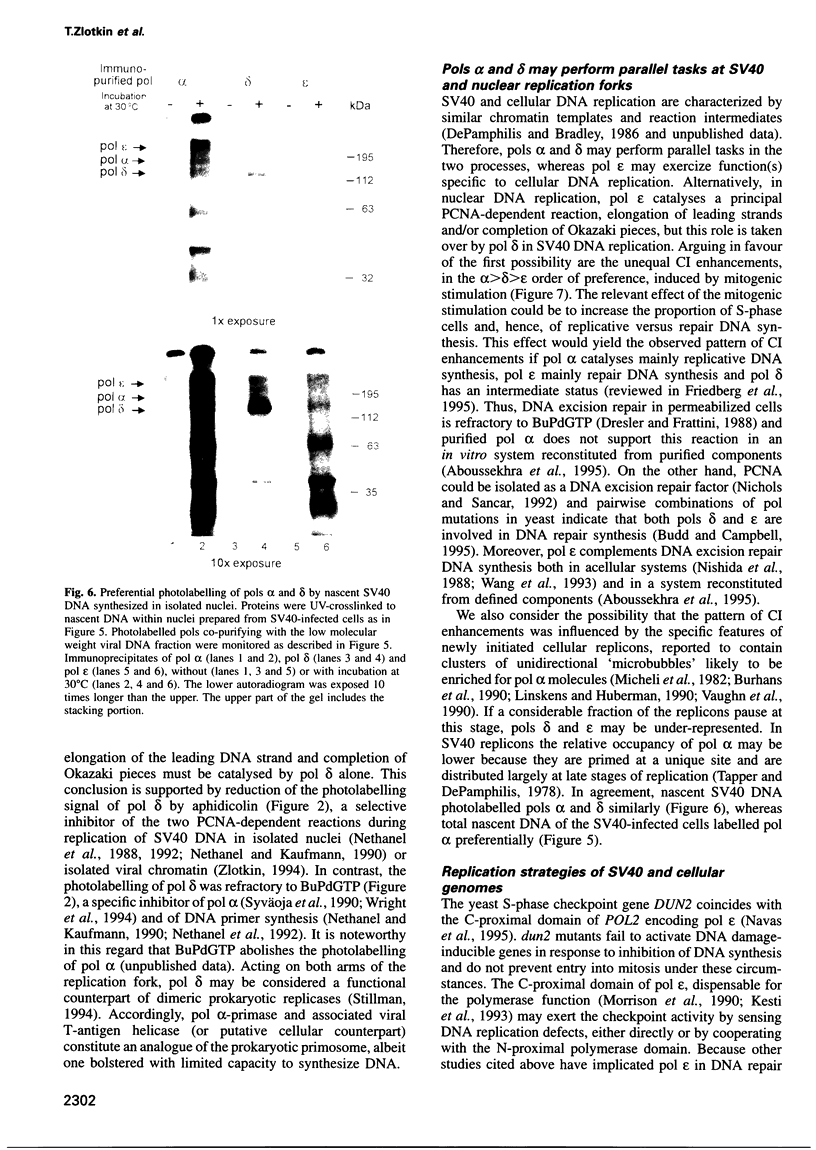
Abstract
The contributions of DNA polymerases alpha, delta, and epsilon to SV40 and nuclear DNA syntheses were evaluated. Proteins were UV-crosslinked to nascent DNA within replicating chromosomes and the photolabelled polymerases were immunopurified. Only DNA polymerases alpha and delta were detectably photolabelled by nascent SV40 DNA, whether synthesized in soluble viral chromatin or within nuclei isolated from SV40-infected cells. In contrast, all three enzymes were photolabelled by the nascent cellular DNA. Mitogenic stimulation enhanced the photolabelling of the polymerases in the alpha>delta>epsilon order of preference. The data agree with the notion that DNA polymerases alpha and delta catalyse the principal DNA polymerisation reactions at the replication fork of SV40 and, perhaps, also of nuclear chromosomes. DNA polymerase epsilon, implicated by others as a cell-cycle checkpoint regulator sensing DNA replication lesions, may be dispensable for replication of the small, fast propagating virus that subverts cell cycle controls.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aboussekhra A., Biggerstaff M., Shivji M. K., Vilpo J. A., Moncollin V., Podust V. N., Protić M., Hübscher U., Egly J. M., Wood R. D. Mammalian DNA nucleotide excision repair reconstituted with purified protein components. Cell. 1995 Mar 24;80(6):859–868. doi: 10.1016/0092-8674(95)90289-9. [DOI] [PubMed] [Google Scholar]
- Araki H., Ropp P. A., Johnson A. L., Johnston L. H., Morrison A., Sugino A. DNA polymerase II, the probable homolog of mammalian DNA polymerase epsilon, replicates chromosomal DNA in the yeast Saccharomyces cerevisiae. EMBO J. 1992 Feb;11(2):733–740. doi: 10.1002/j.1460-2075.1992.tb05106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budd M. E., Campbell J. L. DNA polymerases delta and epsilon are required for chromosomal replication in Saccharomyces cerevisiae. Mol Cell Biol. 1993 Jan;13(1):496–505. doi: 10.1128/mcb.13.1.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budd M. E., Campbell J. L. DNA polymerases required for repair of UV-induced damage in Saccharomyces cerevisiae. Mol Cell Biol. 1995 Apr;15(4):2173–2179. doi: 10.1128/mcb.15.4.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock P. A., Seo Y. S., Hurwitz J. Initiation of simian virus 40 DNA synthesis in vitro. Mol Cell Biol. 1991 May;11(5):2350–2361. doi: 10.1128/mcb.11.5.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgers P. M. Saccharomyces cerevisiae replication factor C. II. Formation and activity of complexes with the proliferating cell nuclear antigen and with DNA polymerases delta and epsilon. J Biol Chem. 1991 Nov 25;266(33):22698–22706. [PubMed] [Google Scholar]
- Burhans W. C., Vassilev L. T., Caddle M. S., Heintz N. H., DePamphilis M. L. Identification of an origin of bidirectional DNA replication in mammalian chromosomes. Cell. 1990 Sep 7;62(5):955–965. doi: 10.1016/0092-8674(90)90270-o. [DOI] [PubMed] [Google Scholar]
