Abstract
The Escherichia coli outer membrane protein FhuA catalyzes the transport of Fe3+(-)ferrichrome and is the receptor of phage T5 and phi 80. The purified protein inserted into planar lipid bilayers showed no channel activity. Binding of phage T5 and FhuA resulted in the appearance of high conductance ion channels. The electrophysiological characteristics of the channels (conductance, kinetic behavior, substates, ion selectivity including the effect of ferrichrome) showed similarities with those of the channel formed by a FhuA derivative from which the 'gating loop' (delta 322-355) had been removed. binding of phage T5 to FhuA in E.coli cells conferred SDS sensitivity to the bacteria, suggesting that such channels also exist in vivo. These data suggest that binding of T5 to loop 322-355 of FhuA, which constitutes the T5 binding site, unmasks an inner channel in FhuA. Both T5 and ferrichrome bind to the closed state of the channel but only T5 can trigger its opening.
Full text
PDF
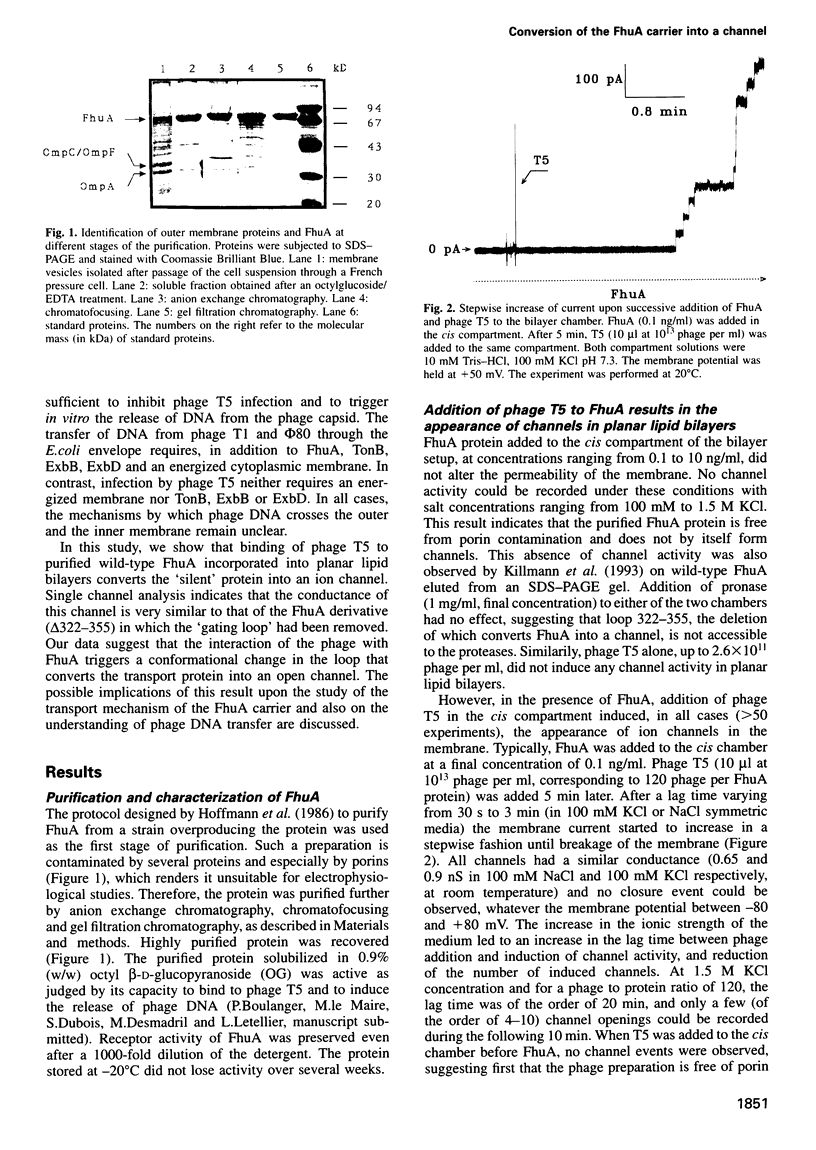
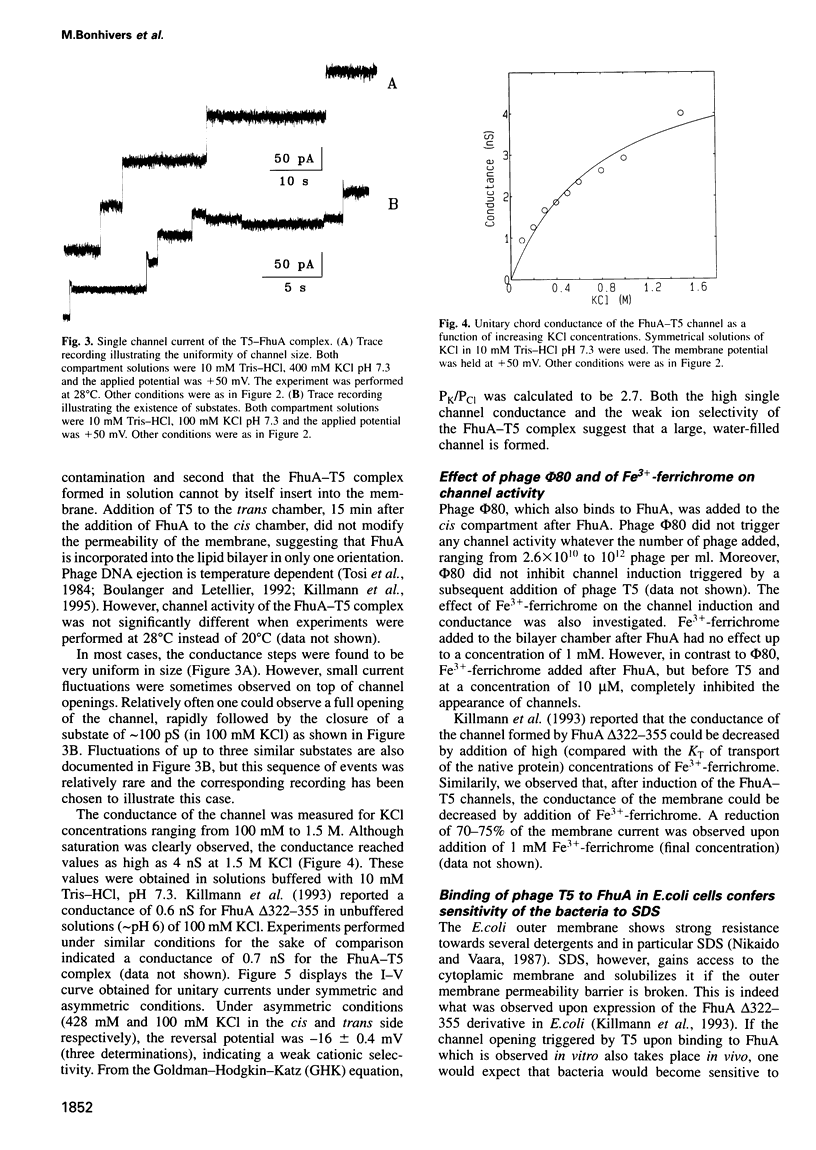
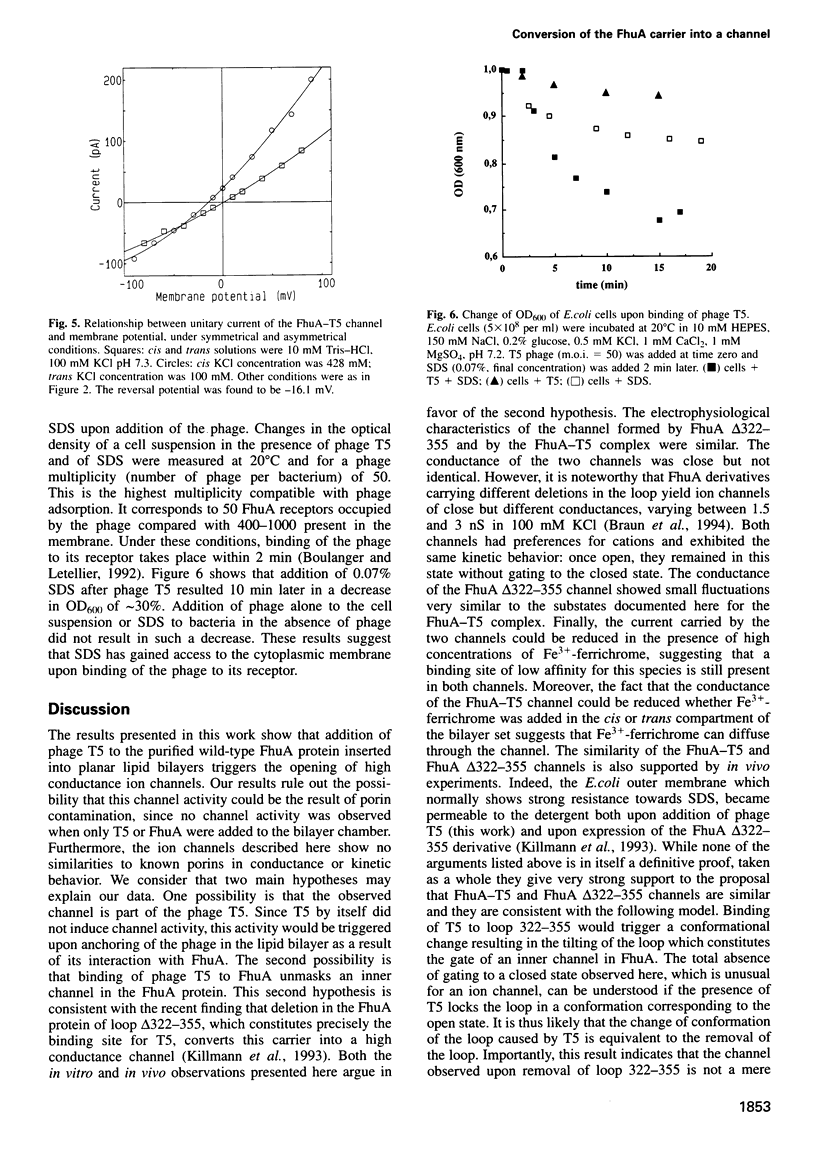



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boulanger P., Letellier L. Ion channels are likely to be involved in the two steps of phage T5 DNA penetration into Escherichia coli cells. J Biol Chem. 1992 Feb 15;267(5):3168–3172. [PubMed] [Google Scholar]
- Braun V. Energy-coupled transport and signal transduction through the gram-negative outer membrane via TonB-ExbB-ExbD-dependent receptor proteins. FEMS Microbiol Rev. 1995 Jul;16(4):295–307. doi: 10.1111/j.1574-6976.1995.tb00177.x. [DOI] [PubMed] [Google Scholar]
- Braun V., Killmann H., Benz R. Energy-coupled transport through the outer membrane of Escherichia coli small deletions in the gating loop convert the FhuA transport protein into a diffusion channel. FEBS Lett. 1994 Jun 6;346(1):59–64. doi: 10.1016/0014-5793(94)00431-5. [DOI] [PubMed] [Google Scholar]
