Abstract
Aims
The enzyme catechol-O-methyl transferase (COMT) plays a primary role in the metabolism of catecholamine neurotransmitters and is implicated in the modulation of cognitive and emotional responses. The best-characterized single nucleotide polymorphism (SNP) of the COMT gene consists of a valine (Val)-to-methionine (Met) substitution at codon 108/158. The Met-containing variant confers a marked reduction in COMT catalytic activity. We recently showed that the activity of recombinant COMT is positively regulated by the enzyme Met sulfoxide reductase (MSR), which counters the oxidation of Met residues of proteins. The current study was designed to assess whether brain COMT activity may be correlated to MSR in an allele-dependent fashion.
Methods
COMT and MSR activities were measured from post-mortem samples of prefrontal cortices, striata and cerebella of 32 subjects, by using catechol and dabsyl-Met sulfoxide as substrates, respectively. Allelic discrimination of COMT Val108/185Met SNP was performed using the Taqman 5’nuclease assay.
Results
Our studies revealed that, in homozygous carriers of Met, but not Val alleles, the activity of COMT and MSR were significantly correlated throughout all tested brain regions.
Discussion
These results suggest that the reduced enzymatic activity of Met-containing COMT may be secondary to Met sulfoxidation, and point to MSR as a key molecular determinant for the modulation of COMT activity.
Keywords: Postmortem human brain, Met oxidation, Catechol-O-methyltransferase, Gene polymorphism, Oxidative stress
INTRODUCTION
Catechol-O-methyltransferase, a key enzyme serving the inactivation of dopamine and norepinephrine [1, 2], is encoded by the COMT gene. The differential transcription of COMT leads to two isoforms of COMT with similar catalytic mechanisms, but divergent cellular localization and substrate affinity [2–6]: a cytosolic soluble form (S-COMT) and a membrane-bound form (MB-COMT), containing an N-terminal, membrane-anchor region with 50 additional amino acids [5, 7, 8]. The best-characterized single nucleotide polymorphism of COMT, rs4680, features the substitution of a valine (Val) for a methionine residue (Met) [9] at positions 108 of S-COMT and 158 of MB-COMT (Val108/158Met) [10]. The Met variant has been shown to exhibit lower enzymatic activity, resulting in reduced catecholamine metabolism and behavioral alterations, including decreased vulnerability for cognitive deficits [11–14] and higher predisposition for aggression in vulnerable individuals [15, 16, 17; see 18 for conflicting results].
Several lines of research have investigated the symptomatic correlates of this polymorphism in schizophrenia and bipolar disorder (BD) [19, 20]. Notably, the Met variant is associated with a slightly lower risk for negative and cognitive symptoms of schizophrenia [21–27], but a greater severity of mania-related symptoms [28–31].
The Val108/158Met substitution has been shown to reduce the thermostability of the COMT enzyme and decrease its activity without affecting its structural properties [10, 32, 33]. Further, this substitution affects protein expression, but not mRNA expression levels, indicating that this polymorphism may lead to post-translational changes in the protein [33, 34]. Nevertheless, the molecular underpinnings of these changes are not fully understood. Given that Met residues undergo spontaneous oxidation into Met sulfoxide, we hypothesized that this process may contribute to the reduction in activity observed in the Met variant. Indeed, we have recently showed that the activity of the recombinant Met108/158 variant was increased by Met sulfoxide reductase (MSR) type A (MSR-A) [35]. The enzymatic reduction of the S- and R- enantiomers of Met sulfoxide is mediated by MSR-A and MSR type B (MSR-B), respectively, and may affect protein function and stability [36, 37]. Accordingly, in this study we tested the activities of COMT and MSR in postmortem brain tissues of schizophrenia and BD patients, as well as non-affected controls, and analyzed this relation with respect to the Val108/158Met genotypes.
MATERIALS AND METHODS
Human Subjects
Postmortem brain tissues from ten (7 males and 3 females) schizophrenia/ schizoaffective disorder patients, thirteen (8 males and 5 females) BD patients and nine (6 males and 3 females) normal (without psychopathology at time of death or significant medical illnesses) control subjects were obtained from the Southwest Brain Bank, Department of Psychiatry, University of Texas Health Science Center at San Antonio, with consent from the next-of-kin. All demographic and clinical characteristics of the individuals are described in Table 1. The three groups did not significantly differ by age at death, postmortem intervals (PMI) (20–45 h) and pH (Table 1).
Table 1.
Characteristics of subjects
| Criteria | Controls | Schizophrenia | Bipolar disorder |
|---|---|---|---|
| Number | 9 | 10 | 13 |
| Mean death age ± SEM | 51.56 ± 5.08 | 54.3 ± 1.81 | 45.77 ± 2.70 |
| Median death age | 54 | 53 | 45 |
| Age range | 20–67 | 49–64 | 25–62 |
| % Suicides | 0.00% | 20.00% | 69.23% |
| % Smokers | 30.00% | 70.00% | 80.00% |
| % Alcohol-dependents | 0.00% | 10.00% | 30.77% |
| Race/ethnicity | |||
| Caucasians | 77.78% | 60.00% | 84.61% |
| Hispanics | 22.22% | 40.00% | 15.39% |
| Gender | |||
| Males | 66.67% | 70.00% | 61.54% |
| Females | 33.33% | 30.00% | 38.46% |
| Other characteristics | |||
| pH | 6.48 ± 0.05 | 6.23 ± 0.14 | 6.56 ± 0.04 |
| Postmortem intervals, PMI | 26.06 ± 1.11 | 29.98 ± 2.21 | 28.22 ± 1.78 |
| Body mass index, BMI | 27.93 ± 2.24 | 29.73 ± 1.23 | 26.34 ± 1.92 |
The Southwest Brain Bank collection of postmortem tissues for research is conducted under the jurisdiction of the State of Texas Anatomical Review Board and the UTHSCSA Institutional Review Board regulates the interviews with the next-of-kin [38]. Trained clinicians interview the next-of-kin about the donor and conduct a DSM-IV-based Mini-International Neuropsychiatric Interview (MINI) [39] to identify Axis 1 and the Structured Clinical Interview for DSM-IV Personality Disorder [40, 41] for Axis 2 psychopathology. Diagnoses were determined by expert diagnostician group consensus (MINI inter-rater reliability = 0.8) after review of the interview materials and available medical records.
Intact fresh brains were transported to the Southwest Brain Bank, and the cerebrum was hemisected. Each hemisphere was cut into 1-cm thick coronal blocks and immediately frozen in 2-methylbutane (Fisher Scientific Co, Fair Lawn, NJ), and stored at −80°C. All tissue samples were analyzed by a neuropathologist, and found to be free of any confounding gross and microscopic neuropathology. The following brain regions were dissected from the right hemisphere: PFC (corresponding to Brodmann area 9); dorsal striatum (rostral caudate); and cerebellum (lateral cerebellar cortex) [38]. The selected brain regions were homogenized in the presence of 25mM Tris-HCl, pH, 7.4 and protease inhibitors cocktail (Roche and Life Technologies, Indianapolis, IN, USA) at 4°C. Following centrifugation at 10,000 g for 20 minutes, the supernatants were collected and stored at −80°C for future analyses.
