrotta V. A Polycomb response element in the Ubx gene that determines an epigenetically inherited state of repression. EMBO J. 1994 Jun 1;13(11):2553–2564. doi: 10.1002/j.1460-2075.1994.tb06545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Tabor S., Struhl K. Distinguishing between mechanisms of eukaryotic transcriptional activation with bacteriophage T7 RNA polymerase. Cell. 1987 Sep 25;50(7):1047–1055. doi: 10.1016/0092-8674(87)90171-1. [DOI] [PubMed] [Google Scholar]
- Chiang A., O'Connor M. B., Paro R., Simon J., Bender W. Discrete Polycomb-binding sites in each parasegmental domain of the bithorax complex. Development. 1995 Jun;121(6):1681–1689. doi: 10.1242/dev.121.6.1681. [DOI] [PubMed] [Google Scholar]
- Cumberledge S., Zaratzian A., Sakonju S. Characterization of two RNAs transcribed from the cis-regulatory region of the abd-A domain within the Drosophila bithorax complex. Proc Natl Acad Sci U S A. 1990 May;87(9):3259–3263. doi: 10.1073/pnas.87.9.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J. J., Krippl B., Bernstein K. E., Westphal H., Studier F. W. Targeting bacteriophage T7 RNA polymerase to the mammalian cell nucleus. Gene. 1988 Sep 7;68(2):259–266. doi: 10.1016/0378-1119(88)90028-5. [DOI] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. Complete nucleotide sequence of bacteriophage T7 DNA and the locations of T7 genetic elements. J Mol Biol. 1983 Jun 5;166(4):477–535. doi: 10.1016/s0022-2836(83)80282-4. [DOI] [PubMed] [Google Scholar]
- Engels W. R., Johnson-Schlitz D. M., Eggleston W. B., Sved J. High-frequency P element loss in Drosophila is homolog dependent. Cell. 1990 Aug 10;62(3):515–525. doi: 10.1016/0092-8674(90)90016-8. [DOI] [PubMed] [Google Scholar]
- Fischer J. A., Giniger E., Maniatis T., Ptashne M. GAL4 activates transcription in Drosophila. Nature. 1988 Apr 28;332(6167):853–856. doi: 10.1038/332853a0. [DOI] [PubMed] [Google Scholar]
- Fuerst T. R., Moss B. Structure and stability of mRNA synthesized by vaccinia virus-encoded bacteriophage T7 RNA polymerase in mammalian cells. Importance of the 5' untranslated leader. J Mol Biol. 1989 Mar 20;206(2):333–348. doi: 10.1016/0022-2836(89)90483-x. [DOI] [PubMed] [Google Scholar]
- Fuerst T. R., Niles E. G., Studier F. W., Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaunt S. J., Singh P. B. Homeogene expression patterns and chromosomal imprinting. Trends Genet. 1990 Jul;6(7):208–212. [PubMed] [Google Scholar]
- Gavis E. R., Lehmann R. Localization of nanos RNA controls embryonic polarity. Cell. 1992 Oct 16;71(2):301–313. doi: 10.1016/0092-8674(92)90358-j. [DOI] [PubMed] [Google Scholar]
- Gindhart J. G., Jr, Kaufman T. C. Identification of Polycomb and trithorax group responsive elements in the regulatory region of the Drosophila homeotic gene Sex combs reduced. Genetics. 1995 Feb;139(2):797–814. doi: 10.1093/genetics/139.2.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloor G. B., Nassif N. A., Johnson-Schlitz D. M., Preston C. R., Engels W. R. Targeted gene replacement in Drosophila via P element-induced gap repair. Science. 1991 Sep 6;253(5024):1110–1117. doi: 10.1126/science.1653452. [DOI] [PubMed] [Google Scholar]
- Gonzy-Tréboul G., Lepesant J. A., Deutsch J. Enhancer-trap targeting at the Broad-Complex locus of Drosophila melanogaster. Genes Dev. 1995 May 1;9(9):1137–1148. doi: 10.1101/gad.9.9.1137. [DOI] [PubMed] [Google Scholar]
- Gyurkovics H., Gausz J., Kummer J., Karch F. A new homeotic mutation in the Drosophila bithorax complex removes a boundary separating two domains of regulation. EMBO J. 1990 Aug;9(8):2579–2585. doi: 10.1002/j.1460-2075.1990.tb07439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S., Ruddell A., Sinclair D., Grigliatti T. Chromosomal structure is altered by mutations that suppress or enhance position effect variegation. Chromosoma. 1990 Oct;99(6):391–400. doi: 10.1007/BF01726690. [DOI] [PubMed] [Google Scholar]
