Abstract
We have demonstrated previously that the majority ( > 90%) of VH12 B cells are absent from the adult peripheral repertoire, and that most that remain have the fourth position at the D-J function (designated 10/G4). We report here that most VH 12-expressing pre-B cells are lost during the transition from the pre-BI to the pre-BII cell stage in normal mice, and that pre-BII cell productive (P) rearrangements ar enriched in 10/G4 CDR3. This coincides with the initial expression of H chain and the generation of the mu/surrogate L chain (SL) receptor. In contrast, there is not enrichment for 10/G4 CDR3 in mu MT mice, and the frequency of P rearrangements is as expected from a random rearrangement mechanism, ruling out a biased rearrangement mechanism unique to VH12. We have also demonstrated that non-10/G4 mu chains can associate with SL and be expressed on the cell surface, suggesting that they are available on the cell surface for selection. Thus, transition of pre-BI to pre-BII cells is dependent on the structure of the VH domain.
Full text
PDF



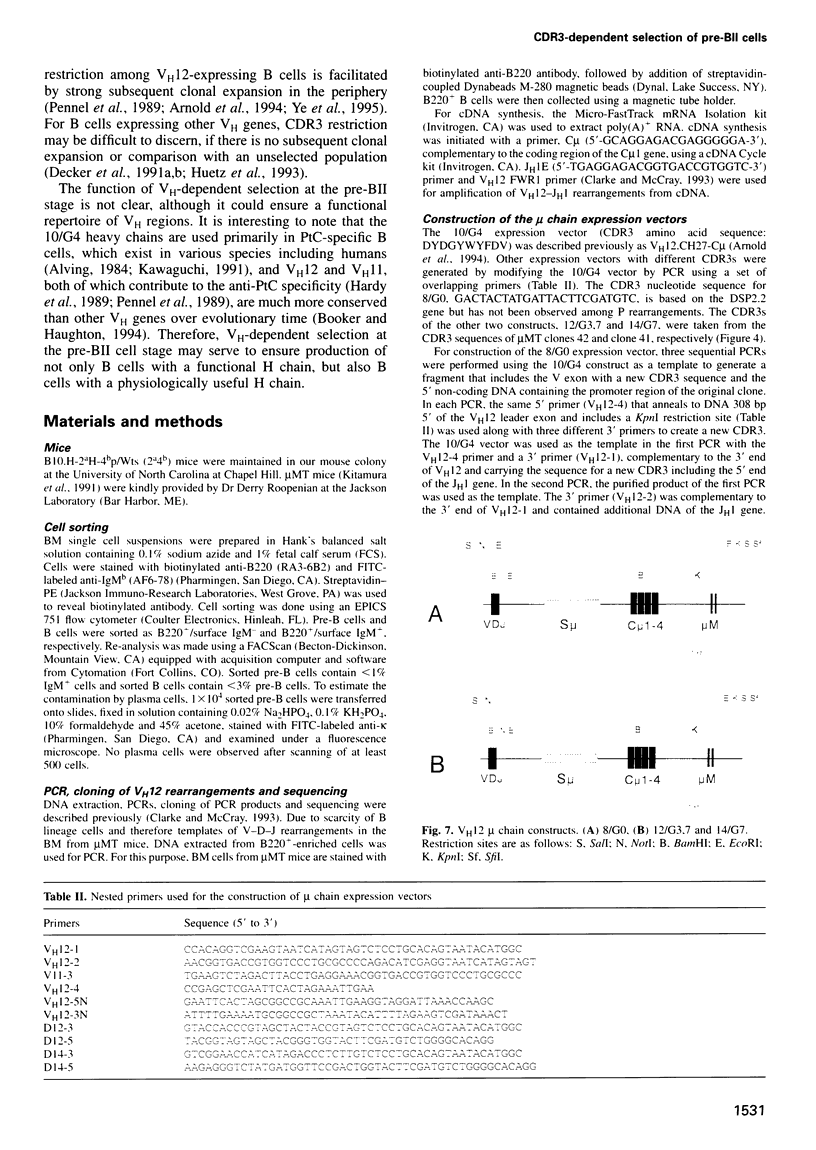


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alessandrini A., Desiderio S. V. Coordination of immunoglobulin DJH transcription and D-to-JH rearrangement by promoter-enhancer approximation. Mol Cell Biol. 1991 Apr;11(4):2096–2107. doi: 10.1128/mcb.11.4.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt F. W., Yancopoulos G. D., Blackwell T. K., Wood C., Thomas E., Boss M., Coffman R., Rosenberg N., Tonegawa S., Baltimore D. Ordered rearrangement of immunoglobulin heavy chain variable region segments. EMBO J. 1984 Jun;3(6):1209–1219. doi: 10.1002/j.1460-2075.1984.tb01955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alving C. R. Natural antibodies against phospholipids and liposomes in humans. Biochem Soc Trans. 1984 Apr;12(2):342–344. doi: 10.1042/bst0120342. [DOI] [PubMed] [Google Scholar]
- Arnold L. W., Pennell C. A., McCray S. K., Clarke S. H. Development of B-1 cells: segregation of phosphatidyl choline-specific B cells to the B-1 population occurs after immunoglobulin gene expression. J Exp Med. 1994 May 1;179(5):1585–1595. doi: 10.1084/jem.179.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booker J. K., Haughton G. Mechanisms that limit the diversity of antibodies. II. Evolutionary conservation of Ig variable region genes which encode naturally occurring autoantibodies. Int Immunol. 1994 Sep;6(9):1427–1436. doi: 10.1093/intimm/6.9.1427. [DOI] [PubMed] [Google Scholar]
- Carlsson L., Overmo C., Holmberg D. Developmentally controlled selection of antibody genes: characterization of individual VH7183 genes and evidence for stage-specific somatic diversification. Eur J Immunol. 1992 Jan;22(1):71–78. doi: 10.1002/eji.1830220112. [DOI] [PubMed] [Google Scholar]
- Chang Y., Paige C. J., Wu G. E. Enumeration and characterization of DJH structures in mouse fetal liver. EMBO J. 1992 May;11(5):1891–1899. doi: 10.1002/j.1460-2075.1992.tb05241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chukwuocha R. U., Nadel B., Feeney A. J. Analysis of homology-directed recombination in VDJ junctions from cytoplasmic Ig- pre-B cells of newborn mice. J Immunol. 1995 Feb 1;154(3):1246–1255. [PubMed] [Google Scholar]
