Abstract
Mast cells originate from the bone marrow and develop into c-kit+ FcɛRI+ cells. Both mast cell progenitors (MCp) and mature mast cells express these cell surface markers, and ways validated to distinguish between the two maturation forms with flow cytometry have been lacking. Here, we show that primary peritoneal MCp from naïve mice expressed high levels of integrin β7 and had a low side scatter (SSC) light profile; whereas mature mast cells expressed lower levels of integrin β7 and had a high SSC light profile. The maturation statuses of the cells were confirmed using three main strategies: (1) MCp, but not mature mast cells, were shown to be depleted by sublethal whole-body γ-irradiation. (2) The MCp were small and immature in terms of granule formation, whereas the mature mast cells were larger and had fully developed metachromatic granules. (3) The MCp had fewer transcripts of mast cell-specific proteases and the enzyme responsible for sulfation of heparin than mature mast cells. Moreover, isolated peritoneal MCp gave rise to mast cells when cultured in vitro. To summarize, we have defined MCp and mature mast cells in naïve mice by flow cytometry. Using this strategy, mast cell maturation can be studied in vivo.
Introduction
Mast cells are c-kit+ FcɛRI+ cells that originate from mast cell progenitors (MCp) that are produced in the bone marrow [1]. In adult mice, progenitors committed to the mast cell lineage have been found at several locations. In the bone marrow, committed MCp are identified as lineage− (Lin−) c-kit+ Sca-1− Ly6c− FcɛRIα− CD27− integrin β7+ T1/ST2+ cells [2]. Once the committed MCp leave the bone marrow, they circulate in the blood as Lin− c-kithi T1/ST2+ integrin β7hi CD16/32hi cells [3]. The majority of these MCp express FcɛRI on the cell surface in BALB/c mice [3]. On entry of the peripheral tissues such as the intestine, the MCp are identified as CD45+ Lin− CD34+ integrin β7hi FcɛRIαlo cells [4]. Once the MCp reach their target organ, they are allowed to mature fully. As both c-kit and FcɛRI expression are found on MCp and mature mast cells in peripheral tissues, these markers are not sufficient to distinguish between the cell types.
In vitro, c-kit+ FcɛRI+ mast cells can be generated by culturing mouse bone marrow cells [5]. Using flow cytometric analysis, c-kit+ FcɛRI+ mast cells with a low side scatter (SSC) light profile can be identified after 2 weeks in the culture [5]. The c-kit+ FcɛRI+ mast cells obtain a high SSC light profile after another 4 to 8 weeks [5]. Even though the SSC light profile can be used as a measure of cells' internal complexity, strategies validated to distinguish MCp from mature mast cells by flow cytometry have been lacking.
In this study, MCp and mature mast cells from peritoneal lavage of mice are distinguished by flow cytometry based on the expression of integrin β7 and the SSC light profiles. The identity of the MCp and the mature mast cells are validated by a number of strategies, including a gene expression microarray analysis. The flow cytometric gating strategy for peritoneal MCp and mature mast cells could be extrapolated to differentiate between these cell types in the lung, making it a valuable tool to quantify the different forms of mast cells in mouse models of various lung diseases.
Materials and Methods
Mice
Female and male BALB/c mice were housed and bred at the Swedish Veterinary Institute and were used at an age of at least 7 weeks. The mice were originally obtained from Bommice (Ry, Denmark). The local ethics committee approved all experiments.
Flow cytometry and cell sorting
The mice were euthanized with an overdose of isoflurane (Schering-Plough, Farum, Denmark). For extraction of peritoneal cells, the abdominal skin was removed and 4 mL of fluorescence-activated cell sorting (FACS) buffer (2% heat-inactivated fetal calf serum in PBS pH 7.4) was injected into the peritoneum. After shaking the abdomen, ∼3 mL of the buffer was extracted and the cells were pelleted by centrifugation (400 g, 10 min). The cells were resuspended in FACS buffer, counted using trypan blue exclusion with a hemocytometer, and stained with antibodies as described in the antibodies section.
For extraction of lung cells, 10 mL of PBS pH 7.4 was injected into the right ventricle of the heart to remove blood from the lung tissue. Cell suspensions were prepared using the mouse lung dissociation kit and the gentleMACS Octo Dissociator with heaters according to the manufacturer's instructions (Miltenyi Biotec, Bergisch Gladbach, Germany). The cell suspensions were filtered through a 70 μm filter. The cells were pelleted by centrifugation (400 g, 10 min), resuspended in FACS buffer, and counted using trypan blue exclusion with a hemocytometer. The cells were stained as described in the antibodies section. Flow cytometric analysis was performed on an FACS Aria III or an LSR II (both from BD Biosciences, Franklin Lakes, NJ). Cell sorting was performed on a FACS Aria III. Flow cytometric data were analyzed using the FlowJo software (Tree Star, Inc., Ashland, OR).
Cell culture
The sorted cells were cultured in Iscove's Modified Dulbecco's Medium supplemented with 20% heat-inactivated fetal calf serum, 2 mM L-glutamine, 10 mM HEPES, 100 U/mL penicillin, 100 μg/mL streptomycin, 10 μg/mL gentamicin, 1×MEM nonessential amino acids, 50 μM 2-mercaptoethanol, 1 mM sodium pyruvate, 20 ng/mL recombinant mouse IL-3, and 20 ng/mL recombinant mouse stem cell factor. The medium and the supplements were from Sigma-Aldrich (St. Louis, MO), except the cytokines that were from Peprotech (Rocky Hill, NJ). Cell culturing was performed at 37°C with 5% CO2.
Image analysis
The sorted cells were transferred onto glass slides using cytospin or Shandon Octospot (Thermo Fisher Scientific, Inc., Waltham, MA) and stained with May-Grünwald Giemsa. Photographs were taken using a Nikon Eclipse 90i microscope with a DS-Fi1 camera or a Nikon Eclipse Ni microscope with a DS-2Mv camera (Nikon, Melville, NY). NIS Elements AR 3.2 or NIS Elements BR 3.2 software were used for capturing the images (Nikon). The images were analyzed using the ImageJ software (NIH, Bethesda, MD). The largest diameter of cells was quantified using the measure function. The metachromatic area of each cell was estimated as the integrated density of the photos converted into binary images.