- Heslip T. R., Hodgetts R. B. Targeted transposition at the vestigial locus of Drosophila melanogaster. Genetics. 1994 Dec;138(4):1127–1135. doi: 10.1093/genetics/138.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslip T. R., Williams J. A., Bell J. B., Hodgetts R. B. A P element chimera containing captured genomic sequences was recovered at the vestigial locus in Drosophila following targeted transposition. Genetics. 1992 Aug;131(4):917–927. doi: 10.1093/genetics/131.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiromi Y., Gehring W. J. Regulation and function of the Drosophila segmentation gene fushi tarazu. Cell. 1987 Sep 11;50(6):963–974. doi: 10.1016/0092-8674(87)90523-x. [DOI] [PubMed] [Google Scholar]
- Hooper Joan E. Homeotic gene function in the muscles of Drosophila larvae. EMBO J. 1986 Sep;5(9):2321–2329. doi: 10.1002/j.1460-2075.1986.tb04500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenuwein T., Forrester W. C., Qiu R. G., Grosschedl R. The immunoglobulin mu enhancer core establishes local factor access in nuclear chromatin independent of transcriptional stimulation. Genes Dev. 1993 Oct;7(10):2016–2032. doi: 10.1101/gad.7.10.2016. [DOI] [PubMed] [Google Scholar]
- Johnson-Schlitz D. M., Engels W. R. P-element-induced interallelic gene conversion of insertions and deletions in Drosophila melanogaster. Mol Cell Biol. 1993 Nov;13(11):7006–7018. doi: 10.1128/mcb.13.11.7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. S., Gelbart W. M. The Drosophila Polycomb-group gene Enhancer of zeste contains a region with sequence similarity to trithorax. Mol Cell Biol. 1993 Oct;13(10):6357–6366. doi: 10.1128/mcb.13.10.6357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch F., Bender W., Weiffenbach B. abdA expression in Drosophila embryos. Genes Dev. 1990 Sep;4(9):1573–1587. doi: 10.1101/gad.4.9.1573. [DOI] [PubMed] [Google Scholar]
- Kirov N., Tsaneva I., Einbinder E., Tsanev R. In vitro transcription through nucleosomes by T7 RNA polymerase. EMBO J. 1992 May;11(5):1941–1947. doi: 10.1002/j.1460-2075.1992.tb05247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon H., Imbalzano A. N., Khavari P. A., Kingston R. E., Green M. R. Nucleosome disruption and enhancement of activator binding by a human SW1/SNF complex. Nature. 1994 Aug 11;370(6489):477–481. doi: 10.1038/370477a0. [DOI] [PubMed] [Google Scholar]
- Laurenson P., Rine J. Silencers, silencing, and heritable transcriptional states. Microbiol Rev. 1992 Dec;56(4):543–560. doi: 10.1128/mr.56.4.543-560.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D. Y., Hayes J. J., Pruss D., Wolffe A. P. A positive role for histone acetylation in transcription factor access to nucleosomal DNA. Cell. 1993 Jan 15;72(1):73–84. doi: 10.1016/0092-8674(93)90051-q. [DOI] [PubMed] [Google Scholar]
- Lewis E. B. A gene complex controlling segmentation in Drosophila. Nature. 1978 Dec 7;276(5688):565–570. doi: 10.1038/276565a0. [DOI] [PubMed] [Google Scholar]
- Lipshitz H. D., Peattie D. A., Hogness D. S. Novel transcripts from the Ultrabithorax domain of the bithorax complex. Genes Dev. 1987 May;1(3):307–322. doi: 10.1101/gad.1.3.307. [DOI] [PubMed] [Google Scholar]
- Locke J. Examination of DNA sequences undergoing chromatin conformation changes at a variegating breakpoint in Drosophila melanogaster. Genetica. 1993;92(1):33–41. doi: 10.1007/BF00057505. [DOI] [PubMed] [Google Scholar]
- Loo S., Rine J. Silencers and domains of generalized repression. Science. 1994 Jun 17;264(5166):1768–1771. doi: 10.1126/science.8209257. [DOI] [PubMed] [Google Scholar]
- McCall K., O'Connor M. B., Bender W. Enhancer traps in the Drosophila bithorax complex mark parasegmental domains. Genetics. 1994 Oct;138(2):387–399. doi: 10.1093/genetics/138.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore G. D., Sinclair D. A., Grigliatti T. A. Histone Gene Multiplicity and Position Effect Variegation in DROSOPHILA MELANOGASTER. Genetics. 1983 Oct;105(2):327–344. doi: 10.1093/genetics/105.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse R. H. Nucleosome disruption by transcription factor binding in yeast. Science. 1993 Dec 3;262(5139):1563–1566. doi: 10.1126/science.8248805. [DOI] [PubMed] [Google Scholar]