- Carmel G., Coulton J. W. Internal deletions in the FhuA receptor of Escherichia coli K-12 define domains of ligand interactions. J Bacteriol. 1991 Jul;173(14):4394–4403. doi: 10.1128/jb.173.14.4394-4403.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan S. W., Schirmer T., Rummel G., Steiert M., Ghosh R., Pauptit R. A., Jansonius J. N., Rosenbusch J. P. Crystal structures explain functional properties of two E. coli porins. Nature. 1992 Aug 27;358(6389):727–733. doi: 10.1038/358727a0. [DOI] [PubMed] [Google Scholar]
- Eick-Helmerich K., Braun V. Import of biopolymers into Escherichia coli: nucleotide sequences of the exbB and exbD genes are homologous to those of the tolQ and tolR genes, respectively. J Bacteriol. 1989 Sep;171(9):5117–5126. doi: 10.1128/jb.171.9.5117-5126.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feucht A., Schmid A., Benz R., Schwarz H., Heller K. J. Pore formation associated with the tail-tip protein pb2 of bacteriophage T5. J Biol Chem. 1990 Oct 25;265(30):18561–18567. [PubMed] [Google Scholar]
- Guihard G., Boulanger P., Letellier L. Involvement of phage T5 tail proteins and contact sites between the outer and inner membrane of Escherichia coli in phage T5 DNA injection. J Biol Chem. 1992 Feb 15;267(5):3173–3178. [PubMed] [Google Scholar]
- Hantke K., Braun V. Functional interaction of the tonA/tonB receptor system in Escherichia coli. J Bacteriol. 1978 Jul;135(1):190–197. doi: 10.1128/jb.135.1.190-197.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller K. J. Molecular interaction between bacteriophage and the gram-negative cell envelope. Arch Microbiol. 1992;158(4):235–248. doi: 10.1007/BF00245239. [DOI] [PubMed] [Google Scholar]
