Abstract
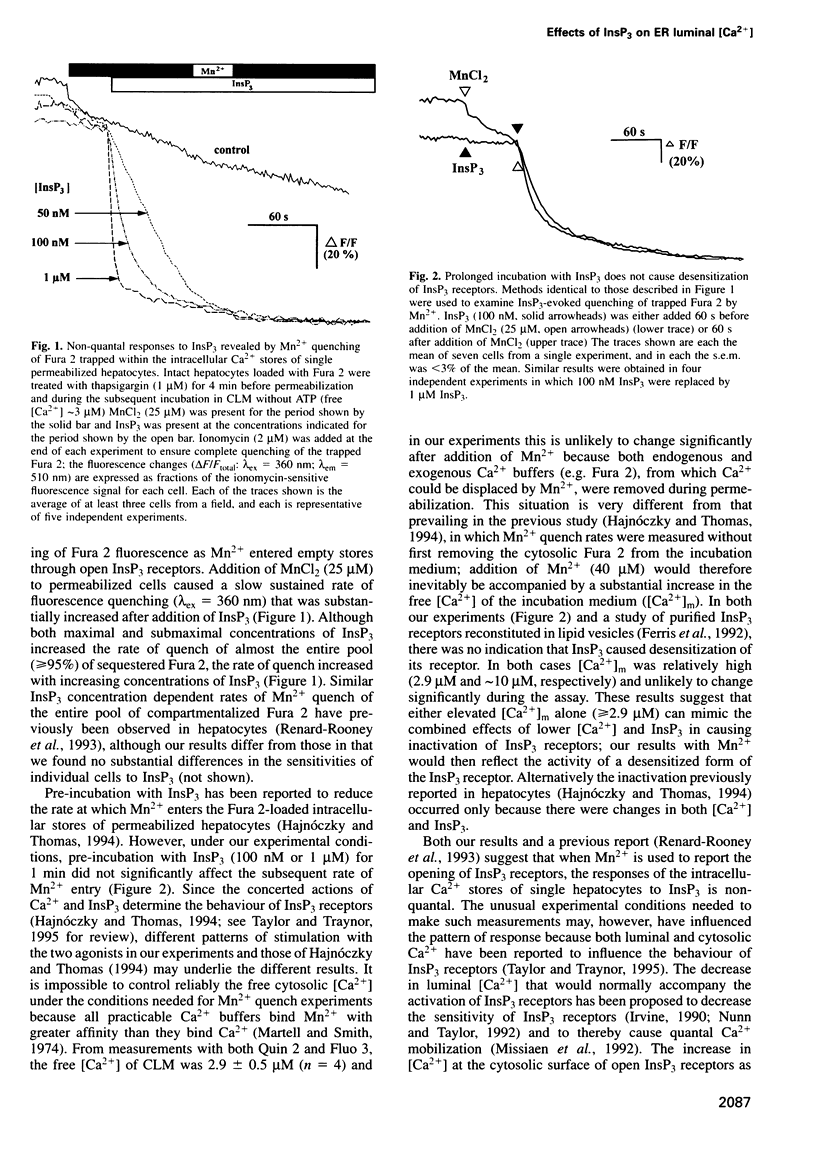
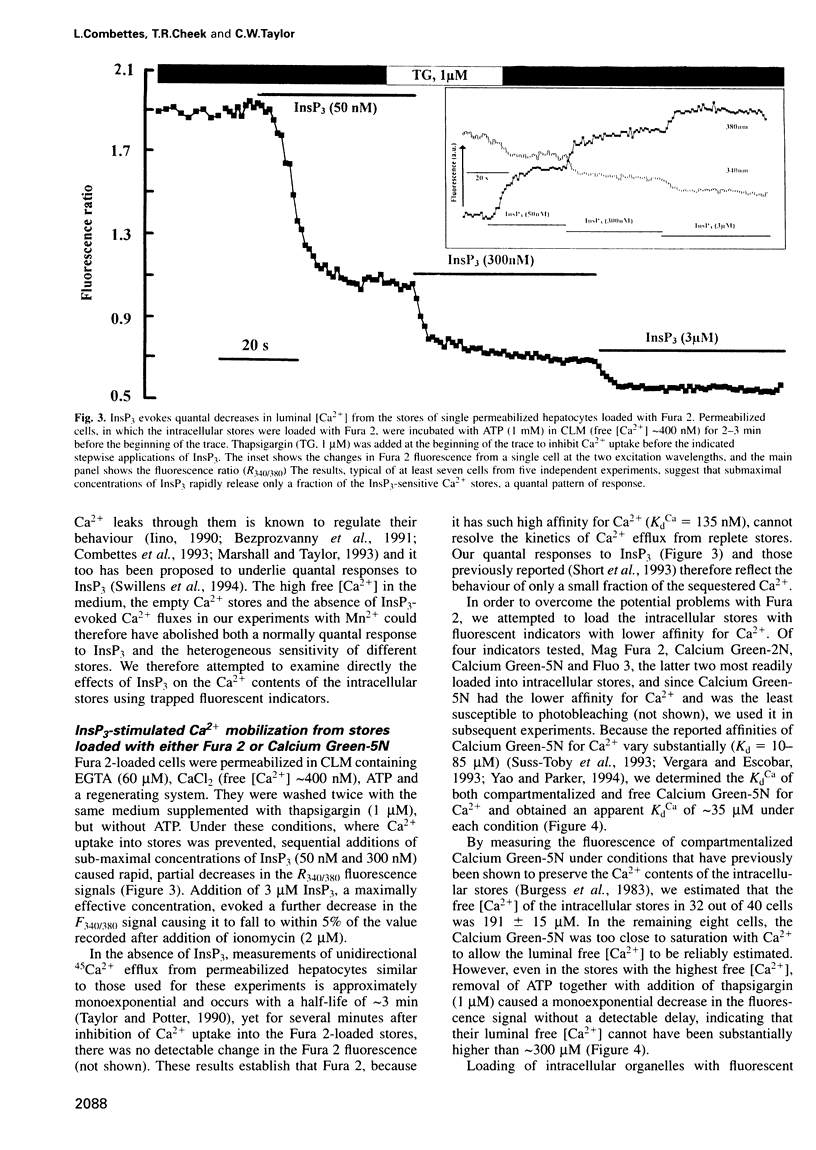
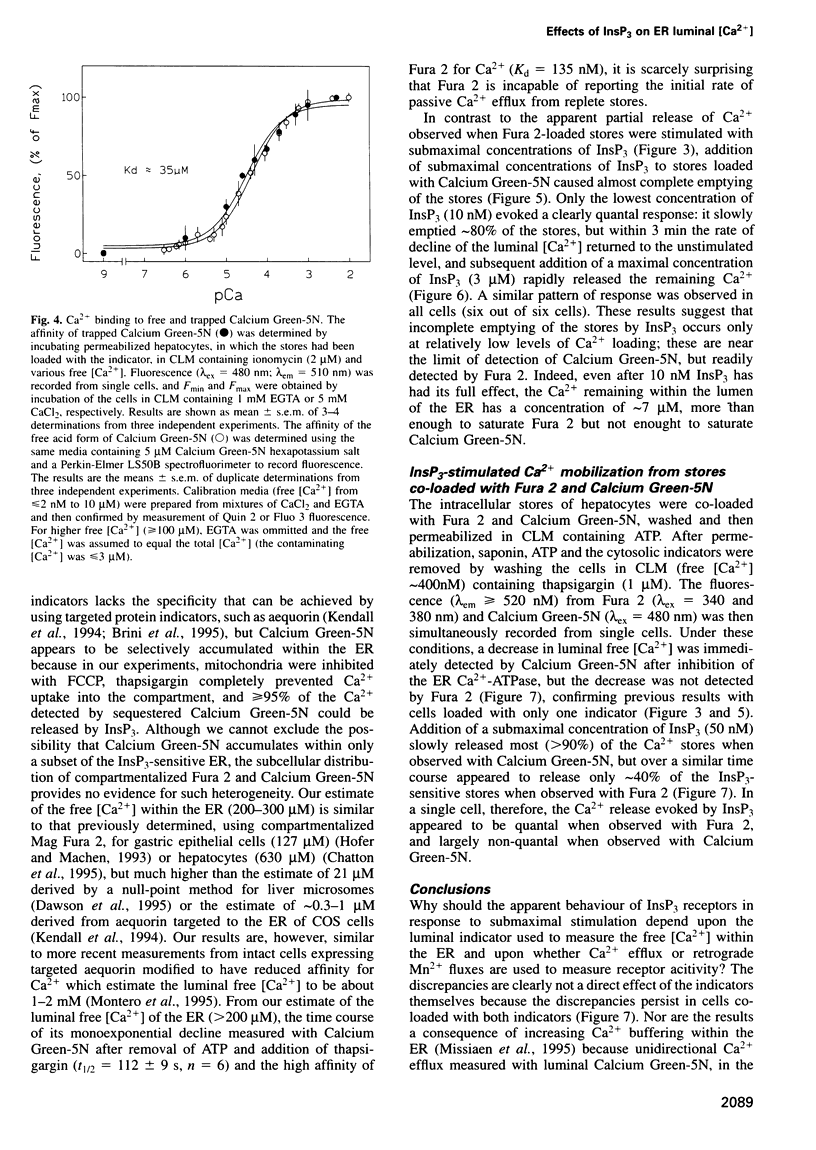
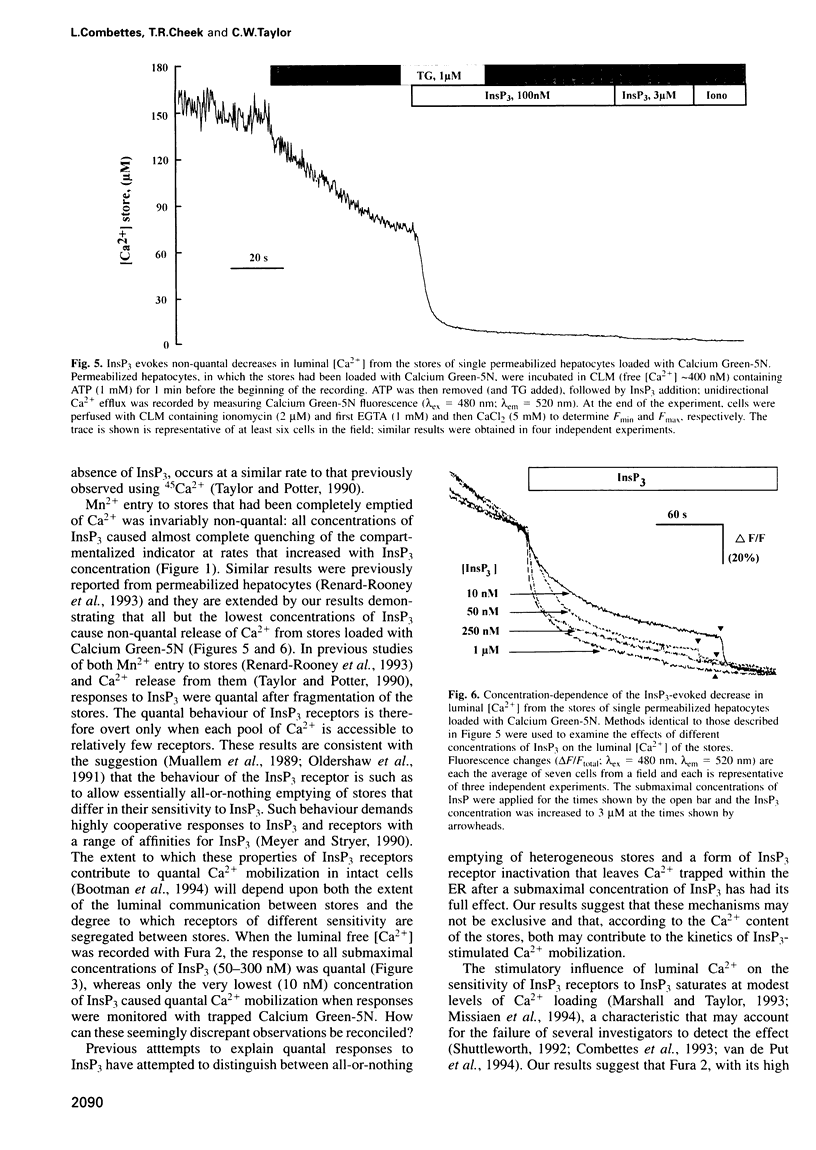
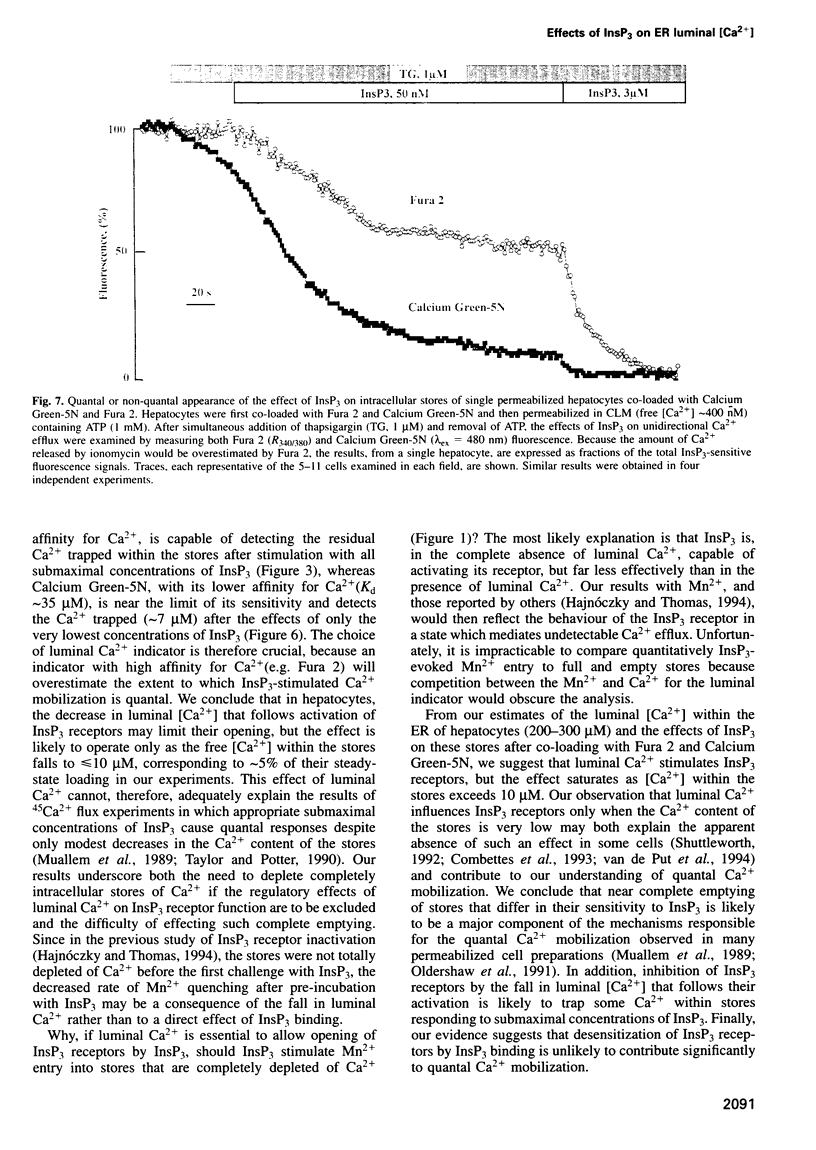
The quantal behaviour of inositol trisphosphate (InsP3) receptors allows rapid graded release of Ca2+ from intracellular stores, but the mechanisms are unknown. In Ca2+-depleted stores loaded with Fura 2, InsP3 caused concentration dependent increases in the rates of fluorescence quench by Mn2+ that were unaffected by prior incubation with InsP3, indicating that InsP3 binding did not cause desensitization. When Fura 2 was used to report the luminal free [Ca2+] after inhibition of further Ca2+ uptake, submaximal concentrations of InsP3 caused rapid, partial decreases in fluorescence ratios. Subsequent addition of a maximal InsP3 concentration caused the fluorescence to fall to within 5% of that recorded after ionomycin. Addition of all but the lowest concentrations of InsP3 to stores loaded with the lower affinity indicator, Calcium Green-5N, caused almost complete emptying of the stores at rates that increased with InsP3 concentration. The lowest concentration of InsP3 (10 nM) slowly emptied approximately 80% of the stores, but within 3 min the rate of Ca2+ release slowed leaving approximately 7 microM Ca2+ within the stores, which was then rapidly released by a maximal InsP3 concentration. In stores co-loaded with both indicators, InsP3-evoked Ca2+ release appeared quantal with Fura 2 and largely non-quantal with