COMT rs4680 genotyping
Genomic DNA was extracted from brain tissue using the Puregene DNA purification kit (Gentra, Minneapolis, MN). Allelic discrimination for the COMT Val108/185Met SNP was performed using the Taqman 5’nuclease assay (SNP ID rs4680, Life Technologies, Grand Island, NY, USA). Genotypes were determined using the ABI 7900HT SDS 2.2.2 software adapted in the ABI 7900HT Sequence Detection System.
Enzymatic activity assay for MSR
MSR activity was measured in cell extracts of the postmortem brains as previously described [42, 43]. Briefly, equal protein amounts of each tissue/cell preparation (measured by Bio-Rad assay) were assayed for MSR activity following incubation in a reaction mixture containing 25mM Tris-HCl, pH, 7.4, 20mM dithiothreitol (DTT), and 200µM dabsyl-Met sulfoxide (total final volume of 100µl) for 30 minutes at 37°C. Then, the reaction was stopped by the addition of 100 µl of acetonitrile. Proteins in each sample were precipitated by a short high-speed centrifugation, and the soluble material was analyzed for the presence of dabsyl-Met following HPLC-based chromatographically separation using C-18 column (Phenomenex, Torrance, CA). MSR activity was expressed as pmol dabsyl-Met formed per mg protein per minute.
Enzymatic activity assay for COMT
Postmortem human brain sections were homogenized as described above and their COMT activity was monitored according to established procedures [26, 44, 45] with minor modifications. Briefly, the 3,4-dihydroxybenzoic acid was used as a catechol substrate to determine the level of COMT- dependent methylation activity. One hundred micrograms of protein from brain extracts or one microgram of pure recombinant S-COMT were added to a final volume of 100 µl of reaction mixture containing: 25mM Tris-HCl, pH 7.4, 1 mM MgCl2, 2.8 µM radioactive S-adenosyl-Met (3H-SAM, 80.7Ci/mMol) (Perkin-Elmer, Waltham, MA), 20 µM non-radioactive SAM, 1 mM 3,4- dihydroxybenzoic acid, 2.0 mM or 20mM DTT as indicated, and 0.7 units of adenosine deaminase. The 2mM and 20 mM concentrations of DTT were used to reduce either cysteine residues alone or both cysteine and Met residues of S-COMT. Accordingly, 20mM DTT was routinely used when measuring S-COMT activity, unless indicated otherwise. The reaction mixture was incubated for 20 minutes at 37°C and the reaction was stopped by the addition of 100 µl of 2 N HCl and 200 µl of ethyl acetate. Samples were then vortexed and centrifuged at 10,000×g for 5 min. Then, 100 µl of the top ethyl acetate layer was mixed with 10ml of scintillation fluid and counted for the 3H-methylated catechol product. S-COMT activity was expressed as pmol 4-hydroxy-3-methoxybenzoic acid (HMBA) formed per mg protein per minute. Given that MSR enzymes are soluble proteins, all analyses on COMT activities were performed on S-COMT, so as to obtain both enzymes from the same soluble cell fraction and maintain reliable and accurate activity correlations.
Statistical analyses
The frequency of COMT Val108/158Met variants was compared across diagnostic groups by Chi-square (χ2) test. Normality and homoscedasticity of data distribution were verified using Kolmogorov-Smirnov and Bartlett’s tests. Comparisons between diagnostic groups and genotypes were performed by ANOVAs, followed by Tukey’s test with Spjøtvoll-Stoline corrections for post-hoc comparisons. Homogeneity-of-slopes ANCOVA designs were used to test for interactions between continuous and categorical predictors. In the absence of significant interactions, correlation analyses were performed by multiple regressions. Significance threshold was set at 0.05. In consideration of the objections to the conceptual limits of Bonferroni corrections for multiple testing [46], significant values were reported uncorrected throughout the study. All statistical analyses were performed by STATISTICA 9 (Statsoft, Tulsa, OK).
RESULTS
Differences in COMT and MSR activities across diagnostic groups, genotypes and genders
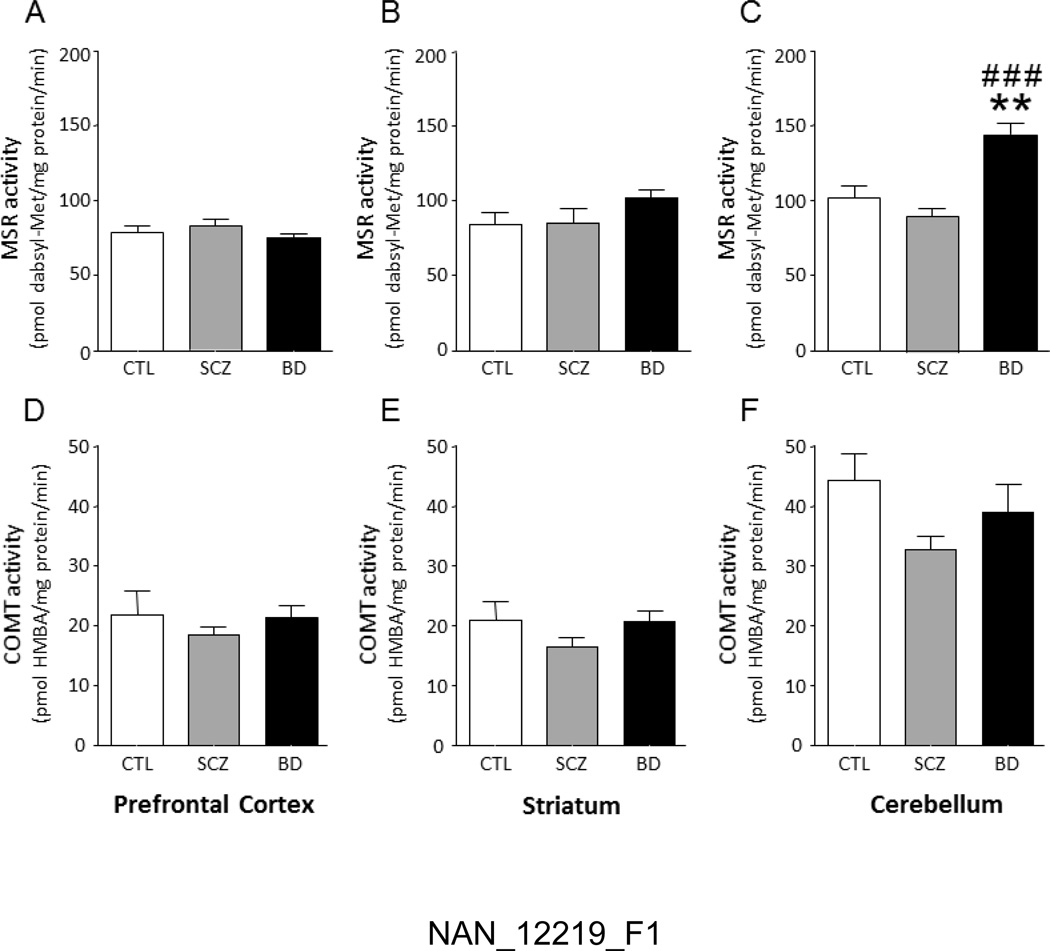
Following genotyping of all subjects, we assessed that the distribution of COMT Val108/158Met genotypes did not differ across diagnostic groups (χ2 =5.26, df= 4, P=0.26, NS; Table 2). Furthermore, the overall genotype distribution was in accordance with Hardy-Weinberg equilibrium, with a predicted frequency of the Val and Met alleles of 0.52 and 0.48, in line with previous data in North American individuals of European origin [47, 48]. We then analyzed the differences in brain-regional COMT and MSR activities between controls, schizophrenia and BD subjects, across the three Val108/158Met genotypes. All values are reported in Table 2. No significant differences in either COMT or MSR activities were observed with respect to genotype. In regards to diagnostic groups, as shown in Fig. 1C, ANOVA detected a significant increase in cerebellar MSR activity in samples from BD-affected subjects (F(2,28)=14.65; P<0.0001), in comparison with both controls (P<0.01) and schizophrenia patients (P<0.001). The increase in MSR activity was not specific to any genotype. No other significant difference was found in MSR activity in the PFC and striatum (Fig.1A–B). Furthermore, COMT activities were not significantly different in any of the tested regions (Fig, 1D–F). Gender-specific comparisons revealed that, in comparison with males, female subjects had a significant increase in MSR activity in the striatum (F(1,30)=4.75, P=0.04), but not in any other region (data not shown).
