Abstract
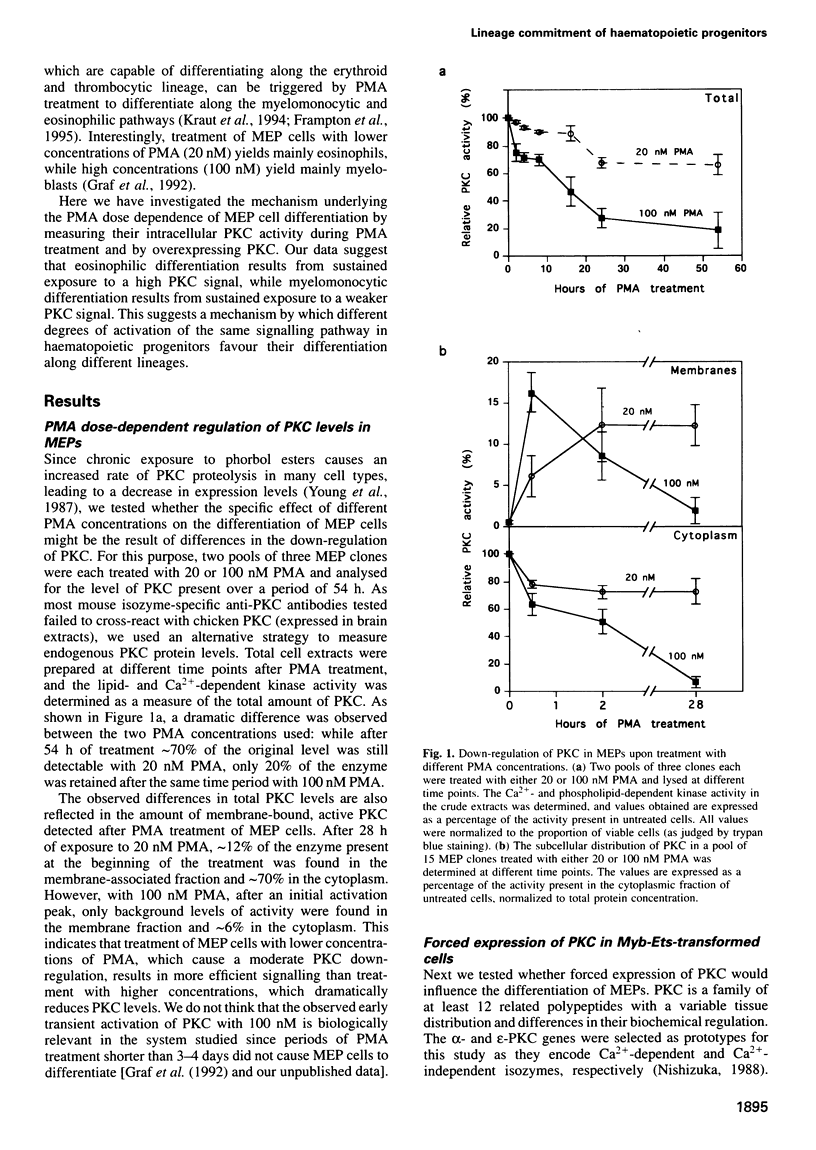
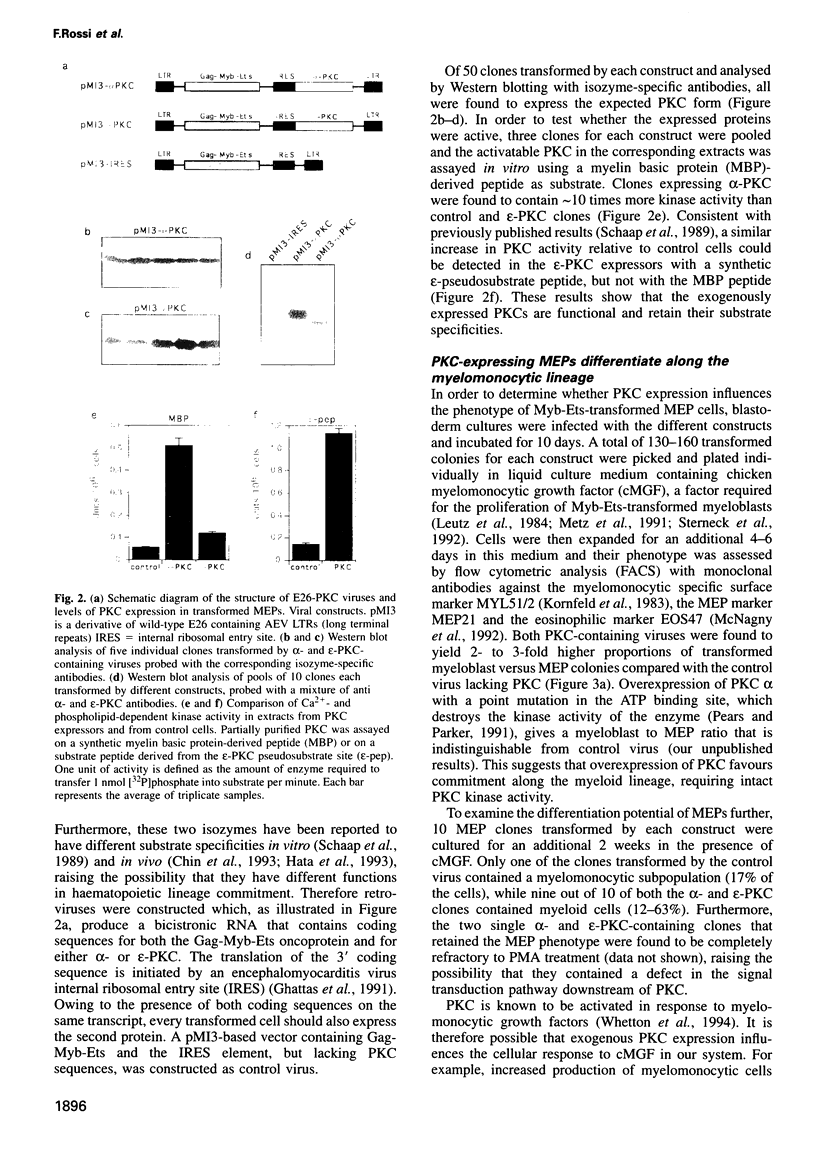
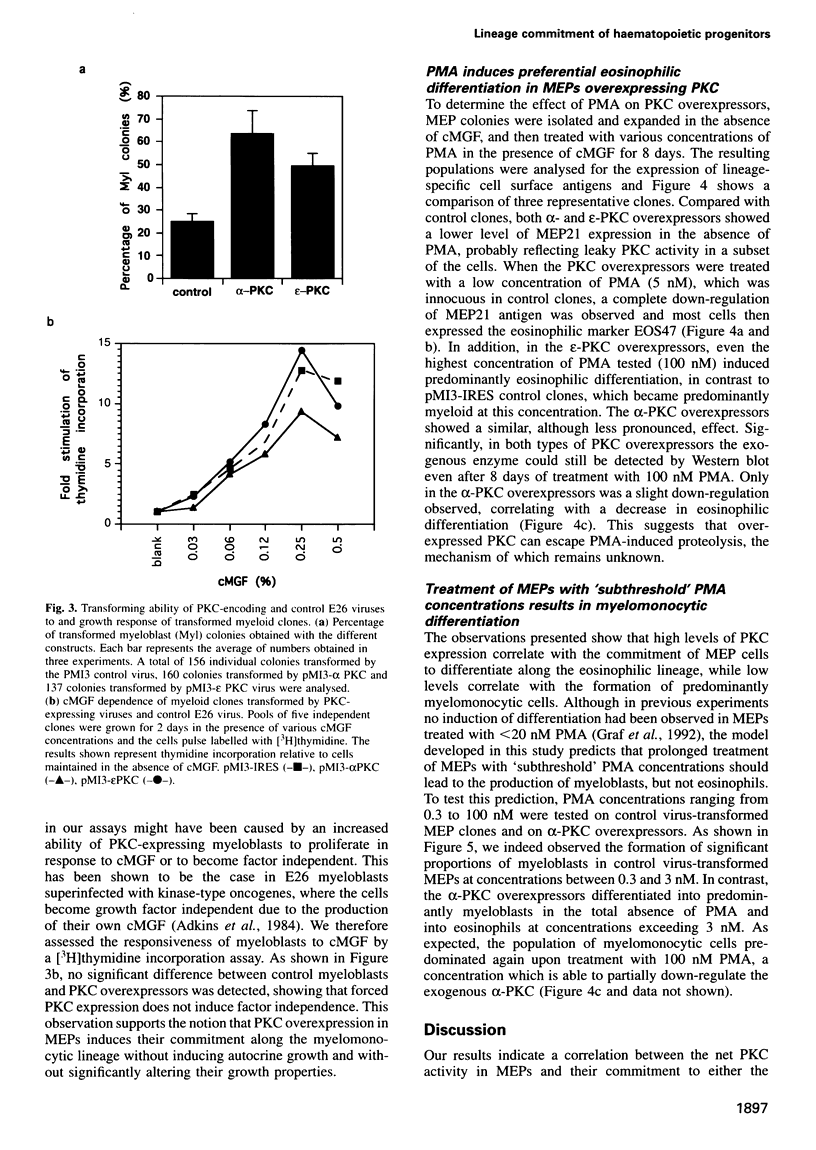
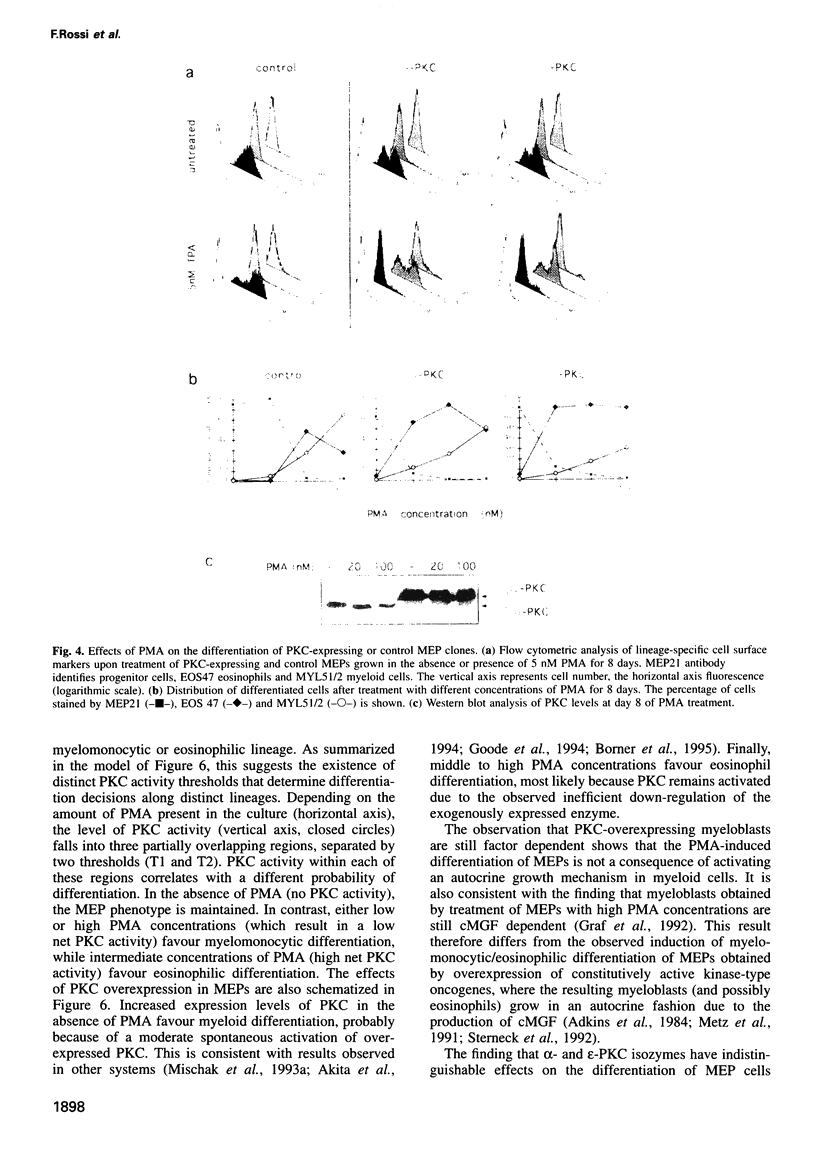
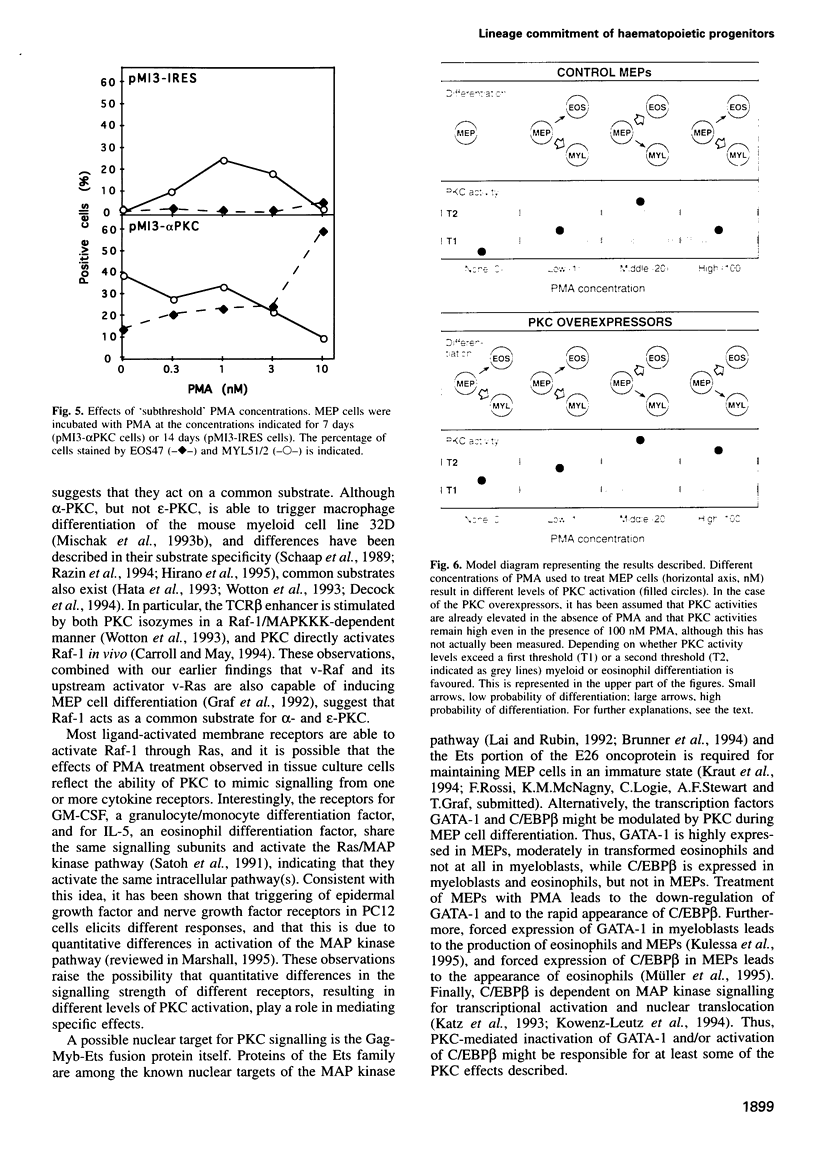
Our previous work showed that haematopoietic precursors transformed by the E26 avian leukemia virus undergo multilineage differentiation in response to the phorbol ester phorbol 12-myristate 13-acetate (PMA). Treatment of the cells with high concentrations of PMA (100 nM) favours myelomonocytic differentiation, while lower concentrations (20 nM) induce predominantly eosinophil differentiation. Here we have investigated the role of protein kinase C (PKC) in this process and found that 100 nM, but not 20 nM, PMA dramatically down-regulates total cellular PKC activity, indicating that high PMA concentrations result in less efficient signalling than lower PMA concentrations. Consistent with these findings is the observation that very low PMA concentrations (1 nM), which presumably only moderately activate PKC, induce myeloid differentiation. This suggests the existence of two PKC thresholds which play a role in lineage commitment. To test the model, alpha- and epsilon-PKC isoforms were expressed in E26-transformed progenitors. These cells exhibited myelomonocytic differentiation even in the absence of PMA, while treatment with concentrations of PMA as high as 100 nM led to the differentiation of predominantly eosinophils and failed to downregulate the exogenous PKC. Our results suggest that different levels of PKC activity result in three different phenotypes: (i) no PKC activity maintains the progenitor phenotype; (ii) low PKC activity favours myelomonocytic differentiation; (iii) high PKC favours eosinophil differentiation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adkins B., Leutz A., Graf T. Autocrine growth induced by src-related oncogenes in transformed chicken myeloid cells. Cell. 1984 Dec;39(3 Pt 2):439–445. doi: 10.1016/0092-8674(84)90451-3. [DOI] [PubMed] [Google Scholar]