Microarray expression analysis
One hundred cells were FACS sorted into 12 μL lysis buffer, provided with the Ovation® One-Direct System (Nugen Technologies, Inc., San Carlos, CA). The following procedures were performed by the Array and Analysis Facility at the Science for Life Laboratory in Uppsala: Amplified and biotinylated sense transcript complementary DNA was generated from the entire expressed genome with the Ovation One-Direct System combined with the Encore Biotin Module (Nugen Technologies, Inc.) according to the manufacturer's protocols (M01312 v1 and M01111 v5). Affymetrix GeneChip® Mouse Gene 2.0 ST Arrays were hybridized at 45°C for 40 h under rotation at 60 rpm. The arrays were washed and stained (according to GeneChip Expression Wash, Stain and Scan Manual, PN702731 Rev 3; Affymetrix, Inc., Santa Clara, CA) using the Fluidics Station 450 and scanned with a GeneChip Scanner 3000 7G. The raw data were normalized using the robust multi-array average method [6,7].
The log2-fold difference in gene expression is shown in Fig. 4B. Heat maps were generated using Genesis 1.7.6 [8]. The data were adjusted by mean centering the gene expression. The gene expression data have been deposited in NCBI's Gene Expression Omnibus, and they are publicly accessible through the GEO Series accession number GSE63507 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE63507).
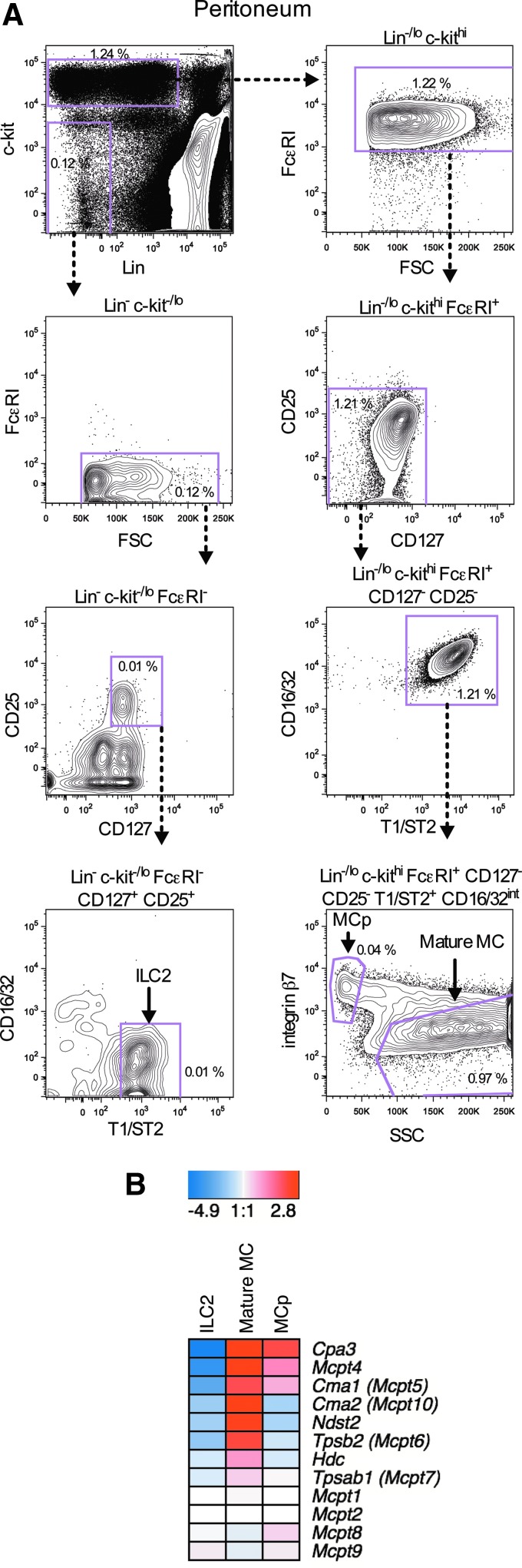
FIG. 4.
(A) Peritoneal lavage cells were analyzed with flow cytometry. ILC2s were gated as Lin− c-kit−/lo FcɛRI− CD127+ CD25+ T1/ST2+ CD16/32− cells, MCp as Lin−/lo c-kithi FcɛRI+ CD127− CD25− T1/ST2+ CD16/32int SSClo integrin β7hi cells, and mature mast cells (MC) as Lin−/lo c-kithi FcɛRI+ CD127− CD25− T1/ST2+ CD16/32int SSChi integrin β7−/lo/int cells. The frequency of the gated cells in each graph was calculated by dividing the gated cell numbers with the total number of peritoneal cells. (B) The ILC2s, mature mast cells, and MCp were doubly sorted. One hundred cells of each population were isolated, and a gene expression microarray analysis was performed. The gene expression profiles of ILC2s, mature mast cells, and MCp are shown as a heat map. The scale shows the log2-fold change in gene expression. Each respective population was sorted from peritoneal lavage cells pooled from five naïve mice. ILC2s, innate lymphoid cells of type 2. Color images available online at www.liebertpub.com/scd
Antibodies
The cells were stained with the following antibodies: phycoerythrin-cyanine 5-conjugated anti-lineage antibodies B220 (RA3-6B2), CD3 (17A2), CD4 (GK1.5), CD8b (ebioH35-17.2), CD11b (M1/70), CD19 (eBio1D3), Gr-1 (RB6-8C5), Ter119 (Ter-119), phycoerythrin-conjugated anti-FcɛRI (MAR1), fluorescein isothiocyanate-conjugated anti-integrin β7 (FIB504), biotinylated anti-T1/ST2 (DJ8) followed by streptavidin-allophycocyanin or streptavidin-allophycocyanin-cyanine 7, Brilliant violet 605-conjugated or Horizon V450-conjugated anti-CD16/32 (2.4G2), Horizon V450 anti-CD127 (SB/199), allophycocyanin-conjugated anti-CD25 (PC61.5), and Alexa 700-conjugated anti-CD45 (30-F11). Cultured cells were pretreated with anti-CD16/32 (2.4G2) before staining to prevent unspecific binding of antibodies. The antibodies were purchased from BD Biosciences, eBioscience (Hatfield, United Kingdom), or MD Bioproducts (Zürich, Switzerland).
