Abstract
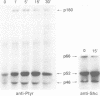
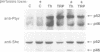
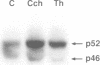
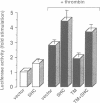
The serine protease thrombin activates G protein signaling systems that lead to Ras activation and, in certain cells, proliferation. Whereas the steps leading to Ras activation by G protein-coupled receptors are not well defined, the mechanisms of Ras activation by receptor tyrosine kinases have recently been elucidated biochemically and genetically. The present study was undertaken to determine whether common signaling components are used by these two distinct classes of receptors. Here we report that the adaptor protein Shc, is phosphorylated on tyrosine residues following stimulation of the thrombin receptor in growth-responsive CCL39 fibroblasts. Shc phosphorylation by thrombin or the thrombin receptor agonist peptide is maximal by 15 min and persists for > or = 2 h. Following thrombin stimulation, phosphorylated Shc is recruited to Grb2 complexes. One or more pertussis toxin-insensitive proteins appear to mediate this effect, since (i) pertussis toxin pre-treatment of cells does not blunt the action of thrombin and (ii) Shc phosphorylation on tyrosine can be stimulated by the muscarinic m1 receptor. Shc phosphorylation does not appear to involve protein kinase C, since the addition of 4-beta-phorbol-12,13-dibutyrate has no effect. Rather, thrombin-induced Shc phosphorylation is enhanced in cells depleted of phorbol ester-sensitive protein kinase C isoforms. Expression of mutant Shc proteins defective in Grb2 binding displays a dominant-negative effect on thrombin-stimulated p44 MAP kinase activation, gene induction and cell growth. From these data, we conclude that Shc represents a crucial point of convergence between signaling pathways activated by receptor tyrosine kinases and G protein-coupled receptors.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baffy G., Yang L., Raj S., Manning D. R., Williamson J. R. G protein coupling to the thrombin receptor in Chinese hamster lung fibroblasts. J Biol Chem. 1994 Mar 18;269(11):8483–8487. [PubMed] [Google Scholar]
- Buday L., Downward J. Epidermal growth factor regulates p21ras through the formation of a complex of receptor, Grb2 adapter protein, and Sos nucleotide exchange factor. Cell. 1993 May 7;73(3):611–620. doi: 10.1016/0092-8674(93)90146-h. [DOI] [PubMed] [Google Scholar]
- Burns L. A., Karnitz L. M., Sutor S. L., Abraham R. T. Interleukin-2-induced tyrosine phosphorylation of p52shc in T lymphocytes. J Biol Chem. 1993 Aug 25;268(24):17659–17661. [PubMed] [Google Scholar]
- Cazaubon S. M., Ramos-Morales F., Fischer S., Schweighoffer F., Strosberg A. D., Couraud P. O. Endothelin induces tyrosine phosphorylation and GRB2 association of Shc in astrocytes. J Biol Chem. 1994 Oct 7;269(40):24805–24809. [PubMed] [Google Scholar]
- Chambard J. C., Paris S., L'Allemain G., Pouysségur J. Two growth factor signalling pathways in fibroblasts distinguished by pertussis toxin. Nature. 1987 Apr 23;326(6115):800–803. doi: 10.1038/326800a0. [DOI] [PubMed] [Google Scholar]
- Chardin P., Camonis J. H., Gale N. W., van Aelst L., Schlessinger J., Wigler M. H., Bar-Sagi D. Human Sos1: a guanine nucleotide exchange factor for Ras that binds to GRB2. Science. 1993 May 28;260(5112):1338–1343. doi: 10.1126/science.8493579. [DOI] [PubMed] [Google Scholar]