- Aihara H., Asaoka Y., Yoshida K., Nishizuka Y. Sustained activation of protein kinase C is essential to HL-60 cell differentiation to macrophage. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11062–11066. doi: 10.1073/pnas.88.24.11062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akita Y., Ohno S., Yajima Y., Konno Y., Saido T. C., Mizuno K., Chida K., Osada S., Kuroki T., Kawashima S. Overproduction of a Ca(2+)-independent protein kinase C isozyme, nPKC epsilon, increases the secretion of prolactin from thyrotropin-releasing hormone-stimulated rat pituitary GH4C1 cells. J Biol Chem. 1994 Feb 11;269(6):4653–4660. [PubMed] [Google Scholar]
- Bell R. M., Burns D. J. Lipid activation of protein kinase C. J Biol Chem. 1991 Mar 15;266(8):4661–4664. [PubMed] [Google Scholar]
- Borner C., Ueffing M., Jaken S., Parker P. J., Weinstein I. B. Two closely related isoforms of protein kinase C produce reciprocal effects on the growth of rat fibroblasts. Possible molecular mechanisms. J Biol Chem. 1995 Jan 6;270(1):78–86. doi: 10.1074/jbc.270.1.78. [DOI] [PubMed] [Google Scholar]
- Carroll M. P., May W. S. Protein kinase C-mediated serine phosphorylation directly activates Raf-1 in murine hematopoietic cells. J Biol Chem. 1994 Jan 14;269(2):1249–1256. [PubMed] [Google Scholar]
- Chin J. E., Dickens M., Tavare J. M., Roth R. A. Overexpression of protein kinase C isoenzymes alpha, beta I, gamma, and epsilon in cells overexpressing the insulin receptor. Effects on receptor phosphorylation and signaling. J Biol Chem. 1993 Mar 25;268(9):6338–6347. [PubMed] [Google Scholar]
