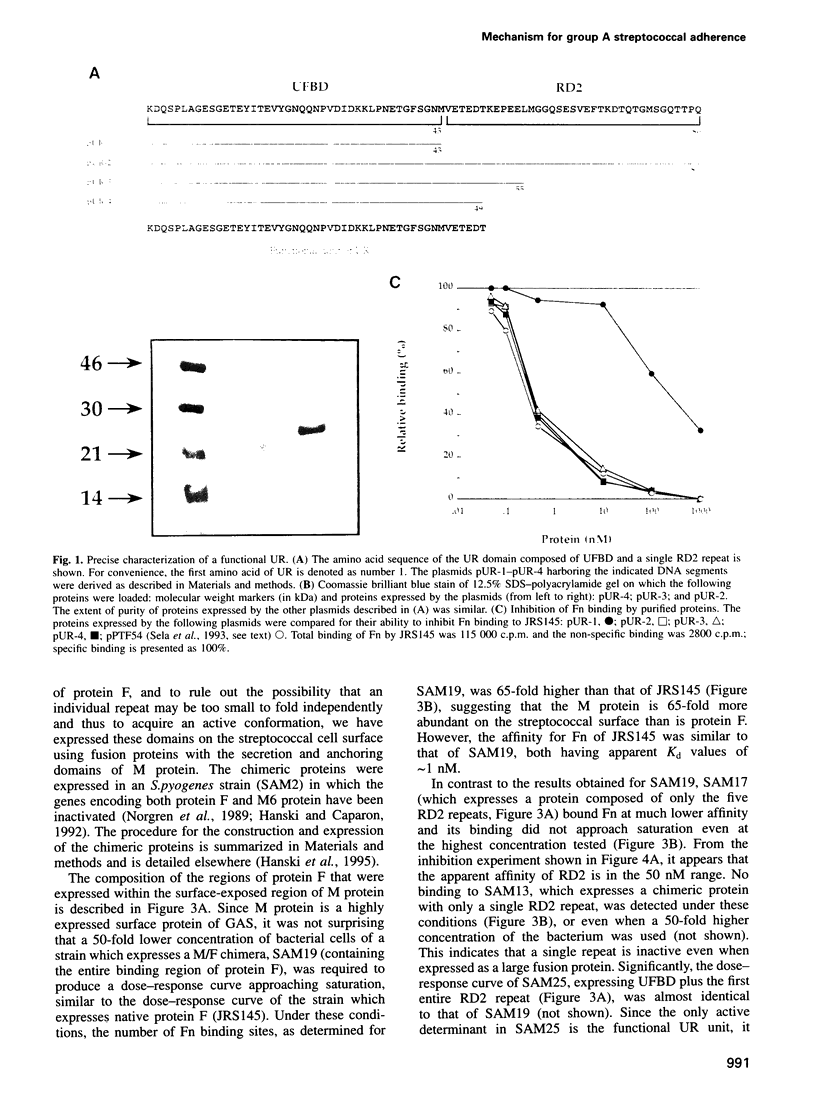
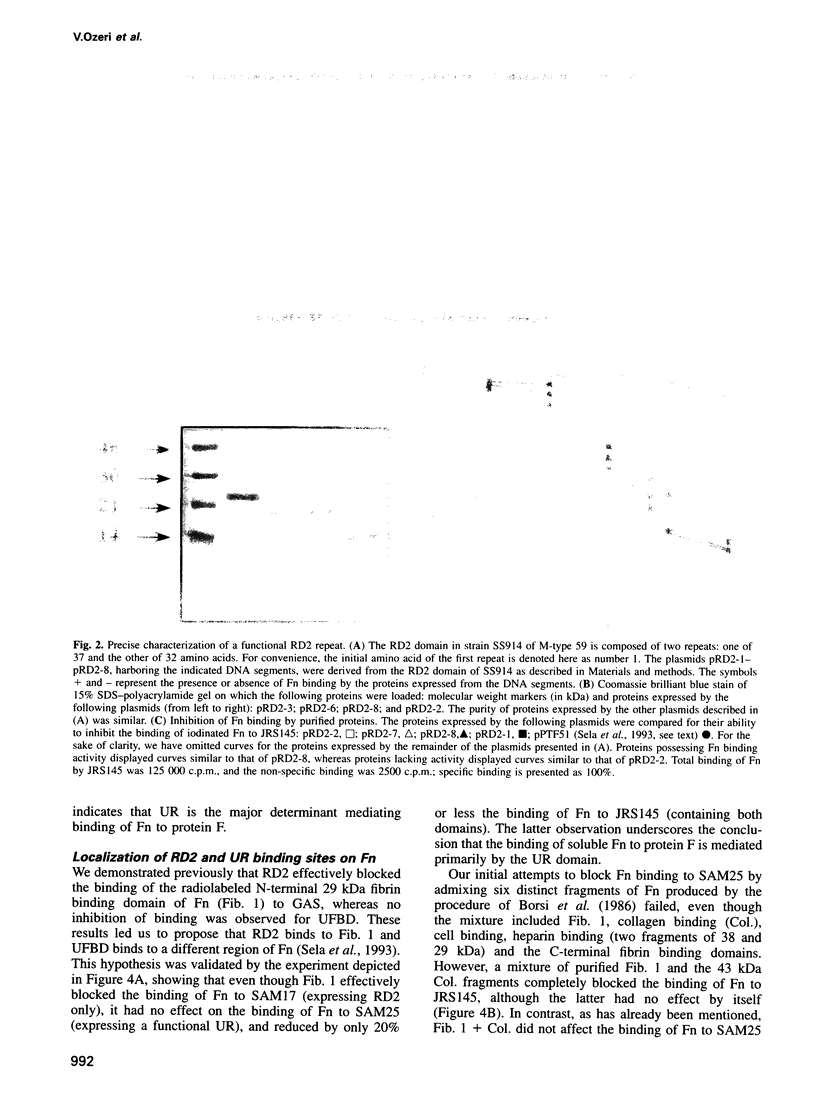
Abstract
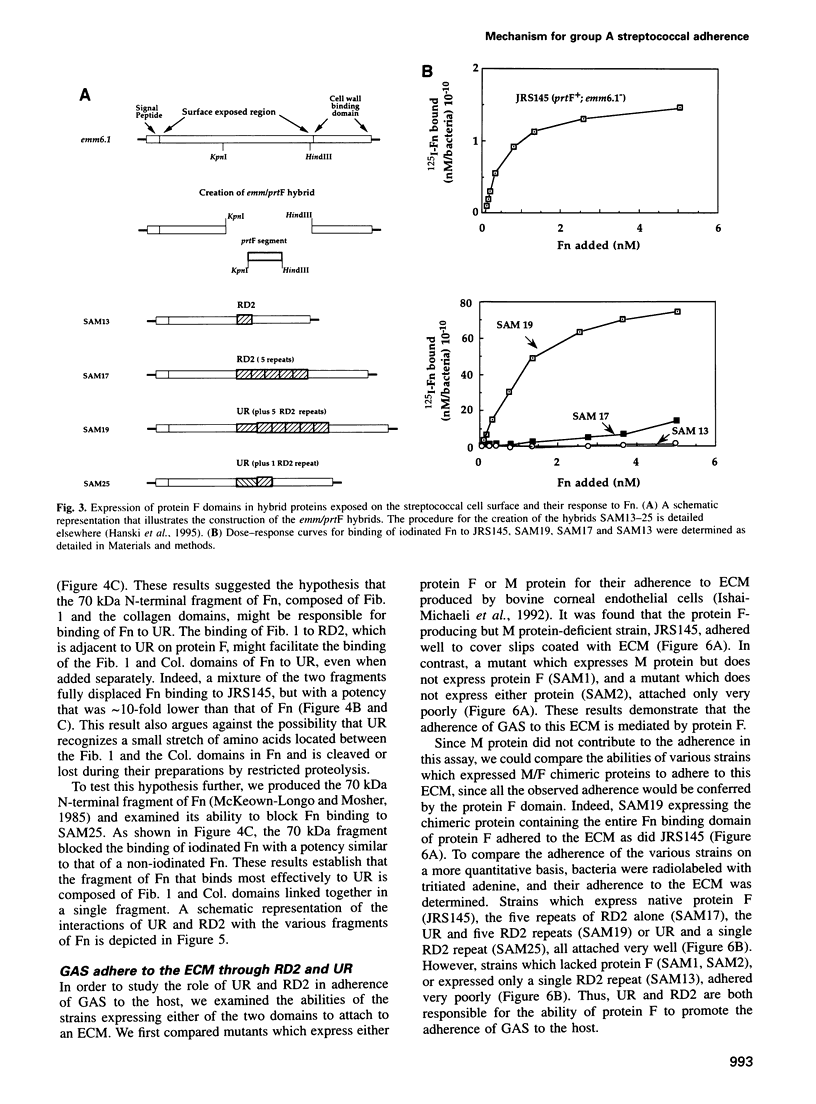
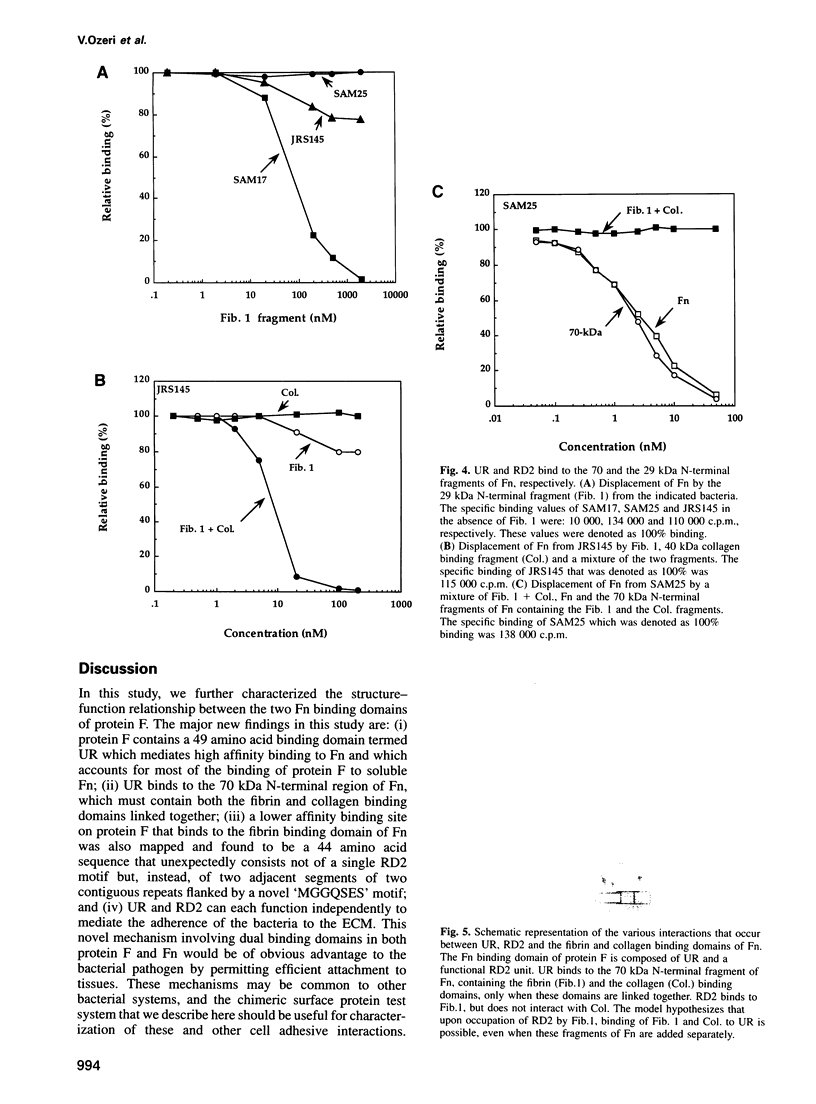
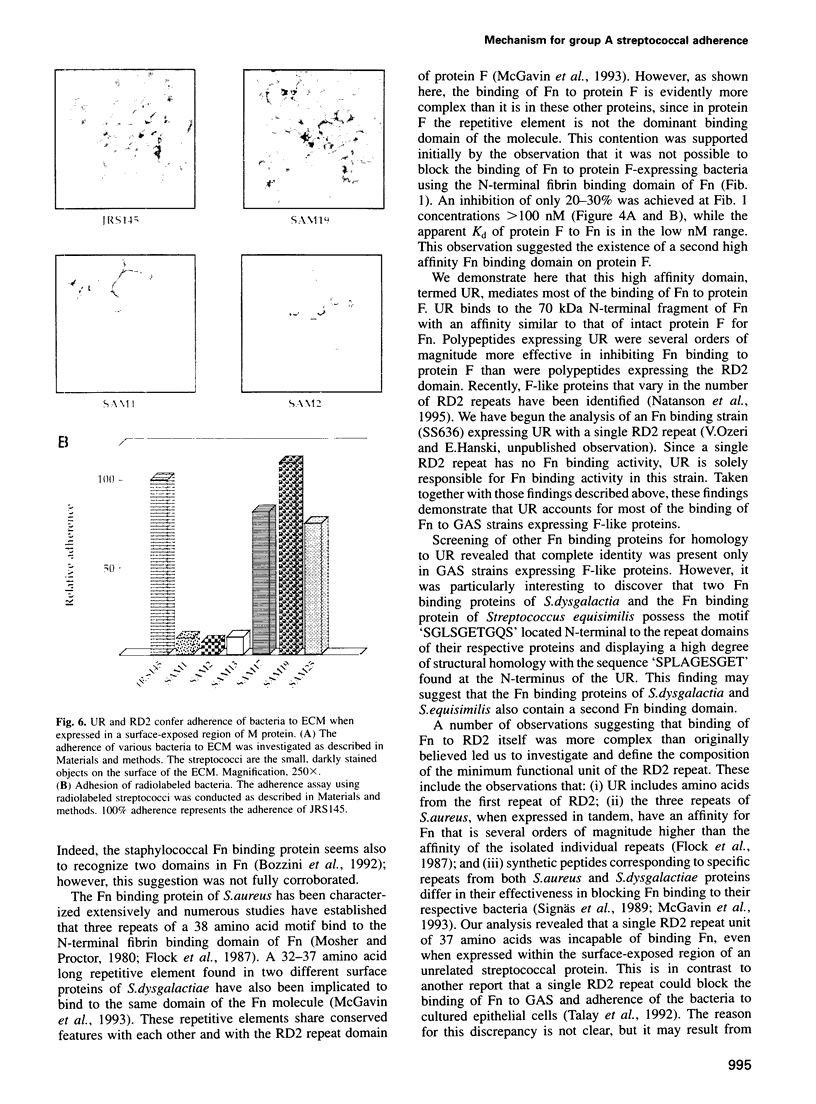
Streptococcus pyogenes binds to the extracellular matrix (ECM) and a variety of host cells and tissues, causing diverse human diseases. Protein F, a S.pyogenes adhesin that binds fibronectin (Fn), contains two binding domains. A repeated domain (RD2) and an additional domain (UR), located immediately N-terminal to RD2. Both domains are required for maximal Fn binding. In this study, we characterize RD2 and UR precisely and compare their functions and binding sites in Fn. The minimal functional unit of RD2 is of 44 amino acids, with contributions from two adjacent RD2 repeats flanked by a novel 'MGGQSES' motif. RD2 binds to the N-terminal fibrin binding domain of Fn. UR contains 49 amino acids, of which six are from the first repeat of RD2. It binds to Fn with higher affinity than RD2, and recognizes a larger fragment that contains fibrin and collagen binding domains. Expression of UR and RD2 independently on the surface-exposed region of unrelated streptococcal protein demonstrates that both mediate adherence of the bacteria to the ECM. We describe here a mechanism of adherence of a pathogen that involves two pairs of sites located on a single adhesin molecule and directed at the same host receptor.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bisno A. L. Group A streptococcal infections and acute rheumatic fever. N Engl J Med. 1991 Sep 12;325(11):783–793. doi: 10.1056/NEJM199109123251106. [DOI] [PubMed] [Google Scholar]