Irradiation of mice
Mice were exposed to a sublethal dose (5 Gy) of whole-body γ-irradiation. Due to ethical considerations, 10,000 bone marrow cells extracted from the femurs and tibiae of naïve mice were transferred intravenously into the irradiated mice. The irradiated and reconstituted mice are referred to as γ-irradiated mice in the text.
Statistics
The data were analyzed with unpaired two-tailed Student's t-tests using Graphpad Prism 5.0d (GraphPad Software, Inc., La Jolla, CA). All P values are shown in the figures. P values less than 0.05 were considered significant.
Results
Two distinct populations of mast cells are present in mouse peritoneum
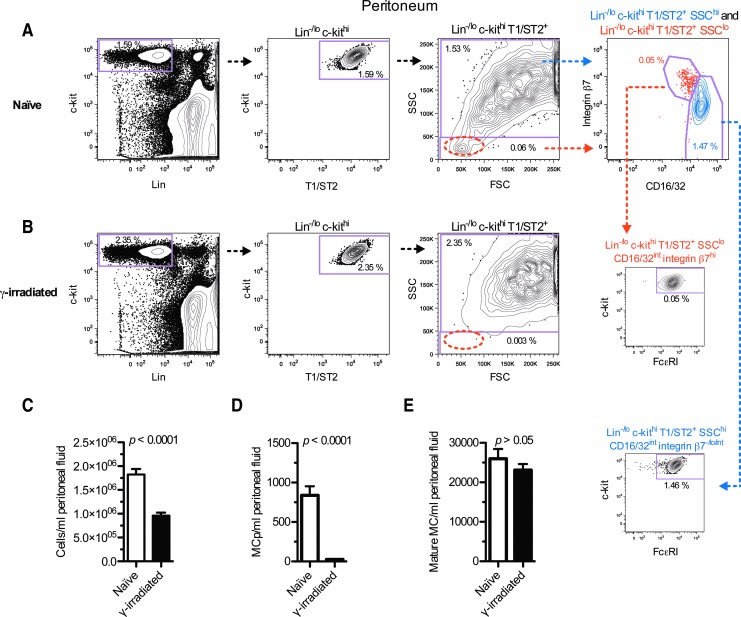
Cells from peritoneal lavage were analyzed with flow cytometry to investigate whether MCp are present at this site. A population of Lin−/lo c-kithi T1/ST2+ cells was found in naïve BALB/c mice (Fig. 1A) that constituted 1.59% of the peritoneal cells. This population could be divided into SSClo and SSChi cells. The Lin−/lo c-kithi T1/ST2+ SSClo cells expressed high levels of integrin β7, intermediate levels of CD16/32, and virtually all were positive for FcɛRI (Fig. 1A). The phenotype of these cells was similar to Lin− c-kithi T1/ST2+ integrin β7hi CD16/32hi MCp in naïve mouse blood [3]. The Lin−/lo c-kithi T1/ST2+ SSChi expressed intermediate levels of CD16/32. Further, they expressed no, low, or intermediate levels of integrin β7 and were positive for FcɛRI (Fig. 1A). Altogether, the Lin−/lo c-kithi T1/ST2+ SSChi CD16/32int integrin β7−/lo/int FcɛRI+ phenotype suggested that these cells were mature mast cells. The Lin−/lo c-kithi T1/ST2+ SSClo CD16/32int integrin β7hi FcɛRI+ and the Lin−/lo c-kithi T1/ST2+ SSChi CD16/32int integrin β7−/lo/int FcɛRI+ cells comprised 0.05% and 1.46% of the total peritoneal cells, respectively (Fig. 1A, small panels).
FIG. 1.
(A) Peritoneal cells from naïve mice were analyzed by flow cytometry. The Lin−/lo c-kithi T1/ST2+ cells were divided into SSClo and SSChi cells. The SSChi cells contained mostly CD16/32int integrin β7−/lo/int cells, whereas the SSClo fraction (encircled with a dashed ellipse) was mainly characterized as CD16/32int integrin β7hi. Virtually all Lin−/lo c-kithi T1/ST2+ SSChi CD16/32int integrin β7−/lo/int and Lin−/lo c-kithi T1/ST2+ SSClo CD16/32int integrin β7hi cells in the peritoneum expressed FcɛRI. (B) Mice were exposed to 5 Gy whole-body γ-irradiation. After 7 days, cells from peritoneal lavage were extracted and analyzed by flow cytometry. The Lin−/lo c-kithi T1/ST2+ SSChi and the Lin−/lo c-kithi T1/ST2+ SSClo (encircled with a dashed ellipse) cells were gated. (C) The total number of cells per milliliter peritoneal fluid was assessed in naïve and γ-irradiated mice. (D) The number of Lin−/lo c-kithi T1/ST2+ SSClo CD16/32int integrin β7hi FcɛRI+ MCp per milliliter peritoneal fluid was quantified in naïve and γ-irradiated mice. (E) The number of Lin−/lo c-kithi T1/ST2+ SSChi CD16/32int integrin β7−/lo/int FcɛRI+ mature mast cells (MC) was quantified in naïve and γ-irradiated mice. The data in (C–E) are derived from three pooled experiments where both groups were evaluated in parallel. Each group consisted of nine animals. The means and SEM are shown. The frequency of the gated cells in each graph was calculated by dividing the gated cell numbers with the total number of peritoneal cells. MCp, mast cell progenitors; SSC, side scatter. Color images available online at www.liebertpub.com/scd
Lin−/lo c-kithi T1/ST2+ SSClo CD16/32int integrin β7hi FcɛRI+ MCp are depleted by γ-irradiation
To provide evidence that Lin−/lo c-kithi T1/ST2+ SSClo CD16/32int integrin β7hi FcɛRI+ cells were MCp and Lin−/lo c-kithi T1/ST2+ SSChi CD16/32int integrin β7−/lo/int FcɛRI+ cells were mature mast cells, their sensitivity to γ-irradiation was studied. The mice were exposed to 5 Gy of whole-body γ-irradiation. After 1 week, the total number of cells per milliliter peritoneal fluid was reduced with 48% in γ-irradiated mice in comparison with naïve mice (Fig. 1C). Mice exposed to 5 Gy whole-body γ-irradiation lost 97% of the Lin−/lo c-kithi T1/ST2+ SSClo CD16/32int integrin β7hi FcɛRI+ MCp per milliliter peritoneal fluid (Fig. 1B, D). In contrast, the number of Lin−/lo c-kithi T1/ST2+ SSChi CD16/32int integrin β7−/lo/int FcɛRI+ mature mast cells in peritoneum was intact (Fig. 1B, E).