- Clemens M. J., Trayner I., Menaya J. The role of protein kinase C isoenzymes in the regulation of cell proliferation and differentiation. J Cell Sci. 1992 Dec;103(Pt 4):881–887. doi: 10.1242/jcs.103.4.881. [DOI] [PubMed] [Google Scholar]
- Collins S. J. The HL-60 promyelocytic leukemia cell line: proliferation, differentiation, and cellular oncogene expression. Blood. 1987 Nov;70(5):1233–1244. [PubMed] [Google Scholar]
- Decock J. B., Gillespie-Brown J., Parker P. J., Sugden P. H., Fuller S. J. Classical, novel and atypical isoforms of PKC stimulate ANF- and TRE/AP-1-regulated-promoter activity in ventricular cardiomyocytes. FEBS Lett. 1994 Dec 19;356(2-3):275–278. doi: 10.1016/0014-5793(94)01283-0. [DOI] [PubMed] [Google Scholar]
- Dikic I., Schlessinger J., Lax I. PC12 cells overexpressing the insulin receptor undergo insulin-dependent neuronal differentiation. Curr Biol. 1994 Aug 1;4(8):702–708. doi: 10.1016/s0960-9822(00)00155-x. [DOI] [PubMed] [Google Scholar]
- Frampton J., McNagny K., Sieweke M., Philip A., Smith G., Graf T. v-Myb DNA binding is required to block thrombocytic differentiation of Myb-Ets-transformed multipotent haematopoietic progenitors. EMBO J. 1995 Jun 15;14(12):2866–2875. doi: 10.1002/j.1460-2075.1995.tb07286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghattas I. R., Sanes J. R., Majors J. E. The encephalomyocarditis virus internal ribosome entry site allows efficient coexpression of two genes from a recombinant provirus in cultured cells and in embryos. Mol Cell Biol. 1991 Dec;11(12):5848–5859. doi: 10.1128/mcb.11.12.5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode N. T., Hajibagheri M. A., Warren G., Parker P. J. Expression of mammalian protein kinase C in Schizosaccharomyces pombe: isotype-specific induction of growth arrest, vesicle formation, and endocytosis. Mol Biol Cell. 1994 Aug;5(8):907–920. doi: 10.1091/mbc.5.8.