- Clarke S. H., McCray S. K. VH CDR3-dependent positive selection of murine VH12-expressing B cells in the neonate. Eur J Immunol. 1993 Dec;23(12):3327–3334. doi: 10.1002/eji.1830231240. [DOI] [PubMed] [Google Scholar]
- Cohen J. J., Duke R. C., Fadok V. A., Sellins K. S. Apoptosis and programmed cell death in immunity. Annu Rev Immunol. 1992;10:267–293. doi: 10.1146/annurev.iy.10.040192.001411. [DOI] [PubMed] [Google Scholar]
- Corcos D., Iglesias A., Dunda O., Bucchini D., Jami J. Allelic exclusion in transgenic mice expressing a heavy chain disease-like human mu protein. Eur J Immunol. 1991 Nov;21(11):2711–2716. doi: 10.1002/eji.1830211110. [DOI] [PubMed] [Google Scholar]
- Decker D. J., Boyle N. E., Klinman N. R. Predominance of nonproductive rearrangements of VH81X gene segments evidences a dependence of B cell clonal maturation on the structure of nascent H chains. J Immunol. 1991 Aug 15;147(4):1406–1411. [PubMed] [Google Scholar]
- Decker D. J., Boyle N. E., Koziol J. A., Klinman N. R. The expression of the Ig H chain repertoire in developing bone marrow B lineage cells. J Immunol. 1991 Jan 1;146(1):350–361. [PubMed] [Google Scholar]
- Ehlich A., Martin V., Müller W., Rajewsky K. Analysis of the B-cell progenitor compartment at the level of single cells. Curr Biol. 1994 Jul 1;4(7):573–583. doi: 10.1016/s0960-9822(00)00129-9. [DOI] [PubMed] [Google Scholar]
- Ehlich A., Schaal S., Gu H., Kitamura D., Müller W., Rajewsky K. Immunoglobulin heavy and light chain genes rearrange independently at early stages of B cell development. Cell. 1993 Mar 12;72(5):695–704. doi: 10.1016/0092-8674(93)90398-a. [DOI] [PubMed] [Google Scholar]
- Feeney A. J. Lack of N regions in fetal and neonatal mouse immunoglobulin V-D-J junctional sequences. J Exp Med. 1990 Nov 1;172(5):1377–1390. doi: 10.1084/jem.172.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeney A. J., Riblet R. DST4: a new, and probably the last, functional DH gene in the BALB/c mouse. Immunogenetics. 1993;37(3):217–221. doi: 10.1007/BF00191888. [DOI] [PubMed] [Google Scholar]
- Freitas A. A., Andrade L., Lembezat M. P., Coutinho A. Selection of VH gene repertoires: differentiating B cells of adult bone marrow mimic fetal development. Int Immunol. 1990;2(1):15–23. doi: 10.1093/intimm/2.1.15. [DOI] [PubMed] [Google Scholar]
- Gu H., Kitamura D., Rajewsky K. B cell development regulated by gene rearrangement: arrest of maturation by membrane-bound D mu protein and selection of DH element reading frames. Cell. 1991 Apr 5;65(1):47–54. doi: 10.1016/0092-8674(91)90406-o. [DOI] [PubMed] [Google Scholar]
- Gu H., Tarlinton D., Müller W., Rajewsky K., Förster I. Most peripheral B cells in mice are ligand selected. J Exp Med. 1991 Jun 1;173(6):1357–1371. doi: 10.1084/jem.173.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy R. R., Carmack C. E., Shinton S. A., Riblet R. J., Hayakawa K. A single VH gene is utilized predominantly in anti-BrMRBC hybridomas derived from purified Ly-1 B cells. Definition of the VH11 family. J Immunol. 1989 May 15;142(10):3643–3651. [PubMed] [Google Scholar]
