Abstract
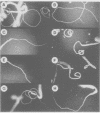
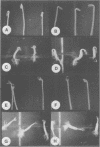
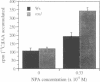
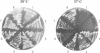
The phytohormone auxin controls processes such as cell elongation, root hair development and root branching. Tropisms, growth curvatures triggered by gravity, light and touch, are also auxin-mediated responses. Auxin is synthesized in the shoot apex and transported through the stem, but the molecular mechanism of auxin transport is not well understood. Naphthylphthalamic acid (NPA) and other inhibitors of auxin transport block tropic curvature responses and inhibit root and shoot elongation. We have isolated a novel Arabidopsis thaliana mutant designated roots curl in NPA (rcn1). Mutant seedlings exhibit altered responses to NPA in root curling and hypocotyl elongation. Auxin efflux in mutant seedlings displays increased sensitivity to NPA. The rcn1 mutation was transferred-DNA (T-DNA) tagged and sequences flanking the T-DNA insert were cloned. Analysis of the RCN1 cDNA reveals that the T-DNA insertion disrupts a gene for the regulatory A subunit of protein phosphatase 2A (PP2A-A). The RCN1 gene rescues the rcn1 mutant phenotype and also complements the temperature-sensitive phenotype of the Saccharomyces cerevisiae PP2A-A mutation, tpd3-1. These data implicate protein phosphatase 2A in the regulation of auxin transport in Arabidopsis.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammerer G. Expression of genes in yeast using the ADCI promoter. Methods Enzymol. 1983;101:192–201. doi: 10.1016/0076-6879(83)01014-9. [DOI] [PubMed] [Google Scholar]
- Ariño J., Pérez-Callejón E., Cunillera N., Camps M., Posas F., Ferrer A. Protein phosphatases in higher plants: multiplicity of type 2A phosphatases in Arabidopsis thaliana. Plant Mol Biol. 1993 Feb;21(3):475–485. doi: 10.1007/BF00028805. [DOI] [PubMed] [Google Scholar]
- Brunn S. A., Muday G. K., Haworth P. Auxin transport and the interaction of phytotropins: probing the properties of a phytotropin binding protein. Plant Physiol. 1992 Jan;98(1):101–107. doi: 10.1104/pp.98.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casamayor A., Pérez-Callejón E., Pujol G., Ariño J., Ferrer A. Molecular characterization of a fourth isoform of the catalytic subunit of protein phosphatase 2A from Arabidopsis thaliana. Plant Mol Biol. 1994 Oct;26(1):523–528. doi: 10.1007/BF00039564. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- Dominov J. A., Stenzler L., Lee S., Schwarz J. J., Leisner S., Howell S. H. Cytokinins and auxins control the expression of a gene in Nicotiana plumbaginifolia cells by feedback regulation. Plant Cell. 1992 Apr;4(4):451–461. doi: 10.1105/tpc.4.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbers C., Simmons C. Approaches to understanding auxin action. Trends Cell Biol. 1994 Jul;4(7):245–250. doi: 10.1016/0962-8924(94)90122-8. [DOI] [PubMed] [Google Scholar]
- Gheysen G., Villarroel R., Van Montagu M. Illegitimate recombination in plants: a model for T-DNA integration. Genes Dev. 1991 Feb;5(2):287–297. doi: 10.1101/gad.5.2.287. [DOI] [PubMed] [Google Scholar]
- Hobbie L., Estelle M. The axr4 auxin-resistant mutants of Arabidopsis thaliana define a gene important for root gravitropism and lateral root initiation. Plant J. 1995 Feb;7(2):211–220. doi: 10.1046/j.1365-313x.1995.7020211.x. [DOI] [PubMed] [Google Scholar]