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf T., McNagny K., Brady G., Frampton J. Chicken "erythroid" cells transformed by the Gag-Myb-Ets-encoding E26 leukemia virus are multipotent. Cell. 1992 Jul 24;70(2):201–213. doi: 10.1016/0092-8674(92)90096-u. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hata A., Akita Y., Suzuki K., Ohno S. Functional divergence of protein kinase C (PKC) family members. PKC gamma differs from PKC alpha and -beta II and nPKC epsilon in its competence to mediate-12-O-tetradecanoyl phorbol 13-acetate (TPA)-responsive transcriptional activation through a TPA-response element. J Biol Chem. 1993 Apr 25;268(12):9122–9129. [PubMed] [Google Scholar]
- Hirano M., Hirai S., Mizuno K., Osada S., Hosaka M., Ohno S. A protein kinase C isozyme, nPKC epsilon, is involved in the activation of NF-kappa B by 12-O-tetradecanoylphorbol-13-acetate (TPA) in rat 3Y1 fibroblasts. Biochem Biophys Res Commun. 1995 Jan 5;206(1):429–436. doi: 10.1006/bbrc.1995.1059. [DOI] [PubMed] [Google Scholar]
- Introna M., Golay J., Frampton J., Nakano T., Ness S. A., Graf T. Mutations in v-myb alter the differentiation of myelomonocytic cells transformed by the oncogene. Cell. 1990 Dec 21;63(6):1289–1297. doi: 10.1016/0092-8674(90)90424-d. [DOI] [PubMed] [Google Scholar]
- Katz S., Kowenz-Leutz E., Müller C., Meese K., Ness S. A., Leutz A. The NF-M transcription factor is related to C/EBP beta and plays a role in signal transduction, differentiation and leukemogenesis of avian myelomonocytic cells. EMBO J. 1993 Apr;12(4):1321–1332. doi: 10.1002/j.1460-2075.1993.tb05777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeffler H. P., Bar-Eli M., Territo M. C. Phorbol ester effect on differentiation of human myeloid leukemia cell lines blocked at different stages of maturation. Cancer Res. 1981 Mar;41(3):919–926. [PubMed] [Google Scholar]