- Borsi L., Castellani P., Balza E., Siri A., Pellecchia C., De Scalzi F., Zardi L. Large-scale procedure for the purification of fibronectin domains. Anal Biochem. 1986 Jun;155(2):335–345. doi: 10.1016/0003-2697(86)90443-4. [DOI] [PubMed] [Google Scholar]
- Bozzini S., Visai L., Pignatti P., Petersen T. E., Speziale P. Multiple binding sites in fibronectin and the staphylococcal fibronectin receptor. Eur J Biochem. 1992 Jul 1;207(1):327–333. doi: 10.1111/j.1432-1033.1992.tb17054.x. [DOI] [PubMed] [Google Scholar]
- Flock J. I., Fröman G., Jönsson K., Guss B., Signäs C., Nilsson B., Raucci G., Hök M., Wadström T., Lindberg M. Cloning and expression of the gene for a fibronectin-binding protein from Staphylococcus aureus. EMBO J. 1987 Aug;6(8):2351–2357. doi: 10.1002/j.1460-2075.1987.tb02511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanski E., Caparon M. Protein F, a fibronectin-binding protein, is an adhesin of the group A streptococcus Streptococcus pyogenes. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6172–6176. doi: 10.1073/pnas.89.13.6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanski E., Fogg G., Tovi A., Okada N., Burstein I., Caparon M. Molecular analysis of Streptococcus pyogenes adhesion. Methods Enzymol. 1995;253:269–305. doi: 10.1016/s0076-6879(95)53025-8. [DOI] [PubMed] [Google Scholar]
