Abstract
Synaptic plasticity at the parallel fiber to Purkinje cell synapse has long been considered a cellular correlate for cerebellar motor learning. Functionally, long-term depression and long-term potentiation at these synapses seem to be the reverse of each other, with both pre- and post- synaptic expression occurring in both. However, different cerebellar motor learning paradigms have been shown to be asymmetric and not equally reversible. Here we discuss the asymmetric reversibility shown in the vestibulo-ocular reflex and eye-blink conditioning, and suggest that different cellular plasticity mechanisms might be recruited under different conditions leading to unequal reversibility.
Keywords: cerebellum, motor learning, synaptic plasticity, Purkinje cells, vestibulo-ocular reflex, eye-blink conditioning
Motor learning in the cerebellum is essential for maintaining and adjusting the precision and accuracy of movements. For example, the vestibulo-ocular reflex (VOR) is a simple reflex that stabilizes gaze during head movements. During vestibulo-visual mismatch training, the gain of the VOR can be increased (gain-up learning) or decreased (gain-down learning) through cerebellum-dependent learning. A long-standing theory of cerebellar function suggests that motor learning may be the result of synaptic plasticity at cerebellar parallel fiber (PF) to Purkinje cell (PC) synapses [1–3]. The pairing of climbing fiber (CF) and PF stimulation is known to cause long-term depression (LTD) at these synapses [3], which is widely viewed to be the cellular correlate of motor learning in the cerebellum, as the reduction in the efficacy of excitatory synapses onto the GABAergic PCs is thought to disinhibit target neurons at the cerebellar and vestibular nuclei [4]. The cellular reverse of LTD is long-term potentiation (LTP). A widespread theory of VOR motor learning proposes that learned gain increases are the result of LTD in the cerebellar flocculus [3, 5–7, but see: 8], while LTP is the mechanism of learned gain decreases [9–12].
Functionally, cerebellar LTD and LTP seem to be cellular inverses of each other. In PCs, the induction of LTP involves the repeated stimulation of PFs, while the additional co-activation of the CF input leads to the induction of LTD. This additional CF stimulation is thought to amplify calcium transients in PC dendrites to a threshold needed to induce LTD. Thus, CF co-activation helps reach the calcium threshold needed for LTD induction and regulates PF-PC bidirectional plasticity [13].
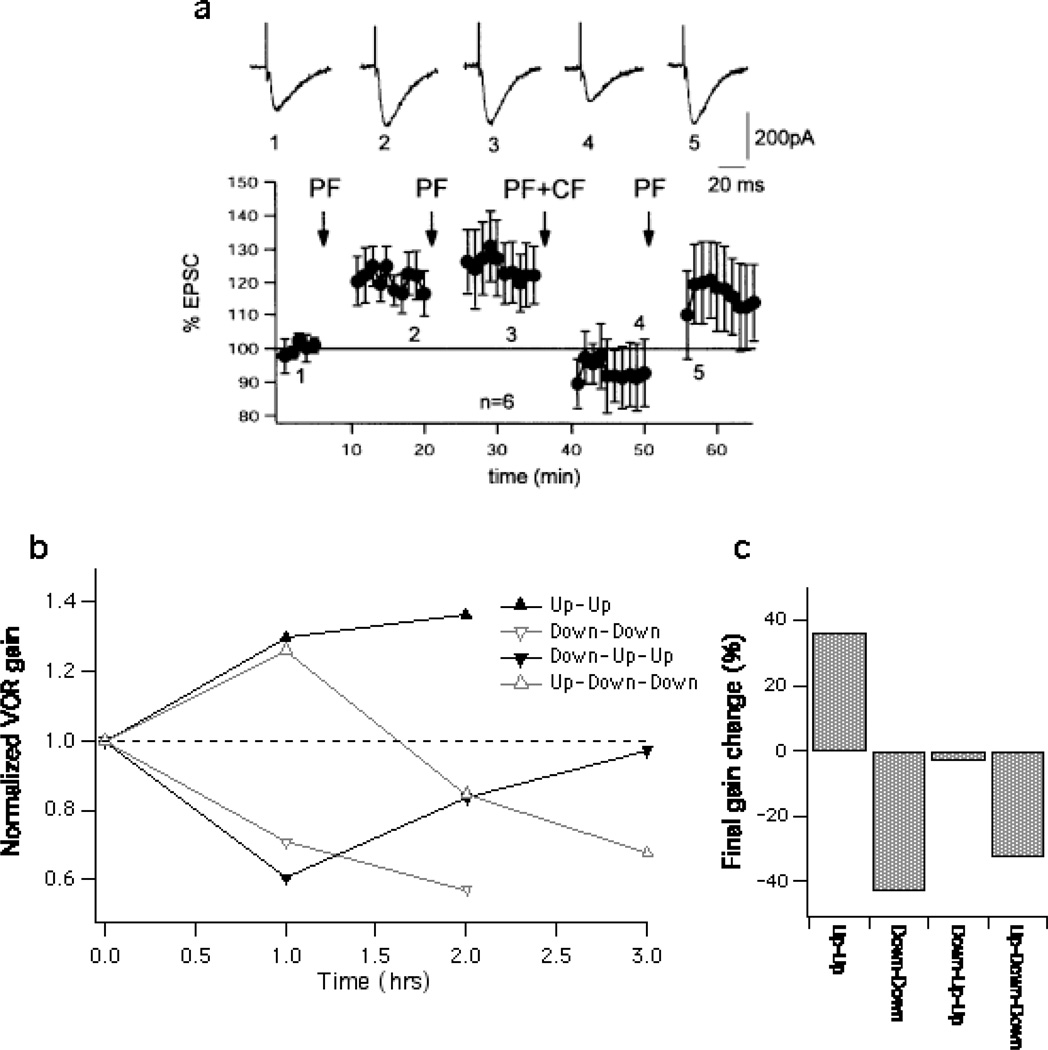
If LTD and LTP are cellular inverses, the expression of one should be able to completely reverse the other if both are expressed post-synaptically. The reversibility of LTP and LTD are confirmed in cerebellar slices. It was shown using both spike probability measurements as well as patch-clamp recordings of EPSCs that PF-LTP induction can be can fully reversed and overcome by LTD, even when LTP was fully saturated [13, 14; Fig 1A]. Furthermore, the reversibility seems to hold true during both low [13] and high [15] frequency PF stimulation induction protocols. Thus, these studies suggest that LTD and LTP are fully reversible, and act upon the same post-synaptic substrates.