- Kornfeld S., Beug H., Doederlein G., Graf T. Detection of avian hematopoietic cell surface antigens with monoclonal antibodies to myeloid cells. Their distribution on normal and leukemic cells of various lineages. Exp Cell Res. 1983 Feb;143(2):383–394. doi: 10.1016/0014-4827(83)90065-4. [DOI] [PubMed] [Google Scholar]
- Kowenz-Leutz E., Twamley G., Ansieau S., Leutz A. Novel mechanism of C/EBP beta (NF-M) transcriptional control: activation through derepression. Genes Dev. 1994 Nov 15;8(22):2781–2791. doi: 10.1101/gad.8.22.2781. [DOI] [PubMed] [Google Scholar]
- Kraut N., Frampton J., McNagny K. M., Graf T. A functional Ets DNA-binding domain is required to maintain multipotency of hematopoietic progenitors transformed by Myb-Ets. Genes Dev. 1994 Jan;8(1):33–44. doi: 10.1101/gad.8.1.33. [DOI] [PubMed] [Google Scholar]
- Kulessa H., Frampton J., Graf T. GATA-1 reprograms avian myelomonocytic cell lines into eosinophils, thromboblasts, and erythroblasts. Genes Dev. 1995 May 15;9(10):1250–1262. doi: 10.1101/gad.9.10.1250. [DOI] [PubMed] [Google Scholar]
- Lai Z. C., Rubin G. M. Negative control of photoreceptor development in Drosophila by the product of the yan gene, an ETS domain protein. Cell. 1992 Aug 21;70(4):609–620. doi: 10.1016/0092-8674(92)90430-k. [DOI] [PubMed] [Google Scholar]
- Leutz A., Beug H., Graf T. Purification and characterization of cMGF, a novel chicken myelomonocytic growth factor. EMBO J. 1984 Dec 20;3(13):3191–3197. doi: 10.1002/j.1460-2075.1984.tb02278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall C. J. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995 Jan 27;80(2):179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- McNagny K. M., Lim F., Grieser S., Graf T. Cell surface proteins of chicken hematopoietic progenitors, thrombocytes and eosinophils detected by novel monoclonal antibodies. Leukemia. 1992 Oct;6(10):975–984. [PubMed] [Google Scholar]