- Chen Y. H., Pouysségur J., Courtneidge S. A., Van Obberghen-Schilling E. Activation of Src family kinase activity by the G protein-coupled thrombin receptor in growth-responsive fibroblasts. J Biol Chem. 1994 Nov 4;269(44):27372–27377. [PubMed] [Google Scholar]
- Cook S. J., Rubinfeld B., Albert I., McCormick F. RapV12 antagonizes Ras-dependent activation of ERK1 and ERK2 by LPA and EGF in Rat-1 fibroblasts. EMBO J. 1993 Sep;12(9):3475–3485. doi: 10.1002/j.1460-2075.1993.tb06022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin S. R., Vu T. K., Hung D. T., Wheaton V. I. Characterization of a functional thrombin receptor. Issues and opportunities. J Clin Invest. 1992 Feb;89(2):351–355. doi: 10.1172/JCI115592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counillon L., Scholz W., Lang H. J., Pouysségur J. Pharmacological characterization of stably transfected Na+/H+ antiporter isoforms using amiloride analogs and a new inhibitor exhibiting anti-ischemic properties. Mol Pharmacol. 1993 Nov;44(5):1041–1045. [PubMed] [Google Scholar]
- Cutler R. L., Liu L., Damen J. E., Krystal G. Multiple cytokines induce the tyrosine phosphorylation of Shc and its association with Grb2 in hemopoietic cells. J Biol Chem. 1993 Oct 15;268(29):21463–21465. [PubMed] [Google Scholar]
- Damen J. E., Liu L., Cutler R. L., Krystal G. Erythropoietin stimulates the tyrosine phosphorylation of Shc and its association with Grb2 and a 145-Kd tyrosine phosphorylated protein. Blood. 1993 Oct 15;82(8):2296–2303. [PubMed] [Google Scholar]
- Egan S. E., Giddings B. W., Brooks M. W., Buday L., Sizeland A. M., Weinberg R. A. Association of Sos Ras exchange protein with Grb2 is implicated in tyrosine kinase signal transduction and transformation. Nature. 1993 May 6;363(6424):45–51. doi: 10.1038/363045a0. [DOI] [PubMed] [Google Scholar]
- Irani K., Herzlinger S., Finkel T. Ras proteins regulate multiple mitogenic pathways in A10 vascular smooth muscle cells. Biochem Biophys Res Commun. 1994 Aug 15;202(3):1252–1258. doi: 10.1006/bbrc.1994.2065. [DOI] [PubMed] [Google Scholar]
- Kavanaugh W. M., Turck C. W., Williams L. T. PTB domain binding to signaling proteins through a sequence motif containing phosphotyrosine. Science. 1995 May 26;268(5214):1177–1179. doi: 10.1126/science.7539155. [DOI] [PubMed] [Google Scholar]
- LaMorte V. J., Goldsmith P. K., Spiegel A. M., Meinkoth J. L., Feramisco J. R. Inhibition of DNA synthesis in living cells by microinjection of Gi2 antibodies. J Biol Chem. 1992 Jan 15;267(2):691–694. [PubMed] [Google Scholar]
- LaMorte V. J., Kennedy E. D., Collins L. R., Goldstein D., Harootunian A. T., Brown J. H., Feramisco J. R. A requirement for Ras protein function in thrombin-stimulated mitogenesis in astrocytoma cells. J Biol Chem. 1993 Sep 15;268(26):19411–19415. [PubMed] [Google Scholar]
- Li N., Batzer A., Daly R., Yajnik V., Skolnik E., Chardin P., Bar-Sagi D., Margolis B., Schlessinger J. Guanine-nucleotide-releasing factor hSos1 binds to Grb2 and links receptor tyrosine kinases to Ras signalling. Nature. 1993 May 6;363(6424):85–88. doi: 10.1038/363085a0. [DOI] [PubMed] [Google Scholar]
- McGlade J., Cheng A., Pelicci G., Pelicci P. G., Pawson T. Shc proteins are phosphorylated and regulated by the v-Src and v-Fps protein-tyrosine kinases. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):8869–8873. doi: 10.1073/pnas.89.19.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloche S., Pagès G., Pouysségur J. Functional expression and growth factor activation of an epitope-tagged p44 mitogen-activated protein kinase, p44mapk. Mol Biol Cell. 1992 Jan;3(1):63–71. doi: 10.1091/mbc.3.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloche S., Seuwen K., Pagès G., Pouysségur J. Biphasic and synergistic activation of p44mapk (ERK1) by growth factors: correlation between late phase activation and mitogenicity. Mol Endocrinol. 1992 May;6(5):845–854. doi: 10.1210/mend.6.5.1603090. [DOI] [PubMed] [Google Scholar]