Table 2.
Brain-regional MSR and COMT activities in diagnostic groups and COMT genotypes
| MSR | COMT | N | ||||||
|---|---|---|---|---|---|---|---|---|
| PFC | STR | CER | PFC | STR | CER | |||
| Controls | Met/Met | 66.9 | 53.65 | 97.48 | 12.19 | 11.44 | 33.15 | 1 |
| Met/Val | 73.37 ± 4.63 | 88.24 ± 13.78 | 88.53 ± 8.09 | 19.29 ± 3.02 | 18.6 ± 2.72 | 34.39 ± 1.77 | 4 | |
| Val/Val | 86.05 ± 2.83 | 101.18 ± 0.94 | 122.42 ± 13 | 20.55 ± 6.3 | 17.8 ± 1.17 | 39.96 ± 21.19 | 4 | |
| Schizophrenia | Met/Met | 75.83 ± 10.49 | 79.68 ± 15.95 | 92.81 ± 8.32 | 14.73 ± 3.19 | 13.12 ± 3.28 | 29.06 ± 1.98 | 4 |
| Met/Val | 80.44 ± 4.04 | 99.41 ± 19.22 | 90.3 ± 10.79 | 19.56 ± 1.63 | 18.78 ± 2.01 | 32.07 ± 2.31 | 4 | |
| Val/Val | 93.27 ± 15.54 | 66.42 ± 5.57 | 85.95 ± 4.43 | 21.26 ± 2.16 | 18.11 ± 2.23 | 39.98 ± 5.85 | 2 | |
| Bipolar disorder | Met/Met | 79.76 ± 7.11 | 96.86 ± 8.07 | 171.41 ± 20.1 | 26.93 ± 4.89 | 19.79 ± 5.58 | 65.02 ± 1.14 | 2 |
| Met/Val | 74.98 ± 4.47 | 104.9 ± 7.33 | 143.37 ± 8.91 | 22.47 ± 3.18 | 31.54 ± 10.72 | 40.55 ± 6.01 | 9 | |
| Val/Val | 86.05 ± 2.83 | 101.18 ± 0.94 | 122.42 ± 13 | 20.55 ± 6.3 | 17.8 ± 1.17 | 39.96 ± 21.19 | 2 | |
All values are means ± SEM. PFC, prefrontal cortex; STR, striatum, CER, cerebellum. COMT’s activity units: pmol HMBA / mg protein / min. Msr’s activity units: pmol dabsyl-Met / mg protein / min.
Figure 1. Enzymatic activities of Msr and COMT in human brain regions.
Differences in MSR (A–C) and COMT (D–F) catalytic activities between post-mortem human tissue samples from the prefrontal cortex, striatum and cerebellum of controls (CTL), schizophrenia subjects (SCZ) and bipolar disorder subjects (BD). Values are displayed as means ± SEM. All analyses were run by 1-way ANOVAs. **, P<0.01 in comparison with controls; ###, P<0.001 in comparison with schizophrenia subjects.
Correlations of COMT and MSR activities
We then analyzed the link between COMT and MSR activities in each of the three tested brain regions, across different diagnostic groups and genotypes. A preliminary homogeneity-of-slope ANCOVA design revealed no significant interactions among COMT activity (dependent variable) and any of the following variables: MSR activity, age, PMI, BMI brain pH (continuous variables), as well as ethnicity, suicidal death, smoking, alcohol dependence and gender (categorical dichotomous variables), across all three regions, diagnostic groups and genotypes.
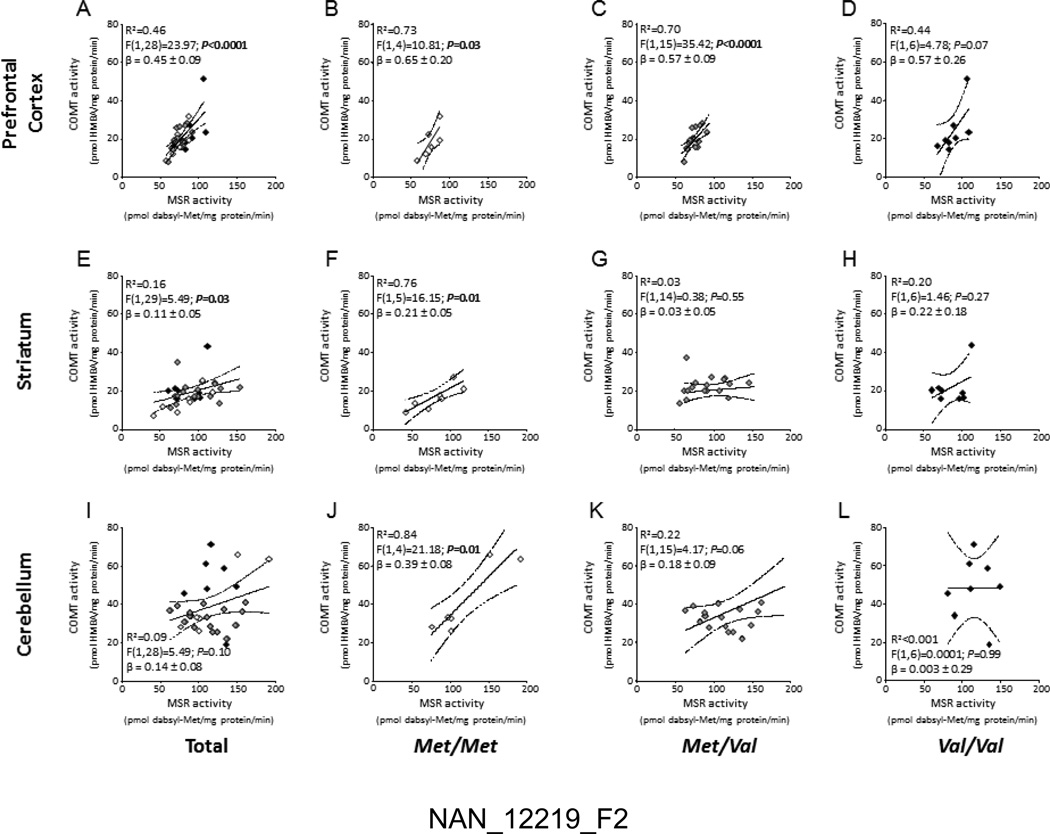
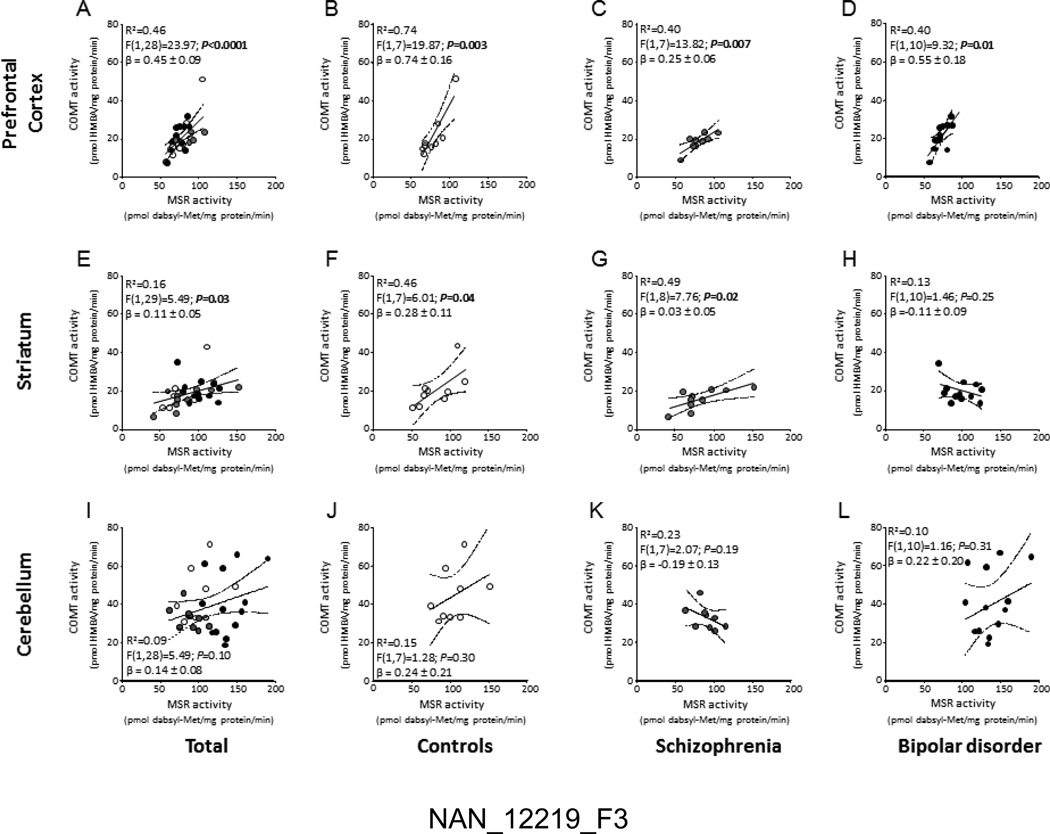
In the PFC (Fig.2A), COMT activity was robustly correlated with MSR activity (F(1,28)=23.97; P<0.0001; R2=0.46). Similar correlations were found for cortical samples of Met/Met (P<0.05; R2=0.73) and Met/Val (P<0.0001; R2=0.70) subjects (Fig. 2B–C); however, MSR and COMT activities were not significantly correlated in the PFCs of Val/Val individuals (Fig. 2D). As shown in Fig. 3A–D, the correlation of COMT and MSR was identified across the PFCs of all diagnostic groups.