Lin−/lo c-kithi T1/ST2+ SSClo CD16/32int integrin β7hi FcɛRI+ MCp have started developing metachromatic granules
To elucidate the appearance of the peritoneal mast cell population, the MCp and the mature mast cells were isolated using FACS and stained with May-Grünwald Giemsa (Fig. 2A, B). The Lin−/lo c-kithi T1/ST2+ SSClo CD16/32int integrin β7hi FcɛRI+ MCp were small, 15.7±0.4 μm (mean±SEM) in diameter, with a round nucleus that filled up a large portion of the cytoplasm (Fig. 2A, C). The Lin−/lo c-kithi T1/ST2+ SSChi CD16/32int integrin β7−/lo/int FcɛRI+ mature mast cells were larger, with a diameter of 22.7±0.3 μm (Fig. 2B, C). The MCp contained metachromatic granules (Fig. 2A). However, the mature mast cells had higher numbers of granules, which appeared more densely stained than those of MCp (compare Fig. 2A and B). The difference in metachromatic granule staining between MCp and mature mast cells was quantified using an image analysis software. The mature mast cells had a larger metachromatic area per cell than MCp (Fig. 2D).
FIG. 2.
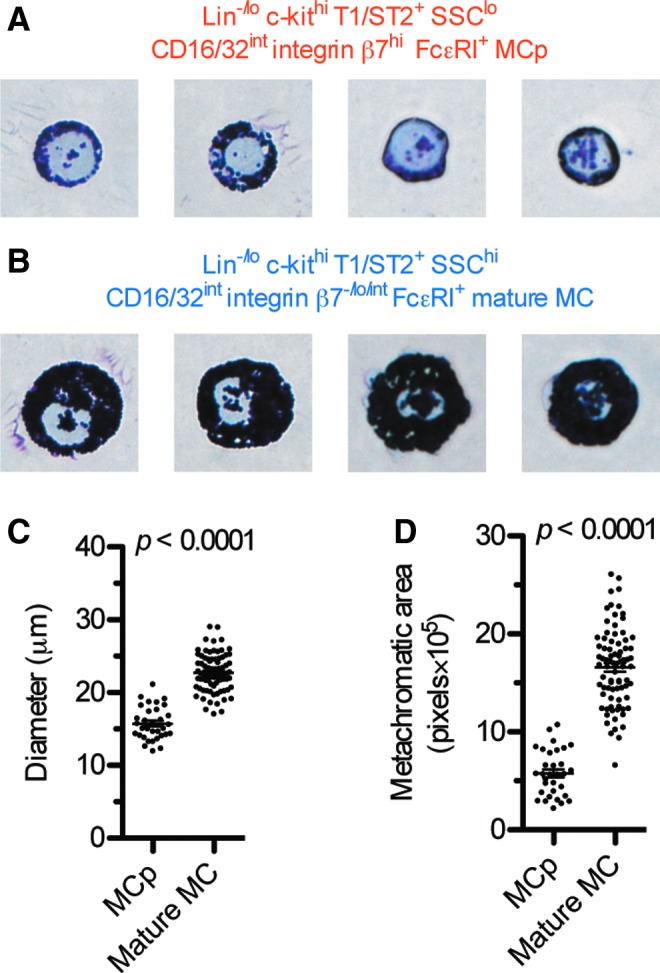
(A, B) Lin−/lo c-kithi T1/ST2+ SSClo CD16/32int integrin β7hi FcɛRI+ MCp and Lin−/lo c-kithi T1/ST2+ SSChi CD16/32int integrin β7−/lo/int FcɛRI+ mature mast cells (MC) were doubly sorted by FACS and stained with May-Grünwald Giemsa. Original magnification, 40×. The width of each photo corresponds to 32 μm. (C, D) The diameter and the metachromatic area of the MCp and the mature mast cells were measured. Each dot in (C, D) represents one cell. The means and SEM are shown. The cells in (A–D) were sorted from three to six mice in two separate experiments. Cells from both experiments are shown in the figure. FACS, fluorescence-activated cell sorting. Color images available online at www.liebertpub.com/scd
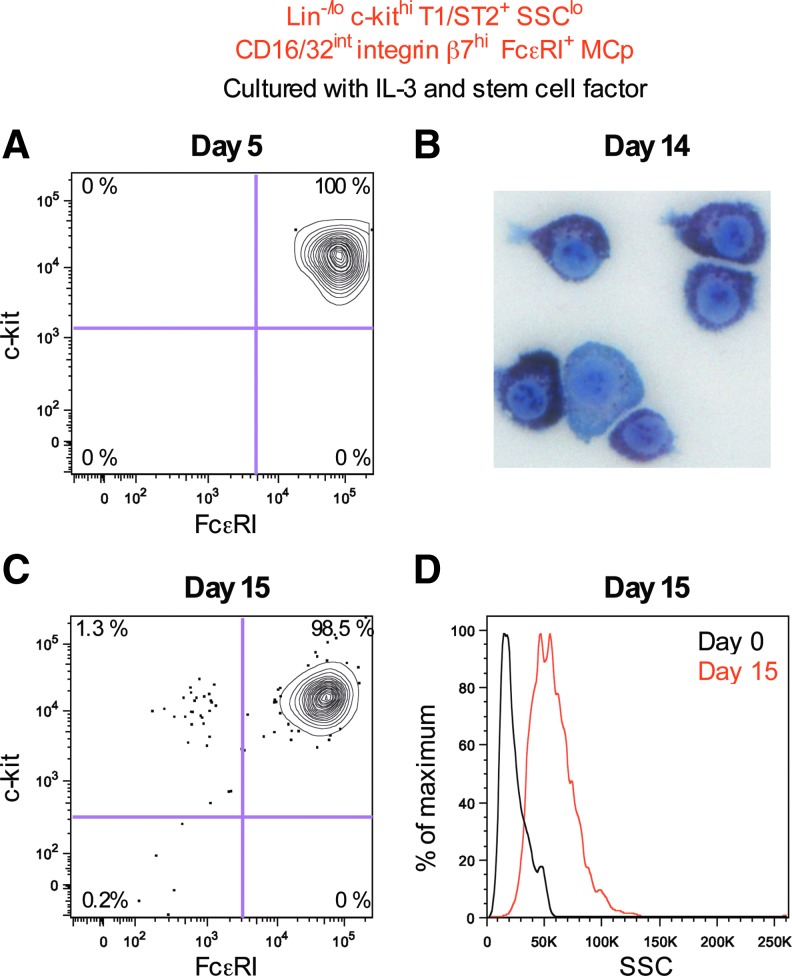
Lin−/lo c-kithi T1/ST2+ SSClo CD16/32int integrin β7hi FcɛRI+ MCp mature in vitro
The Lin−/lo c-kithi T1/ST2+ SSClo CD16/32int integrin β7hi FcɛRI+ MCp were cultured with IL-3 and stem cell factor to verify that the cells give rise to mast cells in vitro. After 5 days in culture, the MCp had proliferated and consisted of 100% c-kit+ FcɛRI+ mast cells (Fig. 3A). The cultured cells had a round nucleus and metachromatic granules after 14 days in culture, characteristics of mast cells (Fig. 3B). After 15 days in culture, 98.5% of the cells were c-kit+ FcɛRI+ mast cells (Fig. 3C). To confirm that MCp cultured in vitro (day 15) were more mature than primary MCp (day 0), the SSC light parameter was studied (Fig. 3D). The gating of the primary MCp was performed without the use of the SSC light parameter, that is, these primary cells were defined as Lin−/lo c-kithi T1/ST2+ CD16/32int integrin β7hi FcɛRI+ cells. After 15 days, the in vitro cultured MCp had a higher SSC light profile than the primary MCp (Fig. 3D).
FIG. 3.