Calcium Green-5N; the discrepancy is not, therefore, a direct effect of the indicators. The fall in luminal [Ca2+] after activation of InsP3 receptors may, therefore, cause their inactivation, but only after the Ca2+ content of the stores has fallen by approximately 95% to < or = 10 microM.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Capacitative calcium entry. Biochem J. 1995 Nov 15;312(Pt 1):1–11. doi: 10.1042/bj3120001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I., Watras J., Ehrlich B. E. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991 Jun 27;351(6329):751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- Bootman M. D., Cheek T. R., Moreton R. B., Bennett D. L., Berridge M. J. Smoothly graded Ca2+ release from inositol 1,4,5-trisphosphate-sensitive Ca2+ stores. J Biol Chem. 1994 Oct 7;269(40):24783–24791. [PubMed] [Google Scholar]
- Brini M., Marsault R., Bastianutto C., Alvarez J., Pozzan T., Rizzuto R. Transfected aequorin in the measurement of cytosolic Ca2+ concentration ([Ca2+]c). A critical evaluation. J Biol Chem. 1995 Apr 28;270(17):9896–9903. doi: 10.1074/jbc.270.17.9896. [DOI] [PubMed] [Google Scholar]
- Burgess G. M., McKinney J. S., Fabiato A., Leslie B. A., Putney J. W., Jr Calcium pools in saponin-permeabilized guinea pig hepatocytes. J Biol Chem. 1983 Dec 25;258(24):15336–15345. [PubMed] [Google Scholar]
- Chatton J. Y., Liu H., Stucki J. W. Simultaneous measurements of Ca2+ in the intracellular stores and the cytosol of hepatocytes during hormone-induced Ca2+ oscillations. FEBS Lett. 1995 Jul 10;368(1):165–168. doi: 10.1016/0014-5793(95)00632-j. [DOI] [PubMed] [Google Scholar]