- Hanski E., Horwitz P. A., Caparon M. G. Expression of protein F, the fibronectin-binding protein of Streptococcus pyogenes JRS4, in heterologous streptococcal and enterococcal strains promotes their adherence to respiratory epithelial cells. Infect Immun. 1992 Dec;60(12):5119–5125. doi: 10.1128/iai.60.12.5119-5125.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasty D. L., Ofek I., Courtney H. S., Doyle R. J. Multiple adhesins of streptococci. Infect Immun. 1992 Jun;60(6):2147–2152. doi: 10.1128/iai.60.6.2147-2152.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishai-Michaeli R., Svahn C. M., Weber M., Chajek-Shaul T., Korner G., Ekre H. P., Vlodavsky I. Importance of size and sulfation of heparin in release of basic fibroblast growth factor from the vascular endothelium and extracellular matrix. Biochemistry. 1992 Feb 25;31(7):2080–2088. doi: 10.1021/bi00122a027. [DOI] [PubMed] [Google Scholar]
- Jönsson K., Signäs C., Müller H. P., Lindberg M. Two different genes encode fibronectin binding proteins in Staphylococcus aureus. The complete nucleotide sequence and characterization of the second gene. Eur J Biochem. 1991 Dec 18;202(3):1041–1048. doi: 10.1111/j.1432-1033.1991.tb16468.x. [DOI] [PubMed] [Google Scholar]
- McGavin M. J., Gurusiddappa S., Lindgren P. E., Lindberg M., Raucci G., Hök M. Fibronectin receptors from Streptococcus dysgalactiae and Staphylococcus aureus. Involvement of conserved residues in ligand binding. J Biol Chem. 1993 Nov 15;268(32):23946–23953. [PubMed] [Google Scholar]