Figure 1.
Post-synaptic LTP and LTD are completely reversible in cerebellar slices, yet cerebellar learning in the VOR shows pronounced asymmetry. a: Post-synaptic LTD can completely reverse prior post-synaptic LTP. Time graph showing 1 Hz LTP (PF stimulation) followed by LTD (PF+CF). Arrows indicate the time points of tetanization. Error bars are mean ± SEM. Panel (a) is taken from Coesmans et al (2004). Copyright 2004 by Elsevier. b and c: In naïve cats, gain-down learning limited subsequent gain-up learning. b: Time course of VOR gain, measured at 0.5 Hz, over 2–3 hours in cats wearing magnifying (x2) or miniaturizing (x0.25) lenses. In the “up-up” and “down-down” protocol, cats wore the magnifying or miniaturizing lenses for 2 hours. In the “up-down-down” and “down-up-up” protocols the lenses were switched after the first hour. c: Summary of final gain change from panel B. In the down-up-up protocol, 2 hours of gain up learning failed to increase the VOR gain above pre-learning values. Panels (b) and (c) are modified from Broussard et al. (2011). Copyright 2011 by Springer.
Since LTD and LTP have been shown to be symmetric correlates of each other in vitro, this would predict that gain-up and gain-down learning in the VOR would also be completely reversible. However, learning in the VOR has been shown to be asymmetric. VOR learning is induced by pairing vestibular motion with a visual error signal. Experimentally, gain-up learning is achieved by rotating the animal in the opposite direction of the visual field, or by wearing magnifying lenses. To decrease the VOR gain the animal can be rotated in the same direction as the visual field, or by wearing miniaturizing lenses [10, for review see: 16]. In naïve animals with no prior learning experience, gain-down learning was shown to be more potent and not easily reversed by repeated gain-up training. Boyden and Raymond have demonstrated in mice, that gain-up learning saturated at a lower value when it was preceded by gain-down learning [9]. Similarly, it was shown that a cat wearing magnifying prisms for 2 hours was still not able to completely overcome 1 hour of prior experience with miniaturizing prisms. In the inverse situation, a gain increase followed by a gain decrease, there was no residual effect, and the animal was able to decrease its VOR completely, to a level comparable to 2 hours of gain-down learning alone [17; Fig 1B, C], in effect showing that gain-up learning is not able to reverse gain-down learning, and may not be as potent. This could suggest that the LTP recruited during gain-down learning was more efficient than the LTD recruited during gain-up learning.
Similarly, it has been shown that the selectivity for head-rotation frequency in the VOR is different for learned gain increases and decreases. Gain changes in the VOR are always greatest when measured at the same rotational frequency at which training occurred, thus showing selectivity for a specific frequency [5, 18–22]. The amount of learning decreases as the training and testing frequencies diverge, thus showing a pattern of generalization, or the ability to transfer learning to a slightly different context. In the VOR, the amount of generalization of learning to other frequencies was significantly less after gain-up learning, showing a much sharper tuning curve compared to gain-down learning [21]. Thus, it is implied that with different patterns of generalization, gain increases and decreases are not exact inverses at the circuit level, and cannot fully reverse each other.
If gain-down learning is more potent than gain-up learning, as evidence suggests, this would indicate that the plasticity at the synapses are not fully reversed when the opposite protocol is induced. If learning were still present or not fully ’reset’ back to a baseline level, this would lead to ‘savings’ of learning at the synapses that could potentially influence or bias behavioral learning such as the VOR. However, it should be noted that learned gain changes in the VOR (both increases and decreases) are shown to be completely reversible in the long-term. Repeated learning trials over months to years, both in the monkey [23] and the cat [7], showed no indication of savings or a decrease in the amount learned over time, indicating that perhaps over time this ‘savings’ passively decays back toward the baseline level.
Perhaps the best example of learned savings is during associative eye-blink conditioning. During delayed eye-blink conditioning, the associative learning of an unconditioned stimulus (such as an air puff or peri-orbital shock) paired with a conditioned stimulus (such as a tone or light), leads to the gradual learning of a conditioned response [eye-blink; for review see: 24]. This learned association has been linked to cerebellar PF-LTD [25, 26]. The repeated non-associative presentation of the conditioned stimulus alone will lead to the extinction of this conditioned response, which is similar to LTP resulting from PF stimulation alone [11, 27]. However, if the unconditioned and conditioned stimuli are paired once again, the re-learning of the conditioned response occurs at a much faster rate [28]. Thus, the initial learning was not actually extinguished or really gone, but perhaps only hidden or masked. This suggests that the associative and non-associative learning mechanisms are asymmetric, and do not fully reverse each other. It is interesting to note that unlike the VOR, here it is the LTD-dependent change that appears dominant, suggesting the possibility that LTP could not fully reverse LTD.