Doubly sorted Lin−/lo c-kithi T1/ST2+ SSClo CD16/32int integrin β7hi FcɛRI+ MCp were cultured with IL-3 and stem cell factor. The cultured cells were analyzed after (A) 5 days, (B) 14 days, and (C, D) 15 days. Flow cytometric analysis was performed in (A, C, D), whereas May-Grünwald Giemsa staining was performed in (B). Primary MCp gated without the use of the SSC light profile (Lin−/lo c-kithi T1/ST2+ CD16/32int integrin β7hi FcɛRI+) were used as day 0 control in (D, black line). The cultured MCp were derived from six naïve mice in (A–D). The day 0 peritoneal MCp gated in (D) were from four naïve mice. The width of the photo in (C) corresponds to 64 μm. Color images available online at www.liebertpub.com/scd
MCp have fewer mast cell-specific protease transcripts than mature mast cells
We performed a gene expression microarray analysis to characterize the peritoneal MCp and to verify that these cells were immature. Two cell populations from the same anatomical site were selected as references: closely related mature mast cells and distantly related innate lymphoid cells of type 2 (ILC2s). ILC2s have been described as Lin− T1/ST2+ CD127+ cells in the mesenteric lymph nodes of IL-25-treated mice [9]. These cells express CD25 [9]. New gates based on CD25 and CD127 expression were, therefore, implemented into the gating strategy of the peritoneal lavage cells. Lin− c-kit−/lo FcɛRI− CD127+ CD25+ T1/ST2+ CD16/32− cells were identified in the peritoneum of naïve mice, a phenotype similar to ILC2s in the mesenteric lymph nodes (Fig. 4A).
The gene expression microarray analysis was focused on a number of mast cell and basophil-related genes coding for proteases, since several of the proteases are located in the granules and the MCp appeared immature in terms of granule formation. The peritoneal ILC2s lacked metachromatic granules, suggesting that these cells were a suitable negative control for these genes (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/scd). Transcripts of the genes Ndst2, which is critical for the synthesis of fully sulfated heparin [10], and Hdc, which is required for the formation of histamine [11], were also studied.
MCp and mature mast cells in the peritoneum were sorted as Lin−/lo c-kithi FcɛRI+ CD127− CD25− T1/ST2+ CD16/32int SSClo integrin β7hi and Lin−/lo c-kithi FcɛRI+ CD127− CD25− T1/ST2+ CD16/32int SSChi integrin β7−/lo/int cells, respectively (Fig. 4A). One hundred cells of the MCp, the mature mast cells, and the ILC2s were isolated and analyzed for gene expression. The mRNA expression of Cpa3, which is known to be upregulated early in cells of the mast cell lineage [12], was high in both MCp and mature mast cells (Fig. 4B). The genes Mcpt4 and Mcpt5, coding for mast cell proteases, were expressed by MCp, but at lower levels than mature mast cells (Fig. 4B). The protease genes Mcpt6, Mcpt7, and Cma2 (Mcpt10) as well as the genes Ndst2 and Hdc were mainly expressed by mature mast cells (Fig. 4B). MCp and mature mast cells in the peritoneum expressed little or no Mcpt1, Mcpt2, Mcpt8, and Mcpt9 (Fig. 4B). In conclusion, the peritoneal MCp had started developing into connective tissue-type mast cells, as they express Mcpt4 and Mcpt5. However, the MCp are immature, as the expression of Mcpt6, Mcpt7, Mcpt10, Ndst2, and Hdc is low or absent in these cells.