- Heller K. J., Schwarz H. Irreversible binding to the receptor of bacteriophages T5 and BF23 does not occur with the tip of the tail. J Bacteriol. 1985 May;162(2):621–625. doi: 10.1128/jb.162.2.621-625.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson H. E., McCorquodale D. J. Genetic and physiological studies of bacteriophage t5 I. An expanded genetic map of t5. J Virol. 1971 May;7(5):612–618. doi: 10.1128/jvi.7.5.612-618.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann H., Fischer E., Kraut H., Braun V. Preparation of the FhuA (TonA) receptor protein from cell envelopes of an overproducing strain of Escherichia coli K-12. J Bacteriol. 1986 May;166(2):404–411. doi: 10.1128/jb.166.2.404-411.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishidate K., Creeger E. S., Zrike J., Deb S., Glauner B., MacAlister T. J., Rothfield L. I. Isolation of differentiated membrane domains from Escherichia coli and Salmonella typhimurium, including a fraction containing attachment sites between the inner and outer membranes and the murein skeleton of the cell envelope. J Biol Chem. 1986 Jan 5;261(1):428–443. [PubMed] [Google Scholar]
- Kadner R. J. Vitamin B12 transport in Escherichia coli: energy coupling between membranes. Mol Microbiol. 1990 Dec;4(12):2027–2033. doi: 10.1111/j.1365-2958.1990.tb00562.x. [DOI] [PubMed] [Google Scholar]
- Kampfenkel K., Braun V. Membrane topology of the Escherichia coli ExbD protein. J Bacteriol. 1992 Aug;174(16):5485–5487. doi: 10.1128/jb.174.16.5485-5487.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampfenkel K., Braun V. Topology of the ExbB protein in the cytoplasmic membrane of Escherichia coli. J Biol Chem. 1993 Mar 15;268(8):6050–6057. [PubMed] [Google Scholar]
- Killmann H., Benz R., Braun V. Conversion of the FhuA transport protein into a diffusion channel through the outer membrane of Escherichia coli. EMBO J. 1993 Aug;12(8):3007–3016. doi: 10.1002/j.1460-2075.1993.tb05969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killmann H., Braun V. An aspartate deletion mutation defines a binding site of the multifunctional FhuA outer membrane receptor of Escherichia coli K-12. J Bacteriol. 1992 Jun;174(11):3479–3486. doi: 10.1128/jb.174.11.3479-3486.