- Metz T., Graf T., Leutz A. Activation of cMGF expression is a critical step in avian myeloid leukemogenesis. EMBO J. 1991 Apr;10(4):837–844. doi: 10.1002/j.1460-2075.1991.tb08016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischak H., Goodnight J. A., Kolch W., Martiny-Baron G., Schaechtle C., Kazanietz M. G., Blumberg P. M., Pierce J. H., Mushinski J. F. Overexpression of protein kinase C-delta and -epsilon in NIH 3T3 cells induces opposite effects on growth, morphology, anchorage dependence, and tumorigenicity. J Biol Chem. 1993 Mar 25;268(9):6090–6096. [PubMed] [Google Scholar]
- Mischak H., Pierce J. H., Goodnight J., Kazanietz M. G., Blumberg P. M., Mushinski J. F. Phorbol ester-induced myeloid differentiation is mediated by protein kinase C-alpha and -delta and not by protein kinase C-beta II, -epsilon, -zeta, and -eta. J Biol Chem. 1993 Sep 25;268(27):20110–20115. [PubMed] [Google Scholar]
- Müller C., Kowenz-Leutz E., Grieser-Ade S., Graf T., Leutz A. NF-M (chicken C/EBP beta) induces eosinophilic differentiation and apoptosis in a hematopoietic progenitor cell line. EMBO J. 1995 Dec 15;14(24):6127–6135. doi: 10.1002/j.1460-2075.1995.tb00303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls S. E., Heyworth C. M., Dexter T. M., Lord J. M., Johnson G. D., Whetton A. D. IL-4 promotes macrophage development by rapidly stimulating lineage restriction of bipotent granulocyte-macrophage colony-forming cells. J Immunol. 1995 Jul 15;155(2):845–853. [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992 Oct 23;258(5082):607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Pears C., Parker P. J. Down-regulation of a kinase defective PKC-alpha. FEBS Lett. 1991 Jun 17;284(1):120–122. doi: 10.1016/0014-5793(91)80776-y. [DOI] [PubMed] [Google Scholar]