Figure 2.
Correlations of MSR and COMT catalytic activities in post-mortem human tissue samples from the (A–D) prefrontal cortex, (E–H) striatum and (I–L) cerebellum across different Val108/158Met COMT genotypes. Empty lozenges, Met/Met carriers; Grey lozenges, Met/Val carriers; Black lozenges, Val/Val carriers.
Figure 3.
Correlations of MSR and COMT catalytic activities in the (A–D) prefrontal cortex, (E–H) striatum and (I–L) cerebellum of controls, schizophrenia subjects and bipolar disorder subjects. Empty circles, controls; Grey circles, schizophrenia subjects; Black circles, bipolar disorder subjects.
In the striatum, COMT and MSR activities were also found to be mildly, yet significantly correlated (F(1,29)=5.49; P=0.03; R2=0.16) (Fig. 2E). The analysis of this correlation across different genotypes revealed significant effects only in the Met/Met group (F(1,5)=16.15; P=0.01; R2=0.76) (Fig. 2F), but not in either Met/Val or Val/Val subjects (Fig.2G–H). The correlation between COMT and MSR was found in the striata of both controls (Fig. 3F) and schizophrenia subjects (Fig. 3G), but not in BD individuals (Fig. 3H).
In the cerebellum, overall COMT activity was not significantly correlated with MSR activity (F(1,28)=5.49; P=0.10; R2=0.10) (Fig. 2I); however, similar to the striatum, a robust correlation between these enzyme activities was identified in the Met/Met group (F(1,4)=21.18; P=0.01; R2=0.84) (Fig. 2J), while no significant correlation was found in either Met/Val or Val/Val subjects (Fig.2K–L). Furthermore, cerebellar MSR and COMT activities were not significantly correlated in any diagnostic group (Fig. 3I–L).
DISCUSSION
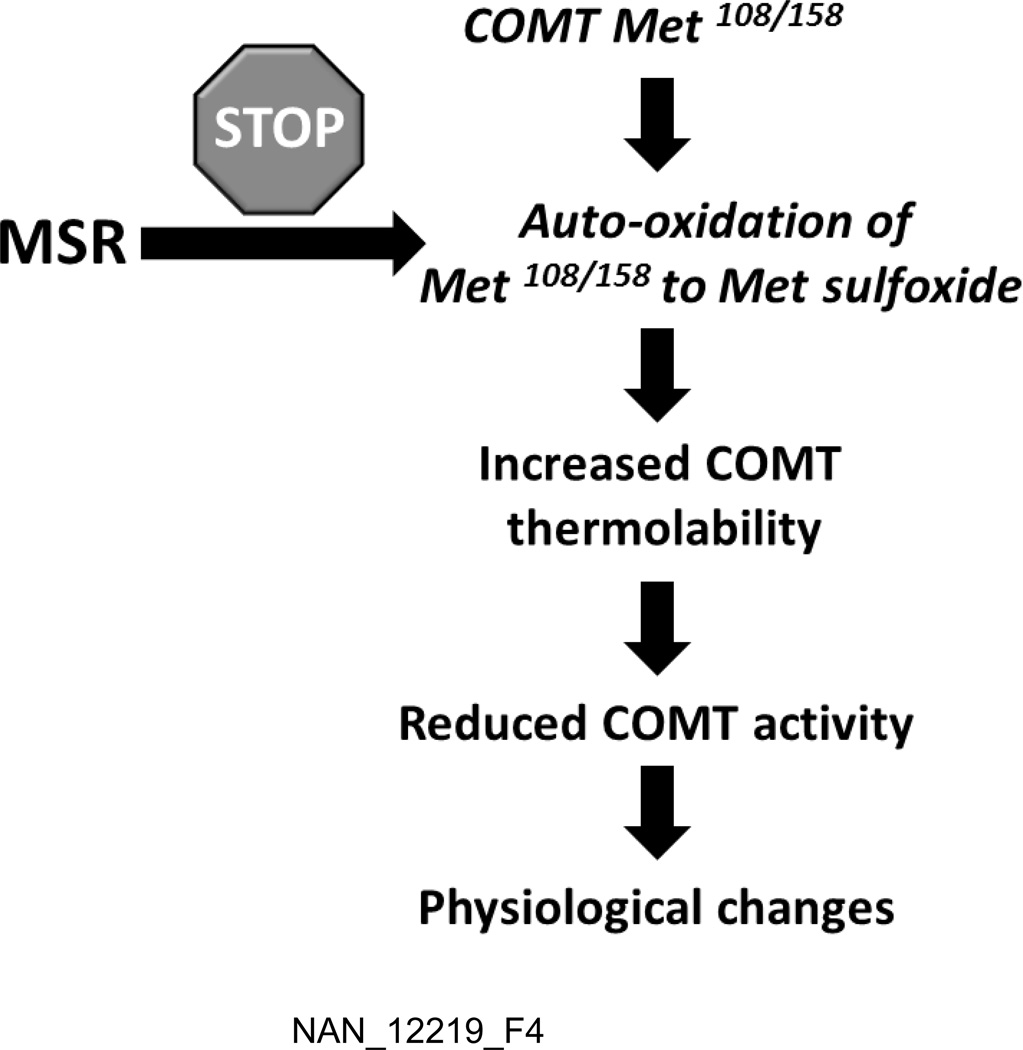
The results of this study showed that, in homozygous carriers of Met, but not Val 108/158 COMT allele, COMT enzyme activity was robustly correlated with MSR across all tested brain regions. In addition, COMT and MSR activities were also correlated in the PFC (but not other brain regions) of Met/Val carriers. These data support our recent findings on the ability of MSR type A to completely restore the activity of the Met108 variant in the absence of oxidative conditions [35]. Collectively, the results of these and our previous analyses suggest that the sulfoxidation of the Met108/158 residue is primarily responsible for the reduction in enzyme activity observed in this variant (Fig. 4). In line with this interpretation, Cotton and colleagues [49] reported the involvement of cysteine, the other sulfur-containing amino acid, in the greater susceptibility of the Met108/158 variant to oxidation.