Recently, we have shown a correlation between the impairment in the acquisition of eye-blink conditioning and PF-LTD in a mouse model of autism. Autistic mice (mouse model for the human 15q11–13 duplication), unlike the wild-type controls, were shown to have a specific impairment in PF-LTD, while LTP was shown to be completely normal. In line with this, these mice were also shown to have impaired learning during the acquisition of the CR, but not the extinction of the response. However, the LTD impairment was ‘rescued’, when the LTD protocol was preceded by prior LTP induction, showing that the LTD was saturated, but not blocked. Similarly, these mice show normal re-acquisition of eye-blink conditioning [15]. Thus, this study supports the hypothesis that LTD contributes to CR acquisition, while LTP is involved with its extinction.
It should be mentioned that it has been recently pointed out that the acquisition of cerebellar eye-blink conditioning requires a very precise delay interval with the CS preceding the US by about 150ms (Wetmore et al, 2014), and that this is incompatible with the original PF-LTD induction protocol involving the simultaneous stimulation of PF and CF inputs (Ito et al, 1982). Indeed, while eye-blink conditioning cannot be induced with the co-activation of the CS and US stimuli, it should be noted that PF-LTD has been shown to have a spike-timing dependency, with the CF stimulus optimally occurring 50–250 ms after PF stimulation (for discussion, see: Piochon et al. 2013). This temporal specificity is in line with the associative requirements of both eye-blink conditioning requiring a PF-CF delay period [30, 31], as well as VOR gain adaptation [32].
Similarly, in our lab we have shown that PF-LTD can be reliably induced after 100 Hz PF stimulation with CF stimulation occurring 120 ms after the PF-stimulation onset [15, 33]. It is thought that this high frequency PF-LTD protocol maybe more physiologically relevant, as PF inputs to the PC are known to fire in bursts of activity and within a wide frequency range [34, 35]. Which may bring up the possibility of multiple LTD and LTP working over a wide frequency range, such as VOR learning at many different frequencies.
In cerebellar slices, LTP can be induced by repeated PF stimulation, while LTD is facilitated by co-activation of the CF. This CF input leads to supralinear calcium transients in PF-PC spines [36], and promotes the induction of LTD, which has a higher calcium threshold than LTP [13]. The large calcium transients are thought to partially result from the activation of type 1 metabotropic glutamate receptors [mGluR1; 25] and subsequent calcium release from internal stores [37], as well as the activation of Nmethyl- D-aspartate (NMDA) receptors [33]. Additionally, the increase in calcium facilitates protein kinase dependent pathways that include protein kinase C [PKC; 5, 38] and α-calcium/calmodulin-dependent protein kinase II [CaMKII; 6]. PKC phosphorylates the AMPA receptor subunit GluR2 at the ser-880 residue, and initiates a clathrinmediated endocytosis of AMPA receptors [39, 40]. Simultaneously, CaMKII facilitates the cGMP/PKG-mediated suppression of protein phosphatase 2A (PP2A), by inhibition of phosphodiesterase 1 [41]. Thus, it is clear that this form of LTD is expressed post-synaptically.
The cellular mechanism of cerebellar LTP seems to be a mirror image of LTD, and shares the same substrate [for review see: 42]. LTP is also calcium dependent, and is prevented when the calcium chelator BAPTA is present in the internal saline, however the calcium threshold for LTP is lower than that for LTD [13]. Unlike LTD which depends on the activity of protein kinases, LTP induction depends on the activation of protein phosphatases [43]. This is similar to the protein phosphatase/kinase switch that controls LTD and LTP at hippocampal synapses. In contrast to LTD, LTP induction does not require glutamate binding to mGluR1 [44] or NMDA receptors [33, 45]. LTP depends on nitric oxide (NO) signaling [46], phospholipase A, and endocannabinoid signaling [47]. Whereas LTD results in the removal of AMPA receptors, LTP results in the insertion of AMPA receptors at the post-synaptic density. It is thought that NO promotes the nitrosylation of the N-ethylmaleimide-sensitive factor [NSF; 48], which in turn binds to the GluR2 AMPA receptor subunits and mediates their membrane insertion [49, see also: 50].
Although it is clear that post-synaptic LTD and LTP are perfectly capable of reversing each other, there is also a pre-synaptically expressed form of LTP (Salin et al., 1996). Pre-synaptic LTP is thought to involve adenylyl cyclase, cAMP, and PKA and is not affected by post-synaptic but rather pre-synaptic calcium signaling. The expression of pre-synaptic LTP has no effect on post-synaptic AMPA receptors, but instead increases the amount of neurotransmitter (glutamate) that is released from the parallel fiber terminals [51]. A potential reverse to this from of LTP, pre-synaptic LTD, has also been described, but seems restricted to conditions that pharmacologically block pre-synaptic LTP. It has been shown in cerebellar slices that pre-synaptic LTD involves endocannabinoid signaling and CB1 receptor activation on PF terminals, and depends on NMDA receptor activation, but not mGluR1 [52]. In urethane-anesthetized mice in vivo, this pre-synaptic LTD was found to be dependent on NMDA, mGuR1, and CB1 receptor activation, and up-regulated by NO synthase [53]. It currently remains unclear under which physiological conditions this type of plasticity is recruited.