Integrin β7 expression and the SSC profile can be used to distinguish between pulmonary MCp and mature mast cells
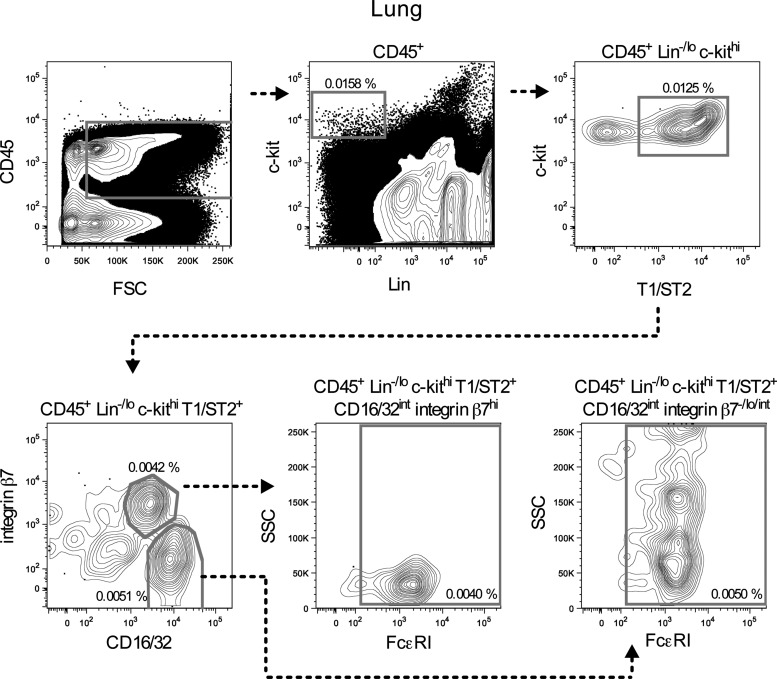
A similar gating strategy to the one used to distinguish peritoneal MCp from mature mast cells was applied to lung cells. In addition to the markers used in peritoneum, CD45 was used to exclude stromal cells from the analysis. In naïve mice, nonapoptotic CD45+ Lin−/lo c-kithi T1/ST2+ CD16/32int integrin β7hi FcɛRI+ SSClo MCp constituted only 0.0040% of the CD45+ lung cells (Fig. 5). Mature mast cells, identified as CD45+ Lin−/lo c-kithi T1/ST2+ CD16/32int integrin β7−/lo/int FcɛRI+ SSChi cells, were also rare and constituted 0.0050% of the CD45+ lung cells (Fig. 5).
FIG. 5.
Cells from naïve mouse lungs were analyzed by flow cytometry. CD45+ Lin−/lo c-kithi T1/ST2+ CD16/32int integrin β7hi cells expressed FcɛRI and had a low SSC light profile, suggesting that these cells were MCp. CD45+ Lin−/lo c-kithi T1/ST2+ CD16/32int integrin β7−/lo/int cells also expressed FcɛRI, but had a high SSC light profile, indicating that that they were mature mast cells. Pooled cells from three to four mice were analyzed. The graphs are derived from one out of two similar experiments. The frequency of the gated cells in each graph was calculated by dividing the gated cell numbers with the number of CD45+ lung cells.
Discussion
Progenitors committed to the mast cell lineage are identified as Lin− c-kithi T1/ST2+ CD16/32hi integrin β7hi cells in the blood of naïve mice [3]. Similar to the blood MCp, we identified a population of Lin−/lo c-kithi T1/ST2+ SSClo CD16/32int integrin β7hi cells in the peritoneum of naïve BALB/c mice. Virtually all these cells expressed FcɛRI (98.8%±0.4%). In contrast, 66% of the committed MCp that circulate in the blood of BALB/c mice express FcɛRI [3]. These data suggest that peritoneal MCp are more mature than blood MCp. The mature mast cells in the peritoneal cavity were identified as Lin−/lo c-kithi T1/ST2+ SSChi CD16/32int integrin β7−/lo/int FcɛRI+ cells. Thus, the SSC light profile and the expression of integrin β7 are two parameters that differ between MCp and mature mast cells in the peritoneum. The SSC profile of the mature mast cells in peritoneum was high, likely due to the granulation. This is consistent with in vitro studies reporting that the SSC light profile is increased with time when bone marrow-derived mast cells mature in culture [5]. Our data also indicate that the mature mast cells in vivo have a reduced integrin β7 expression compared with MCp. Similarly, bone marrow-derived mast cells lose their integrin β7 expression on maturation in vitro [5].
Several lines of evidence support our conclusion that the Lin−/lo c-kithi T1/ST2+ SSClo CD16/32int integrin β7hi FcɛRI+ cells are MCp. (1) They are depleted from the peritoneum by whole-body γ-irradiation. The fact that MCp are sensitive to γ-irradiation was previously shown for intestinal MCp [13]. (2) The isolated MCp were small cells with a mean diameter of 15.7 μm. Their nucleus filled up a large portion of the cytoplasm, and the MCp had a smaller metachromatic area per cell than the mature mast cells. In fact, the metachromatic areas in the mature mast cells were approximately thrice larger than in MCp. Peritoneal MCp from rats have previously been visualized by transmission electron microscopy [14]. Similar to our data, these cells have a large nucleus and contain granules [14]. (3) The isolated peritoneal MCp differentiated into mast cells with a higher SSC light profile on in vitro culture. (4) A gene expression microarray analysis showed that the MCp expressed Cpa3, Mcpt4, and Mcpt5, suggesting that the MCp are committed to the mast cell lineage. The low or absent expression of Mcpt6, Mcpt7, Mcpt10, Ndst2, and Hdc confirms that the MCp are immature. The mature peritoneal mast cells expressed high levels of Cpa3, Ndst2, Mcpt4, Mcpt5, Mcpt6, and Mcpt10. Transcripts of Cpa3, Mcpt4, Mcpt5, Mcpt6, and Mcpt10 have previously been found in gene expression microarrays of total peritoneal cells of wild-type mice [12]. In contrast, expression of the same genes was undetectable in the mast cell-deficient strains Cpa3Cre/+ and KitW/Wv [12]. These data suggest that gene expression of Cpa3, Mcpt4, Mcpt5, Mcpt6, and Mcpt10 is restricted to mast cells, which are of connective tissue type in the peritoneal cavity. The low or absent number of transcripts of Mcpt1, Mcpt2, Mcpt8, and Mcpt9 in peritoneal mast cells and their progenitors is expected, since Mcpt1 and Mcpt2 expression is mainly found in mucosal mast cells in the intestine [15], Mcpt8 in basophils [16], and Mcpt9 in uterine mast cells [17]. In addition, the expression of Mcpt1, Mcpt2, Mcpt8, and Mcpt9 in total peritoneal cells is below the detection limit in wild-type, Cpa3Cre/+, and KitW/Wv mice [12]. Altogether, these data strongly suggest the presence of committed MCp in the peritoneum of naïve mice.