- McKeown-Longo P. J., Mosher D. F. Interaction of the 70,000-mol-wt amino-terminal fragment of fibronectin with the matrix-assembly receptor of fibroblasts. J Cell Biol. 1985 Feb;100(2):364–374. doi: 10.1083/jcb.100.2.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher D. F., Proctor R. A. Binding and factor XIIIa-mediated cross-linking of a 27-kilodalton fragment of fibronectin to Staphylococcus aureus. Science. 1980 Aug 22;209(4459):927–929. doi: 10.1126/science.7403857. [DOI] [PubMed] [Google Scholar]
- Natanson S., Sela S., Moses A. E., Musser J. M., Caparon M. G., Hanski E. Distribution of fibronectin-binding proteins among group A streptococci of different M types. J Infect Dis. 1995 Apr;171(4):871–878. doi: 10.1093/infdis/171.4.871. [DOI] [PubMed] [Google Scholar]
- Norgren M., Caparon M. G., Scott J. R. A method for allelic replacement that uses the conjugative transposon Tn916: deletion of the emm6.1 allele in Streptococcus pyogenes JRS4. Infect Immun. 1989 Dec;57(12):3846–3850. doi: 10.1128/iai.57.12.3846-3850.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada N., Geist R. T., Caparon M. G. Positive transcriptional control of mry regulates virulence in the group A streptococcus. Mol Microbiol. 1993 Mar;7(6):893–903. doi: 10.1111/j.1365-2958.1993.tb01180.x. [DOI] [PubMed] [Google Scholar]