- Hartley S. B., Cooke M. P., Fulcher D. A., Harris A. W., Cory S., Basten A., Goodnow C. C. Elimination of self-reactive B lymphocytes proceeds in two stages: arrested development and cell death. Cell. 1993 Feb 12;72(3):325–335. doi: 10.1016/0092-8674(93)90111-3. [DOI] [PubMed] [Google Scholar]
- Haughton G., Arnold L. W., Whitmore A. C., Clarke S. H. B-1 cells are made, not born. Immunol Today. 1993 Feb;14(2):84–91. doi: 10.1016/0167-5699(93)90064-R. [DOI] [PubMed] [Google Scholar]
- Huetz F., Carlsson L., Tornberg U. C., Holmberg D. V-region directed selection in differentiating B lymphocytes. EMBO J. 1993 May;12(5):1819–1826. doi: 10.1002/j.1460-2075.1993.tb05830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasuyama H., Rolink A., Shinkai Y., Young F., Alt F. W., Melchers F. The expression of Vpre-B/lambda 5 surrogate light chain in early bone marrow precursor B cells of normal and B cell-deficient mutant mice. Cell. 1994 Apr 8;77(1):133–143. doi: 10.1016/0092-8674(94)90241-0. [DOI] [PubMed] [Google Scholar]
- Kawaguchi S. Antiphospholipid antibodies reactive with bromelain-treated mouse erythrocytes in mice, rats and rabbits. Int Arch Allergy Appl Immunol. 1991;96(1):46–50. doi: 10.1159/000235533. [DOI] [PubMed] [Google Scholar]
- Kitamura D., Kudo A., Schaal S., Müller W., Melchers F., Rajewsky K. A critical role of lambda 5 protein in B cell development. Cell. 1992 May 29;69(5):823–831. doi: 10.1016/0092-8674(92)90293-l. [DOI] [PubMed] [Google Scholar]
- Kitamura D., Rajewsky K. Targeted disruption of mu chain membrane exon causes loss of heavy-chain allelic exclusion. Nature. 1992 Mar 12;356(6365):154–156. doi: 10.1038/356154a0. [DOI] [PubMed] [Google Scholar]
- Kitamura D., Roes J., Kühn R., Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991 Apr 4;350(6317):423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- Kurosawa Y., Tonegawa S. Organization, structure, and assembly of immunoglobulin heavy chain diversity DNA segments. J Exp Med. 1982 Jan 1;155(1):201–218. doi: 10.1084/jem.155.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosawa Y., von Boehmer H., Haas W., Sakano H., Trauneker A., Tonegawa S. Identification of D segments of immunoglobulin heavy-chain genes and their rearrangement in T lymphocytes. Nature. 1981 Apr 16;290(5807):565–570. doi: 10.1038/290565a0. [DOI] [PubMed] [Google Scholar]
- Lawler A. M., Lin P. S., Gearhart P. J. Adult B-cell repertoire is biased toward two heavy-chain variable-region genes that rearrange frequently in fetal pre-B cells. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2454–2458. doi: 10.1073/pnas.84.8.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löffert D., Schaal S., Ehlich A., Hardy R. R., Zou Y. R., Müller W., Rajewsky K. Early B-cell development in the mouse: insights from mutations introduced by gene targeting. Immunol Rev. 1994 Feb;137:135–153. doi: 10.1111/j.1600-065x.1994.tb00662.x. [DOI] [PubMed] [Google Scholar]