- Obermeier A., Lammers R., Wiesmüller K. H., Jung G., Schlessinger J., Ullrich A. Identification of Trk binding sites for SHC and phosphatidylinositol 3'-kinase and formation of a multimeric signaling complex. J Biol Chem. 1993 Nov 5;268(31):22963–22966. [PubMed] [Google Scholar]
- Ohmichi M., Sawada T., Kanda Y., Koike K., Hirota K., Miyake A., Saltiel A. R. Thyrotropin-releasing hormone stimulates MAP kinase activity in GH3 cells by divergent pathways. Evidence of a role for early tyrosine phosphorylation. J Biol Chem. 1994 Feb 4;269(5):3783–3788. [PubMed] [Google Scholar]
- Orlowski J., Kandasamy R. A., Shull G. E. Molecular cloning of putative members of the Na/H exchanger gene family. cDNA cloning, deduced amino acid sequence, and mRNA tissue expression of the rat Na/H exchanger NHE-1 and two structurally related proteins. J Biol Chem. 1992 May 5;267(13):9331–9339. [PubMed] [Google Scholar]
- Paris S., Pouysségur J. Guanosine 5'-O-(3-thiotriphosphate) and guanosine 5'-O-(2-thiodiphosphate) activate G proteins and potentiate fibroblast growth factor-induced DNA synthesis in hamster fibroblasts. J Biol Chem. 1990 Jul 15;265(20):11567–11575. [PubMed] [Google Scholar]
- Pawson T. Protein modules and signalling networks. Nature. 1995 Feb 16;373(6515):573–580. doi: 10.1038/373573a0. [DOI] [PubMed] [Google Scholar]
- Pelicci G., Lanfrancone L., Grignani F., McGlade J., Cavallo F., Forni G., Nicoletti I., Grignani F., Pawson T., Pelicci P. G. A novel transforming protein (SHC) with an SH2 domain is implicated in mitogenic signal transduction. Cell. 1992 Jul 10;70(1):93–104. doi: 10.1016/0092-8674(92)90536-l. [DOI] [PubMed] [Google Scholar]
- Pronk G. J., McGlade J., Pelicci G., Pawson T., Bos J. L. Insulin-induced phosphorylation of the 46- and 52-kDa Shc proteins. J Biol Chem. 1993 Mar 15;268(8):5748–5753. [PubMed] [Google Scholar]
- Rivard N., McKenzie F. R., Brondello J. M., Pouysségur J. The phosphotyrosine phosphatase PTP1D, but not PTP1C, is an essential mediator of fibroblast proliferation induced by tyrosine kinase and G protein-coupled receptors. J Biol Chem. 1995 May 5;270(18):11017–11024. doi: 10.1074/jbc.270.18.11017. [DOI] [PubMed] [Google Scholar]
- Rozakis-Adcock M., McGlade J., Mbamalu G., Pelicci G., Daly R., Li W., Batzer A., Thomas S., Brugge J., Pelicci P. G. Association of the Shc and Grb2/Sem5 SH2-containing proteins is implicated in activation of the Ras pathway by tyrosine kinases. Nature. 1992 Dec 17;360(6405):689–692. doi: 10.1038/360689a0. [DOI] [PubMed] [Google Scholar]
- Salcini A. E., McGlade J., Pelicci G., Nicoletti I., Pawson T., Pelicci P. G. Formation of Shc-Grb2 complexes is necessary to induce neoplastic transformation by overexpression of Shc proteins. Oncogene. 1994 Oct;9(10):2827–2836. [PubMed] [Google Scholar]
- Sasaoka T., Rose D. W., Jhun B. H., Saltiel A. R., Draznin B., Olefsky J. M. Evidence for a functional role of Shc proteins in mitogenic signaling induced by insulin, insulin-like growth factor-1, and epidermal growth factor. J Biol Chem. 1994 May 6;269(18):13689–13694. [PubMed] [Google Scholar]