- Razin E., Szallasi Z., Kazanietz M. G., Blumberg P. M., Rivera J. Protein kinases C-beta and C-epsilon link the mast cell high-affinity receptor for IgE to the expression of c-fos and c-jun. Proc Natl Acad Sci U S A. 1994 Aug 2;91(16):7722–7726. doi: 10.1073/pnas.91.16.7722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T., Nakafuku M., Miyajima A., Kaziro Y. Involvement of ras p21 protein in signal-transduction pathways from interleukin 2, interleukin 3, and granulocyte/macrophage colony-stimulating factor, but not from interleukin 4. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3314–3318. doi: 10.1073/pnas.88.8.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaap D., Parker P. J., Bristol A., Kriz R., Knopf J. Unique substrate specificity and regulatory properties of PKC-epsilon: a rationale for diversity. FEBS Lett. 1989 Jan 30;243(2):351–357. doi: 10.1016/0014-5793(89)80160-7. [DOI] [PubMed] [Google Scholar]
- Shearman M. S., Heyworth C. M., Dexter T. M., Haefner B., Owen P. J., Whetton A. D. Haemopoietic stem cell development to neutrophils is associated with subcellular redistribution and differential expression of protein kinase C subspecies. J Cell Sci. 1993 Jan;104(Pt 1):173–180. doi: 10.1242/jcs.104.1.173. [DOI] [PubMed] [Google Scholar]
- Sterneck E., Blattner C., Graf T., Leutz A. Structure of the chicken myelomonocytic growth factor gene and specific activation of its promoter in avian myelomonocytic cells by protein kinases. Mol Cell Biol. 1992 Apr;12(4):1728–1735. doi: 10.1128/mcb.12.4.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker A. W., Bissell M. J. Development of avian sarcoma and leukosis virus-based vector-packaging cell lines. J Virol. 