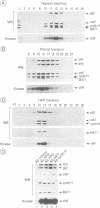
Abstract
MAT1, cyclin H and cdk7 are part of TFIIH, a class II transcription factor which possesses numerous subunits of which several have been shown to be involved in processes other than transcription. Two of them, XPD (ERCC2) and XPB (ERCC3), are helicases involved in nucleotide excision repair (NER), whereas cdk7, cyclin H and MAT1 are thought to participate in cell cycle regulation. MAT1, cyclin H and cdk7 exist as a ternary complex either free or associated with TFIIH from which the latter can be dissociated at high salt concentration. MAT1 is strongly associated with cdk7 and cyclin H. Although not strictly required for the formation and activity of the complex, it stimulates its kinase activity. The kinase activity of TFIIH, which is constant during the cell cycle, is reduced after UV light irradiation.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aboussekhra A., Biggerstaff M., Shivji M. K., Vilpo J. A., Moncollin V., Podust V. N., Protić M., Hübscher U., Egly J. M., Wood R. D. Mammalian DNA nucleotide excision repair reconstituted with purified protein components. Cell. 1995 Mar 24;80(6):859–868. doi: 10.1016/0092-8674(95)90289-9. [DOI] [PubMed] [Google Scholar]
- Carr A. M., Hoekstra M. F. The cellular responses to DNA damage. Trends Cell Biol. 1995 Jan;5(1):32–40. doi: 10.1016/s0962-8924(00)88934-5. [DOI] [PubMed] [Google Scholar]
- Chalut C., Moncollin V., Egly J. M. Transcription by RNA polymerase II: a process linked to DNA repair. Bioessays. 1994 Sep;16(9):651–655. doi: 10.1002/bies.950160910. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E., Hultner M. L. Transcription-related human disorders. Am J Hum Genet. 1995 Jun;56(6):1257–1261. [PMC free article] [PubMed] [Google Scholar]
- Crissman H. A., Hirons G. T. Staining of DNA in live and fixed cells. Methods Cell Biol. 1994;41:195–209. doi: 10.1016/s0091-679x(08)61718-5. [DOI] [PubMed] [Google Scholar]
- Darbon J. M., Devault A., Taviaux S., Fesquet D., Martinez A. M., Galas S., Cavadore J. C., Dorée M., Blanchard J. M. Cloning, expression and subcellular localization of the human homolog of p40MO15 catalytic subunit of cdk-activating kinase. Oncogene. 1994 Nov;9(11):3127–3138. [PubMed] [Google Scholar]
- Devault A., Martinez A. M., Fesquet D., Labbé J. C., Morin N., Tassan J. P., Nigg E. A., Cavadore J. C., Dorée M. MAT1 ('menage à trois') a new RING finger protein subunit stabilizing cyclin H-cdk7 complexes in starfish and Xenopus CAK. EMBO J. 1995 Oct 16;14(20):5027–5036. doi: 10.1002/j.1460-2075.1995.tb00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feaver W. J., Svejstrup J. Q., Henry N. L., Kornberg R. D. Relationship of CDK-activating kinase and RNA polymerase II CTD kinase TFIIH/TFIIK. Cell. 1994 Dec 16;79(6):1103–1109. doi: 10.1016/0092-8674(94)90040-x. [DOI] [PubMed] [Google Scholar]
- Fesquet D., Labbé J. C., Derancourt J., Capony J. P., Galas S., Girard F., Lorca T., Shuttleworth J., Dorée M., Cavadore J. C. The MO15 gene encodes the catalytic subunit of a protein kinase that activates cdc2 and other cyclin-dependent kinases (CDKs) through phosphorylation of Thr161 and its homologues. EMBO J. 1993 Aug;12(8):3111–3121. doi: 10.1002/j.1460-2075.1993.tb05980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R. P., Jin P., Chamberlin H. M., Morgan D. O. Alternative mechanisms of CAK assembly require an assembly factor or an activating kinase. Cell. 1995 Oct 6;83(1):47–57. doi: 10.1016/0092-8674(95)90233-3. [DOI] [PubMed] [Google Scholar]
- Fisher R. P., Morgan D. O. A novel cyclin associates with MO15/CDK7 to form the CDK-activating kinase. Cell. 1994 Aug 26;78(4):713–724. doi: 10.1016/0092-8674(94)90535-5. [DOI] [PubMed] [Google Scholar]
- Gerard M., Fischer L., Moncollin V., Chipoulet J. M., Chambon P., Egly J. M. Purification and interaction properties of the human RNA polymerase B(II) general transcription factor BTF2. J Biol Chem. 1991 Nov 5;266(31):20940–20945. [PubMed] [Google Scholar]