- Ilag L. L., Kumar A. M., Söll D. Light regulation of chlorophyll biosynthesis at the level of 5-aminolevulinate formation in Arabidopsis. Plant Cell. 1994 Feb;6(2):265–275. doi: 10.1105/tpc.6.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M., Gilbert S. F. Basal localization of the presumptive auxin transport carrier in pea stem cells. Science. 1983 Jun 17;220(4603):1297–1300. doi: 10.1126/science.220.4603.1297. [DOI] [PubMed] [Google Scholar]
- Jacobs M., Rubery P. H. Naturally occurring auxin transport regulators. Science. 1988 Jul 15;241(4863):346–349. doi: 10.1126/science.241.4863.346. [DOI] [PubMed] [Google Scholar]
- Katekar G. F., Geissler A. E. Auxin Transport Inhibitors: III. Chemical Requirements of a Class of Auxin Transport Inhibitors. Plant Physiol. 1977 Dec;60(6):826–829. doi: 10.1104/pp.60.6.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung J., Bouvier-Durand M., Morris P. C., Guerrier D., Chefdor F., Giraudat J. Arabidopsis ABA response gene ABI1: features of a calcium-modulated protein phosphatase. Science. 1994 Jun 3;264(5164):1448–1452. doi: 10.1126/science.7910981. [DOI] [PubMed] [Google Scholar]
- Li W., Luan S., Schreiber S. L., Assmann S. M. Evidence for protein phosphatase 1 and 2A regulation of K+ channels in two types of leaf cells. Plant Physiol. 1994 Nov;106(3):963–970. doi: 10.1104/pp.106.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Cm., Xu Zh., Chua N. H. Auxin Polar Transport Is Essential for the Establishment of Bilateral Symmetry during Early Plant Embryogenesis. Plant Cell. 1993 Jun;5(6):621–630. doi: 10.1105/tpc.5.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H., Yanofsky M. F., Klee H. J., Bowman J. L., Meyerowitz E. M. Vectors for plant transformation and cosmid libraries. Gene. 1992 Aug 15;117(2):161–167. doi: 10.1016/0378-1119(92)90725-5. [DOI] [PubMed] [Google Scholar]
- MacKintosh C. Regulation of spinach-leaf nitrate reductase by reversible phosphorylation. Biochim Biophys Acta. 1992 Oct 6;1137(1):121–126. doi: 10.1016/0167-4889(92)90109-o. [DOI] [PubMed] [Google Scholar]
- Mattanovich D., Rüker F., Machado A. C., Laimer M., Regner F., Steinkellner H., Himmler G., Katinger H. Efficient transformation of Agrobacterium spp. by electroporation. Nucleic Acids Res. 1989 Aug 25;17(16):6747–6747. doi: 10.1093/nar/17.16.6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer-Jaekel R. E., Baumgartner S., Bilbe G., Ohkura H., Glover D. M., Hemmings B. A. Molecular cloning and developmental expression of the catalytic and 65-kDa regulatory subunits of protein phosphatase 2A in Drosophila. Mol Biol Cell. 1992 Mar;3(3):287–298. doi: 10.1091/mbc.3.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer-Jaekel R. E., Hemmings B. A. Protein phosphatase 2A--a 'ménage à trois'. Trends Cell Biol. 1994 Aug;4(8):287–291. doi: 10.1016/0962-8924(94)90219-4. [DOI] [PubMed] [Google Scholar]
- Mayer-Jaekel R. E., Ohkura H., Ferrigno P., Andjelkovic N., Shiomi K., Uemura T., Glover D. M., Hemmings B. A. Drosophila mutants in the 55 kDa regulatory subunit of protein phosphatase 2A show strongly reduced ability to dephosphorylate substrates of p34cdc2. J Cell Sci. 1994 Sep;107(Pt 9):2609–2616. doi: 10.1242/jcs.107.9.2609. [DOI] [PubMed] [Google Scholar]
- Mayerhofer R., Koncz-Kalman Z., Nawrath C., Bakkeren G., Crameri A., Angelis K., Redei G. P., Schell J., Hohn B., Koncz C. T-DNA integration: a mode of illegitimate recombination in plants. EMBO J. 1991 Mar;10(3):697–704. doi: 10.1002/j.1460-2075.1991.tb07999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K., Leube M. P., Grill E. A protein phosphatase 2C involved in ABA signal transduction in Arabidopsis thaliana. Science. 1994 Jun 3;264(5164):1452–1455. doi: 10.1126/science.8197457. [DOI] [PubMed] [Google Scholar]