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killmann H., Videnov G., Jung G., Schwarz H., Braun V. Identification of receptor binding sites by competitive peptide mapping: phages T1, T5, and phi 80 and colicin M bind to the gating loop of FhuA. J Bacteriol. 1995 Feb;177(3):694–698. doi: 10.1128/jb.177.3.694-698.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koebnik R., Braun V. Insertion derivatives containing segments of up to 16 amino acids identify surface- and periplasm-exposed regions of the FhuA outer membrane receptor of Escherichia coli K-12. J Bacteriol. 1993 Feb;175(3):826–839. doi: 10.1128/jb.175.3.826-839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreusch A., Neubüser A., Schiltz E., Weckesser J., Schulz G. E. Structure of the membrane channel porin from Rhodopseudomonas blastica at 2.0 A resolution. Protein Sci. 1994 Jan;3(1):58–63. doi: 10.1002/pro.5560030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Rutz J. M., Feix J. B., Klebba P. E. Permeability properties of a large gated channel within the ferric enterobactin receptor, FepA. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10653–10657. doi: 10.1073/pnas.90.22.10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondigler M., Vögele R. T., Heller K. J. Overproduced and purified receptor binding protein pb5 of bacteriophage T5 binds to the T5 receptor protein FhuA. FEMS Microbiol Lett. 1995 Aug 1;130(2-3):293–300. doi: 10.1111/j.1574-6968.1995.tb07734.x. [DOI] [PubMed] [Google Scholar]
- Postle K., Skare J. T. Escherichia coli TonB protein is exported from the cytoplasm without proteolytic cleavage of its amino terminus. J Biol Chem. 1988 Aug 5;263(22):11000–11007. [PubMed] [Google Scholar]
- Postle K. TonB and the gram-negative dilemma. Mol Microbiol. 1990 Dec;4(12):2019–2025. doi: 10.1111/j.1365-2958.1990.tb00561.x. [DOI] [PubMed] [Google Scholar]
- Postle K. TonB protein and energy transduction between membranes. J Bioenerg Biomembr. 1993 Dec;25(6):591–601. doi: 10.1007/BF00770246. [DOI] [PubMed] [Google Scholar]