- Mottus R., Reeves R., Grigliatti T. A. Butyrate suppression of position-effect variegation in Drosophila melanogaster. Mol Gen Genet. 1980;178(2):465–469. doi: 10.1007/BF00270501. [DOI] [PubMed] [Google Scholar]
- Mullins M. C., Rio D. C., Rubin G. M. cis-acting DNA sequence requirements for P-element transposition. Genes Dev. 1989 May;3(5):729–738. doi: 10.1101/gad.3.5.729. [DOI] [PubMed] [Google Scholar]
- Müller J., Bienz M. Sharp anterior boundary of homeotic gene expression conferred by the fushi tarazu protein. EMBO J. 1992 Oct;11(10):3653–3661. doi: 10.1002/j.1460-2075.1992.tb05450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassif N., Penney J., Pal S., Engels W. R., Gloor G. B. Efficient copying of nonhomologous sequences from ectopic sites via P-element-induced gap repair. Mol Cell Biol. 1994 Mar;14(3):1613–1625. doi: 10.1128/mcb.14.3.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill T. E., Roberge M., Bradbury E. M. Nucleosome arrays inhibit both initiation and elongation of transcripts by bacteriophage T7 RNA polymerase. J Mol Biol. 1992 Jan 5;223(1):67–78. doi: 10.1016/0022-2836(92)90716-w. [DOI] [PubMed] [Google Scholar]
- Paro R., Hogness D. S. The Polycomb protein shares a homologous domain with a heterochromatin-associated protein of Drosophila. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):263–267. doi: 10.1073/pnas.88.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paro R. Imprinting a determined state into the chromatin of Drosophila. Trends Genet. 1990 Dec;6(12):416–421. doi: 10.1016/0168-9525(90)90303-n. [DOI] [PubMed] [Google Scholar]
- Paro R. Mechanisms of heritable gene repression during development of Drosophila. Curr Opin Cell Biol. 1993 Dec;5(6):999–1005. doi: 10.1016/0955-0674(93)90084-4. [DOI] [PubMed] [Google Scholar]
- Peterson C. L., Herskowitz I. Characterization of the yeast SWI1, SWI2, and SWI3 genes, which encode a global activator of transcription. Cell. 1992 Feb 7;68(3):573–583. doi: 10.1016/0092-8674(92)90192-f. [DOI] [PubMed] [Google Scholar]
- Pirrotta V., Rastelli L. White gene expression, repressive chromatin domains and homeotic gene regulation in Drosophila. Bioessays. 1994 Aug;16(8):549–556. doi: 10.1002/bies.950160808. [DOI] [PubMed] [Google Scholar]
- Ptashne M. How eukaryotic transcriptional activators work. Nature. 1988 Oct 20;335(6192):683–689. doi: 10.1038/335683a0. [DOI] [PubMed] [Google Scholar]
- Qian S., Capovilla M., Pirrotta V. The bx region enhancer, a distant cis-control element of the Drosophila Ubx gene and its regulation by hunchback and other segmentation genes. EMBO J. 1991 Jun;10(6):1415–1425. doi: 10.1002/j.1460-2075.1991.tb07662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter G., Spierer P. Position effect variegation and chromatin proteins. Bioessays. 1992 Sep;14(9):605–612. doi: 10.1002/bies.950140907. [DOI] [PubMed] [Google Scholar]
- Riggs M. G., Whittaker R. G., Neumann J. R., Ingram V. M. n-Butyrate causes histone modification in HeLa and Friend erythroleukaemia cells. Nature. 1977 Aug 4;268(5619):462–464. doi: 10.1038/268462a0. [DOI] [PubMed] [Google Scholar]
- Robertson H. M., Preston C. R., Phillis R. W., Johnson-Schlitz D. M., Benz W. K., Engels W. R. A stable genomic source of P element transposase in Drosophila melanogaster. Genetics. 1988 Mar;118(3):461–470. doi: 10.1093/genetics/118.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlossherr J., Eggert H., Paro R., Cremer S., Jack R. S. Gene inactivation in Drosophila mediated by the Polycomb gene product or by position-effect variegation does not involve major changes in the accessibility of the chromatin fibre. Mol Gen Genet. 1994 May 25;243(4):453–462. doi: 10.1007/BF00280476. [DOI] [PubMed] [Google Scholar]