The asymmetric reversibility in learning may suggest that different plasticity mechanisms are recruited under different conditions. As expected, pre-synaptically expressed LTP cannot be reversed by post-synaptic LTD [14]. Thus, asymmetric learning in vivo could be the result of the asymmetric recruitment of pre- and post-synaptic mechanisms. For example, during VOR learning the recruitment of both pre- and post-synaptic LTP during a learned gain decrease cannot be completely reversed by gain-up learning if only post-synaptic LTD was recruited. This suggests that pre-synaptic LTD is not being triggered during VOR motor learning. Similarly, it was suggested that short-term behavioral changes apparent in eye-blink conditioning were the result of pre-synaptic LTP and that this form of plasticity faded over the time course of hours as did the behavior, instead of being actively reversed [54]. Again, this suggests that pre-synaptic LTD was not activated.
In should be noted that in vivo, LTP and LTD most likely work in concert with each other. It has been shown that under circumstances when post-synaptic LTD is induced pre-synaptic LTP is actively blocked via retrograde cannabinoid signaling, such as during CF co-activation [55] or cholinergic signaling [56]. This dichotomy is furthered, since under the same conditions that induce high-frequency LTP, a non-synaptic form of plasticity, activity-dependent increase in intrinsic excitability, can also be induced [57]. Thus, intrinsic plasticity can further potentiate the response of the cell and may make the reversal very difficult. Thus, although LTD and LTP are the cellular inverses of each other, the pre- and post-synaptic mechanisms of plasticity are not equally recruited during motor learning, leading to asymmetric behavior and savings of learning.
Clearly, the asymmetries seen in gain decreases and gain increases in the VOR suggest that different mechanisms of synaptic plasticity are behind these types of learning. It should be noted that other plasticity mechanisms and sites of learning could also potentially contribute to cerebellar learning [see: 58, 59], and could also result in asymmetric reversal of learning. For instance, it has been suggested that savings seen during eye-blink conditioning, may in fact be due to multiple learning sites [28], including a LTP-like mechanism at the interpositus nuclei [60]. In addition, many knockout mice with deficits in PF-PC LTD or LTD have shown various or no effect in VOR gain learning [8, 61, 62; and others]. Similarly, experiments have shown that the instructive signals from the CF were sufficient, but not necessary to induce VOR learning [63]. Similarly, the optogenetic stimulation of either only the PC’s or climbing fibers was enough to induce gain-up learning [64], suggesting that PF-LTD might not be the sole mechanism driving these learning behaviors. Although it should be noted, that in these experiments any stimulus that is sufficiently strong to produce dendritic calcium spikes could induce PF-LTD, the involvement of multiple plasticity mechanisms cannot be ruled out.
Although here we mostly focused on the simple idea that bidirectional changes at one potential synapse (PF-PC) contributes to learned changes, more recently it has become obvious that a more complicated network of synapses are involved in cerebellar motor learning [see: 65]. It is now known that most synapses in the cerebellar cortex, are capable of synaptic plasticity, including the CF-PC synapse [66], mossy fiber to granule cell synapses [67–69], molecular layer interneurons to PC synapses [70, 71], and well as plasticity in the cerebellar/vestibular nuclei [72–74]. Furthermore, there is evidence suggesting that the synaptic changes at these additional sites can also play a role in cerebellar motor learning tasks such as in the VOR [75–78]. In addition, these plasticity sites may be recruited at different times during the course of a motor learning task [79]. For instance during the consolidation of long-term memory [22, 80–82], it was been suggested that learning is originally formed in the cerebellar cortex, but is later moved to the cerebellar/vestibular nuclei for long-term storage. It is clear that these different forms and sites of plasticity may work together, and that the asymmetry in the recruitment of these mechanisms may lead to the asymmetry seen in the behavior. However, even with the possibility of many learning sites, the question remains of how or when these different mechanisms are recruited during different behavioral paradigms, and why they are not recruited equally or symmetrically to allow for easy reversal.
LTP and LTD at the PF-PC synapse have long been shown to be involved in VOR learning and remain to be good potential candidates as putative sites of cerebellar learning. Thus, to truly understand learning and how this asymmetry in behavior could arise, we must understand how LTD and LTP work together, and more importantly under what circumstances they are recruited during in vivo learning conditions. The question remains as to why the cellular machinery for the full reversal of LTD and LTP exist (i.e. both pre- and post-synaptic forms of LTP and LTD exist), but these mechanisms are not used during behavioral paradigms.
Acknowledgements
The authors thank D. Broussard and members of the Hansel lab for their many helpful discussions. This work was supported by the National Institute of Neurological Disorders and Stroke (NS062771 to C.H.).
Footnotes
Conflict of Interest:
The authors declare no conflict of interest.