- Dornreiter I., Höss A., Arthur A. K., Fanning E. SV40 T antigen binds directly to the large subunit of purified DNA polymerase alpha. EMBO J. 1990 Oct;9(10):3329–3336. doi: 10.1002/j.1460-2075.1990.tb07533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresler S. L., Frattini M. G. Analysis of butylphenyl-guanine, butylphenyl-deoxyguanosine, and butylphenyl-deoxyguanosine triphosphate inhibition of DNA replication and ultraviolet-induced DNA repair synthesis using permeable human fibroblasts. Biochem Pharmacol. 1988 Mar 15;37(6):1033–1037. doi: 10.1016/0006-2952(88)90506-0. [DOI] [PubMed] [Google Scholar]
- Hockensmith J. W., Kubasek W. L., Vorachek W. R., von Hippel P. H. Laser cross-linking of proteins to nucleic acids. I. Examining physical parameters of protein-nucleic acid complexes. J Biol Chem. 1993 Jul 25;268(21):15712–15720. [PubMed] [Google Scholar]
- Insdorf N. F., Bogenhagen D. F. DNA polymerase gamma from Xenopus laevis. I. The identification of a high molecular weight catalytic subunit by a novel DNA polymerase photolabeling procedure. J Biol Chem. 1989 Dec 25;264(36):21491–21497. [PubMed] [Google Scholar]
- Kesti T., Frantti H., Syväoja J. E. Molecular cloning of the cDNA for the catalytic subunit of human DNA polymerase epsilon. J Biol Chem. 1993 May 15;268(14):10238–10245. [PubMed] [Google Scholar]
- Lee M. Y., Jiang Y. Q., Zhang S. J., Toomey N. L. Characterization of human DNA polymerase delta and its immunochemical relationships with DNA polymerase alpha and epsilon. J Biol Chem. 1991 Feb 5;266(4):2423–2429. [PubMed] [Google Scholar]
- Lee S. H., Kwong A. D., Pan Z. Q., Hurwitz J. Studies on the activator 1 protein complex, an accessory factor for proliferating cell nuclear antigen-dependent DNA polymerase delta. J Biol Chem. 1991 Jan 5;266(1):594–602. [PubMed] [Google Scholar]
- Linskens M. H., Huberman J. A. The two faces of higher eukaryotic DNA replication origins. Cell. 1990 Sep 7;62(5):845–847. doi: 10.1016/0092-8674(90)90258-g. [DOI] [PubMed] [Google Scholar]
- McCarthy S. A., Symonds H. S., Van Dyke T. Regulation of apoptosis in transgenic mice by simian virus 40 T antigen-mediated inactivation of p53. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3979–3983. doi: 10.1073/pnas.91.9.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheli G., Baldari C. T., Carri M. T., Di Cello G., Buongiorno-Nardelli M. An electron microscope study of chromosomal DNA replication in different eukaryotic systems. Exp Cell Res. 1982 Jan;137(1):127–140. doi: 10.1016/0014-4827(82)90015-5. [DOI] [PubMed] [Google Scholar]
- Morrison A., Araki H., Clark A. B., Hamatake R. K., Sugino A. A third essential DNA polymerase in S. cerevisiae. Cell. 1990 Sep 21;62(6):1143–1151. doi: 10.1016/0092-8674(90)90391-q. [DOI] [PubMed] [Google Scholar]
- Murakami Y., Wobbe C. R., Weissbach L., Dean F. B., Hurwitz J. Role of DNA polymerase alpha and DNA primase in simian virus 40 DNA replication in vitro. Proc Natl Acad Sci U S A. 1986 May;83(9):2869–2873. doi: 10.1073/pnas.83.9.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas T. A., Zhou Z., Elledge S. J. DNA polymerase epsilon links the DNA replication machinery to the S phase checkpoint. Cell. 1995 Jan 13;80(1):29–39. doi: 10.1016/0092-8674(95)90448-4. [DOI] [PubMed] [Google Scholar]