- Schorb W., Peeler T. C., Madigan N. N., Conrad K. M., Baker K. M. Angiotensin II-induced protein tyrosine phosphorylation in neonatal rat cardiac fibroblasts. J Biol Chem. 1994 Jul 29;269(30):19626–19632. [PubMed] [Google Scholar]
- Segatto O., Pelicci G., Giuli S., Digiesi G., Di Fiore P. P., McGlade J., Pawson T., Pelicci P. G. Shc products are substrates of erbB-2 kinase. Oncogene. 1993 Aug;8(8):2105–2112. [PubMed] [Google Scholar]
- Seuwen K., Kahan C., Hartmann T., Pouyssegur J. Strong and persistent activation of inositol lipid breakdown induces early mitogenic events but not Go to S phase progression in hamster fibroblasts. Comparison of thrombin and carbachol action in cells expressing M1 muscarinic acetylcholine receptors. J Biol Chem. 1990 Dec 25;265(36):22292–22299. [PubMed] [Google Scholar]
- Treisman R. Ternary complex factors: growth factor regulated transcriptional activators. Curr Opin Genet Dev. 1994 Feb;4(1):96–101. doi: 10.1016/0959-437x(94)90097-3. [DOI] [PubMed] [Google Scholar]
- Van Obberghen-Schilling E., Pouysségur J. Mitogen-potentiating action and binding characteristics of insulin and insulin-like growth factors in Chinese hamster fibroblasts. Exp Cell Res. 1983 Sep;147(2):369–378. doi: 10.1016/0014-4827(83)90219-7. [DOI] [PubMed] [Google Scholar]
- Van Obberghen-Schilling E., Pouysségur J. Signaling pathways of the thrombin receptor. Thromb Haemost. 1993 Jul 1;70(1):163–167. [PubMed] [Google Scholar]
- Van Obberghen-Schilling E., Vouret-Craviari V., Haslam R. J., Chambard J. C., Pouysségur J. Cloning, functional expression and role in cell growth regulation of a hamster 5-HT2 receptor subtype. Mol Endocrinol. 1991 Jul;5(7):881–889. doi: 10.1210/mend-5-7-881. [DOI] [PubMed] [Google Scholar]
- Vouret-Craviari V., Van Obberghen-Schilling E., Rasmussen U. B., Pavirani A., Lecocq J. P., Pouysségur J. Synthetic alpha-thrombin receptor peptides activate G protein-coupled signaling pathways but are unable to induce mitogenesis. Mol Biol Cell. 1992 Jan;3(1):95–102. doi: 10.1091/mbc.3.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vouret-Craviari V., Van Obberghen-Schilling E., Scimeca J. C., Van Obberghen E., Pouysségur J. Differential activation of p44mapk (ERK1) by alpha-thrombin and thrombin-receptor peptide agonist. Biochem J. 1993 Jan 1;289(Pt 1):209–214. doi: 10.1042/bj2890209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu T. K., Hung D. T., Wheaton V. I., Coughlin S. R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991 Mar 22;64(6):1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- Wilson I. A., Niman H. L., Houghten R. A., Cherenson A. R., Connolly M. L., Lerner R. A. The structure of an antigenic determinant in a protein. Cell. 1984 Jul;37(3):767–778. doi: 10.1016/0092-8674(84)90412-4. [DOI] [PubMed] [Google Scholar]
- Yokote K., Mori S., Hansen K., McGlade J., Pawson T., Heldin C. H., Claesson-Welsh L. Direct interaction between Shc and the platelet-derived growth factor beta-receptor. J Biol Chem. 1994 May 27;269(21):15337–15343. [PubMed] [Google Scholar]
- Zhu X., Suen K. L., Barbacid M., Bolen J. B., Fargnoli J. Interleukin-2-induced tyrosine phosphorylation of Shc proteins correlates with factor-dependent T cell proliferation. J Biol Chem. 1994 Feb 25;269(8):5518–5522. [PubMed] [Google Scholar]
- van Corven E. J., Hordijk P. L., Medema R. H., Bos J. L., Moolenaar W. H. Pertussis toxin-sensitive activation of p21ras by G protein-coupled receptor agonists in fibroblasts. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1257–1261. doi: 10.1073/pnas.90.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]