- Combettes L., Berthon B., Binet A., Claret M. Glucagon and vasopressin interactions on Ca2+ movements in isolated hepatocytes. Biochem J. 1986 Aug 1;237(3):675–683. doi: 10.1042/bj2370675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combettes L., Claret M., Champeil P. Calcium control on InsP3-induced discharge of calcium from permeabilised hepatocyte pools. Cell Calcium. 1993 Apr;14(4):279–292. doi: 10.1016/0143-4160(93)90049-c. [DOI] [PubMed] [Google Scholar]
- Dawson A. P., Rich G. T., Loomis-Husselbee J. W. Estimation of the free [Ca2+] gradient across endoplasmic reticulum membranes by a null-point method. Biochem J. 1995 Sep 1;310(Pt 2):371–374. doi: 10.1042/bj3100371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris C. D., Cameron A. M., Huganir R. L., Snyder S. H. Quantal calcium release by purified reconstituted inositol 1,4,5-trisphosphate receptors. Nature. 1992 Mar 26;356(6367):350–352. doi: 10.1038/356350a0. [DOI] [PubMed] [Google Scholar]
- Hajnóczky G., Lin C., Thomas A. P. Luminal communication between intracellular calcium stores modulated by GTP and the cytoskeleton. J Biol Chem. 1994 Apr 8;269(14):10280–10287. [PubMed] [Google Scholar]
- Hajnóczky G., Thomas A. P. The inositol trisphosphate calcium channel is inactivated by inositol trisphosphate. Nature. 1994 Aug 11;370(6489):474–477. doi: 10.1038/370474a0. [DOI] [PubMed] [Google Scholar]