- Perez-Casal J., Price J. A., Maguin E., Scott J. R. An M protein with a single C repeat prevents phagocytosis of Streptococcus pyogenes: use of a temperature-sensitive shuttle vector to deliver homologous sequences to the chromosome of S. pyogenes. Mol Microbiol. 1993 May;8(5):809–819. doi: 10.1111/j.1365-2958.1993.tb01628.x. [DOI] [PubMed] [Google Scholar]
- Sela S., Aviv A., Tovi A., Burstein I., Caparon M. G., Hanski E. Protein F: an adhesin of Streptococcus pyogenes binds fibronectin via two distinct domains. Mol Microbiol. 1993 Dec;10(5):1049–1055. doi: 10.1111/j.1365-2958.1993.tb00975.x. [DOI] [PubMed] [Google Scholar]
- Sheikh-Ali O. D. Ul'trazvukovoi metod issledovaniia serdtsa u zdorovykh lits i u bol'nykh s mitral'nym porokom. Sov Med. 1974;0(4):100–105. [PubMed] [Google Scholar]
- Signäs C., Raucci G., Jönsson K., Lindgren P. E., Anantharamaiah G. M., Hök M., Lindberg M. Nucleotide sequence of the gene for a fibronectin-binding protein from Staphylococcus aureus: use of this peptide sequence in the synthesis of biologically active peptides. Proc Natl Acad Sci U S A. 1989 Jan;86(2):699–703. doi: 10.1073/pnas.86.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens D. L., Tanner M. H., Winship J., Swarts R., Ries K. M., Schlievert P. M., Kaplan E. Severe group A streptococcal infections associated with a toxic shock-like syndrome and scarlet fever toxin A. N Engl J Med. 1989 Jul 6;321(1):1–7. doi: 10.1056/NEJM198907063210101. [DOI] [PubMed] [Google Scholar]