- Nethanel T., Kaufmann G. Two DNA polymerases may be required for synthesis of the lagging DNA strand of simian virus 40. J Virol. 1990 Dec;64(12):5912–5918. doi: 10.1128/jvi.64.12.5912-5918.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nethanel T., Reisfeld S., Dinter-Gottlieb G., Kaufmann G. An Okazaki piece of simian virus 40 may be synthesized by ligation of shorter precursor chains. J Virol. 1988 Aug;62(8):2867–2873. doi: 10.1128/jvi.62.8.2867-2873.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nethanel T., Zlotkin T., Kaufmann G. Assembly of simian virus 40 Okazaki pieces from DNA primers is reversibly arrested by ATP depletion. J Virol. 1992 Nov;66(11):6634–6640. doi: 10.1128/jvi.66.11.6634-6640.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols A. F., Sancar A. Purification of PCNA as a nucleotide excision repair protein. Nucleic Acids Res. 1992 Jul 11;20(13):2441–2446. doi: 10.1093/nar/20.10.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida C., Reinhard P., Linn S. DNA repair synthesis in human fibroblasts requires DNA polymerase delta. J Biol Chem. 1988 Jan 5;263(1):501–510. [PubMed] [Google Scholar]
- Podust V. N., Georgaki A., Strack B., Hübscher U. Calf thymus RF-C as an essential component for DNA polymerase delta and epsilon holoenzymes function. Nucleic Acids Res. 1992 Aug 25;20(16):4159–4165. doi: 10.1093/nar/20.16.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podust V. N., Hübscher U. Lagging strand DNA synthesis by calf thymus DNA polymerases alpha, beta, delta and epsilon in the presence of auxiliary proteins. Nucleic Acids Res. 1993 Feb 25;21(4):841–846. doi: 10.1093/nar/21.4.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prelich G., Stillman B. Coordinated leading and lagging strand synthesis during SV40 DNA replication in vitro requires PCNA. Cell. 1988 Apr 8;53(1):117–126. doi: 10.1016/0092-8674(88)90493-x. [DOI] [PubMed] [Google Scholar]
- Sancar A. DNA repair in humans. Annu Rev Genet. 1995;29:69–105. doi: 10.1146/annurev.ge.29.120195.000441. [DOI] [PubMed] [Google Scholar]
- Schirmbeck R., Deppert W. Structural topography of simian virus 40 DNA replication. J Virol. 1991 May;65(5):2578–2588. doi: 10.1128/jvi.65.5.2578-2588.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman B. Smart machines at the DNA replication fork. Cell. 1994 Sep 9;78(5):725–728. doi: 10.1016/s0092-8674(94)90362-x. [DOI] [PubMed] [Google Scholar]
- Su R. T., DePamphilis M. L. Simian virus 40 DNA replication in isolated replicating viral chromosomes. J Virol. 1978 Oct;28(1):53–65. doi: 10.1128/jvi.28.1.53-65.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syväoja J., Suomensaari S., Nishida C., Goldsmith J. S., Chui G. S., Jain S., Linn S. DNA polymerases alpha, delta, and epsilon: three distinct enzymes from HeLa cells. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6664–6668. doi: 10.1073/pnas.87.17.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapper D. P., DePamphilis M. L. Discontinuous DNA replication: accumulation of Simian virus 40 DNA at specific stages in its replication. J Mol Biol. 1978 Apr 15;120(3):401–422. doi: 10.1016/0022-2836(78)90427-8. [DOI] [PubMed] [Google Scholar]