Figure 4.
Hypothetical relation between MSR and COMT in relation to the post-translational changes of Met108/158-containing COMT variant.
Previous findings have shown that the Met108/158 variant confers lower thermo-stability to COMT, which results in greater vulnerability to proteolytic degradation and reduced protein expression [32, 33]. Several findings support that these phenomena may be contributed by the sulfoxidation of the Met108/158 residue. Indeed, Met oxidation has been shown to alter the hydrophobicity of proteins [50], resulting in conformational changes [51] and higher vulnerability to protease-mediated digestion [52].
It is worth noting that our previous study documented that the enhancement of COMT catalytic activity following addition of MSR-A was observed in both the Met and Val –containing variants after exposure to stress conditions, suggesting that other Met sulfoxide residues in the primary sequence of COMT may also be targeted by MSR [35]. Given that the degree of sulfoxidation of other Met residues on the Met108/158 genotype has yet to be determined, this possibility could not be directly verified in the present study; however, the lack of correlations betw een MSR and COMT in homozygous Val carriers suggests that the oxidation of other Met residues in COMT may need to be accompanied by Met108/158 variant in order to be influential on the catalytic characteristics of this enzyme in vivo.
In substantial agreement with prior evidence [47, 53–58], we failed to identify an association between COMT alleles and either schizophrenia or BD. Nevertheless, studies have indicated a potential association between schizophrenia and polymorphisms within MSRA gene [59]. Thus, epistatic relationships between MSRA and COMT genes may be critical in the functional regulation of catecholamine levels in the PFC and striatum, and play a role in vulnerability for specific behavioral deficits, at least with respect to schizophrenia. An alternative possibility may be that the current diagnostic constructs for schizophrenia and BD may not be adequate to match potential alterations underlying these conditions.
Another important finding of our study was that samples from BD subjects displayed a significant increase in cerebellar MSR activity. Cerebellar atrophy, blood flow reductions and other signs of pathology have been consistently associated with BD [60–67] and secondary mania [9, 68, 69]. Interestingly, cerebellar deficits in BD have been shown to become more prominent with illness progression (63, 68, 69), suggesting that the observed increase in MSR activity in the cerebellum of BD subjects may be secondary to aging-dependent processes, in view of the well-documented action of this enzyme in the protection from these phenomena [36, 70, 71]. In keeping with this idea, cerebellar expression of MSR-A is among the highest in adult humans [72], underscoring the importance of this enzyme in the neuroprotection of this brain region.
This study includes a number of limitations. First, the sample size does not allow for the analysis of complex interactions between genotype and gender across different diagnostic groups, or for the evaluation of potential effects of medications on COMT and MSR activities, which may have interfered with some of the observed correlations. Second, due to our sample size, our analyses could not confirm the impact of COMT genotype on the activity of this enzyme; nevertheless, in keeping with previous post-mortem data collected on larger samples [33], we found that both normal and schizophrenia-affected Met/Met carriers exhibited marked numerical reductions in COMT activity in comparison with their Val/Val counterparts, across all brain regions (Table 2). Third, our analyses of COMT activity were based on S-COMT, in order to maintain reliable correlations with MSR (which is also cytosolic). Although both enzymes display approximately equal amounts and activity [73], S-COMT is less important in catecholamine inactivation than MB-COMT [6, 33, 74]. Nevertheless, it is likely that the results obtained on S-COMT can be generalized to MB-COMT, given their similar catalytic activity [2] and the intracellular localization of MB-COMT [8]; in addition, it should be noted that the action of MSR is based on the availability of Met sulfoxide residues, rather than the specific identity of the protein target. Thus, the action of MSR on S- and MB-COMT should be predictably similar.
These limitations notwithstanding, the present study has highlighted that the actual relation between Met-harboring variants and the reduction in enzyme activity is likely moderated by sulfoxidation of the Met residue, which is directly influenced by MSR. Future studies are required to fully clarify the relationship between COMT and MSR, and its relevance with respect to emotional regulation and the pathophysiology of mental disorders.
Acknowledgements
This research was supported by the Hedwig Miller Fund for Aging Research (to JM), K01MH077777 (to CWB), R24MH076039 (to PMT), R01MH104603 (to MB) as well as by grants from the Tourette Syndrome Association and Kansas Strategic Initiative Grant (to MB). None of the institutions had any further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. The authors wish to thank the Southwest Brain Bank at UTHSCSA for providing tissue for this project and to the donor families that make this research possible.
Abbreviations
- (COMT)
Catechol-O-methyltransferase
- (MSR)
methionine sulfoxide reductase
- (PFC)
prefrontal cortex
- (BD)
Bipolar disorder
Schizophrenia
Footnotes
Author contributions
Study design: JM, MB
Postmortem brain autopsies: DAC, PMT
Genotyping: CWB
Msr and COMT activity assays: JM, JH
Data analysis: JM, MB
Writing: JM, MB
Statement of conflict of Interest: The authors declare no competing financial interests.