MCp circulating in the blood transmigrate into the lung in a mouse model of allergic airway inflammation [18]. At least some of these MCp mature in the lung tissue [19,20]. The number of lung MCp have previously been quantified using a limiting dilution and clonal expansion assay [21]. Here, a gating strategy similar to the one used for peritoneal MCp was applied to identify MCp in the lung. CD45+ Lin−/lo c-kithi T1/ST2+ CD16/32int integrin β7hi FcɛRI+ SSClo cells, similar to the phenotype of MCp in peritoneum, were identified in mouse lungs. These results are in agreement with a study by Bankova et al. that was published during the preparation of this paper [22]. In a mouse model of allergic airway inflammation, they identify lung cells expressing c-kit and FcɛRI that have a low SSC light profile and high expression of integrin β7 as MCp. As the inflammation is resolved, the number of MCp with a high integrin β7 expression decreases, likely since they convert into mast cells with a higher SSC light profile [22]. Mature mast cells have been reported in the lungs by histochemistry, even in mice without induced allergic airway inflammation [23]. Similarly, in our study, mature mast cells were identified as CD45+ Lin−/lo c-kithi T1/ST2+ CD16/32int integrin β7−/lo/int FcɛRI+ SSChi cells in the lungs of naïve mice. No clear population of mature mast cells was observed by flow cytometry in noninflamed lungs in the study by Bankova et al. [22]. The discrepancy is likely explained by the fact that in our report, enormous numbers of events were recorded in the flow cytometric analysis and a large panel of lineage antibodies was used to remove irrelevant cells from the analysis of mature mast cells. It is of interest to note that the relative proportions between MCp and mature mast cells differed substantially between the lung and the peritoneum of naïve mice. MCp in the lung constituted 45% of the total mast cells (the sum of MCp and mature mast cells). In contrast, the MCp constituted only 3% of the total mast cells in the peritoneum. The peritoneal MCp expressed transcripts of Mcpt4 and Mcpt5, which suggest that they are maturing into connective tissue-type mast cells. Future studies will hopefully reveal whether the lung MCp have a similar expression profile, or whether these cells have started developing a mucosal phenotype.
MCp migration to the peritoneal cavity and their development into mature mast cells have been previously studied. Intraperitoneal injection of distilled water into both mice and rats leads to a rapid depletion of peritoneal mast cells [14,24]. In mice, one of the first cell types to repopulate the peritoneal cavity after distilled water injection has the capacity to form large mast cell colonies in vitro [24]. These newly arrived cells have a low density, which suggest that they are MCp with no or few granules [24]. The mature mast cells, which have a high density and form small mast cell colonies in vitro, appear later in the peritoneal cavity, likely due to MCp maturing in situ [24]. In rats, peritoneal mast cell depletion results in a rapid release of MCp from bone marrow into the blood circulation [14]. After the frequency of blood MCp is elevated, MCp repopulate the peritoneal cavity [14]. This is followed by the appearance of more mature mast cells [14]. Altogether, these studies suggest that MCp migrate through the blood, enter the peritoneal cavity, and mature in situ. The blood, the lung, and the peritoneal MCp express similar surface markers, with the exception that the fraction of FcɛRI-expressing MCp is lower in the blood than in the lung and the peritoneal cavity (Figs. 1 and 5) [3]. As FcɛRI can be used as a marker for mast cell maturation, it is tempting to speculate that the blood MCp is a precursor of both lung and peritoneal MCp [3]. However, to verify whether the blood MCp are ancestors of the lung and peritoneal MCp is beyond the scope of this study.
In conclusion, we have provided evidence for how to distinguish between MCp and mature mast cells in mouse peritoneum and lung by flow cytometry. The main criterion is to include integrin β7 in the antibody panel. One population has a high expression of integrin β7 and a low SSC light profile, which is consistent with MCp. The other population consists of mature mast cells, as they have a lower expression of integrin β7 and a high SSC light profile. The gating strategy that we have applied can be used not only to quantify MCp with flow cytometry but also to correctly assess the number of mature mast cells. The gene expression microarray analysis confirms the maturation status of the MCp and the mature mast cells, and it allows data mining to identify novel genes that are specifically expressed by the two separate mast cell populations.