- Tsurimoto T., Melendy T., Stillman B. Sequential initiation of lagging and leading strand synthesis by two different polymerase complexes at the SV40 DNA replication origin. Nature. 1990 Aug 9;346(6284):534–539. doi: 10.1038/346534a0. [DOI] [PubMed] [Google Scholar]
- Tuusa J., Uitto L., Syväoja J. E. Human DNA polymerase epsilon is expressed during cell proliferation in a manner characteristic of replicative DNA polymerases. Nucleic Acids Res. 1995 Jun 25;23(12):2178–2183. doi: 10.1093/nar/23.12.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uitto L., Halleen J., Hentunen T., Höyhtyä M., Syväoja J. E. Structural relationship between DNA polymerases epsilon and epsilon* and their occurrence in eukaryotic cells. Nucleic Acids Res. 1995 Jan 25;23(2):244–247. doi: 10.1093/nar/23.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn J. P., Dijkwel P. A., Hamlin J. L. Replication initiates in a broad zone in the amplified CHO dihydrofolate reductase domain. Cell. 1990 Jun 15;61(6):1075–1087. doi: 10.1016/0092-8674(90)90071-l. [DOI] [PubMed] [Google Scholar]
- Waga S., Bauer G., Stillman B. Reconstitution of complete SV40 DNA replication with purified replication factors. J Biol Chem. 1994 Apr 8;269(14):10923–10934. [PubMed] [Google Scholar]
- Waga S., Stillman B. Anatomy of a DNA replication fork revealed by reconstitution of SV40 DNA replication in vitro. Nature. 1994 May 19;369(6477):207–212. doi: 10.1038/369207a0. [DOI] [PubMed] [Google Scholar]
- Wang Z., Wu X., Friedberg E. C. DNA repair synthesis during base excision repair in vitro is catalyzed by DNA polymerase epsilon and is influenced by DNA polymerases alpha and delta in Saccharomyces cerevisiae. Mol Cell Biol. 1993 Feb;13(2):1051–1058. doi: 10.1128/mcb.13.2.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg D. H., Kelly T. J. Requirement for two DNA polymerases in the replication of simian virus 40 DNA in vitro. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9742–9746. doi: 10.1073/pnas.86.24.9742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright G. E., Hübscher U., Khan N. N., Focher F., Verri A. Inhibitor analysis of calf thymus DNA polymerases alpha, delta and epsilon. FEBS Lett. 1994 Mar 14;341(1):128–130. doi: 10.1016/0014-5793(94)80254-8. [DOI] [PubMed] [Google Scholar]
- Yanai N., Obinata M. Apoptosis is induced at nonpermissive temperature by a transient increase in p53 in cell lines immortalized with temperature-sensitive SV40 large T-antigen gene. Exp Cell Res. 1994 Apr;211(2):296–300. doi: 10.1006/excr.1994.1090. [DOI] [PubMed] [Google Scholar]
- Zeng X. R., Jiang Y., Zhang S. J., Hao H., Lee M. Y. DNA polymerase delta is involved in the cellular response to UV damage in human cells. J Biol Chem. 1994 May 13;269(19):13748–13751. [PubMed] [Google Scholar]