References
- 1.Axelrod J, Tomchick R. Enzymatic O-methylation of epinephrine and other catechols. The. J Biol Chem. 1958;233:702–705. [PubMed] [Google Scholar]
- 2.Männistö PT, Kaakkola S. Catechol-O-methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol Rev. 1999;51:593–628. [PubMed] [Google Scholar]
- 3.Rivett AJ, Francis A, Roth JA. Distinct cellular localization of membrane-bound and soluble forms of catechol-O-methyltransferase in brain. J Neurochem. 1983;40:215–219. doi: 10.1111/j.1471-4159.1983.tb12673.x. [DOI] [PubMed] [Google Scholar]
- 4.Jeffery DR, Roth JA. Characterization of membrane-bound and soluble catechol-O-methyltransferase from human frontal cortex. J Neurochem. 1984;42:826–832. doi: 10.1111/j.1471-4159.1984.tb02755.x. [DOI] [PubMed] [Google Scholar]
- 5.Lundström K, Salminen M, Jalanko A, Savolainen R, Ulmanen I. Cloning and characterization of human placental catechol-O-methyltransferase cDNA. DNA cell biol. 1991;10:181–189. doi: 10.1089/dna.1991.10.181. [DOI] [PubMed] [Google Scholar]
- 6.Tenhunen J, Salminen M, Lundstrom K, Kiviluoto T, Savolainen R, Ulmanen I. Genomic organization of the human catechol O-methyltransferase gene and its expression from two distinct promoters. Eur J Biochem / FEBS. 1994;223:1049–1059. doi: 10.1111/j.1432-1033.1994.tb19083.x. [DOI] [PubMed] [Google Scholar]
- 7.Bertocci B, Miggiano V, Da Prada M, Dembic Z, Lahm HW, Malherbe P. Human catechol-O-methyltransferase: cloning and expression of the membrane-associated form. Proc Natl Acad Sci U S A. 1991;88:1416–1420. doi: 10.1073/pnas.88.4.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ulmanen I, Peranen J, Tenhunen J, Tilgmann C, Karhunen T, Panula P, Bernasconi L, Aubry JP, Lundstrom K. Expression and intracellular localization of catechol O-methyltransferase in transfected mammalian cells. Eur J Biochem / FEBS. 1997;243:452–459. doi: 10.1111/j.1432-1033.1997.0452a.x. [DOI] [PubMed] [Google Scholar]
- 9.Eudo C, Beaufils E, Desmidt T, Constans T, Hommet C, Mondon K, Cottier JP. First manic episode revealing cerebellar stroke. J Am Geriatr Soc. 2012;60:588–589. doi: 10.1111/j.1532-5415.2011.03863.x. [DOI] [PubMed] [Google Scholar]
- 10.Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6:243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Malhotra AK, Kestler LJ, Mazzanti C, Bates JA, Goldberg T, Goldman D. A functional polymorphism in the COMT gene and performance on a test of prefrontal cognition. Am J Psychiatry. 2002;159:652–654. doi: 10.1176/appi.ajp.159.4.652. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg TE, Egan MF, Gscheidle T, Coppola R, Weickert T, Kolachana BS, Goldman D, Weinberger DR. Executive subprocesses in working memory: relationship to catechol-O-methyltransferase Val158Met genotype and schizophrenia. Arch Gen Psychiatry. 2003;60:889–896. doi: 10.1001/archpsyc.60.9.889. [DOI] [PubMed] [Google Scholar]
- 13.Blasi G, Mattay VS, Bertolino A, Elvevag B, Callicott JH, Das S, Kolachana BS, Egan MF, Goldberg TE, Weinberger DR. Effect of catechol-O-methyltransferase val158met genotype on attentional control. J Neurosci. 2005;25:5038–5045. doi: 10.1523/JNEUROSCI.0476-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smolka MN, Schumann G, Wrase J, Grusser SM, Flor H, Mann K, Braus DF, Goldman D, Buchel C, Heinz A. Catechol-O-methyltransferase val158met genotype affects processing of emotional stimuli in the amygdala and prefrontal cortex. J Neurosci. 2005;25:836–842. doi: 10.1523/JNEUROSCI.1792-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strous RD, Bark N, Parsia SS, Volavka J, Lachman HM. Analysis of a functional catechol-O-methyltransferase gene polymorphism in schizophrenia: evidence for association with aggressive and antisocial behavior. Psychiatry Res. 1997;69:71–77. doi: 10.1016/s0165-1781(96)03111-3. [DOI] [PubMed] [Google Scholar]
- 16.Strous RD, Nolan KA, Lapidus R, Diaz L, Saito T, Lachman HM. Aggressive behavior in schizophrenia is associated with the low enzyme activity COMT polymorphism: a replication study. Am Med Genet B Neuropsychiatr Genet. 2003;120B:29–34. doi: 10.1002/ajmg.b.20021. [DOI] [PubMed] [Google Scholar]
- 17.Albaugh MD, Harder VS, Althoff RR, Rettew DC, Ehli EA, Lengyel-Nelson T, Davies GE, Ayer L, Sulman J, Stanger C, Hudziak JJ. COMT Val158Met genotype as a risk factor for problem behaviors in youth. J Am Acad Child Adolesc Psychiatry. 2010;49:841–849. doi: 10.1016/j.jaac.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones G, Zammit S, Norton N, Hamshere ML, Jones SJ, Milham C, Sanders RD, McCarthy GM, Jones LA, Cardno AG, Gray M, Murphy KC, Owen MJ. Aggressive behaviour in patients with schizophrenia is associated with catechol-O-methyltransferase genotype. Br J Psychiatry. 2001;179:351–355. doi: 10.1192/bjp.179.4.351. [DOI] [PubMed] [Google Scholar]
- 19.Van Kammen DP, Kelley M. Dopamine and norepinephrine activity in schizophrenia. An integrative perspective. Schizophr Res. 1991;4:173–191. doi: 10.1016/0920-9964(91)90032-m. [DOI] [PubMed] [Google Scholar]
- 20.Manji HK, Quiroz JA, Payne JL, Singh J, Lopes BP, Viegas JS, Zarate CA. The underlying neurobiology of bipolar disorder. World Psychiatry. 2003;2:136–146. [PMC free article] [PubMed] [Google Scholar]
- 21.Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bilder RM, Volavka J, Czobor P, Malhotra AK, Kennedy JL, Ni X, Goldman RS, Hoptman MJ, Sheitman B, Lindenmayer JP, Citrome L, McEvoy JP, Kunz M, Chakos M, Cooper TB, Lieberman JA. Neurocognitive correlates of the COMT Val(158)Met polymorphism in chronic schizophrenia. Biol Psychiatry. 2002;52:701–707. doi: 10.1016/s0006-3223(02)01416-6. [DOI] [PubMed] [Google Scholar]
- 23.Bray NJ, Buckland PR, Williams NM, Williams HJ, Norton N, Owen MJ, O'Donovan MC. A haplotype implicated in schizophrenia susceptibility is associated with reduced COMT expression in human brain. Am J Hum Genet. 2003;73:152–161. doi: 10.1086/376578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gallinat J, Bajbouj M, Sander T, Schlattmann P, Xu K, Ferro EF, Goldman D, Winterer G. Association of the G1947A COMT (Val(108/158)Met) gene polymorphism with prefrontal P300 during information processing. Biol Psychiatry. 2003;54:40–48. doi: 10.1016/s0006-3223(02)01973-x. [DOI] [PubMed] [Google Scholar]