References
- 1.Marr D. A Theory of cerebellar cortex. J Physiol. 1969;202:437–470. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albus JS. A Theory of cerebellar function. Math Biosci. 1971;10:25–61. [Google Scholar]
- 3.Ito M. Cerebellar control of the vestibulo-ocular reflex - around the flocculus hypothesis. Annu Rev Neurosci. 1982;5:275–296. doi: 10.1146/annurev.ne.05.030182.001423. [DOI] [PubMed] [Google Scholar]
- 4.Ito M, Kawai N, Udo M, Sato N. Cerebellar-evoked disinhibition in dorsal Deiters neurones. Exp brain Res. 1968;6:247–264. doi: 10.1007/BF00235127. [DOI] [PubMed] [Google Scholar]
- 5.De Zeeuw CI, Hansel C, Bian F, Koekkoek SKE, van Alphen AM, Linden DJ, Oberdick J. Expression of a protein kinase C inhibitor in Purkinje cells blocks cerebellar LTD and adaptation of the vestibulo-ocular reflex. Neuron. 1998;20:495–508. doi: 10.1016/s0896-6273(00)80990-3. [DOI] [PubMed] [Google Scholar]
- 6.Hansel C, De Jeu M, Belmeguenai A, Houtman SH, Buitendijk GHS, Andreev D, De Zeeuw CI, Elgersma Y. alphaCaMKII is essential for cerebellar LTD and motor learning. Neuron. 2006;51:835–843. doi: 10.1016/j.neuron.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 7.Titley HK, Heskin-Sweezie R, Broussard DM. The bidirectionality of motor learning in the vestibulo-ocular reflex is a function of cerebellar mGluR1 receptors. J Neurophysiol. 2010;104:3657–3666. doi: 10.1152/jn.00664.2010. [DOI] [PubMed] [Google Scholar]
- 8.Schonewille M, Gao Z, Boele H-JJ, et al. Reevaluating the Role of LTD in Cerebellar Motor Learning. Neuron. 2011;70:43–50. doi: 10.1016/j.neuron.2011.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyden ES, Raymond JL. Active reversal of motor memories reveals rules governing memory encoding. Neuron. 2003;39:1031–1042. doi: 10.1016/s0896-6273(03)00562-2. [DOI] [PubMed] [Google Scholar]
- 10.Boyden ES, Katoh A, Raymond JL. Cerebellum-dependent learning: the role of multiple plasticity mechanisms. Annu Rev Neurosci. 2004;27:581–609. doi: 10.1146/annurev.neuro.27.070203.144238. [DOI] [PubMed] [Google Scholar]
- 11.Jörntell H, Hansel C. Synaptic memories upside down: bidirectional plasticity at cerebellar parallel fiber-Purkinje cell synapses. Neuron. 2006;52:227–238. doi: 10.1016/j.neuron.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 12.Andreescu CE, Milojkovic BA, Haasdijk ED, Kramer P, De Jong FH, Krust A, De Zeeuw CI, De Jeu MTG. Estradiol improves cerebellar memory formation by activating estrogen receptor beta. J Neurosci. 2007;27:10832–10839. doi: 10.1523/JNEUROSCI.2588-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coesmans M, Weber JT, De Zeeuw CI, Hansel C. Bidirectional parallel fiber plasticity in the cerebellum under climbing fiber control. Neuron. 2004;44:691–700. doi: 10.1016/j.neuron.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 14.Lev-Ram V, Mehta SB, Kleinfeld D, Tsien RY. Reversing cerebellar long-term depression. PNAS. 2003;100:15989–15993. doi: 10.1073/pnas.2636935100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piochon C, Kloth AD, Grasselli G, et al. Cerebellar Plasticity and Motor Learning Deficits in a Copy Number Variation Mouse Model of Autism. Nat Commun. 2014;5:5586. doi: 10.1038/ncomms6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broussard DM, Kassardjian CD. Learning in a simple motor system. Learn Mem. 2004;11:127–136. doi: 10.1101/lm.65804. [DOI] [PubMed] [Google Scholar]
- 17.Broussard DM, Titley HK, Antflick J, Hampson DR. Motor learning in the VOR: the cerebellar component. Exp Brain Res. 2011;210:451–463. doi: 10.1007/s00221-011-2589-z. [DOI] [PubMed] [Google Scholar]
- 18.Robinson DA. Adaptive gain control of vestibuloocular reflex by the cerebellum. J Neurophysiol. 1976;39:954–969. doi: 10.1152/jn.1976.39.5.954. [DOI] [PubMed] [Google Scholar]
- 19.Lisberger SG, Miles FA, Optican LM. Frequency-selective adaptation: evidence for channels in the vestibulo-ocular reflex? J Neurosci. 1983;3:1234–1244. doi: 10.1523/JNEUROSCI.03-06-01234.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raymond JL, Lisberger SG. Behavioural analysis of signals that guide learned changes in the amplitude and dynamics of the vestibulo-ocular reflex. J Neurosci. 1996;16:7791–7802. doi: 10.1523/JNEUROSCI.16-23-07791.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimpo RR, Boyden ES, Katoh A, Ke MC, Raymond JL. Distinct patterns of stimulus generalizations of increases and decreases in VOR gain. J Neurophysiol. 2005;94:3092–3100. doi: 10.1152/jn.00048.2005. [DOI] [PubMed] [Google Scholar]
- 22.Titley HK, Heskin-Sweezie R, Broussard DM. Consolidation and disruption of motor memory generalize across stimulus conditions in the vestibulo-ocular reflex. Brain Res. 2009;1267:37–43. doi: 10.1016/j.brainres.2009.02.041. [DOI] [PubMed] [Google Scholar]