Supplementary Material
Acknowledgments
J.S.D. is supported by grants from Agnes and Mac Rudberg Foundation, Lennander's Foundation, the Royal Swedish Academy of Sciences, and Ellen, Walter, and Lennart Hesselman Foundation. J.H. is supported by grants from the Swedish Research Council, Bror Hjerpstedt Foundation, and Malin and Lennart Philipson Foundation. Z.D. is supported by grants from the Swedish Research Council given to Prof. Birgitta Heyman (Uppsala University). The authors would like to thank Prof. Birgitta Heyman for critically reading this article. The Array and Analysis Facility at the Science for Life Laboratory [Uppsala Biomedical Center (BMC), Uppsala] performed the gene expression microarray analysis. We performed the flow cytometric analysis and the cell sorting on instruments provided by the BioVis facility at the Science for Life Laboratory.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Kitamura Y, Shimada M, Hatanaka K. and Miyano Y. (1977). Development of mast cells from grafted bone marrow cells in irradiated mice. Nature 268:442–443 [DOI] [PubMed] [Google Scholar]
- 2.Chen CC, Grimbaldeston MA, Tsai M, Weissman IL. and Galli SJ. (2005). Identification of mast cell progenitors in adult mice. Proc Natl Acad Sci U S A 102:11408–11413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dahlin JS, Heyman B. and Hallgren J. (2013). Committed mast cell progenitors in mouse blood differ in maturity between Th1 and Th2 strains. Allergy 68:1333–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arinobu Y, Iwasaki H, Gurish MF, Mizuno S, Shigematsu H, Ozawa H, Tenen DG, Austen KF. and Akashi K. (2005). Developmental checkpoints of the basophil/mast cell lineages in adult murine hematopoiesis. Proc Natl Acad Sci U S A 102:18105–18110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weller CL, Collington SJ, Brown JK, Miller HR, Al Kashi A, Clark P, Jose PJ, Hartnell A. and Williams TJ. (2005). Leukotriene B4, an activation product of mast cells, is a chemoattractant for their progenitors. J Exp Med 201:1961–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li C. and Wong WH. (2001). Model-based analysis of oligonucleotide arrays: expression index computation and outlier detection. Proc Natl Acad Sci U S A 98:31–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U. and Speed TP. (2003). Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4:249–264 [DOI] [PubMed] [Google Scholar]
- 8.Sturn A, Quackenbush J. and Trajanoski Z. (2002). Genesis: cluster analysis of microarray data. Bioinformatics 18:207–208 [DOI] [PubMed] [Google Scholar]
- 9.Saenz SA, Siracusa MC, Monticelli LA, Ziegler CG, Kim BS, Brestoff JR, Peterson LW, Wherry EJ, Goldrath AW, Bhandoola A. and Artis D. (2013). IL-25 simultaneously elicits distinct populations of innate lymphoid cells and multipotent progenitor type 2 (MPPtype2) cells. J Exp Med 210:1823–1837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forsberg E, Pejler G, Ringvall M, Lunderius C, Tomasini-Johansson B, Kusche-Gullberg M, Eriksson I, Ledin J, Hellman L. and Kjellen L. (1999). Abnormal mast cells in mice deficient in a heparin-synthesizing enzyme. Nature 400:773–776 [DOI] [PubMed] [Google Scholar]
- 11.Tran VT. and Snyder SH. (1981). Histidine decarboxylase. Purification from fetal rat liver, immunologic properties, and histochemical localization in brain and stomach. J Biol Chem 256:680–686 [PubMed] [Google Scholar]
- 12.Feyerabend TB, Weiser A, Tietz A, Stassen M, Harris N, Kopf M, Radermacher P, Moller P, Benoist C, et al. (2011). Cre-mediated cell ablation contests mast cell contribution in models of antibody- and T cell-mediated autoimmunity. Immunity 35:832–844 [DOI] [PubMed] [Google Scholar]
- 13.Gurish MF, Tao H, Abonia JP, Arya A, Friend DS, Parker CM. and Austen KF. (2001). Intestinal mast cell progenitors require CD49dβ7 (α4β7 integrin) for tissue-specific homing. J Exp Med 194:1243–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jamur MC, Moreno AN, Mello LF, Souza Junior DA, Campos MR, Pastor MV, Grodzki AC, Silva DC. and Oliver C. (2010). Mast cell repopulation of the peritoneal cavity: contribution of mast cell progenitors versus bone marrow derived committed mast cell precursors. BMC Immunol 11:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friend DS, Ghildyal N, Austen KF, Gurish MF, Matsumoto R. and Stevens RL. (1996). Mast cells that reside at different locations in the jejunum of mice infected with Trichinella spiralis exhibit sequential changes in their granule ultrastructure and chymase phenotype. J Cell Biol 135:279–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ugajin T, Kojima T, Mukai K, Obata K, Kawano Y, Minegishi Y, Eishi Y, Yokozeki H. and Karasuyama H. (2009). Basophils preferentially express mouse mast cell protease 11 among the mast cell tryptase family in contrast to mast cells. J Leukoc Biol 86:1417–1425 [DOI] [PubMed] [Google Scholar]
- 17.Hunt JE, Friend DS, Gurish MF, Feyfant E, Sali A, Huang C, Ghildyal N, Stechschulte S, Austen KF. and Stevens RL. (1997). Mouse mast cell protease 9, a novel member of the chromosome 14 family of serine proteases that is selectively expressed in uterine mast cells. J Biol Chem 272:29158–29166 [DOI] [PubMed] [Google Scholar]
- 18.Abonia JP, Hallgren J, Jones T, Shi T, Xu Y, Koni P, Flavell RA, Boyce JA, Austen KF. and Gurish MF. (2006). Alpha-4 integrins and VCAM-1, but not MAdCAM-1, are essential for recruitment of mast cell progenitors to the inflamed lung. Blood 108:1588–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dahlin JS, Feinstein R, Cui Y, Heyman B. and Hallgren J. (2012). CD11c+ cells are required for antigen-induced increase of mast cells in the lung. J Immunol 189:3869–3877 [DOI] [PubMed] [Google Scholar]
- 20.Hallgren J, Jones TG, Abonia JP, Xing W, Humbles A, Austen KF. and Gurish MF. (2007). Pulmonary CXCR2 regulates VCAM-1 and antigen-induced recruitment of mast cell progenitors. Proc Natl Acad Sci U S A 104:20478–20483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dahlin JS. and Hallgren J. (2015). Mast cell progenitors: Origin, development and migration to tissues. Mol Immunol 63:9–17 [DOI] [PubMed] [Google Scholar]
- 22.Bankova LG, Dwyer DF, Liu AY, Austen KF. and Gurish MF. (2014). Maturation of mast cell progenitors to mucosal mast cells during allergic pulmonary inflammation in mice. Mucosal Immunol [Epub ahead of print]; DOI: 10.1038/mi.2014.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xing W, Austen KF, Gurish MF. and Jones TG. (2011). Protease phenotype of constitutive connective tissue and of induced mucosal mast cells in mice is regulated by the tissue. Proc Natl Acad Sci U S A 108:14210–14215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanakura Y, Kuriu A, Waki N, Nakano T, Asai H, Yonezawa T. and Kitamura Y. (1988). Changes in numbers and types of mast cell colony-forming cells in the peritoneal cavity of mice after injection of distilled water: evidence that mast cells suppress differentiation of bone marrow-derived precursors. Blood 71:573–580 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.