- Mumby M. C., Walter G. Protein serine/threonine phosphatases: structure, regulation, and functions in cell growth. Physiol Rev. 1993 Oct;73(4):673–699. doi: 10.1152/physrev.1993.73.4.673. [DOI] [PubMed] [Google Scholar]
- Okada K., Ueda J., Komaki M. K., Bell C. J., Shimura Y. Requirement of the Auxin Polar Transport System in Early Stages of Arabidopsis Floral Bud Formation. Plant Cell. 1991 Jul;3(7):677–684. doi: 10.1105/tpc.3.7.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallas D. C., Shahrik L. K., Martin B. L., Jaspers S., Miller T. B., Brautigan D. L., Roberts T. M. Polyoma small and middle T antigens and SV40 small t antigen form stable complexes with protein phosphatase 2A. Cell. 1990 Jan 12;60(1):167–176. doi: 10.1016/0092-8674(90)90726-u. [DOI] [PubMed] [Google Scholar]
- Raz V., Fluhr R. Ethylene Signal Is Transduced via Protein Phosphorylation Events in Plants. Plant Cell. 1993 May;5(5):523–530. doi: 10.1105/tpc.5.5.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruediger R., Roeckel D., Fait J., Bergqvist A., Magnusson G., Walter G. Identification of binding sites on the regulatory A subunit of protein phosphatase 2A for the catalytic C subunit and for tumor antigens of simian virus 40 and polyomavirus. Mol Cell Biol. 1992 Nov;12(11):4872–4882. doi: 10.1128/mcb.12.11.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundle S. J., Hartung A. J., Corum J. W., 3rd, O'Neill M. Characterization of a cDNA encoding the 55 kDa B regulatory subunit of Arabidopsis protein phosphatase 2A. Plant Mol Biol. 1995 May;28(2):257–266. doi: 10.1007/BF00020245. [DOI] [PubMed] [Google Scholar]
- Simmons C., Migliaccio F., Masson P., Caspar T., Soll D. A novel root gravitropism mutant of Arabidopsis thaliana exhibiting altered auxin physiology. Physiol Plant. 1995;93:790–798. [PubMed] [Google Scholar]
- Slabas A. R., Fordham-Skelton A. P., Fletcher D., Martinez-Rivas J. M., Swinhoe R., Croy R. R., Evans I. M. Characterisation of cDNA and genomic clones encoding homologues of the 65 kDa regulatory subunit of protein phosphatase 2A in Arabidopsis thaliana. Plant Mol Biol. 1994 Nov;26(4):1125–1138. doi: 10.1007/BF00040694. [DOI] [PubMed] [Google Scholar]
- Sussman M. R., Gardner G. Solubilization of the receptor for N-1-naphthylphthalamic Acid. Plant Physiol. 1980 Dec;66(6):1074–1078. doi: 10.1104/pp.66.6.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter G., Ruediger R., Slaughter C., Mumby M. Association of protein phosphatase 2A with polyoma virus medium tumor antigen. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2521–2525. doi: 10.1073/pnas.87.7.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zettl R., Feldwisch J., Boland W., Schell J., Palme K. 5'-Azido-[3,6-3H2]-1-napthylphthalamic acid, a photoactivatable probe for naphthylphthalamic acid receptor proteins from higher plants: identification of a 23-kDa protein from maize coleoptile plasma membranes. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):480–484. doi: 10.1073/pnas.89.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zyl W. H., Wills N., Broach J. R. A general screen for mutant of Saccharomyces cerevisiae deficient in tRNA biosynthesis. Genetics. 1989 Sep;123(1):55–68. doi: 10.1093/genetics/123.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zyl W., Huang W., Sneddon A. A., Stark M., Camier S., Werner M., Marck C., Sentenac A., Broach J. R. Inactivation of the protein phosphatase 2A regulatory subunit A results in morphological and transcriptional defects in Saccharomyces cerevisiae. Mol Cell Biol. 1992 Nov;12(11):4946–4959. doi: 10.1128/mcb.12.11.4946. [DOI] [PMC free article] [PubMed] [Google Scholar]