- Malynn B. A., Yancopoulos G. D., Barth J. E., Bona C. A., Alt F. W. Biased expression of JH-proximal VH genes occurs in the newly generated repertoire of neonatal and adult mice. J Exp Med. 1990 Mar 1;171(3):843–859. doi: 10.1084/jem.171.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P., Iacomini J., Johnson R. S., Herrup K., Tonegawa S., Papaioannou V. E. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992 Mar 6;68(5):869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- Nemazee D., Buerki K. Clonal deletion of autoreactive B lymphocytes in bone marrow chimeras. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8039–8043. doi: 10.1073/pnas.86.20.8039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmond D. G. Proliferation kinetics and the lifespan of B cells in central and peripheral lymphoid organs. Curr Opin Immunol. 1991 Apr;3(2):179–185. doi: 10.1016/0952-7915(91)90047-5. [DOI] [PubMed] [Google Scholar]
- Pennell C., McCray S., Clarke S. VH12 rearrangements in adult peritoneal B cells. Ann N Y Acad Sci. 1992 May 4;651:311–315. doi: 10.1111/j.1749-6632.1992.tb24629.x. [DOI] [PubMed] [Google Scholar]
- Rolink A., Karasuyama H., Haasner D., Grawunder U., Mårtensson I. L., Kudo A., Melchers F. Two pathways of B-lymphocyte development in mouse bone marrow and the roles of surrogate L chain in this development. Immunol Rev. 1994 Feb;137:185–201. doi: 10.1111/j.1600-065x.1994.tb00665.x. [DOI] [PubMed] [Google Scholar]
- Roth D. B., Menetski J. P., Nakajima P. B., Bosma M. J., Gellert M. V(D)J recombination: broken DNA molecules with covalently sealed (hairpin) coding ends in scid mouse thymocytes. Cell. 1992 Sep 18;70(6):983–991. doi: 10.1016/0092-8674(92)90248-b. [DOI] [PubMed] [Google Scholar]
- Sakano H., Maki R., Kurosawa Y., Roeder W., Tonegawa S. Two types of somatic recombination are necessary for the generation of complete immunoglobulin heavy-chain genes. Nature. 1980 Aug 14;286(5774):676–683. doi: 10.1038/286676a0. [DOI] [PubMed] [Google Scholar]
- Shinkai Y., Rathbun G., Lam K. P., Oltz E. M., Stewart V., Mendelsohn M., Charron J., Datta M., Young F., Stall A. M. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992 Mar 6;68(5):855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- Spanopoulou E., Roman C. A., Corcoran L. M., Schlissel M. S., Silver D. P., Nemazee D., Nussenzweig M. C., Shinton S. A., Hardy R. R., Baltimore D. Functional immunoglobulin transgenes guide ordered B-cell differentiation in Rag-1-deficient mice. Genes Dev. 1994 May 1;8(9):1030–1042. doi: 10.1101/gad.8.9.1030. [DOI] [PubMed] [Google Scholar]
- Winkler T. H., Rolink A., Melchers F., Karasuyama H. Precursor B cells of mouse bone marrow express two different complexes with the surrogate light chain on the surface. Eur J Immunol. 1995 Feb;25(2):446–450. doi: 10.1002/eji.1830250221. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Ye J., McCray S. K., Clarke S. H. The majority of murine VH12-expressing B cells are excluded from the peripheral repertoire in adults. Eur J Immunol. 1995 Sep;25(9):2511–2521. doi: 10.1002/eji.1830250916. [DOI] [PubMed] [Google Scholar]
- Young F., Ardman B., Shinkai Y., Lansford R., Blackwell T. K., Mendelsohn M., Rolink A., Melchers F., Alt F. W. Influence of immunoglobulin heavy- and light-chain expression on B-cell differentiation. Genes Dev. 1994 May 1;8(9):1043–1057. doi: 10.1101/gad.8.9.1043. [DOI] [PubMed] [Google Scholar]