- Hargreaves A. C., Lummis S. C., Taylor C. W. Ca2+ permeability of cloned and native 5-hydroxytryptamine type 3 receptors. Mol Pharmacol. 1994 Dec;46(6):1120–1128. [PubMed] [Google Scholar]
- Hirose K., Iino M. Heterogeneity of channel density in inositol-1,4,5-trisphosphate-sensitive Ca2+ stores. Nature. 1994 Dec 22;372(6508):791–794. doi: 10.1038/372791a0. [DOI] [PubMed] [Google Scholar]
- Hofer A. M., Machen T. E. Technique for in situ measurement of calcium in intracellular inositol 1,4,5-trisphosphate-sensitive stores using the fluorescent indicator mag-fura-2. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2598–2602. doi: 10.1073/pnas.90.7.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M. Biphasic Ca2+ dependence of inositol 1,4,5-trisphosphate-induced Ca release in smooth muscle cells of the guinea pig taenia caeci. J Gen Physiol. 1990 Jun;95(6):1103–1122. doi: 10.1085/jgp.95.6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall J. M., Badminton M. N., Dormer R. L., Campbell A. K. Changes in free calcium in the endoplasmic reticulum of living cells detected using targeted aequorin. Anal Biochem. 1994 Aug 15;221(1):173–181. doi: 10.1006/abio.1994.1394. [DOI] [PubMed] [Google Scholar]
- Lechleiter J. D., Clapham D. E. Molecular mechanisms of intracellular calcium excitability in X. laevis oocytes. Cell. 1992 Apr 17;69(2):283–294. doi: 10.1016/0092-8674(92)90409-6. [DOI] [PubMed] [Google Scholar]
- Marshall I. C., Taylor C. W. Biphasic effects of cytosolic Ca2+ on Ins(1,4,5)P3-stimulated Ca2+ mobilization in hepatocytes. J Biol Chem. 1993 Jun 25;268(18):13214–13220. [PubMed] [Google Scholar]