- 25.Tunbridge EM, Harrison PJ, Weinberger DR. Catechol-o-methyltransferase, cognition, and psychosis: Val158Met and beyond. Biol Psychiatry. 2006;60:141–151. doi: 10.1016/j.biopsych.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 26.Ehlis AC, Reif A, Herrmann MJ, Lesch KP, Fallgatter AJ. Impact of catechol-O-methyltransferase on prefrontal brain functioning in schizophrenia spectrum disorders. Neuropsychopharmacology : official publication of the American College of. Neuropsychopharmacology. 2007;32:162–170. doi: 10.1038/sj.npp.1301151. [DOI] [PubMed] [Google Scholar]
- 27.Godar SC, Bortolato M. Gene-sex interactions in schizophrenia: focus on dopamine neurotransmission. Front Behav Neurosci. 2014;8:71. doi: 10.3389/fnbeh.2014.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirov G, Murphy KC, Arranz MJ, Jones I, McCandles F, Kunugi H, Murray RM, McGuffin P, Collier DA, Owen MJ, Craddock N. Low activity allele of catechol-O-methyltransferase gene associated with rapid cycling bipolar disorder. Mol Psychiatry. 1998;3:342–345. doi: 10.1038/sj.mp.4000385. [DOI] [PubMed] [Google Scholar]
- 29.Benedetti F, Dallaspezia S, Colombo C, Lorenzi C, Pirovano A, Smeraldi E. Association between catechol-O-methyltransferase Val(108/158)Met polymorphism and psychotic features of bipolar disorder. J Affect Disord. 2010;125:41–344. doi: 10.1016/j.jad.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Benedetti F, Dallaspezia S, Locatelli C, Radaelli D, Poletti S, Lorenzi C, Pirovano A, Colombo C, Smeraldi E. Recurrence of bipolar mania is associated with catechol-O-methyltransferase Val(108/158)Met polymorphism. J Affect Disord. 2011;132:293–296. doi: 10.1016/j.jad.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 31.Lelli-Chiesa G, Kempton MJ, Jogia J, Tatarelli R, Girardi P, Powell J, Collier DA, Frangou S. The impact of the Val158Met catechol-O-methyltransferase genotype on neural correlates of sad facial affect processing in patients with bipolar disorder and their relatives. Psycholog Med. 2011;41:779–788. doi: 10.1017/S0033291710001431. [DOI] [PubMed] [Google Scholar]
- 32.Lotta T, Vidgren J, Tilgmann C, Ulmanen I, Melen K, Julkunen I, Taskinen J. Kinetics of human soluble and membrane-bound catechol O-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry. 1995;34:4202–4210. doi: 10.1021/bi00013a008. [DOI] [PubMed] [Google Scholar]
- 33.Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, Kolachana BS, Hyde TM, Herman MM, Apud J, Egan MF, Kleinman JE, Weinberger DR. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75:807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsumoto M, Weickert CS, Beltaifa S, Kolachana B, Chen J, Hyde TM, Herman MM, Weinberger DR, Kleinman JE. Catechol O-methyltransferase (COMT) mRNA expression in the dorsolateral prefrontal cortex of patients with schizophrenia. Neuropsychopharmacology. 2003;28:1521–1530. doi: 10.1038/sj.npp.1300218. [DOI] [PubMed] [Google Scholar]
- 35.Moskovitz J, Walss-Bass C, Cruz DA, Thompson PM, Bortolato M. Methionine sulfoxide reductase regulates brain catechol-O-methyl transferase activity. Int J Neuropsychopharmacol. 2014;17:1707–1713. doi: 10.1017/S1461145714000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moskovitz J. Methionine sulfoxide reductases: ubiquitous enzymes involved in antioxidant defense, protein regulation, and prevention of aging-associated diseases. Biochim Biophys Acta. 2005;1703:213–219. doi: 10.1016/j.bbapap.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 37.Oien DB, Moskovitz J. Substrates of the methionine sulfoxide reductase system and their physiological relevance. Curr Top Dev Biol. 2008;80:93–133. doi: 10.1016/S0070-2153(07)80003-2. [DOI] [PubMed] [Google Scholar]
- 38.Thompson PM, Bernardo CG, Cruz DA, Ketchum NS, Michalek JE. Concordance of psychiatric symptom ratings between a subject and informant, relevancy to post-mortem research. Transl Psychiatry. 2013;3:e214. doi: 10.1038/tp.2012.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- 40.First MB, Gibbon M, Spitzer RL, Williams JB, Benjamin LS. Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II) 4th edn. Washington (DC): American Psychiatric Association; [Google Scholar]
- 41.Schneider B, Maurer K, Sargk D, Heiskel H, Weber B, Frolich L, Georgi K, Fritze J, Seidler A. Concordance of DSM-IV Axis I and II diagnoses by personal and informant's interview. Psychiatry Res. 2004;127:121–136. doi: 10.1016/j.psychres.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 42.Moskovitz J, Flescher E, Berlett BS, Azare J, Poston JM, Stadtman ER. Overexpression of peptide-methionine sulfoxide reductase in Saccharomyces cerevisiae and human T cells provides them with high resistance to oxidative stress. Proc Natl Acad Sci U S A. 1998;95:14071–14075. doi: 10.1073/pnas.95.24.14071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moskovitz J, Malik A, Hernandez A, Band M, Avivi A. Methionine sulfoxide reductases and methionine sulfoxide in the subterranean mole rat (Spalax): characterization of expression under various oxygen conditions. Comp Biochem Physiol A Mol Integr Physiol. 2012;161:406–414. doi: 10.1016/j.cbpa.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 44.Lipson RS, Clarke SG. S-adenosylmethionine-dependent protein methylation in mammalian cytosol via tyrphostin modification by catechol-O-methyltransferase. J Biol Chem. 2007;282:1094–1102. doi: 10.1074/jbc.M705456200. [DOI] [PubMed] [Google Scholar]
- 45.Paquette B, Fortier PK, Héroux J, Thibodeau PA, Wagner R, Liu J, Cantin A. Oestrogen metabolism in lymphangioleiomyomatosis: catechol-O-methyltransferase pathway is not involved. Thorax. 2000;55(7):574–578. doi: 10.1136/thorax.55.7.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perneger TV. What's wrong with Bonferroni adjustments. BMJ. 1998;316:1236–1238. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karayiorgou M, Gogos JA, Galke B, Wolyniec PS, Nestadt G, Antonarakis SE, Kazazian HH, Housman DE, Pulver AE. Identification of sequence variants and analysis of the role of the catechol-O-methyl-transferase gene in schizophrenia susceptibility. Biol Psychiatry. 1998;43:425–431. doi: 10.1016/s0006-3223(97)00202-3. [DOI] [PubMed] [Google Scholar]
- 48.Palmatier MA, Kang AM, Kidd KK. Global variation in the frequencies of functionally different catechol-O-methyltransferase alleles. Biol Psychiatry. 1999;46:557–567. doi: 10.1016/s0006-3223(99)00098-0. [DOI] [PubMed] [Google Scholar]
- 49.Cotton NJ, Stoddard B, Parson WW. Oxidative inhibition of human soluble catechol-O-methyltransferase. J Biol Chem. 2004;279:23710–23718. doi: 10.1074/jbc.M401086200. [DOI] [PubMed] [Google Scholar]