- Rutz J. M., Liu J., Lyons J. A., Goranson J., Armstrong S. K., McIntosh M. A., Feix J. B., Klebba P. E. Formation of a gated channel by a ligand-specific transport protein in the bacterial outer membrane. Science. 1992 Oct 16;258(5081):471–475. doi: 10.1126/science.1411544. [DOI] [PubMed] [Google Scholar]
- Saigo K. Isolation of high-density mutants and identification of nonessential structural proteins in bacteriophage T5; dispensability of L-shaped tail fibers and a secondary major head protein. Virology. 1978 Apr;85(2):422–433. doi: 10.1016/0042-6822(78)90449-x. [DOI] [PubMed] [Google Scholar]
- Schirmer T., Keller T. A., Wang Y. F., Rosenbusch J. P. Structural basis for sugar translocation through maltoporin channels at 3.1 A resolution. Science. 1995 Jan 27;267(5197):512–514. doi: 10.1126/science.7824948. [DOI] [PubMed] [Google Scholar]
- Schöffler H., Braun V. Transport across the outer membrane of Escherichia coli K12 via the FhuA receptor is regulated by the TonB protein of the cytoplasmic membrane. Mol Gen Genet. 1989 Jun;217(2-3):378–383. doi: 10.1007/BF02464907. [DOI] [PubMed] [Google Scholar]
- Tosi F., Labedan B., Legault-Démare J. Analysis of the coliphage T5 DNA ejection process with free and liposome-associated TonA protein. J Virol. 1984 Apr;50(1):213–219. doi: 10.1128/jvi.50.1.213-219.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M. S., Abele U., Weckesser J., Welte W., Schiltz E., Schulz G. E. Molecular architecture and electrostatic properties of a bacterial porin. Science. 1991 Dec 13;254(5038):1627–1630. doi: 10.1126/science.1721242. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]
- Zweig M., Cummings D. J. Cleavage of head and tail proteins during bacteriophage T5 assembly: selective host involvement in the cleavage of a tail protein. J Mol Biol. 1973 Nov 5;80(3):505–518. doi: 10.1016/0022-2836(73)90418-x. [DOI] [PubMed] [Google Scholar]