- Talay S. R., Ehrenfeld E., Chhatwal G. S., Timmis K. N. Expression of the fibronectin-binding components of Streptococcus pyogenes in Escherichia coli demonstrates that they are proteins. Mol Microbiol. 1991 Jul;5(7):1727–1734. doi: 10.1111/j.1365-2958.1991.tb01921.x. [DOI] [PubMed] [Google Scholar]
- Talay S. R., Valentin-Weigand P., Jerlström P. G., Timmis K. N., Chhatwal G. S. Fibronectin-binding protein of Streptococcus pyogenes: sequence of the binding domain involved in adherence of streptococci to epithelial cells. Infect Immun. 1992 Sep;60(9):3837–3844. doi: 10.1128/iai.60.9.3837-3844.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talay S. R., Valentin-Weigand P., Timmis K. N., Chhatwal G. S. Domain structure and conserved epitopes of Sfb protein, the fibronectin-binding adhesin of Streptococcus pyogenes. Mol Microbiol. 1994 Aug;13(3):531–539. doi: 10.1111/j.1365-2958.1994.tb00448.x. [DOI] [PubMed] [Google Scholar]
- Vuento M., Vaheri A. Purification of fibronectin from human plasma by affinity chromatography under non-denaturing conditions. Biochem J. 1979 Nov 1;183(2):331–337. doi: 10.1042/bj1830331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannamaker L. W. Differences between streptococcal infections of the throat and of the skin. I. N Engl J Med. 1970 Jan 1;282(1):23–31. doi: 10.1056/NEJM197001012820106. [DOI] [PubMed] [Google Scholar]
- Westerlund B., Korhonen T. K. Bacterial proteins binding to the mammalian extracellular matrix. Mol Microbiol. 1993 Aug;9(4):687–694. doi: 10.1111/j.1365-2958.1993.tb01729.x. [DOI] [PubMed] [Google Scholar]