- 23.Miles FA, Eighmy BB. Long-term adaptive changes in primate vestibuloocular reflexIBehavioral observations. J Neurophysiol. 1980;43:1406–1425. doi: 10.1152/jn.1980.43.5.1406. [DOI] [PubMed] [Google Scholar]
- 24.Freeman JH, Steinmetz AB. Neural circuitry and plasticity mechanisms underlying delay eyeblink conditioning. Learn Mem. 2011;18:666–677. doi: 10.1101/lm.2023011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aiba A, Kano M, Chen C, Stanton ME, Fox GD, Herrup K, Zwingman TA, Tonegawa S. Deficient cerebellar long-term depression and impaired motor learning in mGluR1 mutant mice. Cell. 1994;79:377–388. [PubMed] [Google Scholar]
- 26.Kim JJ, Thompson RF. Cerebellar circuits and synaptic mechanisms involved in classical eyeblink conditioning. Trends Neurosci. 1997;20:177–181. doi: 10.1016/s0166-2236(96)10081-3. [DOI] [PubMed] [Google Scholar]
- 27.Perrett SP, Mauk MD. Extinction of conditioned eyelid responses requires the anterior lobe of cerebellar cortex. J Neurosci. 1995;15:2074–2080. doi: 10.1523/JNEUROSCI.15-03-02074.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Medina JF, Garcia KS, Mauk MD. A mechanism for savings in the cerebellum. J Neurosci. 2001;21:4081–4089. doi: 10.1523/JNEUROSCI.21-11-04081.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piochon C, Kruskal P, Maclean J, Hansel C. Non-Hebbian spike-timing-dependent plasticity in cerebellar circuits. Front Neural Circuits. 2013;6:124. doi: 10.3389/fncir.2012.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson RF, Krupa DJ. Organization of memory traces in the mammalian brain. Annu Rev Neurosci. 1994;17:519–549. doi: 10.1146/annurev.ne.17.030194.002511. [DOI] [PubMed] [Google Scholar]
- 31.Wetmore DZ, Jirenhed D-A, Rasmussen A, Johansson F, Schnitzer MJ, Hesslow G. Bidirectional plasticity of Purkinje cells matches temporal features of learning. J Neurosci. 2014;34:1731–1737. doi: 10.1523/JNEUROSCI.2883-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raymond JL, Lisberger SG. Neural learning rules for the vestibulo-ocular reflex. J Neurosci. 1998;18:9112–9129. doi: 10.1523/JNEUROSCI.18-21-09112.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piochon C, Levenes C, Ohtsuki G, Hansel C. Purkinje cell NMDA receptors assume a key role in synaptic gain control in the mature cerebellum. J Neurosci. 2010;30:15330–15335. doi: 10.1523/JNEUROSCI.4344-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kase M, Miller DC, Noda H. Discharges of Purkinje cells and mossy fibres in the cerebellar vermis of the monkey during saccadic eye movements and fixation. J Physiol. 1980;300:539–555. doi: 10.1113/jphysiol.1980.sp013178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pellerin JP, Lamarre Y. Local field potential oscillations in primate cerebellar cortex during voluntary movement. J Neurophysiol. 1997;78:3502–3507. doi: 10.1152/jn.1997.78.6.3502. [DOI] [PubMed] [Google Scholar]
- 36.Wang SS, Denk W, Häusser M. Coincidence detection in single dendritic spines mediated by calcium release. Nat Neurosci. 2000;3:1266–1273. doi: 10.1038/81792. [DOI] [PubMed] [Google Scholar]
- 37.Finch EA, Augustine GJ. Local calcium signalling by inositol-1,4,5-trisphosphate in Purkinje cell dendrites. Nature. 1998;396:753–756. doi: 10.1038/25541. [DOI] [PubMed] [Google Scholar]
- 38.Linden DJ, Connor JA. Participation of postsynaptic PKC in cerebellar long-term depression in culture. Science (80- ) 1991;254:1656–1659. doi: 10.1126/science.1721243. [DOI] [PubMed] [Google Scholar]
- 39.Wang YT, Linden DJ. Expression of cerebellar long-term depression requires postsynaptic clathrin-mediated endocytosis. Neuron. 2000;25:635–647. doi: 10.1016/s0896-6273(00)81066-1. [DOI] [PubMed] [Google Scholar]
- 40.Chung HJ, Steinberg JP, Huganir RL, Linden DJ. Requirement of AMPA receptor GluR2 phosphorylation for cerebellar long-term depression. Science (80- ) 2003;300:1751–1755. doi: 10.1126/science.1082915. [DOI] [PubMed] [Google Scholar]
- 41.Kawaguchi S, Hirano T. Gating of long-term depression by Ca2+/calmodulin-dependent protein kinase II through enhanced cGMP signalling in cerebellar Purkinje cells. J Physiol. 2013;591:1707–1730. doi: 10.1113/jphysiol.2012.245787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grasselli G, Hansel C. Cerebellar long-term potentiation: cellular mechanisms and role in learning. Int Rev Neurobiol. 2014;117:39–51. doi: 10.1016/B978-0-12-420247-4.00003-8. [DOI] [PubMed] [Google Scholar]