- Meyer T., Stryer L. Transient calcium release induced by successive increments of inositol 1,4,5-trisphosphate. Proc Natl Acad Sci U S A. 1990 May;87(10):3841–3845. doi: 10.1073/pnas.87.10.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezna M., Michelangeli F. Opening up Ca2+ stores with InsP3. Nature. 1995 Jul 27;376(6538):300–301. doi: 10.1038/376300a0. [DOI] [PubMed] [Google Scholar]
- Missiaen L., De Smedt H., Droogmans G., Casteels R. Ca2+ release induced by inositol 1,4,5-trisphosphate is a steady-state phenomenon controlled by luminal Ca2+ in permeabilized cells. Nature. 1992 Jun 18;357(6379):599–602. doi: 10.1038/357599a0. [DOI] [PubMed] [Google Scholar]
- Missiaen L., De Smedt H., Parys J. B., Casteels R. Co-activation of inositol trisphosphate-induced Ca2+ release by cytosolic Ca2+ is loading-dependent. J Biol Chem. 1994 Mar 11;269(10):7238–7242. [PubMed] [Google Scholar]
- Missiaen L., Parys J. B., De Smedt H., Sienaert I., Henning R. H., Casteels R. Opening up Ca2+ stores with InsP3. Nature. 1995 Jul 27;376(6538):299–301. doi: 10.1038/376299b0. [DOI] [PubMed] [Google Scholar]
- Montero M., Brini M., Marsault R., Alvarez J., Sitia R., Pozzan T., Rizzuto R. Monitoring dynamic changes in free Ca2+ concentration in the endoplasmic reticulum of intact cells. EMBO J. 1995 Nov 15;14(22):5467–5475. doi: 10.1002/j.1460-2075.1995.tb00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muallem S., Pandol S. J., Beeker T. G. Hormone-evoked calcium release from intracellular stores is a quantal process. J Biol Chem. 1989 Jan 5;264(1):205–212. [PubMed] [Google Scholar]