- Shimell M. J., Simon J., Bender W., O'Connor M. B. Enhancer point mutation results in a homeotic transformation in Drosophila. Science. 1994 May 13;264(5161):968–971. doi: 10.1126/science.7909957. [DOI] [PubMed] [Google Scholar]
- Simon J., Chiang A., Bender W. Ten different Polycomb group genes are required for spatial control of the abdA and AbdB homeotic products. Development. 1992 Feb;114(2):493–505. doi: 10.1242/dev.114.2.493. [DOI] [PubMed] [Google Scholar]
- Simon J. Locking in stable states of gene expression: transcriptional control during Drosophila development. Curr Opin Cell Biol. 1995 Jun;7(3):376–385. doi: 10.1016/0955-0674(95)80093-x. [DOI] [PubMed] [Google Scholar]
- Singh J., Klar A. J. Active genes in budding yeast display enhanced in vivo accessibility to foreign DNA methylases: a novel in vivo probe for chromatin structure of yeast. Genes Dev. 1992 Feb;6(2):186–196. doi: 10.1101/gad.6.2.186. [DOI] [PubMed] [Google Scholar]
- Smith A. V., Orr-Weaver T. L. The regulation of the cell cycle during Drosophila embryogenesis: the transition to polyteny. Development. 1991 Aug;112(4):997–1008. doi: 10.1242/dev.112.4.997. [DOI] [PubMed] [Google Scholar]
- Soto M. C., Chou T. B., Bender W. Comparison of germline mosaics of genes in the Polycomb group of Drosophila melanogaster. Genetics. 1995 May;140(1):231–243. doi: 10.1093/genetics/140.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staveley B. E., Hodgetts R. B., O'Keefe S. L., Bell J. B. Targeting of an enhancer trap to vestigial. Dev Biol. 1994 Sep;165(1):290–293. doi: 10.1006/dbio.1994.1254. [DOI] [PubMed] [Google Scholar]
- Struhl G., Akam M. Altered distributions of Ultrabithorax transcripts in extra sex combs mutant embryos of Drosophila. EMBO J. 1985 Dec 1;4(12):3259–3264. doi: 10.1002/j.1460-2075.1985.tb04075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Herrero E., Akam M. Spatially ordered transcription of regulatory DNA in the bithorax complex of Drosophila. Development. 1989 Oct;107(2):321–329. doi: 10.1242/dev.107.2.321. [DOI] [PubMed] [Google Scholar]
- Tamkun J. W., Deuring R., Scott M. P., Kissinger M., Pattatucci A. M., Kaufman T. C., Kennison J. A. brahma: a regulator of Drosophila homeotic genes structurally related to the yeast transcriptional activator SNF2/SWI2. Cell. 1992 Feb 7;68(3):561–572. doi: 10.1016/0092-8674(92)90191-e. [DOI] [PubMed] [Google Scholar]
- Taylor I. C., Workman J. L., Schuetz T. J., Kingston R. E. Facilitated binding of GAL4 and heat shock factor to nucleosomal templates: differential function of DNA-binding domains. Genes Dev. 1991 Jul;5(7):1285–1298. doi: 10.1101/gad.5.7.1285. [DOI] [PubMed] [Google Scholar]
- Turner B. M. Histone acetylation and control of gene expression. J Cell Sci. 1991 May;99(Pt 1):13–20. doi: 10.1242/jcs.99.1.13. [DOI] [PubMed] [Google Scholar]
- Wallrath L. L., Elgin S. C. Position effect variegation in Drosophila is associated with an altered chromatin structure. Genes Dev. 1995 May 15;9(10):1263–1277. doi: 10.1101/gad.9.10.1263. [DOI] [PubMed] [Google Scholar]
- Wedeen C., Harding K., Levine M. Spatial regulation of Antennapedia and bithorax gene expression by the Polycomb locus in Drosophila. Cell. 1986 Mar 14;44(5):739–748. doi: 10.1016/0092-8674(86)90840-8. [DOI] [PubMed] [Google Scholar]
- Wolffe A. P., Drew H. R. Initiation of transcription on nucleosomal templates. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9817–9821. doi: 10.1073/pnas.86.24.9817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink D., Paro R. Drosophila Polycomb-group regulated chromatin inhibits the accessibility of a trans-activator to its target DNA. EMBO J. 1995 Nov 15;14(22):5660–5671. doi: 10.1002/j.1460-2075.1995.tb00253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]