- 43.Belmeguenai A, Hansel C. A role for protein phosphatases 1, 2A, and 2B in cerebellar long-term potentiation. J Neurosci. 2005;25:10768–10772. doi: 10.1523/JNEUROSCI.2876-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Belmeguenai A, Botta P, Weber JT, Carta M, De Ruiter M, De Zeeuw CI, Valenzuela CF, Hansel C. Alcohol impairs long-term depression at the cerebellar parallel fiber-Purkinje cell synapse. J Neurophysiol. 2008;100:3167–3174. doi: 10.1152/jn.90384.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He Q, Titley H, Grasselli G, Piochon C, Hansel C. Ethanol affects NMDA receptor signaling at climbing fiber-Purkinje cell synapses in mice and impairs cerebellar LTD. J Neurophysiol. 2013;109:1333–1342. doi: 10.1152/jn.00350.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lev-Ram V, Wong ST, Storm DR, Tsien RY. A new form of cerebellar long-term potentiation is postsynaptic and depends on nitric oxide but not cAMP. PNAS. 2002;99:8389–8393. doi: 10.1073/pnas.122206399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang D-J, Su L-D, Wang Y-N, Yang D, Sun C-L, Zhou L, Wang X-X, Shen Y. Long-term potentiation at cerebellar parallel fiber-Purkinje cell synapses requires presynaptic and postsynaptic signaling cascades. J Neurosci. 2014;34:2355–2364. doi: 10.1523/JNEUROSCI.4064-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang Y, Man H-Y, Sekine-Aizawa Y, Han Y, Juluri K, Luo H, Cheah J, Lowenstein C, Huganir RL, Snyder SH. S-nitrosylation of N-ethylmaleimide sensitive factor mediates surface expression of AMPA receptors. Neuron. 2005;46:533–540. doi: 10.1016/j.neuron.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 49.Kakegawa W, Yuzaki M. A mechanism underlying AMPA receptor trafficking during cerebellar long-term potentiation. PNAS. 2005;102:17846–17851. doi: 10.1073/pnas.0508910102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hansel C. When the B-team runs plasticity: GluR2 receptor trafficking in cerebellar long-term potentiation. PNAS. 2005;102:18245–18246. doi: 10.1073/pnas.0509686102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salin PA, Malenka RC, Nicoll RA. Cyclic AMP mediates a presynaptic form of LTP at cerebellar parallel fiber synapses. Neuron. 1996;16:797–803. doi: 10.1016/s0896-6273(00)80099-9. [DOI] [PubMed] [Google Scholar]
- 52.Qiu D, Knöpfel T. Presynaptically expressed long-term depression at cerebellar parallel fiber synapses. Eur J Physiol. 2009;457:865–875. doi: 10.1007/s00424-008-0555-9. [DOI] [PubMed] [Google Scholar]
- 53.Chu C-P, Zhao G-Y, Jin R, Zhao S-N, Sun L, Qiu D-L. Properties of 4 Hz stimulation-induced parallel fiber-Purkinje cell presynaptic long-term plasticity in mouse cerebellar cortex in vivo. Eur J Neurosci. 2014;39:1624–1631. doi: 10.1111/ejn.12559. [DOI] [PubMed] [Google Scholar]
- 54.Ohyama T, Voicu H, Kalmbach B, Mauk MD. A decrementing form of plasticity apparent in cerebellar learning. J Neurosci. 2010;30:16993–17003. doi: 10.1523/JNEUROSCI.2455-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van Beugen BJ, Nagaraja RY, Hansel C. Climbing fiber-evoked endocannabinoid signaling heterosynaptically suppresses presynaptic cerebellar long-term potentiation. J Neurosci. 2006;26:8289–8294. doi: 10.1523/JNEUROSCI.0805-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rinaldo L, Hansel C. Muscarinic acetylcholine receptor activation blocks long-term potentiation at cerebellar parallel fiber-Purkinje cell synapses via cannabinoid signaling. PNAS. 2013;110:11181–11186. doi: 10.1073/pnas.1221803110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Belmeguenai A, Hosy E, Bengtsson F, et al. Intrinsic plasticity complements long-term potentiation in parallel fiber input gain control in cerebellar Purkinje cells. J Neurosci. 2010;30:13630–13643. doi: 10.1523/JNEUROSCI.3226-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hansel C, Linden DJ, D’Angelo E. Beyond parallel fiber LTD: The diversity of synaptic and non-synaptic plasticity in the cerebellum. Nat Neurosci. 2001;4:467–475. doi: 10.1038/87419. [DOI] [PubMed] [Google Scholar]
- 59.Gao Z, van Beugen BJ, De Zeeuw CI. Distributed synergistic plasticity and cerebellar learning. Nat Rev Neurosci. 2012;13:619–635. doi: 10.1038/nrn3312. [DOI] [PubMed] [Google Scholar]
- 60.Ohyama T, Nores WL, Medina JF, Riusech FA, Mauk MD. Learning-induced plasticity in deep cerebellar nucleus. J Neurosci. 2006;26:12656–12663. doi: 10.1523/JNEUROSCI.4023-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schonewille M, Belmeguenai A, Koekkoek SKE, et al. Purkinje Cell-Specific Knockout of the Protein Phosphatase PP2B Impairs Potentiation and Cerebellar Motor Learning. Neuron. 2010;67:618–628. doi: 10.1016/j.neuron.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ly R, Bouvier G, Schonewille M, Arabo A, Rondi-Reig L, Léna C, Casado M, De Zeeuw CI, Feltz A. T-type channel blockade impairs long-term potentiation at the parallel fiber-Purkinje cell synapse and cerebellar learning. PNAS. 2013;110:20302–20307. doi: 10.1073/pnas.1311686110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ke MC, Guo CC, Raymond JL. Elimination of climbing fiber instructive signals during motor learning. Nat Neurosci. 2009;12:1171–1179. doi: 10.1038/nn.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nguyen-Vu TDB, Kimpo RR, Rinaldi JM, Kohli A, Zeng H, Deisseroth K, Raymond JL. Cerebellar Purkinje cell activity drives motor learning. Nat Neurosci. 2013;16:1734–1736. doi: 10.1038/nn.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Clopath C, Badura A, De Zeeuw CI, Brunel N. A cerebellar learning model of vestibulo-ocular reflex adaptation in wild-type and mutant mice. J Neurosci. 2014;34:7203–7215. doi: 10.1523/JNEUROSCI.2791-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hansel C, Linden DJ. Long-term depression of the cerebellar climbing fiber--Purkinje neuron synapse. Neuron. 2000;26:473–482. doi: 10.1016/s0896-6273(00)81179-4. [DOI] [PubMed] [Google Scholar]