- Gerber H. P., Hagmann M., Seipel K., Georgiev O., West M. A., Litingtung Y., Schaffner W., Corden J. L. RNA polymerase II C-terminal domain required for enhancer-driven transcription. Nature. 1995 Apr 13;374(6523):660–662. doi: 10.1038/374660a0. [DOI] [PubMed] [Google Scholar]
- Gottesfeld J. M., Wolf V. J., Dang T., Forbes D. J., Hartl P. Mitotic repression of RNA polymerase III transcription in vitro mediated by phosphorylation of a TFIIIB component. Science. 1994 Jan 7;263(5143):81–84. doi: 10.1126/science.8272869. [DOI] [PubMed] [Google Scholar]
- Hanawalt P. C. Heterogeneity of DNA repair at the gene level. Mutat Res. 1991 Apr;247(2):203–211. doi: 10.1016/0027-5107(91)90016-h. [DOI] [PubMed] [Google Scholar]
- Humbert S., van Vuuren H., Lutz Y., Hoeijmakers J. H., Egly J. M., Moncollin V. p44 and p34 subunits of the BTF2/TFIIH transcription factor have homologies with SSL1, a yeast protein involved in DNA repair. EMBO J. 1994 May 15;13(10):2393–2398. doi: 10.1002/j.1460-2075.1994.tb06523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffrey P. D., Russo A. A., Polyak K., Gibbs E., Hurwitz J., Massagué J., Pavletich N. P. Mechanism of CDK activation revealed by the structure of a cyclinA-CDK2 complex. Nature. 1995 Jul 27;376(6538):313–320. doi: 10.1038/376313a0. [DOI] [PubMed] [Google Scholar]
- Li Y., Kornberg R. D. Interplay of positive and negative effectors in function of the C-terminal repeat domain of RNA polymerase II. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2362–2366. doi: 10.1073/pnas.91.6.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H., Zawel L., Fisher L., Egly J. M., Reinberg D. Human general transcription factor IIH phosphorylates the C-terminal domain of RNA polymerase II. Nature. 1992 Aug 20;358(6388):641–645. doi: 10.1038/358641a0. [DOI] [PubMed] [Google Scholar]
- Morgan D. O. Principles of CDK regulation. Nature. 1995 Mar 9;374(6518):131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- Mäkelä T. P., Parvin J. D., Kim J., Huber L. J., Sharp P. A., Weinberg R. A. A kinase-deficient transcription factor TFIIH is functional in basal and activated transcription. Proc Natl Acad Sci U S A. 1995 May 23;92(11):5174–5178. doi: 10.1073/pnas.92.11.5174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkelä T. P., Tassan J. P., Nigg E. A., Frutiger S., Hughes G. J., Weinberg R. A. A cyclin associated with the CDK-activating kinase MO15. Nature. 1994 Sep 15;371(6494):254–257. doi: 10.1038/371254a0. [DOI] [PubMed] [Google Scholar]
- Nigg E. A. Cyclin-dependent protein kinases: key regulators of the eukaryotic cell cycle. Bioessays. 1995 Jun;17(6):471–480. doi: 10.1002/bies.950170603. [DOI] [PubMed] [Google Scholar]
- Roy R., Adamczewski J. P., Seroz T., Vermeulen W., Tassan J. P., Schaeffer L., Nigg E. A., Hoeijmakers J. H., Egly J. M. The MO15 cell cycle kinase is associated with the TFIIH transcription-DNA repair factor. Cell. 1994 Dec 16;79(6):1093–1101. doi: 10.1016/0092-8674(94)90039-6. [DOI] [PubMed] [Google Scholar]
- Schaeffer L., Moncollin V., Roy R., Staub A., Mezzina M., Sarasin A., Weeda G., Hoeijmakers J. H., Egly J. M. The ERCC2/DNA repair protein is associated with the class II BTF2/TFIIH transcription factor. EMBO J. 1994 May 15;13(10):2388–2392. doi: 10.1002/j.1460-2075.1994.tb06522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer L., Roy R., Humbert S., Moncollin V., Vermeulen W., Hoeijmakers J. H., Chambon P., Egly J. M. DNA repair helicase: a component of BTF2 (TFIIH) basic transcription factor. Science. 1993 Apr 2;260(5104):58–63. doi: 10.1126/science.8465201. [DOI] [PubMed] [Google Scholar]
- Schwabe J. W., Klug A. Zinc mining for protein domains. Nat Struct Biol. 1994 Jun;1(6):345–349. doi: 10.1038/nsb0694-345. [DOI] [PubMed] [Google Scholar]