- 50.Chao CC, Ma YS, Stadtman ER. Modification of protein surface hydrophobicity and methionine oxidation by oxidative systems. Proc Natl Acad Sci U S A. 1997;94:2969–2974. doi: 10.1073/pnas.94.7.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berlett BS, Friguet B, Yim MB, Chock PB, Stadtman ER. Peroxynitrite-mediated nitration of tyrosine residues in Escherichia coli glutamine synthetase mimics adenylylation: relevance to signal transduction. Proc Natl Acad Sci U S A. 1996;93:1776–1780. doi: 10.1073/pnas.93.5.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Levine RL, Mosoni L, Berlett BS, Stadtman ER. Methionine residues as endogenous antioxidants in proteins. Proc Natl Acad Sci U S A. 1996;93:15036–15040. doi: 10.1073/pnas.93.26.15036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Daniels JK, Williams NM, Williams J, Jones LA, Cardno AG, Murphy KC, Spurlock G, Riley B, Scambler P, Asherson P, McGuffin P, Owen MJ. No evidence for allelic association between schizophrenia and a polymorphism determining high or low catechol O-methyltransferase activity. Am J Psychiatry. 1996;153:268–270. doi: 10.1176/ajp.153.2.268. [DOI] [PubMed] [Google Scholar]
- 54.Riley B, Mogudi-Carter M, Jenkins T, Williamson R. No evidence for linkage of chromosome 22 markers to schizophrenia in southern African Bantu-speaking families. Am J Med Genet A. 1996;67:515–522. doi: 10.1002/(SICI)1096-8628(19961122)67:6<515::AID-AJMG2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 55.Gutierrez B, Bertranpetit J, Guillamat R, Valles V, Arranz MJ, Kerwin R, Fananas L. Association analysis of the catechol O-methyltransferase gene and bipolar affective disorder. Am J Psychiatry. 1997;154:113–115. doi: 10.1176/ajp.154.1.113. [DOI] [PubMed] [Google Scholar]
- 56.Kunugi H, Vallada H, Hoda F, Kirov G, Gill M, Aitchison KJ, Ball D, Arranz MJ, Murray RM, Collier DA. No evidence for an association of affective disorders with high- or low-activity allele of catechol-o-methyltransferase gene. Biol Psychiatry. 1997;42:282–285. doi: 10.1016/S0006-3223(96)00366-6. [DOI] [PubMed] [Google Scholar]
- 57.Lachman HM, Kelsoe J, Moreno L, Katz S, Papolos DF. Lack of association of catechol-O-methyltransferase (COMT) functional polymorphism in bipolar affective disorder. Psychiatr Genet. 1997;7:13–17. doi: 10.1097/00041444-199700710-00002. [DOI] [PubMed] [Google Scholar]
- 58.Strous RD, Lapidus R, Viglin D, Kotler M, Lachman HM. Analysis of an association between the COMT polymorphism and clinical symptomatology in schizophrenia. Neurosci Lett. 2006;393:170–173. doi: 10.1016/j.neulet.2005.09.067. [DOI] [PubMed] [Google Scholar]
- 59.Walss-Bass C, Soto-Bernardini MC, Johnson-Pais T, Leach RJ, Ontiveros A, Nicolini H, Mendoza R, Jerez A, Dassori A, Chavarria-Siles I, Escamilla MA, Raventos H. Methionine sulfoxide reductase: a novel schizophrenia candidate gene. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:219–225. doi: 10.1002/ajmg.b.30791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nasrallah HA, Jacoby CG, McCalley-Whitters M. Cerebellar atrophy in schizophrenia and mania. Lancet. 1981;1:1102. doi: 10.1016/s0140-6736(81)92266-2. [DOI] [PubMed] [Google Scholar]
- 61.Hamilton NG, Frick RB, Takahashi T, Hopping MW. Psychiatric symptoms and cerebellar pathology. Am J Psychiatry. 1983;140:1322–1326. doi: 10.1176/ajp.140.10.1322. [DOI] [PubMed] [Google Scholar]
- 62.Yadalam KG, Jain AK, Simpson GM. Mania in two sisters with similar cerebellar disturbance. Am J Psychiatry. 1985;142:1067–1069. doi: 10.1176/ajp.142.9.1067. [DOI] [PubMed] [Google Scholar]
- 63.Yates WR, Jacoby CG, Andreasen NC. Cerebellar atrophy in schizophrenia and affective disorder. Am J Psychiatry. 1987;144:465–467. doi: 10.1176/ajp.144.4.465. [DOI] [PubMed] [Google Scholar]
- 64.Lauterbach EC. Bipolar disorders, dystonia, and compulsion after dysfunction of the cerebellum, dentatorubrothalamic tract, and substantia nigra. Biol Psychiatry. 1996;40:726–730. doi: 10.1016/0006-3223(96)82516-9. [DOI] [PubMed] [Google Scholar]
- 65.DelBello MP, Strakowski SM, Zimmerman ME, Hawkins JM, Sax KW. MRI analysis of the cerebellum in bipolar disorder: a pilot study. Neuropsychopharmacology. 1999;21:63–68. doi: 10.1016/S0893-133X(99)00026-3. [DOI] [PubMed] [Google Scholar]
- 66.Konarski JZ, McIntyre RS, Grupp LA, Kennedy SH. Is the cerebellum relevant in the circuitry of neuropsychiatric disorders? J Psychiatry Neurosci. 2005;30:178–186. [PMC free article] [PubMed] [Google Scholar]
- 67.Baldaçara L, Borgio JG, Lacerda AL, Jackowski AP. Cerebellum and psychiatric disorders. Rev Bras Psiquiatr. 2008;30:281–289. doi: 10.1590/s1516-44462008000300016. [DOI] [PubMed] [Google Scholar]
- 68.Jagadesan V, Thiruvengadam KR, Muralidharan R. Cerebellar Stroke-manifesting as Mania. Indian J Psychol Med. 2014;36:338–340. doi: 10.4103/0253-7176.135396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim D, Cho HB, Dager SR, Yurgelun-Todd DA, Yoon S, Lee JH, Lee SH, Lee S, Renshaw PF, Lyoo IK. Posterior cerebellar vermal deficits in bipolar disorder. J Affect Disord. 2013;150:499–506. doi: 10.1016/j.jad.2013.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moskovitz J, Bar-Noy S, Williams WM, Requena J, Berlett BS, Stadtman ER. Methionine sulfoxide reductase (MsrA) is a regulator of antioxidant defense and lifespan in mammals. Proc Natl Acad Sci U S A. 2001;98:12920–12925. doi: 10.1073/pnas.231472998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pal R, Oien DB, Ersen FY, Moskovitz J. Elevated levels of brain-pathologies associated with neurodegenerative diseases in the methionine sulfoxide reductase A knockout mouse. Exp Brain Res. 2007;180:765–774. doi: 10.1007/s00221-007-0903-6. [DOI] [PubMed] [Google Scholar]
- 72.Kuschel, Hansel A, Schonherr R, Weissbach H, Brot N, Hoshi T, Heinemann SH. Molecular cloning and functional expression of a human peptide methionine sulfoxide reductase (hMsrA) FEBS Lett. 1999;456:17–21. doi: 10.1016/s0014-5793(99)00917-5. [DOI] [PubMed] [Google Scholar]
- 73.Myöhänen TT, Männistö PT. Distribution and functions of catechol-O-methyltransferase proteins: do recent findings change the picture? Int Rev Neurobiol. 2010;95:29–47. doi: 10.1016/B978-0-12-381326-8.00003-X. [DOI] [PubMed] [Google Scholar]
- 74.Roth JA. Membrane-bound catechol-O-methyltransferase: a reevaluation of its role in the O-methylation of the catecholamine neurotransmitters. Rev Physiol Biochem Pharmacol. 1992;120:1–29. doi: 10.1007/BFb0036121. [DOI] [PubMed] [Google Scholar]