1988 Mar;62(3):1008–1015. doi: 10.1128/jvi.62.3.1008-1015.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traverse S., Seedorf K., Paterson H., Marshall C. J., Cohen P., Ullrich A. EGF triggers neuronal differentiation of PC12 cells that overexpress the EGF receptor. Curr Biol. 1994 Aug 1;4(8):694–701. doi: 10.1016/s0960-9822(00)00154-8. [DOI] [PubMed] [Google Scholar]
- Trayner I. D., Clemens M. J. Stimulation of proliferation of HL60 cells by low concentrations of 12-O-tetradecanoylphorbol-13-acetate and its relationship to the mitogenic effects of insulin. Exp Cell Res. 1992 Mar;199(1):154–161. doi: 10.1016/0014-4827(92)90473-l. [DOI] [PubMed] [Google Scholar]
- Ways D. K., Dodd R. C., Earp H. S. Dissimilar effects of phorbol ester and diacylglycerol derivative on protein kinase activity in the monoblastoid U937 cell. Cancer Res. 1987 Jul 1;47(13):3344–3350. [PubMed] [Google Scholar]
- Whetton A. D., Heyworth C. M., Nicholls S. E., Evans C. A., Lord J. M., Dexter T. M., Owen-Lynch P. J. Cytokine-mediated protein kinase C activation is a signal for lineage determination in bipotential granulocyte macrophage colony-forming cells. J Cell Biol. 1994 May;125(3):651–659. doi: 10.1083/jcb.125.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wotton D., Ways D. K., Parker P. J., Owen M. J. Activity of both Raf and Ras is necessary for activation of transcription of the human T cell receptor beta gene by protein kinase C, Ras plays multiple roles. J Biol Chem. 1993 Aug 25;268(24):17975–17982. [PubMed] [Google Scholar]
- Young S., Parker P. J., Ullrich A., Stabel S. Down-regulation of protein kinase C is due to an increased rate of degradation. Biochem J. 1987 Jun 15;244(3):775–779. doi: 10.1042/bj2440775. [DOI] [PMC free article] [PubMed] [Google Scholar]