- 67.D’Angelo E, Rossi P, Armano S, Taglietti V. Evidence for NMDA and mGlu receptor-dependent long-term potentiation of mossy fiber-granule cell transmission in rat cerebellum. J Neurophysiol. 1999;81:277–287. doi: 10.1152/jn.1999.81.1.277. [DOI] [PubMed] [Google Scholar]
- 68.Armano S, Rossi P, Taglietti V, D’Angelo E. Long-term potentiation of intrinsic excitability at the mossy fiber-granule cell synapse of rat cerebellum. J Neurosci. 2000;20:5208–5216. doi: 10.1523/JNEUROSCI.20-14-05208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gall D, Prestori F, Sola E, D’Errico A, Roussel C, Forti L, Rossi P, D’Angelo E. Intracellular calcium regulation by burst discharge determines bidirectional long-term synaptic plasticity at the cerebellum input stage. J Neurosci. 2005;25:4813–4822. doi: 10.1523/JNEUROSCI.0410-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kano M, Rexhausen U, Dreessen J, Konnerth A. Synaptic excitation produces a long-lasting rebound potentiation of inhibitory synaptic signals in cerebellar Purkinje cells. Nature. 1992;356:601–604. doi: 10.1038/356601a0. [DOI] [PubMed] [Google Scholar]
- 71.Duguid IC, Smart TG. Retrograde activation of presynaptic NMDA receptors enhances GABA release at cerebellar interneuron-Purkinje cell synapses. Nat Neurosci. 2004;7:525–533. doi: 10.1038/nn1227. [DOI] [PubMed] [Google Scholar]
- 72.Aizenman CD, Manis PB, Linden DJ. Polarity of long-term synaptic gain change is related to postsynaptic spike firing at a cerebellar inhibitory synapse. Neuron. 1998;21:827–835. doi: 10.1016/s0896-6273(00)80598-x. [DOI] [PubMed] [Google Scholar]
- 73.Pugh JR, Raman IM. Potentiation of mossy fiber EPSCs in the cerebellar nuclei by NMDA receptor activation followed by postinhibitory rebound current. Neuron. 2006;51:113–123. doi: 10.1016/j.neuron.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 74.Du Lac S, Raymond JL, Sejnowski TJ, Lisberger SG. Learning and memory in the vestibulo-ocular reflex. Annu Rev Neurosci. 1995;18:409–441. doi: 10.1146/annurev.ne.18.030195.002205. [DOI] [PubMed] [Google Scholar]
- 75.Prestori F, Bonardi C, Mapelli L, et al. Gating of long-term potentiation by nicotinic acetylcholine receptors at the cerebellum input stage. PLoS One. 2013;8:e64828. doi: 10.1371/journal.pone.0064828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boele H-J, Koekkoek SKE, De Zeeuw CI, Ruigrok TJH. Axonal sprouting and formation of terminals in the adult cerebellum during associative motor learning. J Neurosci. 2013;33:17897–17907. doi: 10.1523/JNEUROSCI.0511-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wulff P, Schonewille M, Renzi M, et al. Synaptic inhibition of Purkinje cells mediates consolidation of vestibulo-cerebellar motor learning. Nat Neurosci. 2009;12:1042–1049. doi: 10.1038/nn.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Andreescu CE, Prestori F, Brandalise F, et al. NR2A subunit of the N-methyl D-aspartate receptors are required for potentiation at the mossy fiber to granule cell synapse and vestibulo-cerebellar motor learning. Neuroscience. 2011;176:274–283. doi: 10.1016/j.neuroscience.2010.12.024. [DOI] [PubMed] [Google Scholar]
- 79.Yang Y, Lisberger SG. Role of plasticity at different sites across the time course of cerebellar motor learning. J Neurosci. 2014;34:7077–7090. doi: 10.1523/JNEUROSCI.0017-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Titley HK, Heskin-Sweezie R, Chung JJ, Kassardjian CD, Razik F, Broussard DM. Rapid Consolidation of Motor Memory in the Vestibuloocular Reflex. J Neurophysiol. 2007;98:3809–3812. doi: 10.1152/jn.01056.2007. [DOI] [PubMed] [Google Scholar]
- 81.Kassardjian CD, Tan Y-F, Chung J-YJ, Heskin R, Peterson MJ, Broussard DM. The site of a motor memory shifts with consolidation. J Neurosci. 2005;25:7979–7985. doi: 10.1523/JNEUROSCI.2215-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shutoh F, Ohki M, Kitazawa H, Itohara S, Nagao S. Memory trace of motor learning shifts transsynaptically from cerebellar cortex to nuclei for consolidation. Neuroscience. 2006;139:767–777. doi: 10.1016/j.neuroscience.2005.12.035. [DOI] [PubMed] [Google Scholar]