- Nicotera P., Bellomo G., Orrenius S. Calcium-mediated mechanisms in chemically induced cell death. Annu Rev Pharmacol Toxicol. 1992;32:449–470. doi: 10.1146/annurev.pa.32.040192.002313. [DOI] [PubMed] [Google Scholar]
- Nunn D. L., Taylor C. W. Luminal Ca2+ increases the sensitivity of Ca2+ stores to inositol 1,4,5-trisphosphate. Mol Pharmacol. 1992 Jan;41(1):115–119. [PubMed] [Google Scholar]
- Oldershaw K. A., Nunn D. L., Taylor C. W. Quantal Ca2+ mobilization stimulated by inositol 1,4,5-trisphosphate in permeabilized hepatocytes. Biochem J. 1991 Sep 15;278(Pt 3):705–708. doi: 10.1042/bj2780705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard-Rooney D. C., Hajnóczky G., Seitz M. B., Schneider T. G., Thomas A. P. Imaging of inositol 1,4,5-trisphosphate-induced Ca2+ fluxes in single permeabilized hepatocytes. Demonstration of both quantal and nonquantal patterns of Ca2+ release. J Biol Chem. 1993 Nov 5;268(31):23601–23610. [PubMed] [Google Scholar]
- Short A. D., Klein M. G., Schneider M. F., Gill D. L. Inositol 1,4,5-trisphosphate-mediated quantal Ca2+ release measured by high resolution imaging of Ca2+ within organelles. J Biol Chem. 1993 Dec 5;268(34):25887–25893. [PubMed] [Google Scholar]
- Shuttleworth T. J. Ca2+ release from inositol trisphosphate-sensitive stores is not modulated by intraluminal [Ca2+]. J Biol Chem. 1992 Feb 25;267(6):3573–3576. [PubMed] [Google Scholar]