- Serizawa H., Conaway J. W., Conaway R. C. Phosphorylation of C-terminal domain of RNA polymerase II is not required in basal transcription. Nature. 1993 May 27;363(6427):371–374. doi: 10.1038/363371a0. [DOI] [PubMed] [Google Scholar]
- Serizawa H., Mäkelä T. P., Conaway J. W., Conaway R. C., Weinberg R. A., Young R. A. Association of Cdk-activating kinase subunits with transcription factor TFIIH. Nature. 1995 Mar 16;374(6519):280–282. doi: 10.1038/374280a0. [DOI] [PubMed] [Google Scholar]
- Shiekhattar R., Mermelstein F., Fisher R. P., Drapkin R., Dynlacht B., Wessling H. C., Morgan D. O., Reinberg D. Cdk-activating kinase complex is a component of human transcription factor TFIIH. Nature. 1995 Mar 16;374(6519):283–287. doi: 10.1038/374283a0. [DOI] [PubMed] [Google Scholar]
- Solomon M. J., Harper J. W., Shuttleworth J. CAK, the p34cdc2 activating kinase, contains a protein identical or closely related to p40MO15. EMBO J. 1993 Aug;12(8):3133–3142. doi: 10.1002/j.1460-2075.1993.tb05982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassan J. P., Jaquenoud M., Fry A. M., Frutiger S., Hughes G. J., Nigg E. A. In vitro assembly of a functional human CDK7-cyclin H complex requires MAT1, a novel 36 kDa RING finger protein. EMBO J. 1995 Nov 15;14(22):5608–5617. doi: 10.1002/j.1460-2075.1995.tb00248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassan J. P., Schultz S. J., Bartek J., Nigg E. A. Cell cycle analysis of the activity, subcellular localization, and subunit composition of human CAK (CDK-activating kinase). J Cell Biol. 1994 Oct;127(2):467–478. doi: 10.1083/jcb.127.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usheva A., Maldonado E., Goldring A., Lu H., Houbavi C., Reinberg D., Aloni Y. Specific interaction between the nonphosphorylated form of RNA polymerase II and the TATA-binding protein. Cell. 1992 May 29;69(5):871–881. doi: 10.1016/0092-8674(92)90297-p. [DOI] [PubMed] [Google Scholar]
- Vermeulen W., van Vuuren A. J., Chipoulet M., Schaeffer L., Appeldoorn E., Weeda G., Jaspers N. G., Priestley A., Arlett C. F., Lehmann A. R. Three unusual repair deficiencies associated with transcription factor BTF2(TFIIH): evidence for the existence of a transcription syndrome. Cold Spring Harb Symp Quant Biol. 1994;59:317–329. doi: 10.1101/sqb.1994.059.01.036. [DOI] [PubMed] [Google Scholar]
- White R. J., Gottlieb T. M., Downes C. S., Jackson S. P. Mitotic regulation of a TATA-binding-protein-containing complex. Mol Cell Biol. 1995 Apr;15(4):1983–1992. doi: 10.1128/mcb.15.4.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaizumi M., Sugano T. U.v.-induced nuclear accumulation of p53 is evoked through DNA damage of actively transcribed genes independent of the cell cycle. Oncogene. 1994 Oct;9(10):2775–2784. [PubMed] [Google Scholar]
- Yankulov K., Yamashita K., Roy R., Egly J. M., Bentley D. L. The transcriptional elongation inhibitor 5,6-dichloro-1-beta-D-ribofuranosylbenzimidazole inhibits transcription factor IIH-associated protein kinase. J Biol Chem. 1995 Oct 13;270(41):23922–23925. doi: 10.1074/jbc.270.41.23922. [DOI] [PubMed] [Google Scholar]
- Zawel L., Kumar K. P., Reinberg D. Recycling of the general transcription factors during RNA polymerase II transcription. Genes Dev. 1995 Jun 15;9(12):1479–1490. doi: 10.1101/gad.9.12.1479. [DOI] [PubMed] [Google Scholar]
- Zehring W. A., Lee J. M., Weeks J. R., Jokerst R. S., Greenleaf A. L. The C-terminal repeat domain of RNA polymerase II largest subunit is essential in vivo but is not required for accurate transcription initiation in vitro. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3698–3702. doi: 10.1073/pnas.85.11.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieve G. W., Turnbull D., Mullins J. M., McIntosh J. R. Production of large numbers of mitotic mammalian cells by use of the reversible microtubule inhibitor nocodazole. Nocodazole accumulated mitotic cells. Exp Cell Res. 1980 Apr;126(2):397–405. doi: 10.1016/0014-4827(80)90279-7. [DOI] [PubMed] [Google Scholar]