- Swillens S., Combettes L., Champeil P. Transient inositol 1,4,5-trisphosphate-induced Ca2+ release: a model based on regulatory Ca(2+)-binding sites along the permeation pathway. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):10074–10078. doi: 10.1073/pnas.91.21.10074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. W. Kinetics of inositol 1,4,5-trisphosphate-stimulated Ca2+ mobilization. Adv Second Messenger Phosphoprotein Res. 1992;26:109–142. [PubMed] [Google Scholar]
- Taylor C. W., Potter B. V. The size of inositol 1,4,5-trisphosphate-sensitive Ca2+ stores depends on inositol 1,4,5-trisphosphate concentration. Biochem J. 1990 Feb 15;266(1):189–194. doi: 10.1042/bj2660189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. W., Traynor D. Calcium and inositol trisphosphate receptors. J Membr Biol. 1995 May;145(2):109–118. doi: 10.1007/BF00237369. [DOI] [PubMed] [Google Scholar]
- Taylor C. W. Why do hormones stimulate Ca2+ mobilization? Biochem Soc Trans. 1995 Aug;23(3):637–642. doi: 10.1042/bst0230637. [DOI] [PubMed] [Google Scholar]
- Yao Y., Parker I. Ca2+ influx modulation of temporal and spatial patterns of inositol trisphosphate-mediated Ca2+ liberation in Xenopus oocytes. J Physiol. 1994 Apr 1;476(1):17–28. [PMC free article] [PubMed] [Google Scholar]
- van de Put F. H., De Pont J. J., Willems P. H. Heterogeneity between intracellular Ca2+ stores as the underlying principle of quantal Ca2+ release by inositol 1,4,5-trisphosphate in permeabilized pancreatic acinar cells. J Biol Chem. 1994 Apr 29;269(17):12438–12443. [PubMed] [Google Scholar]



